Abstract
Background
Transient postnatal exposure of rodents to the selective serotonin (5-HT) reuptake inhibitor (SSRI) fluoxetine alters behavior and brain 5-HT neurotransmission during adulthood, and also reduces brain arachidonic (ARA) metabolic consumption and protein level of the ARA metabolizing enzyme, cytochrome P4504A (CYP4A).
Hypothesis
Brain 20-hydroxyeicosatetraenoic acid (20-HETE), converted by CYP4A from ARA, will be reduced in adult mice treated transiently and postnatally with fluoxetine.
Methods
Male mice pups were injected i.p. daily with fluoxetine (10 mg/kg) or saline during P4-P21. At P90 their brain was high-energy microwaved and analyzed for 20-HETE and six other ARA metabolites by enzyme immunoassay.
Results
Postnatal fluoxetine vs. saline significantly decreased brain concentrations of 20-HETE (−70.3%) and 15-epi-lipoxin A4 (−60%) in adult mice, but did not change other eicosanoid concentrations.
Conclusions
Transient postnatal administration of fluoxetine to mice results in reduced brain ARA metabolism involving CYP4A and 20-HETE formation during their adulthood.
Keywords: fluoxetine, arachidonic acid, mouse, neurodevelopment, postnatal, serotonin, 5-HT, SSRI, cytochrome P450 4A, metabolism, brain, 20-HETE, 15-epi-LXA4, ARA, PUFA, antidepressant
Introduction
Selective serotonin (5-HT) reuptake inhibitors (SSRIs) such as fluoxetine (Prozac®) are approved for treating depression, anxiety and personality disorders in women during pregnancy and lactation, and in children and adolescents. Their use during pregnancy has been associated in offspring with premature birth, neonatal cardiovascular abnormalities, pulmonary hypertension, disturbed behavior, and increased risk for autism spectrum disorder [1, 2]. Infants that receive SSRIs via the mother’s breast milk also may be at risk [3]. SSRIs were reported to increase risk for suicide in pediatric patients [4], but a recent review called this into question [5]. In contrast, several studies suggest that antidepressant use during pregnancy has no major long-term effects on neurodevelopment and behavior in the offspring [6, 7]. Thus the issue remains controversial.
One way to understand potential pathological mechanisms of early exposure to SSRIs may be to study rodents. The P1 to P21 postnatal period in rodents coincides with a brain growth spurt, rapid dendritic and axonal outgrowth, synaptogenesis and myelination, peak establishment of neural connections and susceptibility to xenobiotics [8, 9]. This period corresponds to the period of maturation of the human brain in the third trimester of pregnancy and through the first year of postnatal life [10, 11].
Adult rodents that have been exposed transiently and postnatally to an SSRI show increased brain density of the presynaptic 5-HT reuptake transporter (5-HTT) [12, 13], and structurally abnormal serotonergic neurons [14] and dendritic spines [15]. They also demonstrate depressive-like [16–19] and anxiety-like [16, 20] behaviors, and altered circadian rhythm [21]. These long-term effects depend on the specific SSRI administered, since early exposure to escitalopram (Lexapro) but not to fluoxetine reduced the 5-HT concentration in the mouse hippocampus and the two drugs caused different behaviors in the adult mice [22]. On the other hand, exposure of Ts65Dn mice, an animal model for Down syndrome, to fluoxetine from P3 to P15 rescued abnormalities in behavior, neurogenesis, and beta-amyloidogenic processing of amyloid precursor protein noted in untreated adult Ts65Dn mice [23].
Changes in behavior and brain integrity in adult rodents following transient postnatal fluoxetine may be associated with disturbed neurotransmission and metabolism involving the polyunsaturated fatty acid, arachidonic acid (ARA, 20:4n-6) [24]. Unesterified ARA can be released as a second messenger from synaptic membrane phospholipid during neurotransmission involving 5-HT2A/2C and other neuroreceptors that are coupled to activation of calcium-dependent cytosolic phospholipase A2 (cPLA2) type IVA, and ARA release can be modified by therapeutic levels of chronic lithium in rodents [25–30]. Unesterified ARA can modify multiple aspects of brain function and structure, and it is a precursor of a many bioactive eicosanoid products within the brain ARA “metabolic cascade” [31–33].
Supporting ARA cascade changes in adult rodents following transient postnatal fluoxetine, we used neuroimaging with [1-14C]ARA to show that incorporation coefficients k* and rates Jin of unesterified ARA from plasma into brain were decreased widely in adult unanaesthetized mice that had been injected i.p. daily with a clinically relevant dose of fluoxetine (10 mg/kg) [16] during postnatal days P4-P21, compared with saline-injected control mice [24]. Jin represents the rate of brain metabolic consumption of ARA, since ARA cannot be synthesized de novo in vertebrates nor elongated significantly in brain from its circulating shorter-chain n-6 precursors [34, 35]. Transient postnatal fluoxetine compared with saline in mice also reduced the adult brain protein level of CYP4A (4A1 plus 4A2 plus 4A3) by 74% (p = 0.004), suggesting a quasi-permanent effect independent of drug presence. Fluoxetine did not change protein levels of several other measured ARA metabolizing enzymes, namely cyclooxygenase (COX)-1, COX-2, 5-lipoxygenase (LOX), 12-LOX, 15-LOX, cytochrome P450 (CYP) 2C9 or membrane-associated PGE synthase (mPGES).
CYP4A can convert ARA to 20-hydroxyeicosatrienoic acid (20-HETE) [36], an autacoid that can influence cerebrovascular function and be formed following stimulation of 5-HT1B receptors [37–40]. Based on our finding reduced brain CYP4A protein in adult mice subjected to transient postnatal fluoxetine [24], we hypothesized that 20-HETE would be reduced as well. To test this hypothesis, in the present study we used enzyme immunoassay (EIA) to measure concentrations of 20-HETE and of six other ARA metabolites in high-energy microwaved brain, critical for minimizing postmortem changes in these concentrations [41, 42], from 90-day old mice that had been injected at P4-P21 with a clinically relevant dose [16] of fluoxetine or saline.
Materials and Methods
Chemicals
Fluoxetine and EDTA were purchased from Sigma-Aldrich (Saint Louis, MO, USA). HPLC-grade hexane and methanol were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ultra-pure water was purchased from KD Medical (Columbia, MD, USA). Saline (bacteriostatic 0.9% NaCl injection, USP) was purchased from Hospira (Lake Forest, IL, USA). EIA buffer was obtained from Oxford Biochemical (Oxford, MI, USA). Strata-X 33μ polymeric reversed phase cartridges (200 mg, 6 ml) for solid phase extraction were purchased from Phenomenex (Torrance, CA, USA).
Animals
The experimental protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institute of Health Publication 86-23). Untimed pregnant C57BL/6 mice (Charles River Laboratories International, Wilmington, MA, USA) were housed singly until they gave birth. Dams and their litters were housed in separate cages, in a temperature-controlled facility with a 12-h/12-h light/dark cycle. The mice had free access to water and Purina Lab Chow, which contained (as % of total fatty acids), 30.6% saturated, 33.5% monounsaturated, 47.1% linoleic, 4.9% α-linolenic, 0.27% ARA, 1.68% eicosapentaenoic, and 2.2% docosahexaenoic acid [24].
On postnatal day 4 (P4), male pups were assigned randomly to a saline control group (n = 4) or fluoxetine (10 mg/kg dissolved in saline) treatment group (n = 11). The pups were injected i.p. once per day on P4 through P21. This dosing regimen is reported to produce therapeutically relevant blood concentrations of fluoxetine (360 ± 123 ng/ml) and of its metabolite norfluoxetine (708 ± 168 ng/ml) at P22 [16]. Pups were weaned at P28, their dams were removed, and the pups were housed into adulthood. At P90, the mice were anesthetized lightly and rapidly subjected to head-focused microwave fixation (5.5 kW, 3.4 s, 90% power output) (Cober Electronics, Norwalk CT, USA). Brains were removed and stored at −80 °C until analysis.
Brain sample preparation
Two volumes of ice-cold methanol containing 0.01% acetic acid, 0.1% butylated hydroxytoluene (BHT) and 20 μL of 0.5 mM EDTA were added to a whole brain sample. The sample was homogenized for 30 sec with a Bullet blender (Next Advance, Averill Park, New York, USA), and homogenization was repeated for 30 sec if the first procedure appeared incomplete. Homogenized samples were incubated at −80 °C for 30 min, followed by centrifugation at 14,000 rpm for 5 min. The supernatant was transferred to a new Kimble glass centrifuge tube. The pellets were subjected to homogenization, incubation, and centrifugation another time as described above. The combined supernatant was evaporated to dryness under nitrogen. The samples were re-dissolved in methanol and placed in the icebox for solid-phase extraction.
Solid-phase extraction
Solid phase extraction was performed using a vacuum manifold (Agilent Technologies, Santa Clara, CA, USA) and Strata X (Phenomenex) solid phase extraction cartridges that were preconditioned with 6 mL methanol followed by 6 mL of water. The brain samples were diluted with water to bring the methanol concentration to approximately 10% (v/v) and kept on ice. The samples were acidified to pH 3.5 with 2 M HCl and immediately loaded into an extraction cartridge. The cartridge was washed with 6 mL of 10% methanol in water. Elution was performed with 6 mL of methanol. The organic solvent was evaporated to dryness under a fine stream of nitrogen. Evaporated samples were reconstituted in EIA buffer, flushed with nitrogen and stored at −80 °C for performing enzyme immunoassays.
Enzyme immunoassay
Eicosanoid concentrations were measured using specific enzyme immunoassay (EIA) kits according to the manufacturer’s instructions. Prostaglandin (PG)E2, thromboxane (TX)B2, leukotriene (LT)B4, 15-HETE, and lipoxin (LX)A4 kits were purchased from Cayman Chemicals (Ann Arbor, MI, USA). 12-HETE and 15-epi-LXA4 kits were purchased from Enzo Life Sciences (Farmingdale, NY, USA) and Oxford Biochemical (Oxford, MI, USA), respectively. The 20-HETE kit was from Eagle Biosciences (Nashua, NH, USA).
Data analysis
All data are expressed as mean ± standard error of mean (SEM). Statistical analysis was conducted with GraphPad Prism version 5.02 (GraphPad, La Jolla, CA, USA). Unpaired t tests were used for statistical comparison between the two groups. Initially, p < 0.05 was considered statistically significant, then corrections were considered based on the hypothesis-driven and exploratory nature of this study.
Results
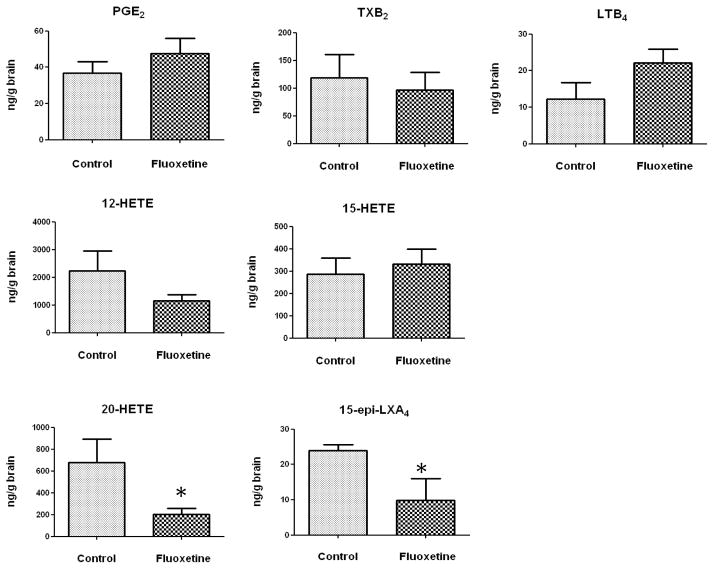
Figure 1 summarizes means and SEM’s of concentrations of each of seven ARA metabolites in high-energy microwaved brain of 3-month old mice, injected between P4 and P21 with either fluoxetine or saline (control). Asterisks indicate signicant difference between means at p < 0.05, using a two-tailed unpaired t test.
Figure 1. Effects of early exposure to chronic fluoxetine on brain concentrations of PGE2, TXB2, LTB4, 12-HETE, 15-HETE, 15-epi-LXA4, and 20-HETE in 3 month old rats.
Data are means ± SEM (n = 10–11 for fluoxetine-treated group, and n = 4 for control group). Data were analyzed using a two-tailed unpaired t test. * p < 0.05.
Postnatal fluoxetine treatment compared with saline did not alter brain concentrations of PGE2, TXB2, LXB4, 12-HETE, or 15-HETE (p > 0.05). However, the mean 20-HETE concentration was significantly reduced by 70.3% in the fluoxetine-treated compared to control group (201 ± 59.8 ng/g brain vs. 676.3 ± 219.1 ng/g brain, p = 0.012), and the concentration of 15-epi-LXA4 was reduced by 59.1% (9.8 ± 6.1 ng/g brain vs. 24.0 ± 1.6 ng/g brain, p = 0.046). The concentration of 15-epi-LXA4 was at least 10-fold less than that of 20-HETE in the saline and fluoxetine exposed adult mice.
Discussion
Transient postnatal fluoxetine compared with saline, administered i.p. daily from P4 to P21, significantly decreased baseline high energy-microwaved brain concentrations in adult male mice at P90 of two of seven eicosanoids that were measured, 20-HETE by 70.3% (p = 0.012) and 15-epi-LXA4 by 59% (p = 0.046), using two-sided unpaired t-tests. Concentrations of PGE2, TXB2, LXB4, 12-HETE, or 15-HETE were not changed significantly (p > 0.05).
While the results do not survive correction for multiple comparisons on a simple two-sided test, the significant 20-HETE reduction was specifically hypothesized from our prior finding of a 73% reduction in CYP4A protein in mice treated in an identical way with chronic fluoxetine, and would survive multiple correction comparison were the more appropriate one-sided t test used. Furthermore, this is an exploratory analysis, and should be considered informative even without Bonferroni correction [43]. Finally, the data are not truly independent, as all of the eicosanoids are metabolic products of ARA once released.
20-HETE is an ARA metabolite synthesized mainly by CYP4A, but CYP4F also can contribute to its formation [36, 44, 45]. Its concentration together with that of ARA is elevated by brain ischemia [46, 47]. 20-HETE influences neurovascular and cardiovascular function and can activate NF-κB to produce inflammatory changes [37–40, 48, 49]. It also can activate protein kinase C-alpha (PKC-alpha), which cross-talks with the extracellular signal-regulated kinase (ERK1/2) pathway. Both pathways regulate smooth muscle contraction, which was prevented in the aorta of CYP4A knockout (KO) mice or mice treated with a CYP4A inhibitor [50].
CYP4A gene expression is regulated by nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) and the retinoid X receptor (RXR) [51, 52]. Since 20-HETE is a potent PPARα ligand that induces the heterodimer binding to peroxisome proliferator response element and consequently activates CYP4A transcription [53, 54], a reduction in 20-HETE concentration would downregulate its own production in a negative feedback loop. After postnatal fluoxetine exposure, the decreased 20-HETE level thus could be related to falling activation of CYP4A transcriptional regulation caused by reduced 20-HETE.
Postnatal fluoxetine compared with saline also decreased the 15-epi-LXA4 concentration in the adult brain, uncorrected for multiple comparisons. 15-epi-LXA4 has an anti-inflammatory action and can inhibit a range of inflammatory mediators and proinflammatory cytokines, as well as NF-κB activation [55, 56]. 15-epi-LXA4 can be generated by CYP enzymes through hydroxylation of ARA to form 15R-HETE, which subsequently is converted by 5-LOX and LXA4 synthase [57]. A study using adult human liver microsomes showed formation of 15-HETE, with the R configuration predominant [58]. One group reported that CYP2C9 can produce 15R-HETE [59], but we did not find a significantly altered protein level of CYP2C9 or of 5-LOX in adult mice treated with fluoxetine at P4-P21 [24].
Although postnatally fluoxetine-treated adult mice did not show reduced hippocampal 5-HT in one study, whole brain 5-HT levels have not been examined as far as we know in such mice [22]. They do show other markers of 5-HT dysfunction, however, since they have increased brain 5-HTT density [12, 13], and structurally abnormal serotonergic neurons [14] and dendritic spines [15]. In comparison, increased brain extracellular 5-HT was reported in adult mice treated with chronic fluoxetine during adulthood [60, 61], and in adult 5-HTT KO mice [62]. Chronic fluoxetine and 5-HTT KO rodent preparations show elevated incorporation of plasma unesterified ARA into brain on neuroimaging [63, 64]. The 5-HTT KO mouse has anxiety-like [65] and less-consistently depression-like behaviors [66, 67], while rodents treated with fluoxetine during adulthood show behavioral sensitization to amphetamine and ethanol [68, 69].
It is possible that reduced brain ARA metabolism on neuroimaging and downregulation of the CYP4A pathway producing 20-HETE contributes to reported depressive-like [16–19, 70–72] and anxiety-like behaviors [16, 20] in adult mice given fluoxetine postnatally. These long-term quasi-permanent effects of transient postnatal fluoxetine suggest epigenetic or other neuroplastic mechanisms, since 5-HT and its metabolites are absent from brain at the time of measurement [73] and epigenetic changes associated with SSRI administration to neonatal or adult rats have been reported [74–76]. These different mechanisms remain to be studied in more detail. But the new evidence of long-term effects on brain ARA metabolism – reduced incorporation from plasma of unesterified ARA, reduced expression of CYP4A and reduced concentrations of 20-HETE and 15-epi-LXA4 – points to a role of ARA in altered behavior and disturbed neurotransmission in the postnatally treated adult mice [16–20, 70–72]. In brain, ARA is released as a second messenger following activation of 5-HT2A/2C, dopaminegic D2, chloinergic muscarinic M1,3,5, glutamatergic NMDA and other neuroreceptors during neurotransmission [31, 77].
In summary, transient postnatal fluoxetine exposure of mice reduced brain concentrations 20-HETE and 15-epi-LXA4 during adulthood, but not of PGE2, TXB2, LXB4, 12-HETE, or 15-HETE. The reduced 20-HETE was hypothesized by our prior reports that the brain protein level of CYP4A was reduced, and that net brain ARA consumption was downregulated [24]. A long-term reported behavioral consequence of early SSRI exposure thus may involve disturbed brain ARA metabolism In the future, PET scanning with [1-11C]ARA or [18F]ARA might be used to see if brain ARA metabolism is reduced in adult humans exposed during early life to fluoxetine, as it is in rodents [24, 78, 79]. Additionally, behavioral studies in rodents, following pharmacologic stimulation or inhibition, or genetic manipulation, of CYP4A, might be considered [50, 80–82].
Highlights.
Adolescents given antidepressant fluoxetine may develop behavioral disturbances.
Cytochrome P450 (CYP450) converts arachidonic acid (ARA) to 20-HETE.
Rodents given postnatal fluoxetine have reduced brain CYP450 4A at 3 months.
We now confirm that brain 20-HETE also is reduced at 3 months in pre-treated mice.
Behavioral changes by early fluoxetine may involve reduced CYP450 4A and 20-HETE.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute on Aging and the National Institute of Mental Health at the National Institutes of Health. We thank Ms. Lisa Chang and Mei Chen for the helpful contributions with animal preparation and injection.
Footnotes
No author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 2.Olivier JD, Akerud H, Kaihola H, Pawluski JL, Skalkidou A, Hogberg U, Sundstrom-Poromaa I. The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Frontiers in cellular neuroscience. 2013;7:73. doi: 10.3389/fncel.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri R, Stowe ZN, Hendrick V, Hostetter A, Widawski M, Altshuler LL. Estimates of nursing infant daily dose of fluoxetine through breast milk. Biological psychiatry. 2002;52:446–451. doi: 10.1016/s0006-3223(02)01368-9. [DOI] [PubMed] [Google Scholar]
- 4.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 5.Haliburn J. Adolescent suicide and SSRI antidepressants. Australasian psychiatry: bulletin of Royal Australian and New Zealand College of Psychiatrists. 2010;18:587. doi: 10.3109/10398562.2010.502574. [DOI] [PubMed] [Google Scholar]
- 6.Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102:1054–1059. doi: 10.1111/apa.12379. [DOI] [PubMed] [Google Scholar]
- 7.Riggin L, Frankel Z, Moretti M, Pupco A, Koren G. The fetal safety of fluoxetine: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2013;35:362–369. doi: 10.1016/S1701-2163(15)30965-8. [DOI] [PubMed] [Google Scholar]
- 8.Tabata H, Bell JM, Miller JC, Rapoport SI. Incorporation of plasma palmitate into the brain of the rat during development. Brain Res. 1986;394:1–8. doi: 10.1016/0165-3806(86)90076-3. [DOI] [PubMed] [Google Scholar]
- 9.Alm H, Kultima K, Scholz B, Nilsson A, Andren PE, Fex-Svenningsen A, Dencker L, Stigson M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology. 2008;29:628–637. doi: 10.1016/j.neuro.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 12.Bock N, Quentin DJ, Huther G, Moll GH, Banaschewski T, Rothenberger A. Very early treatment with fluoxetine and reboxetine causing long-lasting change of the serotonin but not the noradrenaline transporter in the frontal cortex of rats. World J Biol Psychiatry. 2005;6:107–112. doi: 10.1080/15622970510029731. [DOI] [PubMed] [Google Scholar]
- 13.Haskell SE, Hermann GM, Reinking BE, Volk KA, Peotta VA, Zhu V, Roghair RD. Sertraline exposure leads to small left heart syndrome in adult mice. Pediatric research. 2013;73:286–293. doi: 10.1038/pr.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva CM, Goncalves L, Manhaes-de-Castro R, Nogueira MI. Postnatal fluoxetine treatment affects the development of serotonergic neurons in rats. Neurosci Lett. 2010;483:179–183. doi: 10.1016/j.neulet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Xu DF, Li K, Wang HT, Shen PC, Lin M, Cao XH, Wang R. Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons. Int J Clin Exp Pathol. 2011;4:162–168. [PMC free article] [PubMed] [Google Scholar]
- 16.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 17.Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? The Journal of pharmacology and experimental therapeutics. 1997;283:1333–1341. [PubMed] [Google Scholar]
- 18.Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JW, Ahn HS, Baik JH, Yoon BJ. Administration of clomipramine to neonatal mice alters stress response behavior and serotonergic gene expressions in adult mice. Journal of psychopharmacology. 2013;27:171–180. doi: 10.1177/0269881112460107. [DOI] [PubMed] [Google Scholar]
- 20.Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Kiryanova V, Smith VM, Dyck RH, Antle MC. The effects of perinatal fluoxetine treatment on the circadian system of the adult mouse. Psychopharmacology (Berl) 2013;225:743–751. doi: 10.1007/s00213-012-2861-3. [DOI] [PubMed] [Google Scholar]
- 22.Altieri SC, Yang H, O’Brien HJ, Redwine HM, Senturk D, Hensler JG, Andrews AM. Perinatal vs. Genetic Programming of Serotonin States Associated with Anxiety. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagni F, Giacomini A, Guidi S, Ciani E, Ragazzi E, Filonzi M, De Iasio R, Rimondini R, Bartesaghi R. Long-term effects of neonatal treatment with fluoxetine on cognitive performance in Ts65Dn mice. Neurobiology of disease. 2014;74C:204–218. doi: 10.1016/j.nbd.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Ramadan E, Blanchard H, Cheon Y, Fox MA, Chang L, Chen M, Ma K, Rapoport SI, Basselin M. Transient postnatal fluoxetine leads to decreased brain arachidonic acid metabolism and cytochrome P450 4A in adult mice. Prostaglandins Leukot Essent Fatty Acids. 2014;90:191–197. doi: 10.1016/j.plefa.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 27.Basselin M, Ramadan E, Rapoport SI. Imaging brain signal transduction and metabolism via arachidonic and docosahexaenoic acid in animals and humans. Brain Res Bull. 2012;87:154–171. doi: 10.1016/j.brainresbull.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CR, Arai T, Bell JM, Rapoport SI. Preferential in vivo incorporation of [3H]arachidonic acid from blood into rat brain synaptosomal fractions before and after cholinergic stimulation. J Neurochem. 1996;67:822–829. doi: 10.1046/j.1471-4159.1996.67020822.x. [DOI] [PubMed] [Google Scholar]
- 29.Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonic-specific phospholipase A2. J Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5:459–467. doi: 10.1021/cn500058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapoport SI. Arachidonic acid and the brain. The Journal of nutrition. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzpatrick F, Soberman R. Regulated formation of eicosanoids. J Clin Invest. 2001;107:1347–1351. doi: 10.1172/JCI13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 34.DeMar JCJ, Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- 36.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochimica et biophysica acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circulation research. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 38.Stromstedt M, Warner M, Gustafsson JA. Cytochrome P450s of the 4A subfamily in the brain. Journal of neurochemistry. 1994;63:671–676. doi: 10.1046/j.1471-4159.1994.63020671.x. [DOI] [PubMed] [Google Scholar]
- 39.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 40.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2003;34:1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 41.Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochemical research. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- 42.Murphy EJ. Brain fixation for analysis of brain lipid-mediators of signal transduction and brain eicosanoids requires head-focused microwave irradiation: An historical perspective. Prostaglandins Other Lipid Mediat. 2010;91:63–67. doi: 10.1016/j.prostaglandins.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Morgan JF. p Value fetishism and use of the Bonferroni adjustment. Evidence-based mental health. 2007;10:34–35. doi: 10.1136/ebmh.10.2.34. [DOI] [PubMed] [Google Scholar]
- 44.Kroetz DL, Xu F. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu Rev Pharmacol Toxicol. 2005;45:413–438. doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- 45.Xu F, Falck JR, Ortiz de Montellano PR, Kroetz DL. Catalytic activity and isoform-specific inhibition of rat cytochrome p450 4F enzymes. J Pharmacol Exp Ther. 2004;308:887–895. doi: 10.1124/jpet.103.059626. [DOI] [PubMed] [Google Scholar]
- 46.Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki T, Marumo T, Shirakami K, Mori T, Doi H, Suzuki M, Watanabe Y, Chaki S, Nakazato A, Ago Y, Hashimoto H, Matsuda T, Baba A, Onoe H. Increase of 20-HETE synthase after brain ischemia in rats revealed by PET study with 11C-labeled 20-HETE synthase-specific inhibitor. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1737–1746. doi: 10.1038/jcbfm.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-hydroxyeicosatetraenoic acid stimulates nuclear factor-kappa B activation and the production of inflammatory cytokines in human endothelial cells. Journal of Pharmacology and Experimental Therapeutics. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 49.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 50.Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-alpha, and ERK1/2. Journal of cardiovascular pharmacology. 2013;62:78–83. doi: 10.1097/FJC.0b013e3182919591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson EF, Palmer CN, Griffin KJ, Hsu MH. Role of the peroxisome proliferator-activated receptor in cytochrome P450 4A gene regulation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1996;10:1241–1248. doi: 10.1096/fasebj.10.11.8836037. [DOI] [PubMed] [Google Scholar]
- 52.Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochimica et biophysica acta. 2012;1821:57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 55.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A(4) and aspirin-triggered 15-epi-lipoxin A(4) inhibit human neutrophil migration: Comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. Journal of Immunology. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 56.Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci U S A. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliw EH. bis-Allylic hydroxylation of linoleic acid and arachidonic acid by human hepatic monooxygenases. Biochimica et biophysica acta. 1993;1166:258–263. doi: 10.1016/0005-2760(93)90106-j. [DOI] [PubMed] [Google Scholar]
- 59.Bylund J, Ericsson J, Oliw EH. Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Analytical biochemistry. 1998;265:55–68. doi: 10.1006/abio.1998.2897. [DOI] [PubMed] [Google Scholar]
- 60.Benfield P, Heel RC, Lewis SP. Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs. 1986;32:481–508. doi: 10.2165/00003495-198632060-00002. [DOI] [PubMed] [Google Scholar]
- 61.Stenfors C, Ross SB. Evidence for involvement of 5-hydroxytryptamine(1B) autoreceptors in the enhancement of serotonin turnover in the mouse brain following repeated treatment with fluoxetine. Life Sci. 2002;71:2867–2880. doi: 10.1016/s0024-3205(02)02138-0. [DOI] [PubMed] [Google Scholar]
- 62.Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Qu Y, Chang L, Klaff J, Seemann R, Greenstein D, Rapoport SI. Chronic fluoxetine upregulates arachidonic acid incorporation into the brain of unanesthetized rats. Eur Neuropsychopharmacol. 2006;16:561–571. doi: 10.1016/j.euroneuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Basselin M, Fox M, Chang L, Bell JM, Greenstein D, Chen M, Murphy DL, Rapoport SI. Imaging elevated brain arachidonic acid signaling in unanesthetized serotonin transporter (5-HTT)-deficient mice. Neuropsychopharmacology. 2009;34:1695–1709. doi: 10.1038/npp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Line SJ, Barkus C, Coyle C, Jennings KA, Deacon RM, Lesch KP, Sharp T, Bannerman DM. Opposing alterations in anxiety and species-typical behaviours in serotonin transporter overexpressor and knockout mice. Eur Neuropsychopharmacol. 2011;21:108–116. doi: 10.1016/j.euroneuro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 68.Kaneko Y, Kashiwa A, Ito T, Ishii S, Umino A, Nishikawa T. Selective serotonin reuptake inhibitors, fluoxetine and paroxetine, attenuate the expression of the established behavioral sensitization induced by methamphetamine. Neuropsychopharmacology. 2007;32:658–664. doi: 10.1038/sj.npp.1301111. [DOI] [PubMed] [Google Scholar]
- 69.Goeldner FO, Pigatto G, Ribeiro AF, Machado HB, Boerngen-Lacerda R. Influence of fluoxetine and paroxetine in behavioral sensitization induced by ethanol in mice. Pharmacol Biochem Behav. 2005;82:388–396. doi: 10.1016/j.pbb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neuroscience and biobehavioral reviews. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- 71.Velazquez-Moctezuma J, Diaz Ruiz O. Neonatal treatment with clomipramine increased immobility in the forced swim test: an attribute of animal models of depression. Pharmacol Biochem Behav. 1992;42:737–739. doi: 10.1016/0091-3057(92)90022-8. [DOI] [PubMed] [Google Scholar]
- 72.Ribas VR, Aniceto HK, Martins HA, Ribas KH, de Guerra-Ribas RM, do Fraga SN, Ribeiro-Ribas V, Vasconcelos CM, Viana MT, Manhaes-de-Castro R. Neonatal administration of fluoxetine did not alter the anxiety indicators, but decreased the locomotor activity in adult rats in the elevated plus-maze. Arquivos de neuro-psiquiatria. 2008;66:844–847. doi: 10.1590/s0004-282x2008000600013. [DOI] [PubMed] [Google Scholar]
- 73.Caccia S, Cappi M, Fracasso C, Garattini S. Influence of dose and route of administration on the kinetics of fluoxetine and its metabolite norfluoxetine in the rat. Psychopharmacology (Berl) 1990;100:509–514. doi: 10.1007/BF02244004. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melas PA, Rogdaki M, Lennartsson A, Bjork K, Qi H, Witasp A, Werme M, Wegener G, Mathe AA, Svenningsson P, Lavebratt C. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol. 2012;15:669–679. doi: 10.1017/S1461145711000940. [DOI] [PubMed] [Google Scholar]
- 76.Clinton SM, Glover ME, Pugh PC, Cohen J, Akil H. Neonatal SSRI exposure alters neurodevelopment and risk for depression in model rats. Neuropsychopharmacology. 2013;38:S435–S593. [Google Scholar]
- 77.Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC, Herscovitch P, Eckelman WC, Rapoport SI, Carson RE. Brain incorporation of [11C]arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004;45:1471–1479. [PubMed] [Google Scholar]
- 79.Pichika R, Taha AY, Gao F, Kotta K, Cheon Y, Chang L, Kiesewetter D, Rapoport SI, Eckelman WC. The synthesis and in vivo pharmacokinetics of fluorinated arachidonic Acid: implications for imaging neuroinflammation. J Nucl Med. 2012;53:1383–1391. doi: 10.2967/jnumed.112.105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park EC, Kim SI, Hong Y, Hwang JW, Cho GS, Cha HN, Han JK, Yun CH, Park SY, Jang IS, Lee ZW, Choi JS, Kim S, Kim GH. Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology. 2014;147:860–869. doi: 10.1053/j.gastro.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol. 1999;276:R1691–1700. doi: 10.1152/ajpregu.1999.276.6.R1691. [DOI] [PubMed] [Google Scholar]
- 82.Lukaszewicz KM, Lombard JH. Role of the CYP4A/20-HETE pathway in vascular dysfunction of the Dahl salt-sensitive rat. Clin Sci (Lond) 2013;124:695–700. doi: 10.1042/CS20120483. [DOI] [PMC free article] [PubMed] [Google Scholar]