Abstract
Introduction
Although nicotine is the primary reinforcing constituent in cigarettes, there is evidence that other constituents in cigarette smoke may interact with nicotine to reinforce smoking behavior.
Methods
The present experiments investigated whether a novel combination of these cigarette smoke constituents would increase nicotine self-administration in adult male rats. The constituents included five minor alkaloids (anabasine, nornicotine, cotinine, myosmine, and anatabine), two β-carbolines (harman and norharman), and acetaldehyde. All doses were indexed to be proportional to concentrations in cigarette smoke given a standard dose of nicotine used in rodent self-administration, or ten times higher than this standard. To model MAO inhibition seen in chronic smokers, some groups received separate injections of tranylcypromine prior to each self-administration session.
Results
Tranylcypromine increased low-dose nicotine self-administration independent of other smoke constituents, which had no effect on self-administration behavior. The effect of tranylcypromine was confirmed across a large range of reinforcement schedules. The effect of tranylcypromine on low-dose nicotine self-administration was observed regardless of whether the injection was delivered 1-hr or 23-hrs prior to the self-administration session, consistent with the interpretation that MAO inhibition was responsible for the increase in self-administration, instead of acute off-target effects.
Conclusions
These data suggest that this cocktail of constituents does not significantly alter the primary reinforcing effects of nicotine, but constituents that inhibit MAO may increase the primary reinforcing effects of nicotine, especially at low doses.
Keywords: Non-nicotine tobacco constituents, Nicotine, Monoamine oxidase inhibition, Tobacco policy, Self-administration, Regulatory science
1. INTRODUCTION
Nicotine is the primary reinforcing constituent in cigarettes (Stolerman and Jarvis, 1995; USDHHS, 2010; West, 1992). However, there are over 8,000 other constituents in cigarette smoke (Rodgman and Perfetti, 2013), and it is unclear whether other cigarette constituents might interact with the reinforcing strength of nicotine to maintain smoking behavior. As the Food and Drug Administration (FDA) has the authority to regulate cigarette constituents, including nicotine, to any non-zero level (US Congress, 2009), determining whether non-nicotine constituents interact with the reinforcing effects of nicotine is important for FDA policy aimed at improving public health.
Research on non-nicotine constituents is scarce (Hoffman and Evans, 2013) and choosing an approach for investigating their reinforcing potential is challenging. Researchers have studied self-administration of individual constituents (Bardo et al., 1999; Caine et al., 2014), self-administration of nicotine in combination with one or more constituent (Belluzzi et al., 2005; Clemens et al., 2009; DeNoble and Mele, 1983), or self-administration of a cigarette smoke extract (Brennan et al., 2013a, 2013b; Costello et al., 2014). For studies investigating one or more constituent, choosing doses is problematic. Some researchers have chosen doses to be proportional to the concentrations in cigarette smoke given a dose of nicotine that maintains intravenous nicotine self-administration in rodent models (Belluzzi et al., 2005; Clemens et al., 2009). However, even then, determining the concentrations of the substances in cigarette smoke poses a dilemma, as these concentrations vary considerably across type of cigarette and assay method (Rodgman and Perfetti, 2013).
Although existing research is scarce and complicated, studies suggest that at least four classes of constituents deserve particular attention (Donny et al., 2012). First, although nicotine makes up the majority of the alkaloid content of tobacco, other alkaloids, with chemical structures similar to nicotine, are also present, and some have been shown to reinforce behavior at high doses (Bardo et al., 1999). Most notably these include nornicotine, cotinine, myosmine, anatabine, and anabasine. Second, acetaldehyde has been shown to support self-administration on its own at high doses (Takayama and Uyeno, 1985) and to increase nicotine self-administration under some circumstances when doses are indexed to those in cigarette smoke (Belluzzi et al., 2005; DeNoble and Mele, 1983). Third, two β-carbolines, norharman and harman, may partially contribute to inhibition of monoamine oxidase (MAO; Herraiz et al., 2008) and have other effects on the brain independent of MAO inhibition (Abu Ghazaleh et al., 2015; Arib et al., 2010; Touiki et al., 2005). Fourth, unknown cigarette smoke constituents are presumed to be largely responsible for the 30–40% inhibition of MAO seen in chronic smokers (Berlin et al., 1995; Fowler et al., 1996; Lewis et al., 2007). Research has shown that injections of known MAO inhibitors, such as tranylcypromine (TCP), increase nicotine self-administration at low doses of nicotine (Guillem et al., 2005). However, more recent research has questioned whether the cause of this increase is MAO inhibition or more acute, off-target effects of TCP, such as the release of serotonin (Lotfipour et al., 2011; Villegier et al., 2011).
The present experiments examined, for the first time, whether the constituents discussed above would, in combination, alter intravenous (i.v.) self-administration of nicotine in adult male rats. This formulation has been used in two recent papers from our research lab (Smith et al., 2013, 2014a), so an investigation of its effect on nicotine self-administration was warranted. Groups of rats were also included that were pre-treated with TCP, an irreversible MAO inhibitor. Previous research suggests that any effect of smoke constituents on nicotine self-administration may be dependent on dose and schedule parameters (Clemens et al., 2009; Guillem et al., 2005), so the present experiments included both low and high doses of nicotine, two concentrations of the cocktail constituents, and multiple schedules of reinforcement. We also measured sensitivity to cost, a measure of reinforcing efficacy not previously applied to the study of other cigarette constituents (Hursh and Silberberg, 2008). Sensitivity to cost has translational validity, as smokers experience increasing monetary, social, and health costs for their cigarette use. The results show that this formulation of constituents does not increase nicotine self-administration, but extends previous literature by demonstrating that chronic injections of TCP prior to nicotine self-administration sessions can have a large effect, especially on acquisition of low-dose nicotine self-administration. Previous research has suggested that TCP may increase nicotine self-administration through serotonin release, a short-lasting effect of TCP, instead of through MAO-inhibition, a longer-lasting effect (Lotfipour et al., 2011; Villegier et al., 2011). Because in the present study we replicated the large effect of TCP injections on low-dose nicotine self-administration, an additional aim emerged: to test whether the acute off-target effects or long-lasting effects of TCP are responsible for the increase in nicotine self-administration. The results show that TCP increases low-dose nicotine self-administration, regardless of whether it is delivered shortly before or many hours before the self-administration session, consistent with the interpretation that MAO inhibition is responsible for the increase in low-dose nicotine self-administration. These data suggest that reinforcement by low-dose nicotine is likely to be increased by cigarette smoke constituents that inhibit MAO.
2. METHODS
2.1 Subjects
Male Sprague-Dawley rats (Harlan-Farms, IN) weighing between 175 and 225 g on arrival were used as subjects. Rats were housed individually in wire-mesh, hanging cages in a temperature-controlled room (68 to 70° F). Rats were kept on a reverse light-dark 12:12 hour schedule, and testing occurred during the dark phase. Rats had unlimited access to water in their home cages. Rats received ad libitum chow for the first seven days while habituating to individual home cages. At least eight days after arrival, rats were implanted with jugular catheters and were changed to a feeding schedule where 20 g/day was delivered after each session. Rats were allowed at least five days of recovery following surgery.
2.2 Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline (doses expressed as free base). The cocktail of other cigarette constituents contained acetaldehyde, harman, norharman, anabasine, nornicotine, myosmine, cotinine, (Sigma, St. Louis, MO), and anatabine (Toronto Research Chemicals, Inc), which were all dissolved in 0.9% saline (See supplementary methods for a description of the method for creating the cocktail1). The concentrations of the non-nicotine constituents in the “standard” cocktail were as follows: 16 μg/kg/infusion (acetaldehyde), 0.1 μg/kg/infusion (harman), 0.3 μg/kg/infusion (norharman), 0.9 μg/kg/infusion (anabasine, nornicotine), and 0.09 μg/kg/infusion (myosmine, cotinine, and anatabine), and were chosen either to be proportional to the content found in cigarette smoke given 30 μg/kg/infusion nicotine (Herraiz, 2004) and/or because of their use in prior literature (Belluzzi et al., 2005; Clemens et al., 2009). Table S12 shows the doses of each constituent and their percentage relative to the nicotine doses used in these experiments. Groups in one experiment (Experiment 1) responded for doses of the cocktail ten times those in the standard solution. During sessions, infusions were delivered in less than 1 s at a volume of 0.1 ml/kg/infusion. Tranylcypromine hydrochloride (Sigma, St Louis, MO, TCP) was delivered intraperitoneally (i.p.) 1-hr before each self-administration session, except in Experiment 3 where some rats received the injection 23 hrs before each session, at a dose of 1 mg/kg and in a volume of 1 ml/kg. This is a relatively large dose of TCP which we show here produces near complete inhibition of MAO. All solutions were sterilized by being passed through a 0.22 μm filter.
2.3 Procedures
2.3.1 General self-administration procedures
Thirty-eight standard self-administration operant chambers (ENV-008 CT; Med-Associates) were configured as previously described (Smith et al., 2013), and included two nose poke holes below two stimulus lights on one side of the chamber. Procedures for jugular catheterization and daily flushing solutions were as previously described (Smith et al., 2013). Only data from rats that passed a patency test consisting of rapid loss of muscle tone to either chloral hydrate (up to 60 mg/rat i.v.) or methohexital (5 mg/kg i.v.) at the end of the experiment are included. Rats were given the opportunity to respond via nosepokes for i.v. infusions. The active nosepoke hole was counterbalanced across rats as either the left or right hole. Active pokes resulted in a simultaneous onset of an intravenous infusion, a 15-s cue light presentation, and a time out lasting one minute (not cued) according to the reinforcement schedule in effect (Smith et al., 2013). Active nosepokes during time out and inactive nosepokes were recorded, but had no consequence. Sessions lasted one hour and were conducted seven days per week.
2.3.2 Experiment 1: Effects of a “standard cocktail” and “10X cocktail” of cigarette constituents on low-dose nicotine self-administration with and without a pre-session injection of tranylcypromine
2.3.2.1 Locomotor Response to a Novel Environment
Previous research suggests that a rat’s locomotor activity in a novel environment might predict the increase in self-administration associated with MAO inhibition (Guillem et al., 2005, but see Guillem et al., 2006). Specifically, rats that have high levels of activity in the locomotor chambers tested prior to self-administration showed a greater increase in nicotine self-administration when a pre-session injection of TCP is delivered than rats that have low levels of activity (Guillem et al., 2005). Prior to surgery, rats in this experiment were tested for their locomotor response in a novel environment for two hours. The novel environment consisted of a clear, acrylic open field chamber, which measured distance traveled (standard Med Associates chambers, 43.2 cm × 43.2 cm × 30.5 cm).
2.3.2.2 Self-Administration
Rats had the opportunity to respond via nosepokes (fixed-ratio [FR] 2 schedule of reinforcement) for i.v. infusions of a low nicotine dose (10 μg/kg/infusion). This dose of nicotine is in a range that is expected to produce minimal acquisition of self-administration on its own (Smith et al., 2014a), allowing for the cocktail or TCP to increase acquisition. Nicotine was delivered alone (“nicotine only”), or in combination with a “standard” cocktail (“nicotine + standard cocktail,” described above), or with a cocktail of other constituents ten times more concentrated than the standard (“nicotine + 10X cocktail”). Rats received a pre-session (1-hr prior) i.p. injection of either saline or TCP (1.0 mg/kg) (TCP groups indicated by “+ TCP”). Rats were given 16 sessions to acquire self-administration.
Following Experiment 1, 6–8 rats from each non-TCP group were anesthetized with isoflurane, decapitated and the left dorsal striatum was dissected and flash frozen before being assayed for MAO activity. The primary purpose was to assess whether the cocktail solution, without TCP, inhibited MAO. MAO activity was measured in vitro in homogenates of the left dorsal striatum using an absorbance-based assay involving the oxidation of p-tyramine coupled to the conversion of Ampliflu Red to resorufin in the presence of horseradish peroxidase (See supplementary information for detailed assay methods). Because the tissue is homogenized, this assay likely only assesses irreversible inhibition. In a separate assay, cocktail was added directly to brain homogenates to determine whether the cocktail has the potential to reversibly or irreversibly inhibit MAO activity. Cocktail was added to assay wells, in concentrations ranging from 1:20 to 1:20,000. A 1:20 dilution is estimated to be the maximum concentration to which a rat in the 10X cocktail group may have been exposed. A solution without harman and norharman was also included to assess the contribution of the two β-carbolines to MAO inhibition.
2.3.3 Experiment 2: Effects of a cocktail of cigarette smoke constituents with and without tranylcypromine combined with a low and a high nicotine dose on progressive-ratio self-administration and elasticity of demand
2.3.3.1 Acquisition
A new group of rats had the opportunity to respond via nosepokes (10 sessions, FR2 schedule of reinforcement) for i.v. infusions of one of two nicotine doses (15 μg/kg/infusion or 60 μg/kg/infusion). Compared to Experiment 1, a slightly higher nicotine dose was chosen for the low nicotine dose because these rats were tested for sensitivity to increases in fixed-ratio (FR) schedule, making it advantageous for a higher proportion of rats in all groups to acquire self-administration. Rats in each of the two nicotine dose conditions received nicotine alone and a pre-session i.p. injection of saline (“nicotine only”), nicotine along with a standard cocktail of other constituents and a pre-session injection of saline (“nicotine + cocktail”), or nicotine along with a standard cocktail of other constituents and a pre-session i.p. injection of TCP (1.0 mg/kg) (“nicotine + cocktail + TCP”).
2.3.3.2 Progressive-ratio performance
All rats were tested on a progressive-ratio (PR) schedule for four hours in which the number of responses required increased with each infusion (1, 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 179, 219, 268, 328, 402, 492) (Depoortere et al., 1993). Each rat experienced PR sessions on three consecutive days. The extended length of the session prevented the testing of all rats on this procedure on the same days, but all rats experienced the three consecutive PR sessions within a nine day period. Rats not experiencing the PR during any given session remained on the FR2 schedule.
2.3.3.3 FR Escalation/Elasticity of demand
All rats then experienced four sessions each at escalating fixed ratios (2, 3, 5, 7, 10, 15, 20, 25, 35, 50, 70, 100, 150). These increases in FR might be thought of as increases in cost, and a demand curve analysis is particularly useful for data like these (Hursh and Silberberg, 2008; Smith et al., 2014b). Demand curves characterize change in consumption of a reinforcer as a function of cost according to the following equation (Hursh and Silberberg, 2008):
| [1] |
in which Q is consumption at a given cost (C), k is a scaling parameter that describes the range of dependent variable, and e is the base of the natural logarithm. The two free parameters, Q0, and α, estimate consumption if the reinforcer were free (often thought of as the intensity of demand), and sensitivity to increases in cost (often thought of as elasticity of demand), respectively. α has been described as an inverse measure of the “essential value” of any reinforcer (Hursh and Silberberg, 2008). Two other parameters, Omax and Pmax, can be estimated from a best-fitting equation and represent the maximum level of responding across the curve, and the price at which that responding is emitted, respectively. An example demand curve, including the best fitting function and estimated parameter values is shown in Figure S13.
2.3.4 Experiment 3: Timing of tranylcypromine injection on enhancement of self-administration
Previous research has suggested that low-dose nicotine self-administration may be enhanced by TCP through an acute mechanism other than long-lasting inhibition of MAO (Lotfipour et al., 2011; Villegier et al., 2011). To test whether the TCP effect seen in the first two experiments is due to acute or long-lasting actions of TCP, the timing of the TCP injection relative to the session was manipulated in a new group of rats. In the two critical groups, rats responded for nicotine (10 μg/kg/infusion, 15 sessions, FR2 schedule of reinforcement) and received TCP either 1-hr or 23-hrs before the session. Rats received saline at the alternate time. In three control groups rats either: self-administered nicotine and received saline at both time points, self-administered saline and received saline at both time points, or self-administered saline and received saline 23-hrs before the session and TCP 1 hr before the session. Prior literature has shown that MAO remains almost completely inhibited 20-hrs following injection, but the off-target effects, such as serotonin release, are absent (Lotfipour et al., 2011; Villegier et al., 2011, 2007). On the last day of self-administration, the dorsal striatum was collected from 6 rats each from the 1-hr and 23-hr TCP injection groups to assess MAO activity (as described in the supplementary methods). MAO activity was compared in the same assay to the MAO activity of 10 rats pre-treated with saline that had previously finished a separate experiment (rats had a variety of drug histories including no drug history, systemic yohimbine injections, or intravenous cocaine infusions).
2.4 Data Analysis
Analysis of Variance omnibus tests were conducted. In the case of significant tests of Mauchly’s Sphericity, tests were Greenhouse Geisser controlled. See Figure Captions for more detailed information than presented in the results. Only infusion data are presented in the primary text, but analyses were conducted on both active and inactive responding. Full statistical information, including active and inactive analyses, is shown in Table S24. To describe the proportion of rats acquiring self-administration, criteria for self-administration were set at an average of five infusions over the last three sessions and twice as many active as inactive responses over the same time period. We have previously shown that rats given the opportunity to respond for saline along with the same stimulus light rarely meet these criteria (Smith et al., 2014a). Pairwise contrasts were conducted using Bonferonni corrections to control for Type 1 Error and are displayed in the figures. When three or six pairwise comparisons were required, alpha was set at 0.017 or 0.008, respectively.
For Experiment 2, follow-up tests were conducted in the case of significant omnibus tests to further delineate group differences. These analyses consisted of three 2 × 2 ANOVAs to test three effects. 1) To test the effects of adding cocktail to the nicotine solution, a 2 × 2 ANOVA compared nicotine dose as one factor and nicotine only groups vs. nicotine + cocktail groups as the other factor. 2) To test the effects of adding TCP to the cocktail solution, a 2 × 2 ANOVA compared nicotine dose as one factor and nicotine + cocktail groups vs. nicotine + cocktail + TCP groups as the other factor. 3) To test the effects of adding cocktail and TCP to the nicotine solution, a 2 × 2 ANOVA compared nicotine dose as one factor and nicotine only groups vs. nicotine + cocktail + TCP groups as the other factor. Significant main effect of nicotine dose from these 2 × 2 ANOVAs are not reported in the text, but are available in Table S25.
2.4.1 Behavioral economics analysis
For each rat, the average number of infusions earned over the last two sessions at each FR was fit to Equation 1 through the last price at which behavior was maintained at or above 10% of the FR2 baseline. Rats that had two or less data points meeting this criterion (n=1), or that did not earn an average of at least five infusions at baseline were excluded (n=7). k was set to 2, the lowest integer that was greater than all logarithmic infusion values. Fits of Equation 1 were good, with the median R2 = 0.87 (mean = 0.74), only 6% of R2 values less than 0.4, and 78% of R2 values above 0.7. Microsoft Excel Solver was used to obtain Pmax and Omax after the best fitting equation was obtained.
3. RESULTS
3.1 Experiment 1: Effects of a “standard cocktail” and “10X cocktail” of cigarette constituents on low-dose nicotine self-administration with and without a pre-session injection of tranylcypromine
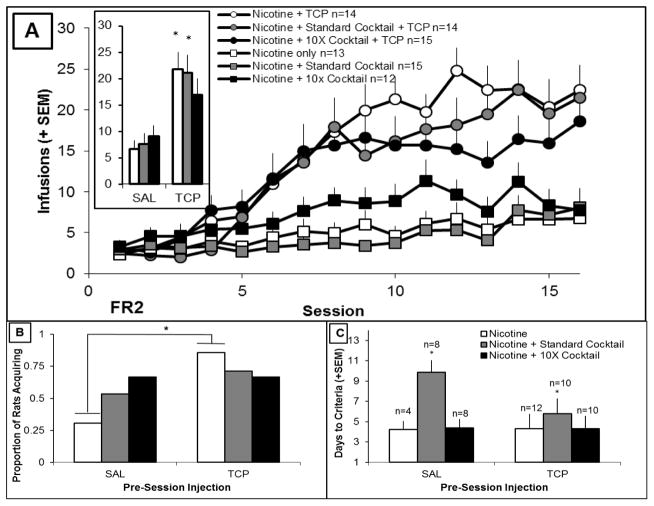
Figure 1A shows number of infusions earned over the 16 sessions for each group. There was a significant effect of day, a significant effect of TCP, and a significant day X TCP interaction. Cocktail did not have a significant effect on number of infusions earned. TCP significantly increased the number of infusions earned in the nicotine only and nicotine + standard cocktail groups compared to the same groups receiving pre-session saline injections. Figure 1B shows the proportion of rats in each group meeting the criteria for acquisition of self-administration. There was a significant effect of TCP. The interaction between TCP and cocktail group did not reach significance, (p= 0.158). TCP significantly increased the proportion of rats that acquired self-administration for rats receiving nicotine only. Cocktail did not significantly affect the proportion of rats treated with either TCP or saline that met acquisition criteria. Excluding rats that did not meet the self-administration criteria does not shift the pattern seen in Figure 1A. The number of days to reach criteria for each group (computed using a 3-day moving average) is shown in Figure 1C. There was a significant effect of cocktail (log transformed for positive skew, but no effect of TCP or interaction, and follow up t-tests confirmed that nicotine + standard cocktail groups took longer to meet the criteria than rats in the nicotine or nicotine + 10X cocktail groups.
Figure 1.
A) Number of infusions earned over 16 sessions of 10 μg/kg/infusion nicotine self-administration. Average infusions over the last three sessions are shown in inset. * represents significant difference (p<0.05) compared to saline pre-session injection counterpart. B) Proportion of rats acquiring self-administration in each group. * represents a significant difference between saline and TCP rats receiving nicotine only that met criteria for acquiring nicotine self-administration. C) Number of days to meet the criteria for self-administration. * represents significant difference (p<0.05) in the number of days to meet criteria compared to rats in the nicotine or nicotine + 10X cocktail groups. Error bars represent standard error.
Previous research has suggested that the TCP-induced increase in nicotine self-administration may be selective to rats that have high activity levels prior to self-administration. To investigate this variable, data were reanalyzed after dividing rats into high and low activity groups (median split, 6–7 rats/activity group) using the total distance traveled during a two hour period in a novel environment prior to the start of self-administration. Figure S26 shows distance traveled and the average number of earned infusions over the last three self-administration sessions as a function of group. There was a significant main effect of TCP and a significant main effect of activity group, but no main effect of cocktail and no interactions, suggesting that TCP increased nicotine self-administration similarly in low activity and high activity rats.
Neither the standard nor the 10X cocktail significantly reduced MAO activity (Figure S37). When cocktail was added directly to the homogenate in vitro, only the highest concentration of cocktail tested, a 20-fold dilution of the standard cocktail solution, inhibited MAO, and the extent of MAO inhibition was ~33% (Figure S48). This concentration is likely much higher than the brain concentration that would be expected in rats responding for the “standard” cocktail (see Discussion). In separate samples (n=3), the highest concentration of cocktail tested in vivo inhibited MAO-A by 28% and MAO-B by 42%. Also, the highest concentration of cocktail without harman and norharman did not significantly inhibit MAO (data from this smaller sample not shown).
3.2 Experiment 2: Effects of a cocktail of cigarette smoke constituents with and without TCP combined with low and high nicotine doses on progressive-ratio self-administration and elasticity of demand
3.2.1 Acquisition
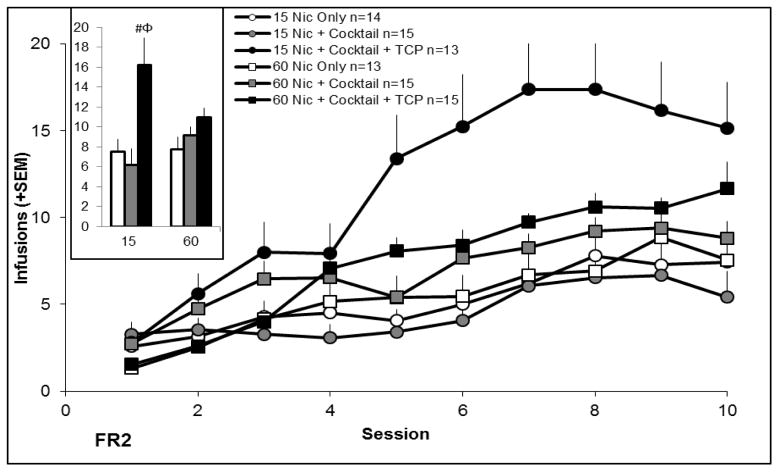
Figure 2 shows infusions earned across the 10 acquisition sessions, and the average number of infusions earned over the final three sessions of acquisition (inset). There were significant main effects of day and group, and several significant interactions: group X nicotine dose, day X group, and day X group X nicotine dose. The 2×2 ANOVAs did not reveal a significant effect of adding cocktail to the nicotine solution (comparison between nicotine only and nicotine + cocktail), but did confirm a significant effect of adding TCP to the cocktail solution that interacted with nicotine dose (comparison between nicotine + cocktail and nicotine + cocktail + TCP) and a significant effect of adding cocktail along with TCP to the nicotine solution that did not depend upon nicotine dose (comparison between nicotine only and nicotine + cocktail + TCP). Pairwise comparisons revealed no significant effects of dose. Adding TCP to the cocktail solution significantly increased infusions earned at the low nicotine dose, as did adding cocktail along with TCP.
Figure 2.
Number of infusions earned over 10 acquisition sessions of nicotine self-administration. A 3 × 2 × 10 ANOVA testing the effects of group, nicotine dose, and day revealed significant main effects of group (p < 0.05) and day (p < 0.05), and several significant interactions [group X day (p < 0.05), group X nicotine dose (p < 0.05), and day X group X nicotine dose interaction (p < 0.05)]. Follow-up 2 × 2 ANOVAs to further delineate the effect of group as described in section 2.4 were conducted on the last three sessions. These analyses failed to reveal a significant effect of adding cocktail to the nicotine solution; however, adding TCP to the cocktail solution significantly increased infusions earned (p < 0.05) in a manner that depended on nicotine dose (p < 0.05). Nicotine + cocktail + TCP also differed from nicotine alone (p < 0.05), although this was not significantly altered by nicotine dose (p > 0.05). Pairwise comparisons revealed no significant effects of dose. Significant pairwise comparison to nicotine + cocktail is indicated by Φ, p < 0.05, and significant pairwise comparison to nicotine only indicated by #, p < 0.05. Error bars represent standard error.
3.2.2 Progressive-ratio performance
Figure 3 shows average number of infusions earned over the last two PR sessions. There was a significant main effect of nicotine dose, and a significant main effect of group, but no interaction. There was no effect of adding cocktail to the nicotine solution. However, there was a significant effect of adding TCP to the cocktail solution and a significant effect of adding cocktail along with TCP to the nicotine solution; neither effect interacted with nicotine dose. Pairwise comparisons revealed that the higher dose of nicotine produced increased infusions earned compared to the lower dose of nicotine in both the nicotine only and the nicotine + cocktail groups. Adding TCP to the cocktail solution significantly increased earned infusions for both the low and the high dose of nicotine, and adding cocktail along with TCP significantly increased infusions earned for the low dose of nicotine only.
Figure 3.
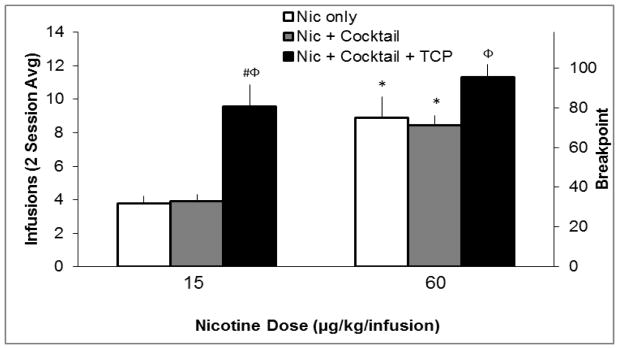
Average number of earned infusions over the final two sessions of a progressive-ratio (PR) schedule of reinforcement. The 3 × 2 ANOVA revealed a significant main effect of nicotine dose (p < 0.05), and a significant main effect of group (p < 0.05), but no significant interaction (p > 0.05). Follow-up 2 × 2 ANOVAs were conducted to further delineate the effect of group as described in section 2.4. These analyses revealed that there was no effect of adding cocktail to the nicotine solution; however, adding TCP to the cocktail solution significantly increased infusions (p<0.05; interaction with nicotine dose failed to reach significance). Likewise, adding cocktail along with TCP increased infusions relative to nicotine alone (p < 0.05; interaction with nicotine dose failed to reach significance). Significant pairwise comparison to low nicotine dose is indicated by *, p < 0.05. Significant pairwise comparison to nicotine + cocktail is indicated by Φ, p < 0.05, and significant pairwise comparison to nicotine only indicated by #, p < 0.05. Error bars represent standard error.
3.2.3 FR Escalation/Elasticity of demand
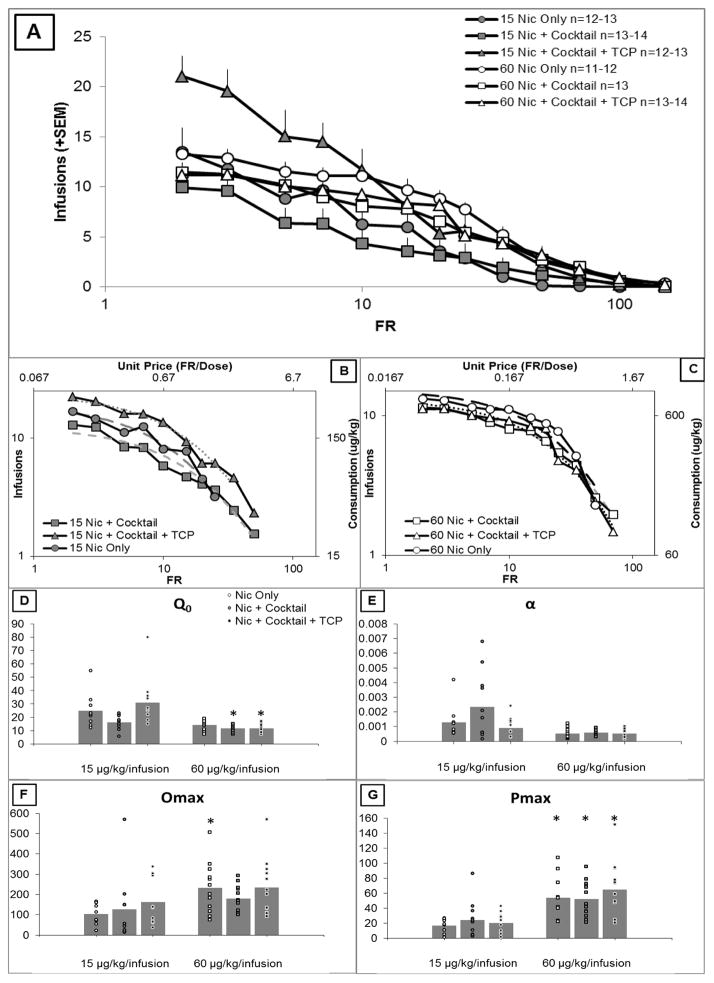
Figure 4A shows the average number of earned infusions for each group as a function of FR (average over last two sessions). A descriptive presentation of the data is followed by a statistical analysis of the behavioral economics parameters. At low FRs, rats responding for 15 μg/kg/infusion nicotine + cocktail and receiving TCP injections earned a greater number of infusions than all other groups, but this effect was FR dependent, and disappeared at higher FRs. Although the remaining groups responded similarly at low FRs, all groups responding for 60 μg/kg/infusion nicotine earned more infusions across the middle range of FRs. Any differences between rats responding for nicotine only compared to nicotine + cocktail were small and suggest that cocktail may decrease responding for nicotine across a range of FRs.
Figure 4.
A) Average number of earned infusions on the last two sessions of each FR for each group. B and C) Average demand curve for each group responding along with the best fit of Equation 1. C–F) Demand parameters for all six groups. Bars represent group averages and data points represent individual rats. A 3×2 ANOVA (nicotine dose X group) was conducted for each parameter. Follow-up 2 × 2 ANOVAs were conducted to further delineate the effect of group as described in section 2.4. Significant pairwise comparison to low nicotine dose is indicated by *, p < 0.05. C) Q0 is a free parameter representative of estimated consumption if the drug were free. There was a significant main effect of nicotine dose (p < 0.05), and a significant main effect of group (p < 0.05) that interacted with nicotine dose (p < 0.05). There was no effect of adding cocktail to the nicotine solution. However, adding TCP to the cocktail solution significantly increased demand intensity (p < 0.05), and this effect interacted with nicotine dose (p < 0.05). Adding cocktail along with TCP did not affect demand intensity. D) α is a free parameter estimating sensitivity to cost and might be thought of as inversely related to essential value of the reinforcer. There was a significance main effect of nicotine dose (p < 0.05), and there was a significant effect of group (p < 0.05), but no nicotine dose X group interaction. Adding cocktail to the nicotine solution had no effect on sensitivity to cost. Adding TCP to the cocktail solution decreased sensitivity to cost, and this effect significantly interacted with nicotine dose (p < 0.05). Adding cocktail along with TCP to the nicotine solution had no effect on sensitivity to cost. E) Omax is an estimate of maximum reinforcer consumption. There was a significant main effect of nicotine dose (p < 0.05), but no effect of group or nicotine dose X group interaction. F) Pmax is an estimate of the price that produces maximum consumption. There was a significant main effect of nicotine dose (p < 0.05), but no effect of group or nicotine dose X group interaction. Follow-up 2 × 2 ANOVAs did not reveal any significant effects between groups or interactions with dose.
Panels B and C of Figure 4 show average demand curves for each group along with the best fit from Equation 1. Panels D-G show the four parameter values for each of the six groups. Q0 estimates consumption if the reinforcer was free (intensity of demand). Rats responding for 60 μg/kg/infusion had lower demand intensity than rats responding for 15 ug/kg/infusion, and there was a significant effect of group that interacted with nicotine dose. Follow-up tests showed that adding cocktail to the nicotine solution had no effect. Adding TCP to the cocktail solution significantly increased demand intensity, and this effect interacted with nicotine dose. Adding cocktail along with TCP did not significantly affect demand intensity. Demand intensity was significantly lower for the 60 μg/kg/infusion nicotine + cocktail and 60 μg/kg/infusion nicotine + cocktail + TCP groups compared to their low nicotine dose counterparts. None of the pairwise group comparisons within nicotine dose met the Bonferonni criteria for significance.
α is an estimate of elasticity of demand (i.e., sensitivity to cost), and has been described as inversely related to the “essential value” of the reinforcer. Rats responding for 60 μg/kg/infusion were less sensitive to cost, and there was a significant effect of group, but no nicotine dose X group interaction. Adding cocktail to the nicotine solution had no effect on sensitivity to cost. Adding TCP to the cocktail solution decreased sensitivity to cost, and there was a significant interaction with nicotine dose. Adding cocktail along with TCP to the nicotine solution had no effect on sensitivity to cost. None of the pairwise dose or group comparisons met the Bonferonni criteria for significance.
Omax is an estimate of maximum reinforcer consumption. There was a significant main effect of nicotine dose, but no effect of group or nicotine dose X group interaction. Pairwise comparisons of dose within each group revealed that Omax was significantly higher in the 60 μg/kg/infusion nicotine only group than in the 15 μg/kg/infusion nicotine only group.
Pmax is an estimate of the price that produces maximum consumption. There was a significant main effect of nicotine dose, but no effect of group or nicotine dose X group interaction. Follow-up tests did not reveal any significant effects between groups or interactions with dose. Rats responding for 60 ug/kg/infusion had higher Pmax scored in all three groups than their low nicotine dose counterparts.
3.3 Experiment 3: Timing of TCP injection on enhancement of self-administration
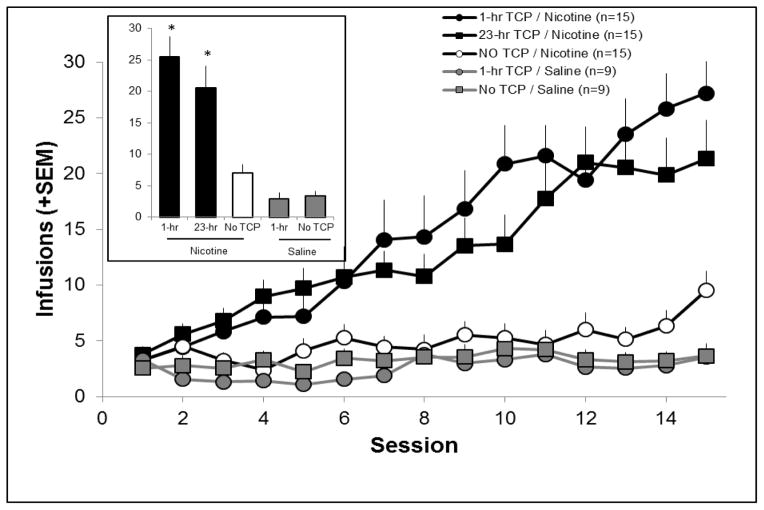
Experiment 3 investigated whether the increase in low-dose nicotine self-administration caused by TCP was due to off target effects, as suggested by Lotfipour et al. (2011) and Villegier et al. (2011), or the more long lasting effect of MAO inhibition. In the two critical groups, rats received injections of TCP either 1-hr before the self-administration session, as was done in Experiments 1 and 2, or 23-hrs before the session, such that MAO is still inhibited at the time of the self-administration session, but the off-target effects would be expected to be absent. Figure 5 shows number of infusions earned over the 15 sessions (average of the last 3 sessions shown in the inset). Follow-up independent samples t-tests conducted on the average of the last three sessions confirmed that the number of infusions earned for both the 1-hr and 23-hr TCP groups were significantly greater than the number of infusions earned for all other groups, but the 1-hr and 23-hr groups did not differ from each other, and no other comparisons were significant.
Figure 5.
A) Number of earned infusions across 15 acquisition sessions for rats receiving pre-session i.p. injections of either TCP (1 mg/kg) or saline 1-hr and 23-hrs before the self-administration session. Rats responded for either 10 μg/kg/infusion nicotine or saline. There was a significant main effect of day (p < 0.05), a main effect of group (p < 0.05), and day X group interaction (p < 0.05). Average infusions over the last three sessions of self-administration (shown in inset) revealed that rats responding for nicotine and receiving TCP 1-hr or 23-hrs before the session earned significantly more infusions than all other groups (p < 0.05, represented by *), except each other.
MAO activity was significantly suppressed in both the 1-hr and 23-hr TCP pretreatment groups (Figure S59), though MAO activity was suppressed by a lesser extent in the 23-hr group. This was true for both MAO-A and MAO-B, as well as total activity (Figure S510). MAO activity was similar in control rats regardless of drug history (i.e.g, yohimbine, cocaine, no drug history).
4. DISCUSSION
4.1 Summary
The present results show that a novel cocktail of cigarette smoke constituents, containing five minor alkaloids (nornicotine, cotinine, myosmine, anatabine, and anabasine), two β-carbolines (harman and norharman), and acetaldehyde, did not significantly enhance nicotine self-administration across a range of nicotine doses, two different doses of these constituents, and a variety of schedules of reinforcement. TCP, an irreversible inhibitor of both MAO-A and MAO-B, had a large impact on low-dose nicotine self-administration, but the effect on higher doses of nicotine was smaller, if present at all.
4.2 Lack of an effect for a cocktail containing the minor alkaloids, β-carbolines, and acetaldehyde
These experiments are the first to test this combination of cigarette smoke constituents, and the data show that at these doses and using these procedures, the constituents do not interact with the reinforcing strength of nicotine at relatively low (10 and 15 μg/kg/infusion) or relatively high (60 μg/kg/infusion) nicotine doses. The only exception is that cocktail significantly increased the number of days required to acquire self-administration in Experiment 1. These data contrast a previous report from Clemens et al. (2009) suggesting that the five minor alkaloids used here, in these same doses, significantly increased nicotine self-administration. However, the effect presented by Clemens et al., (2009) was relatively small, only present when the FR was escalated to an FR5, and also occurred with a similarly large increase in responding on the inactive nosepoke (~75% of responding on the active nosepoke). In the present experiments, the effect of cocktail was not significant for active or inactive responding. Thus, the interaction with nicotine reinforcement by minor alkaloids may not be robust or specific to the reinforced behavior. Furthermore, the present cocktail contained three additional important constituents of cigarette smoke not included by Clemens et al., (2009), so there may be competing effects of some of these constituents, which combined to show no effect in the present studies. While the inclusion of these additional constituents may be masking an effect of the minor alkaloids, the minor alkaloids are present along with these constituents in tobacco smoke, so their study as a mixture is important.
The present results are the first to show that these cigarette smoke constituents do not, in combination, increase self-administration when combined with nicotine. Nonetheless, we cannot rule out that these constituents may increase nicotine self-administration under different conditions (e.g., rats escalating intake due to intermittent periods of withdrawal; Cohen et al., 2012, 2015). We have not tested whether this cocktail of constituents would support self-administration without nicotine, though this seems unlikely given the low level of responding in rats receiving cocktail along with a low dose of nicotine. Previous research on nornicotine shows that it can support self-administration, but not in the dose range used in the present paper, which better reflects the doses relevant to cigarette smoke. The dose range for self-administration of nornicotine is even higher than the dose range for self-administration of nicotine (Bardo et al., 1999; Caine et al., 2014), whereas the doses used in the present experiments were much lower than the nicotine doses. Previous research has also shown that the dose of acetaldehyde used in our cocktail, when combined with nicotine, did not increase self-administration in adult rats (Belluzzi et al., 2005). One recent report on self-administration of norharman on its own and with nicotine suggested that norharman may have some reinforcing properties at a dose similar to the one used in our 10X cocktail (Arnold et al., 2014). However, in that report responding for norharman on its own was statistically different from inactive responding, but not statistically different from saline, and responding for norharman along with nicotine was only different from responding for nicotine on one reinforcement schedule. The present experiments suggest that, even when these constituents are combined and presented along with nicotine, they do not increase self-administration.
As noted in the introduction, selection of doses for self-administration of cigarette smoke constituents is complicated. In the present experiments, doses of the minor alkaloids were based on Clemens et al., (2009), but closer examination of the papers they cited, specifically Liu et al. (2008) and Wu et al. (2002), suggests that the concentrations of anabasine and anatabine might be more appropriate if they were reversed. Furthermore, creating a solution of constituents requires researchers to rely on the accuracy of the chemicals obtained from commercial suppliers. A standard cocktail solution created in the way described in the methods here was assayed by HPLC, LC/MS, or GC/MS (see supplementary methods) and, although there was some variability, constituent concentrations were within an order of magnitude of expectations (Table S311). In the present set of studies, one group of rats received a cocktail with concentrations ten times higher than the ones used by Clemens et al., (2009). The group receiving these higher doses did not have markedly different self-administration behavior from the group receiving nicotine alone, suggesting that a reversal between anabasine and anatabine concentrations or small variances in drug concentrations would be unlikely to have a substantially different result. Furthermore, we recently completed a study in which rats responded for either nicotine alone (60 ug/kg/infusion) or nicotine along with a revised cocktail in which all of the constituent doses were the same as in the standard cocktail, except the doses anatabine or anabasine were reversed. Across 20 sessions, there were no differences between the groups (Figure S612).
Previous data suggest that two constituents in the cocktail, harman and norharman, can inhibit MAO (Herraiz and Chaparro, 2005). MAO activity was assessed in a subset of rats not receiving TCP from Experiment 1, and there was no significant MAO inhibition. However, at least some of the inhibition caused by harman and norharman is likely reversible, and is not maintained following homogenization of the tissue. MAO activity was also assessed across various concentrations of cocktail added to tissue in vitro. These data show that the highest concentration of cocktail tested significantly inhibited MAO compared to saline. This concentration was chosen to conservatively estimate the maximum exposure a rat might experience in a self-administration session. While it is unlikely that a rat in the “standard” cocktail would reach a brain concentration of cocktail high enough to inhibit MAO, rats in the “10X” cocktail group could potentially have brain concentrations of cocktail high enough to partially inhibit MAO. With the highest concentration of cocktail tested on MAO activity in vitro, the harman and norharman concentrations in the assay were 0.275 uM and 0.9 uM, respectively. At these concentrations, it would be expected that some inhibition of MAO-A and MAO-B would be observed (Herraiz and Chaparro, 2005). Together, these data suggest that while MAO activity in the 10X cocktail group may have been partially inhibited, this inhibition was not large enough to produce a substantial increase in nicotine self-administration.
More research investigating the reinforcing efficacy of cigarette smoke constituents is needed. The eight constituents tested in the current experiments were chosen from over 8,000 constituents present in cigarette smoke because they are thought to have significant psychopharmacological actions (Rodgman and Perfetti, 2013). However, the limited ability of individually selected constituents to model tobacco smoke is why some researchers have turned to the use of smoke extracts (Brennan et al., 2013a, 2013b; Costello et al., 2014; Harris et al., 2012, 2015). Both approaches have advantages and disadvantages. The approach taken here allows for the precise control of concentrations and the identification of constituents that are included. In contrast, the smoke extract approach may allow thousands more constituents to be included, but it is not clear exactly which of the 8,000 constituents are dissolved into solution and at what concentrations. The best strategy may be for researchers to continue to take a variety of approaches, covering as much ground as possible until a larger body of knowledge about the constituents can be created.
4.3 Enhancement of nicotine self-administration in rats receiving pre-session TCP injections
These experiments show that pre-session injections of TCP increase nicotine self-administration, especially at low nicotine doses. TCP injections sometimes also slightly increased inactive responding, but this effect was much smaller than the increase observed in active responding (Supplementary Results13). The increase in active responding for low doses of nicotine is consistent with reports by Guillem et al. (2005) and Villegier et al. (2007). However, Lotfipour et al. (2011) and Villegier et al. (2011) more recently suggested the enhancement of nicotine self-administration may be due to a mechanism other than MAO inhibition. These reports showed that the enhancement is not present if injections are delivered 20-hrs before the session, in contrast to the data presented here. However, Lotfipour et al. (2011) reported that rats receiving TCP 20-hrs before the session did acquire self-administration (compared to rats self-administering saline), while rats not receiving TCP at all did not, suggesting that these injections 20 hrs prior to the session may have enhanced self-administration, although not to the same degree that the 1-hr injections did. Furthermore, Lotfipour et al. (2011) and Villegier et al. (2011) used a larger dose of TCP (3 mg/kg) than the dose used here (1 mg/kg) and observed an effect of TCP on the first or second day of use. The effect reported here consistently took 4–7 days to develop. Taken together, the effects observed in previous reports are likely different from the one observed here, and those may indeed be driven by off-target, acute effects of large TCP doses, while the one seen here is primarily driven by chronic MAO inhibition produced using a lower TCP dose.
The mechanism by which TCP increases nicotine self-administration is unclear. A single dose of TCP would be expected to rapidly inhibit MAO, but the increase in nicotine self-administration consistently took 4–7 days to develop. The delay may be due to time required for the rat to learn the behavioral response (rats were not food trained), or it may be that the increase in nicotine self-administration is due to a downstream effect of MAO inhibition. An additional possibility is that the increase in nicotine self-administration is due to a decrease in the threshold for nicotine’s reinforcement enhancing effects. Nicotine can noncontingently increase responding for other rewards (Barrett and Bevins, 2012; Caggiula et al., 2009; Donny et al., 2003; Palmatier et al., 2006), an effect known as reinforcement enhancement. Over the first 4–7 days, the cue light likely becomes a mild conditioned reinforcer, and TCP may have decreased the nicotine dose necessary for enhancing the value of that reinforcer, causing rats receiving TCP to respond more for the nicotine/cue combination.
The effect of TCP on nicotine self-administration shown here warrants future research. The administered dose of TCP results in near complete MAO inhibition. However, MAO is inhibited by ~ 30–40% in chronic smokers, and future research should aim to determine whether inhibition in this range produces an enhancement of nicotine self-administration. TCP injections delivered 23-hrs prior to the self-administration session inhibited MAO by ~66%, and rats in this group responded significantly more than rats receiving injections of saline, suggesting that partial MAO inhibition does enhance nicotine self-administration. Additionally, TCP is a nonselective inhibitor, and so determination of whether inhibition of MAO-A or MAO-B selectively produces the enhancement of nicotine self-administration may help indicate which smoke constituents might contribute to maintaining smoking behavior (Guillem et al., 2006). Importantly, while these data indicate that MAO inhibition may alter the reinforcing efficacy of low-dose nicotine self-administration, the constituents that contribute to MAO inhibition seen in smokers are unknown.
4.4 Behavioral Economics Approach
The behavioral economics procedure employed here has several advantages over traditional measures of testing self-administration across a small range of low FRs. First, application of Equation 1 to demand curves for each animal allows for the estimation of parameters that summarize sensitivity to these increases in cost without requiring interpretation of differences in self-administration at individual FRs. Second, a behavioral economics analysis revealed effects that would not have been obvious in a more traditional procedure. Rats receiving a low nicotine dose + cocktail were more sensitive to cost and had lower estimated consumption of free nicotine compared to rats receiving the same solution and daily TCP injections. The analysis also revealed that the increase in low-dose nicotine self-administration produced by TCP is abolished if FR is escalated. These data are informative regarding the impact of MAO inhibition on cigarette smoking. For example, MAO inhibition may be most important when the dose of nicotine is reduced and cigarettes are inexpensive. Finally, behavioral economics posits that consumption of a reinforcer is a function of the unit price of that reinforcer. Increase in cost (or work requirement) should be functionally equivalent to decreases in reinforcer magnitude (or nicotine dose). Thus, a behavioral economics procedure such as the one employed here provides information about sensitivity to increases in the unit price of the intravenous solution, and may be used to predict whether cigarette smoke constituents would alter the impact of a reduction in nicotine content, such as a regulatory policy requiring the nicotine in cigarettes to be reduced (Smith et al., 2014b).
4.5 Implications
These data suggest that this combination of cigarette smoke constituents, at least in the manner in which they were studied in the present paper, do not appear to increase the reinforcing efficacy of nicotine. However, cigarette smoke constituents that inhibit MAO, whatever those may be, may have a large effect on behavior that results in low-dose nicotine delivery. This effect could become important if regulatory action is taken to decrease the content of nicotine in cigarettes because variation in MAO inhibition across products or individuals could be an important determinant of behavior.
Supplementary Material
Highlights.
We tested whether tobacco constituents would alter nicotine self-administration
Rats were tested across several doses and schedules of reinforcement
A variety of tobacco smoke constituents did not alter nicotine self-administration
An injection of an MAO inhibitor increased low-dose nicotine self-administration
Acknowledgments
Role of Funding Source
Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659 awarded to E.C.D.) The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Funding for Tracy Smith was provided by the National Institute on Drug Abuse (F31 DA037643).
The authors would like to thank Maysa Gharib, Emily Pitzer, and Josh Alberts for their extensive help in conducting experimental sessions. Thanks to all undergraduate students in the lab with special thanks to Samantha N. Cwalina, Alexandre Kenefake, Jessica Pelland, Hangil Seo, Marisa Wallas, and Matthew Onimus.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Portions of these data were also presented as part of two posters at the annual meeting for the Society for Nicotine and Tobacco Research in Seattle, WA on February 6th, 2014 and a symposium at the annual meeting for the Association for Behavior Analysis in Chicago, IL on May 26th, 2014. Dr. Buffalari is now at Westminster College, Department of Psychology.
Contributors
All authors have read and agreed on the content of this version of the submitted manuscript and the order of authorship, with Dr. Alan Sved and Dr. Eric Donny serving as co-senior authors.
Conflict of Interest
The authors have no competing interests to declare.
Contributors
Tracy T. Smith was the primary graduate student responsible for designing and overseeing the experiments described in the manuscript. She also analyzed the data, created the figures, and wrote the manuscript. Matthew B. Schaff was the undergraduate student responsible for conducting Experiment 1, which served as his Undergraduate Honors Thesis. Laura E. Rupprecht was the graduate student responsible for designing and overseeing the experiment shown in Figure S6, and she provided feedback on the manuscript. Rachel L. Schassburger and Deanne M. Buffalari were involved in design decisions and provided feedback on the manuscript. Sharon E. Murphy conducted the assay evaluating the levels of each constituent in the cocktail solution. Alan F. Sved and Eric C. Donny were the Principal Investigators responsible for the studies, contributed to design decisions, and provided feedback on the data analyses and the manuscript. All authors have read and approved of submission of this paper to Drug and Alcohol Dependence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tracy T. Smith, Department of Psychology, University of Pittsburgh
Matthew B. Schaff, Department of Neuroscience, University of Pittsburgh
Laura E. Rupprecht, Department of Neuroscience, University of Pittsburgh
Rachel L. Schassburger, Department of Neuroscience, University of Pittsburgh
Deanne M. Buffalari, Department of Neuroscience, University of Pittsburgh
Sharon E. Murphy, College of Biological Sciences, University of Minnesota
Alan F. Sved, Departments of Psychology and Neuroscience, University of Pittsburgh.
Eric C. Donny, Department of Psychology, University of Pittsburgh.
References
- Abu Ghazaleh H, Lalies MD, Nutt DJ, Hudson AL. The modulatory action of harmane on serotonergic neurotransmission in rat brain. Brain Res. 2015;1597:57–64. doi: 10.1016/j.brainres.2014.11.056. [DOI] [PubMed] [Google Scholar]
- Arib O, Rat P, Molimard R, Chait A, Faure P, de Beaurepaire R. Electrophysiological characterization of harmane-induced activation of mesolimbic dopamine neurons. Eur J Pharmacol. 2010;629:47–52. doi: 10.1016/j.ejphar.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85:293–304. doi: 10.1016/j.neuropharm.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behav Pharmacol. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase A and B activities in heavy smokers. Biol Psychiatry. 1995;38:756–761. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict Biol. 2013a;20:227–235. doi: 10.1111/adb.12099. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Putt F, Truman P. Nicotine-, tobacco particulate matter- and methamphetamine-produced locomotor sensitisation in rats. Psychopharmacology (Berl) 2013b;228:659–672. doi: 10.1007/s00213-013-3071-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. Nebraska Symposium on Motivation: The Motivational Impact of Nicotine and its Role in Tobacco Use. Vol. 55. Springer Science + Business Media; New York, NY: 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp Clin Psychopharmacol. 2014;22:9–22. doi: 10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12:1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37:2153–2160. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Soleiman MT, Talia R, Koob GF, George O, Mandyam CD. Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology (Berl) 2015;232:453–463. doi: 10.1007/s00213-014-3685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Behavioral Pharmacology Annual Report. Philip Morris Tobacco Resolution. 1983 Bates Number 20605661 ( http://www.pmdocs.com/getallimg.asp?if=avpidx&DOCID=1003060364/0441)
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, Lesage MG, Levin M, Buffalari DM, Joel D, Sved AF. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Harris AC, Stepanov I, Pentel PR, Lesage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl) 2012;220:565–576. doi: 10.1007/s00213-011-2514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Schmidt CE, Muelken P, Stepanov I, Saha S. Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend. 2015;147:60–67. doi: 10.1016/j.drugalcdep.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T. Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit Contam. 2004;21:1041–1050. doi: 10.1080/02652030400019844. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun. 2005;326:378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Guillen H, Aran VJ. Oxidative metabolism of the bioactive and naturally occurring beta-carboline alkaloids, norharman and harman, by human cytochrome P450 enzymes. Chem Res Toxicol. 2008;21:2172–2180. doi: 10.1021/tx8002565. [DOI] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen C, Wu D, Su Q. Enantiomeric analysis of anatabine, nornicotine and anabasine in commercial tobacco by multi-dimensional gas chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865:13–17. doi: 10.1016/j.jchromb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM. The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology. 2011;61:95–104. doi: 10.1016/j.neuropharm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. CRC Press, Taylor and Francis Group, LLC; Boca Raton, FL: 2013. [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15:1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naive to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014a;22:453–459. doi: 10.1037/a0037396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Sved AF, Hatsukami DK, Donny EC. Nicotine reduction as an increase in the unit price of cigarettes: a behavioral economics approach. Prev Med. 2014b doi: 10.1016/j.ypmed.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Takayama S, Uyeno ET. Intravenous self-administration of ethanol and acetaldehyde by rats. Yakubutsu Seishin Kodo. 1985;5:329–334. [PubMed] [Google Scholar]
- Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Harmane inhibits serotonergic dorsal raphe neurons in the rat. Psychopharmacology (Berl) 2005;182:562–569. doi: 10.1007/s00213-005-0118-0. [DOI] [PubMed] [Google Scholar]
- USDHHS. How Tobacco Smoking Causes Disease: The Biological And Behavioral Basis For Smoking-Attributable Disease: A Report Of The Surgeon General (Executive Summary) USDHHS; Washington, D.C: 2010. [Google Scholar]
- Villegier AS, Belluzzi JD, Leslie FM. Serotonergic mechanism underlying tranylcypromine enhancement of nicotine self-administration. Synapse. 2011;65:479–489. doi: 10.1002/syn.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- West R. Nicotine addiction: a re-analysis of the arguments. Psychopharmacology (Berl) 1992;108:408–410. doi: 10.1007/BF02247413. discussion 411–406. [DOI] [PubMed] [Google Scholar]
- Wu W, Ashley DL, Watson CH. Determination of nicotine and other minor alkaloids in international cigarettes by solid-phase microextraction and gas chromatography/mass spectrometry. Anal Chem. 2002;74:4878–4884. doi: 10.1021/ac020291p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.