Abstract
Purpose
We compared smartphone fundus photography, nonmydriatic fundus photography, and 7-field mydriatic fundus photography for their abilities to detect and grade diabetic retinopathy (DR).
Design
This was a prospective, comparative study of 3 photography modalities.
Participants
Diabetic patients (n = 300) were recruited at the ophthalmology clinic of a tertiary diabetes care center in Chennai, India.
Methods
Patients underwent photography by all 3 modalities, and photographs were evaluated by 2 retina specialists.
Main Outcome Measures
The sensitivity and specificity in the detection of DR for both smartphone and nonmydriatic photography were determined by comparison with the standard method, 7-field mydriatic fundus photography.
Results
The sensitivity and specificity of smartphone fundus photography, compared with 7-field mydriatic fundus photography, for the detection of any DR were 50% (95% confidence interval [CI], 43–56) and 94% (95% CI, 92–97), respectively, and of nonmydriatic fundus photography were 81% (95% CI, 75–86) and 94% (95% CI, 92–96%), respectively. The sensitivity and specificity of smartphone fundus photography for the detection of vision-threatening DR were 59% (95% CI, 46–72) and 100% (95% CI, 99–100), respectively, and of nonmydriatic fundus photography were 54% (95% CI, 40–67) and 99% (95% CI, 98–100), respectively.
Conclusions
Smartphone and nonmydriatic fundus photography are each able to detect DR and sight-threatening disease. However, the nonmydriatic camera is more sensitive at detecting DR than the smartphone. At this time, the benefits of the smartphone (connectivity, portability, and reduced cost) are not offset by the lack of sufficient sensitivity for detection of DR in most clinical circumstances.
Worldwide, the prevalence of diabetes is increasing rapidly. Although diabetes was previously considered a disease of affluence, it is now estimated that 85% of those with undiagnosed diabetes live in low- or middle-income countries.1,2 Asia is now home to approximately 80% of the world's diabetic population,1,3 including more than 60 million Indians, and the total number of diabetic persons is expected to increase to more than 100 million by 2030.2,4 Alongside this rapidly increasing disease incidence is an increase in the associated complications, including diabetic retinopathy (DR), which is estimated to affect more than 93 million people.5 Although the burden of DR is significant, early treatment is effective, and an estimated 90% of severe vision loss can be prevented.6 Early detection and management of DR require an effective screening program. The current clinical practice guidelines recommend annual or biennial comprehensive eye examinations.1,7–9
Unfortunately, there are many barriers to screening and compliance with current screening recommendations, even in high-resource settings.10 Access to care for screening represents a significant obstacle. Presently, there is a shortfall in the number of ophthalmologists worldwide, with the most significant lack of providers in developing countries despite the rapidly increasing disease burden in these same regions.11 Cost is another significant barrier. Both direct and indirect costs, such as transportation and time away from work, represent additional barriers to regular eye care for many patients.12–14
A commonly used alternative to the comprehensive eye examination is remotely interpreted fundus photography, also known as “telemedicine” or “teleretinal screening.”15 Programs using these remote screening techniques have been successful in various high- and low-resource settings, including India.14,16–20 In particular, nonmydriatic fundus photography offers a noninvasive, fast, and convenient method of screening that does not require pupillary dilation.21 Although these programs offer an appealing solution for DR screening, a typical fundus camera may be beyond the means for many resource-limited areas, with costs ranging from $20 000 to $50 000, thus making this equipment unaffordable in many developing countries.14
A newly described technique using a smartphone camera for fundus photography could offer low-cost screening, especially with personnel shortages and limited photographic equipment, even in low- and middle-income countries.22 The smartphone offers a new alternative that is cheaper, is portable, and has image transmission capability. Reports thus far have been limited to descriptions of technique22–25 or third-party attachments to cellphone cameras.26 To date, this alternative has not been examined in a systematic study that compares imaging methods with the standard techniques of dilated, 7-field fundus photography. We compare the effectiveness of smartphone fundus photography, the nonmydriatic fundus camera, and standard 7-field mydriatic photography in detecting and grading DR in a retina specialty clinic in south India.
Methods
All patients provided signed informed consent before participation in the study. This study was approved by the Madras Diabetes Research Foundation Ethics Committee and the Emory University Institutional Review Board, and the research adhered to the tenets of the declaration of Helsinki.
A total of 300 patients were recruited at the Eye Department at Dr. Mohan's Diabetes Specialties Centre in Chennai, India. This sample size was selected on the basis of feasibility, given the limited time and resources to complete the study. To examine across multiple stages of DR, people were recruited on the basis of the duration of diabetes (<18 months in 100 people, 18 months to 15 years in 100 people, and >15 years in 100 people) to enhance the diversity of DR severity. The inclusion criteria for patients included age ranging from 18 to 65 years, a diagnosis of type 2 diabetes, and a willingness to undergo photography with all 3 cameras. People were excluded from the study if they had a medical condition that was a contraindication to dilation, had an overt media opacity, or had gestational diabetes. Patient eligibility was determined by review of medical records on presentation to the clinic, and patients were recruited over a period of 5 months.
Patients underwent all 3 imaging techniques on the same day as their regular eye appointment. Before beginning the study procedures, clinic staff obtained written consent from the patients and then administered a health questionnaire containing basic questions about diabetes management and past ocular health. After initial evaluation of visual acuity and a slit-lamp examination of the anterior segment of the eye, nonmydriatic photography was performed using the Nidek Model AFC-230 (Nidek Inc., Fremont, CA). Patients sat in a darkened room for approximately 1 to 3 minutes to achieve physiologic mydriasis before photography. Three 45-degree field images were taken of each eye, 1 view centered on the fovea, 1 nasal view centered on the optic disc, and 1 temporal with the optic disc at the edge of the field. The photographer evaluated each photograph immediately for clarity and focus. If the image was not satisfactory, the images were reacquired. The patient's eyes were then dilated using 0.5% tropicamide drops.
After adequate dilatation was achieved, the smartphone was used to take a video of each eye. To perform this examination, a 20 diopter condensing lens was held in the photographer's left hand and the iPhone 5 (Apple Inc., Cupertino, CA) was held in the right hand. The images were captured on the 3264 × 2488 pixels of the camera sensor using previously reported techniques.22 The FilmIc Pro application (Cinegenix, LLC, Seattle, WA) allows the smartphone camera focus and zoom to be independently adjusted with an active light source from the phone. The light was set to the lowest intensity to minimize patient discomfort. The lens was held approximately 6 cm from the patient's eye, and the camera was held approximately 12 cm from the eye. While filming, the photographer observed the on screen video display and adjusted the distance of the lens–phone relationship to both focus and optimize the field of view to the macula and optic disc. After confirmation of adequate video segments from both eyes, the video was stopped. The photographer reviewed the video, and representative screen shots were acquired to obtain the best images of the optic nerve and macula.
Last, the patient had standard 7-field fundus photography performed by a trained optometrist using the Zeiss FF450 Plus (Carl Zeiss Meditec, Inc., Dublin, CA). Immediately after each photograph, image quality was assessed and images were reacquired as necessary. One photographer performed all image acquisition for each individual modality to ensure a standard technique.
All photographs were coded with an identification number and uploaded to a secure database. The photographs were assessed, and if no lesions of DR were seen, the absence of DR was recorded. If any lesions were seen, the presence of DR was recorded and the severity assigned on the basis of scoring according to the following modified Early Treatment Diabetic Retinopathy Study (ETDRS) criteria for the grading of DR compared with standards.27 A modified grading scale based on ETDRS criteria was used to simplify categorization. The ETDRS level 20 to 35 is labeled as mild nonproliferative DR, 43 to 47 is labeled as moderate nonproliferative DR, >53 to 60 is labeled as severe, and >60 is labeled as proliferative DR. The presence of panretinal photocoagulation (PRP) laser burns was classified as proliferative DR. Macular edema was judged to be present with visible cystoid spaces, retinal thickening, hard exudates, or laser photocoagulation scars within the macula. Two retina specialists (V.P. and A.M.H.) assessed the images independently. Images were reviewed on a Dell 24-inch monitor (Model P1424H; Dell Inc., Round Rock, TX) at 1920 × 1080 resolution. The graders were masked to the fellow grader's assessment and any patient-related data. Disagreement in the severity of retinopathy between the 2 graders was adjudicated by a third retina specialist (R.R.). The presence of macular edema or a grade of severe nonproliferative DR or worse was considered vision-threatening diabetic retinopathy (VTDR). The quality of the photograph was graded on a 1 to 5 scale (excellent, good, satisfactory, poor, unreadable). The quality was dependent on the visibility of detail and the acquisition of the entire macular region.
The sensitivity and specificity for diagnosing DR and VTDR were calculated for the smartphone and for nonmydriatic fundus photography assuming that dilated, 7-field fundus photography gave the true diagnosis and was the gold standard. The 95% confidence intervals (CIs) for sensitivity and specificity were calculated using normal approximations. Similar calculations were done for nonmydriatic fundus photography. In addition, the agreement between dilated, 7-field fundus photography and both the smartphone and nonmydriatic fundus photography was assessed using the kappa statistic. These analyses were carried out on an eye-specific basis.
Given the strong inter-eye correlation (the phi coefficients for the association of the presence of DR and of VTDR between left and right eyes for each of the devices are reported in the “Results” section), estimates of sensitivity and specificity were also calculated using a method that accounted for this correlation.28 Percentile bootstrap 95% CIs for these estimates were constructed on the basis of 10 000 bootstrap samples.28 All statistical analyses were performed using SAS 9.3 (SAS Inc., Cary NC).
Results
A total of 300 patients (600 eyes) completed all 3 methods of photography, and demographic data are presented in Table 1. The mean (± standard deviation) age at examination was 48±11 years, 201 patients (67%) were male, the mean hemoglobin A1c was 8.7% (72 mmol/mol) ± 2.1, and 103 patients (34%) had insulin-dependent diabetes. For the low disease duration (<18 months) group (N = 100), the median disease duration was 0.3 years and the interquartile range (IQR) was 0.7 years. For the medium disease duration group (N = 100), the median disease duration was 8.25 years and the IQR was 6 years. For the long (<15 years) disease duration group (N = 100), the median was 20 years and the IQR was 7. For all patients, the minimum disease duration was 0.1 years and the maximum was 37.2 years. Of the 600 eyes, 204 (34%) had DR, 54 (9%) had VTDR, and 26 (4%) had a diagnosis of macular edema based on the images produced by dilated, 7-field fundus photography.
Table 1.
Demographics (by Person)
| Mean (SD) or N (%) | |
|---|---|
| Age (yrs) | 47.94 (11.01), (n = 300) |
| Male sex | 201 (67.0%) |
| HBA1C (%) | 8.74 (2.13), (n = 295) |
| HBA1C (mmol/mol) | 72 (n = 295) |
| Duration of insulin use (yrs) | 2.33 (5.43), (n = 298) |
| Hypertension duration (yrs) | 2.09 (4.64), (n = 298) |
| Diabetes duration range (yrs) | 0.1–37.2 |
HBA1C = hemoglobin A1C; SD = standard deviation.
Image Quality
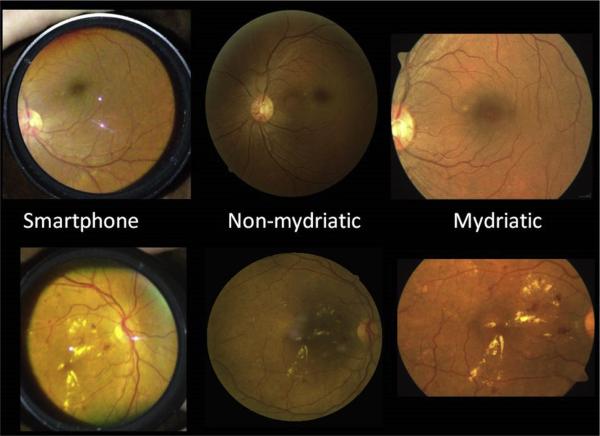
Representative images of the photograph quality for each modality are presented in Figure 1. Results of image quality are summarized in Table 2. Photographs were ungradable for DR in 11 (1.8%) photographs by smartphone fundus photography, 9 (1.5%) photographs by nonmydriatic fundus photography, and 0 photographs by mydriatic fundus photography. The mydriatic camera images had the highest quality.
Figure 1.
Representative photographs from each image modality. Top: Series taken from the same eye demonstrates no background retinopathy. Bottom: Images demonstrate diabetic macular edema in the same eye with each modality.
Table 2.
Photographic Quality Assessment
| Smartphone Fundus Photography (No. of Eyes, %) | Nonmydriatic Fundus Photography (No. of Eyes, %) | Mydriatic Fundus Photography (No. of Eyes, %) | |
|---|---|---|---|
| Not gradable | 11 (1.8%) | 9 (1.5%) | 0 (0%) |
| Satisfactory quality | 508 (84.7%) | 534 (89%) | 595 (99.2%) |
Detection of Diabetic Retinopathy
The device performance in DR detection is summarized in Table 3. The sensitivity and specificity of smartphone photographic detection of DR compared with the mydriatic photographs were 50% (95% CI, 43–56) and 94% (95% CI, 92–97), respectively. The kappa was 0.48 (95% CI, 0.41–0.56), indicating moderate agreement between the smartphone and the 7-field mydriatic photographs. The sensitivity and specificity of nonmydriatic photographic detection of any DR compared with the mydriatic photographs were 81% (95% CI, 75–86) and 94% (95% CI, 92–96), respectively. The kappa was 0.76 (95% CI, 0.71–0.82), indicating substantial agreement with the mydriatic photography.
Table 3.
Sensitivity, Specificity, and Kappa for Any Diabetic Retinopathy and Vision-Threatening Diabetic Retinopathy
| Diabetic Retinopathy |
Vision-Threatening Diabetic Retinopathy |
||||||
|---|---|---|---|---|---|---|---|
| Camera | Estimate Type | Sensitivity (95% CI) | Specificity (95% CI) | Kappa (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Kappa (95% CI) |
| Nonmydriatic | Raw | 81 (75–86) | 94 (92–96) | 0.76 (0.71–0.82) | 54 (40–67) | 99 (98–100) | 64 (52–76) |
| Correlated | 84 (76–91) | 96 (93–98) | 52 (33–71) | 98 (97–100) | |||
| IPhone (Apple Inc., Cupertino, CA) | Raw | 50 (43–56) | 94 (92–97) | 0.48 (0.41–0.56) | 59 (46–72) | 100 (99–100) | 71 (60–82) |
| Correlated | 51 (41–61) | 93 (89–96) | 56 (36–74) | 100 (99–100) | |||
CI = confidence interval.
The correlation between nonmydriatic and smartphone regarding DR diagnosis based on the 7-field mydriatic photographs had a phi coefficient of 0.74, indicating a strong positive association. When controlling for this correlation, the sensitivity and specificity were 51% (95% CI, 41–61) and 93% (95% CI, 89–96), respectively, between the smartphone and the 7-field mydriatic photographs. The sensitivity and specificity were 84% (95% CI, 76–91) and 96% (95% CI, 93–98), respectively, between the nonmydriatic photographs and mydriatic photographs.
Detection of Vision-Threatening Diabetic Retinopathy
The device performance for VTDR detection is summarized in Table 3. The sensitivity and specificity of smartphone photographic detection of VTDR compared with the mydriatic photographs were 59% (95% CI, 46–72) and 100% (95% CI, 99–100), respectively. The kappa was calculated to be 0.71 (95% CI, 0.60–0.82), indicating substantial agreement with the 7-field mydriatic photography. The sensitivity and specificity of nonmydriatic photographic detection of VTDR compared with the mydriatic photographs were 54% (95% CI, 40–67) and 99% (95% CI, 98–100), respectively. The kappa value was calculated to be 0.64 (95% CI, 0.52–0.76), indicating substantial agreement with the 7-field mydriatic photography.
The correlation of VTDR between the eyes was 0.92, based on the diagnosis by 7-field mydriatic camera, indicating a strong correlation. When controlling for this correlation, the diagnostic sensitivity and specificity between the smartphone and the mydriatic photograph modalities were 56% (95% CI, 36–74) and 100% (95% CI, 99–100), respectively. When controlling for this correlation using the nonmydriatic photographs and the 7-field mydriatic photographs, the sensitivity and specificity were 52% (95% CI, 33–71) and 98% (95% CI, 97–100), respectively.
Discussion
Much excitement surrounds the potential of this technology as a diagnostic instrument. This study is noteworthy in the systematic evaluation of smartphone fundus photography in detecting and grading DR. Also, this study is the first to compare the use of a smartphone-generated image to more commonly used imaging modalities.
The 20 diopter lens-assisted smartphone photographs had a low rate of ungradable images, and the majority of images were at least of satisfactory quality. The kappa statistic demonstrated “moderate” or “substantial” range of grader agreement between devices in both groups: presence of DR and vision-threatening DR. The smartphone was less sensitive than nonmydriatic photography in detecting the presence of any DR. However, both methods were similar for detecting vision-threatening disease. Although both methods showed robust specificity, teleretinal screening is intended to serve as a tool that enables detection of disease in patients who may not have access to ophthalmologic care. A screening tool with low sensitivity to detect the presence of DR will result in an underdiagnosis and ultimately missed opportunity to prevent permanent vision loss.
A comparative smartphone fundus photographic screening has not been previously reported for comparison. However, the nonmydriatic photography screening results are similar to other published data.29–34 Our report demonstrates that nonmydriatic photography had a sensitivity and specificity of 81% and 94%, respectively, for the detection of any DR. These data are within range of the 78% to 92% and 86% to 99%, respectively, previously established29–33 and better than the 58% and 69%, respectively, recently reported by Gupta et al34 in a similar population.
In this study, nonmydriatic fundus photography lost sensitivity for the detection of VTDR compared with the detection of any DR. We believe there are 2 reasons that account for the disparity. First, we postulate that when the image quality is relatively compromised, it limits detection of subtle pathology such as fine neovascularization or macular edema. Reduction in image quality is likely explained by comorbidities of advanced DR, such as cataract and reduced physiologic dilation. Second, the 7-field mydriatic photography inherently captures a wider view of the periphery with a greater chance to detect VTDR than the nonmydriatic and smartphone methods chosen in this study. Slight variations in the technique may improve the visualization of more peripheral fundus.
Furthermore, this was a comparative clinical study performed in South India, not a community screening program. Patients were already under the care of retina expertise, and nearly all who had VTDR had received laser photocoagulation. Our data were gathered to evaluate device performance and maximize the event rate of detecting DR. Our data also reflect many unique qualities of this patient population. It is possible that device performance in the community may be different than in this study. We anticipate that factors such as longer diabetes duration, darker irides, and older age detrimentally affect the image quality and device performance, and these will be evaluated in subsequent analyses.
On the basis of these data, we conclude that smartphone fundus photography does not yet have a role in screening for DR at this time. However, we believe that many reasons exist to be optimistic for the future potential of this technology. Smartphone camera technology will continue to improve, interest will grow, and add-on optical devices may provide simplification and standardization to the methods of acquisition. With technical improvements, we envision that nonophthalmic personnel could accomplish a similar technique in primary healthcare settings and send the images for remote interpretation. In this study, a medical student (M.E.R.) performed the photography for both the smart-phone and nonmydriatic images and had limited training. One particular area for improvement using this technique will be to enable a more peripheral view of the fundus. Third-party adapters or contact lenses, such as the Koeppe lens, have been suggested for these purposes in prior publications.22,26
There are compelling reasons to continue to consider the use of smartphone technology. Smartphones are ubiquitous, inexpensive, portable, and “connected.” These attributes enable a substantial benefit for this technology to be used in screening for DR, especially in low resource settings where a trade-off in device performance in exchange for improved cost and availability may be acceptable. Smartphones are not Food and Drug Administration–approved diagnostic devices, and we are unaware of any approval being sought in the United States. As such, data transmission will need to adhere to privacy regulations with encryption. Our study demonstrates both the limitations and the feasibility of using this technology in India, where the prevalence of diabetes has reached epidemic proportions and substantial obstacles to routine screening for DR exist.2
Acknowledgments
The authors thank the staff of the Eye Department at Dr. Mohan's Diabetes Specialities Centre for assistance and support throughout the study, as well as the participating patients who have made this work possible. Martha E. Ryan, BS, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by an unrestricted departmental grant from Research to Prevent Blindness, an Emory Ophthalmology departmental core grant (National Institutes of Health /National Eye Institute: P30 EY006360), the GO-Emory program, and National Institutes of Health Research Training Grant R25 TW009337 funded by the Fogarty International Center and the National Institute of Mental Health. The sponsors had no role in the design or conduct of this research.
Abbreviations and Acronyms
- CI
confidence interval
- DR
diabetic retinopathy
- ETDRS
Early Treatment Diabetic Retinopathy Study
- IQR
interquartile range
- VTDR
vision-threatening diabetic retinopathy
Footnotes
Presented at: the Association for Research in Vision and Ophthalmology meeting, May 3–7, 2015, Denver, Colorado.
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author Contributions:
Conception and design: Ryan, Rajalakshmi, Narayan, Mohan, Olsen, Hendrick
Data collection: Ryan, Rajalakshmi, Prathiba, Anjana, Ranjani, Mohan, Hendrick
Analysis and interpretation: Ryan, Rajalakshmi, Ward, Lynn, Hendrick
Obtained funding: Not applicable
Overall responsibility: Ryan, Rajalakshmi, Ranjani, Olsen, Hendrick
References
- 1.IDF . Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation; 2013. [November 18, 2013]. Available at: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full.pdf. [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–57. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjana RM, Pradeepa R, Deepa M, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMRINDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 5.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris FL., 3rd How effective are retinopathy treatments. JAMA. 1993;269:2. [PubMed] [Google Scholar]
- 7.Prevention of blindness from diabetes mellitus: report of a WHO consultation in Geneva, Switzerland. World Health Organization; Geneva: Nov 9–11, 2005. Report of a WHO consultation. [Google Scholar]
- 8.American Academy of Ophthalmology Retina/Vitreous Panel . Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. American Academy of Ophthalmology; San Francisco, CA: 2014. [July 6, 2015]. Available at: www.aao.org/ppp. [Google Scholar]
- 9.Association AD. Standards of medical care in diabetese—2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 10.Bressler NM, Varma R, Doan QV, et al. Underuse of the health care system by persons with diabetes mellitus and diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:168–73. doi: 10.1001/jamaophthalmol.2013.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resnikoff S, Felch W, Gauthier T-M, Spivey B. The number of ophthalmologists in practice and training worldwide: a growing gap despite more than 200,000 practitioners. Br J Ophthalmol. 2012;96:783–7. doi: 10.1136/bjophthalmol-2011-301378. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher AE, Donoghue M, Devavaram J, et al. Low uptake of eye services in rural India: a challenge for programs of blindness prevention. Arch Ophthalmol. 1999;117:1393–9. doi: 10.1001/archopht.117.10.1393. [DOI] [PubMed] [Google Scholar]
- 13.Namperumalsamy P, Nirmalan PK, Ramasamy K. Developing a screening program to detect sight-threatening diabetic retinopathy in South India. Diabetes Care. 2003;26:1831–5. doi: 10.2337/diacare.26.6.1831. [DOI] [PubMed] [Google Scholar]
- 14.Rachapelle S, Legood R, Alavi Y, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology. 2013;120:566–73. doi: 10.1016/j.ophtha.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Williams GA, Scott IU, Haller JA, et al. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:1055–62. doi: 10.1016/j.ophtha.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Cuadros J, Bresnick G. EyePACS: an adaptable telemedicine system for diabetic retinopathy screening. J Diabetes Sci Technol. 2009;3:509–16. doi: 10.1177/193229680900300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogunyemi O, Terrien E, Eccles A, et al. Teleretinal screening for diabetic retinopathy in six Los Angeles urban safety-net clinics: initial findings. AMIA Annu Symp Proc. 2011;2011:1027–35. [PMC free article] [PubMed] [Google Scholar]
- 18.Hautala N, Aikkila R, Korpelainen J, et al. Marked reductions in visual impairment due to diabetic retinopathy achieved by efficient screening and timely treatment. Acta Ophthalmol. 2013;92:582–7. doi: 10.1111/aos.12278. [DOI] [PubMed] [Google Scholar]
- 19.Mansberger SL, Gleitsmann K, Gardiner S, et al. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: a randomized controlled trial. Telemed J E Health. 2013;19:942–8. doi: 10.1089/tmj.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan V, Prathiba V, Pradeepa R. Tele-diabetology to screen for diabetes and associated complications in rural India: The Chunampet Rural Diabetes Prevention Project Model. J Diabetes Sci Technol. 2014;8:256–61. doi: 10.1177/1932296814525029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallerano JD, Aiello LP, Cavallerano AA, et al. Nonmydriatic digital imaging alternative for annual retinal examination in persons with previously documented no or mild diabetic retinopathy. Am J Ophthalmol. 2005;140:667–73. doi: 10.1016/j.ajo.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 22.Haddock LJ, Kim DY, Mukai S. Simple, inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. J Ophthalmol. 2013;2013:518479. doi: 10.1155/2013/518479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord RK, Shah VA, Filipino ANS, Krishna R. Novel uses of smartphones in ophthalmology. Ophthalmology. 2010;117:1274, e3. doi: 10.1016/j.ophtha.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Bastawrous A. Smartphone fundoscopy. Ophthalmology. 2012;119:432–3. doi: 10.1016/j.ophtha.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Kim DY, Delori F, Mukai S. Smartphone photography safety. Ophthalmology. 2012;119:2200–1. doi: 10.1016/j.ophtha.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Maamari RN, Keenan JD, Fletcher DA, Margolis TP. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol. 2014;98:438–41. doi: 10.1136/bjophthalmol-2013-303797. [DOI] [PubMed] [Google Scholar]
- 27.Group ETDRSR Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 28.Leon ARd, Soo A, Bonzo DC, Rudnisky CJ. Joint estimation of diagnostic accuracy measures for paired organs–application in ophthalmology. Biom J. 2009;51:837–50. doi: 10.1002/bimj.200800123. [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Blumenkranz MS, Brothersa RJ, et al. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134:204–13. doi: 10.1016/s0002-9394(02)01522-2. [DOI] [PubMed] [Google Scholar]
- 30.Mizrachi Y, Knyazer B, Guigui S, et al. Evaluation of diabetic retinopathy screening using a non-mydriatic retinal digital camera in primary care settings in south Israel. Int Ophthalmol. 2014;34:831–7. doi: 10.1007/s10792-013-9887-3. [DOI] [PubMed] [Google Scholar]
- 31.Aptel F, Denis P, Rouberol F, Thivolet C. Screening of diabetic retinopathy: effect of field number and mydriasis on sensitivity and specificity of digital fundus photography. Diabetes Metab. 2008;34:290–3. doi: 10.1016/j.diabet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Baeza M, Orozco-Beltrån D, Gil-Guillen VF, et al. Screening for sight threatening diabetic retinopathy using non-mydriatic retinal camera in a primary care setting: to dilate or not to dilate? Int J Clin Pract. 2009;63:433–8. doi: 10.1111/j.1742-1241.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 33.Vujosevic S, Benetti E, Massignan F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol. 2009;148:111–8. doi: 10.1016/j.ajo.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Gupta V, Bansal R, Gupta A, Bhansali A. Sensitivity and specificity of nonmydriatic digital imaging in screening diabetic retinopathy in Indian eyes. Indian J Ophthalmol. 2014;62:851–6. doi: 10.4103/0301-4738.141039. [DOI] [PMC free article] [PubMed] [Google Scholar]