Abstract
Background
The prevalence and characteristics of fetal alcohol syndrome (FAS) and partial FAS (PFAS) in the United States (US) are not well known.
Methods
This active case ascertainment study in a Rocky Mountain Region City assessed the prevalence and traits of children with FAS and PFAS and linked them to maternal risk factors. Diagnoses made by expert clinical dysmorphologists in multidisciplinary case conferences utilized all components of the study: dysmorphology and physical growth; neurobehavior; and maternal risk interviews.
Results
Direct parental (active) consent was obtained for 1,278 children. Averages for key physical diagnostic traits and several other minor anomalies were significantly different among FAS, PFAS, and randomly-selected, normal controls. Cognitive tests and behavioral checklists discriminated the diagnostic groups from controls on 12 of 14 instruments. Mothers of children with FAS and PFAS were significantly lower in educational attainment, shorter, later in pregnancy recognition, and suffered more depression, and used marijuana and methamphetamine during their pregnancy. Most pre-pregnancy and pregnancy drinking measures were worse for mothers of FAS and PFAS. Excluding a significant difference in simply admitting drinking during the index pregnancy (FAS and PFAS = 75% vs. 39.4% for controls), most quantitative intergroup differences merely approached significance. This community’s prevalence of FAS is 2.9 to 7.5 per 1,000, PFAS is 7.9 to 17.7 per 1,000, and combined prevalence is 10.9 to 25.2 per 1,000 or 1.1% to 2.5%.
Conclusions
Comprehensive, active case ascertainment methods produced rates of FAS and PFAS higher than predicted by long-standing, popular estimates.
Keywords: fetal alcohol spectrum disorders, alcohol use and abuse, maternal risk factors, prenatal alcohol use, prevalence, children with FAS and PFAS, United States
1. INTRODUCTION
The rate of fetal alcohol syndrome (FAS) in the United States (US) was estimated for many years, and believed by many, to be 0.33 to 3.0 per 1000 children (Abel, 1998; Abel and Sokol, 1987, 1991; CDC, 1995, 1997; Fox et al., 2015; May and Gossage, 2001; Stratton et al., 1996). However, given the lack of active case ascertainment studies (which seek to identify cases in a general population) of any fetal alcohol spectrum disorders (FASD) in the US and other developed countries (May et al., 2009), many people believed that the above estimates were substantial under estimates. FASD are rarely recognized or diagnosed in the general population or in general clinical settings (Chasnoff et al., 2015). Therefore, surveillance and clinic-based studies are unlikely to produce a true prevalence (Fox et al., 2015). Active case ascertainment studies undertaken in schools have proven to be accurate and to produce high rates of FASD (May et al., 2009). In-school study methods for large communities have been employed mostly in South Africa and Italy (May et al., 2000, 2006, 2007, 2011, 2013a; Urban et al., 2008; Viljoen et al., 2005).
Prior to 2014, only three articles had been published that utilized active case ascertainment methods in schools in the US general population. Clarren et al. (2001) reported a rate of 3.1 FAS cases per 1,000 in a county in Washington State. In that study, only one of the seven FAS cases found in the schools had been diagnosed previously. Burd et al. (1999) and Poitra et al. (2003) have reported on active case ascertainment methods used in multiple years in Head Start classrooms using a screening tool and follow up dysmorphology exam. Burd et al. (1999) reported six cases of FAS out of 1,013 children, 5.9 per 1,000. Poitra et al. (2003) included more children from the same community and 4.3 children per 1,000 had FAS. The authors do mention that some of the children with FAS identified in the Head Start studies had been diagnosed prior to the study, but this community benefited from an active dysmorphology consultation service. Many undiagnosed cases of FAS and other FASD exist in the US population, and active case ascertainment studies are designed to find them and to estimate the true prevalence of FASD.
1.1 Purpose
The purpose of this study was to: 1.) determine the feasibility of using these particular active case ascertainment methods in a US community to identify children with FAS and partial fetal alcohol syndrome (PFAS) in public and private schools; 2.) ascertain maternal risk factors for FASD in a general US population; and 3.) determine the prevalence and characteristics of children with FAS and PFAS in this community. Data were collected in three samples of first grade students in this Rocky Mountain Region City (RMRC). This study was initiated at the invitation of the city/county health department, endorsed by school administrators, and approved by the Board of Trustees of the City Schools. The studies utilized research methods pioneered in South Africa and Italy.
1.2 The Study Community
The population of RMRC is 59,000 in a county of 85,000. The composition of the population is 88.5% White, 5.0% American Indian, 3.8% mixed race, 1.1% Black, and 3.4% of Hispanic ethnicity (US Census, 2015). Compared to the US population, the study community was more White (+10.8%), less Black (−12.1%), more American Indian (+3.8%) and less Hispanic (−13.7%). RMRC residents are predominantly middle class, with average economic indicators similar to small cities and counties in the region and state. The averages in the county are slightly below US averages on a number of economic indicators: per capita income is $24,100 (US average is $28,155), median household income is $43,800 (US = $53,000), and 16.5% are below the poverty level (US = 15.4%) (US Census, 2015). Per capita alcohol consumption in this state was 2.99 gallons (11.3 liters) of ethanol per year in 2009 compared to the US average of 2.3 gallons (8.7 liters) (LaVallee and Yi, 2011). The overall health rank of this state by the United Health Foundation (2014) is well in the upper middle tier, between 20 and 25 of 50 states. County data from the CDC Behavioral Risk Factor Surveillance System (BRFSS) indicates less binge drinking overall (13.3%) than in either the state (16.9%) or the US (15.5%); however among adults ages 18 – 44, 18.3% binge drink which compares to 24.5% for the state (“Western” County, 2011). Heavy drinking was reported for this county at 4.9%, compared to 5.9% for the state, and 5.1% for the US (“Western” County, 2011). Among adults 18 – 44 years, heavy drinking was 5.6% in the County and 6.9% for the State (“Western” County, 2011). Binge drinking among women (four or more drinks per occasion) was lower at 14.3% in this state than among males (five or more drinks per occasion) at 27.3% (“State” BRFSS, 2013), but bingeing among women was 2.2% higher than US averages (12.5%) (CDC, 2013). Therefore, this site has slightly lower economic status than US averages, overall good health, but indicators of heavy drinking and binge drinking are lower than the home state but higher than US averages.
2. METHODS
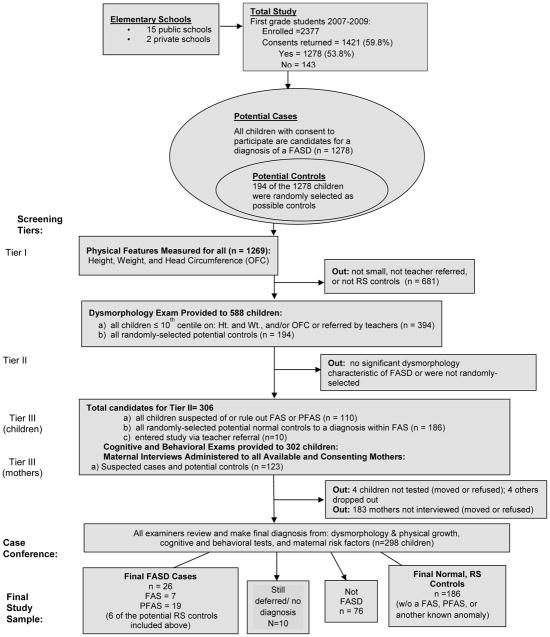
The diagnostic criteria used for FAS and PFAS (see Figure 1) were originally set forth by the FAS advisory group of the U.S. Institute of Medicine (Stratton et al., 1996), and operationalized and updated from clinical experience (Hoyme et al., 2005). The classification of the children is based on: (1) specific facial dysmorphology, (2) diminished physical stature and/or weight, (3) defined intellectual, developmental, social, and neuropsychological assessments, and (4) multiple measures of maternal alcohol consumption. Data for all of the diagnostic domains were collected and analyzed by members of a multidisciplinary clinical team, each member (physicians, psychologists, and interviewers) working blinded to any prior knowledge of the diagnostic findings in other parts of the study and the child and family. The data and findings were summarized for each child, and structured case conferences were held among the specialists. Highly experienced pediatric dysmorphologists/clinical geneticists assigned final diagnoses after considering all of the relevant data and the opinions of team members involved in the examination, testing, and interview process. The diagnoses considered in this study were: FAS, PFAS, normal/not FAS, another known disorder, or deferred for further testing. Due to limited resources and a short time frame, the team focused on only FAS and PFAS diagnoses, as these conditions exhibit the most clearly defined dysmorphic features in the FASD continuum. Detailed numbers of sampling and the flow of the study are found in Figure 2.
Figure 1.
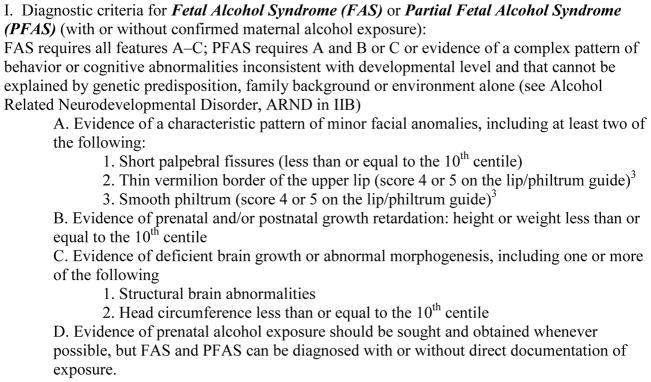
Diagnostic Guidelines for Specific Fetal Alcohol Spectrum Disorders (FASD), According to the Institute of Medicine, as Clarified by Hoyme et al., 2005
1Stratton KR, Howe CJ & Battaglia FC (1996) Fetal alcohol syndrome diagnosis, epidemiology, prevention, and treatment. Institute of Medicine. National Academy Press, Washington, D.C.
2Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller J, Khaole N, Viljoen DL, Jones KL, Robinson LK (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 115:39–47.
3Astley SJ, Clarren, SK (2000) Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol 35:400–10.
Figure 2.
Methodology of the Rocky Mountain Region City (USA) FASD Pilot Studies with Sampling Procedures and Numbers
2.1 Child Data
The overall study population was all children enrolled in first grade (n=2377) in all of the 17 elementary schools (15 public and 2 private) during school years beginning in 2007, 2008, and 2009. Children who participated in the study did so via active (direct written) parental consent. While almost 60% of the parents returned the consent forms for the combined three samples (n=1421), 143 checked the box that declined participation; so the total consented into the study was 1278 (53.8%) (see Figure 2). The return of forms was lowest in the first sample (53%), better in the second (57%), and extra effort by the staff (multiple mailings and phone calls) in sample 3 led to a 70% return rate.
Data on the growth, development, and dysmorphology of the children were collected via a three-tier method. In Tier I all consented children were screened for height, weight, and occipitofrontal (head) circumference (OFC) in their respective schools by project staff. Then, if the child had measurements ≤ 10th centile on OFC and/or both height and weight on the Centers for Disease Control and Prevention (CDC) growth charts, he/she was referred for Tier II screening, a full dysmorphology exam, (n = 588). Because children with FASD are often not recognized or identified (Chasnoff et al., 2015), special referrals of children experiencing problems in school were also accepted from the teachers (n=10). For the dysmorphology exams, all qualifying children were seen by the research team in their respective schools (in specially-arranged rooms). Three pediatric clinical geneticists/dysmorphologists who are highly trained in and experienced with FASD diagnoses provided the exams. Each child was examined individually and independent of any prior knowledge (e.g. reason for entry to the study). Each physician-led team collected independent measures of growth, physical development, and dysmorphology and recorded them on a structured inventory form for child data salient to FASD and other known birth defects. This form includes a weighted FAS dysmorphology score (Hoyme et al., 2005). In the first sample of this study, each child received identical exams from two dysmorphologists; in samples 2 and 3, only one exam. At the end of each day, after the dysmorphology assessments, a preliminary diagnosis of FAS, PFAS, deferred, another diagnosis, or not FASD was assigned by the examining physicians. Those children who received a preliminary diagnosis of a FASD or deferred were advanced to cognitive and behavioral testing as part of Tier III of the study.
2.2 Normal Controls
Candidates for normal comparison/control children were picked by random methods (without replacement) from the school rolls. By drawing the children from the same first grade population, the final control children were well matched with the subject children by age, sex, and general environment. Identical physical exams and cognitive/behavioral testing were performed on the control candidates. One hundred-ninety four (194) of the 1,278 children entered the study randomly (see Figure 2).
2.3 Developmental Testing
Tier III developmental testing over the three samples included the following battery: 1) Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) was used as a measure of verbal, nonverbal and overall intelligence; the Digit Span, coding and mazes subtests of the Wechsler Intelligence Scale for Children (WISC, 3rd ed.) were used as specific measures of memory, processing speed and visual/planning respectively (Wechsler, 1991); the Wide Range Achievement Test (WRAT; Wilkinson, 1993); the Vineland Adaptive Behavior Scales (Classroom Edition; Sparrow et al., 2006); and the Personal Behavior Checklist (PBCL; Streissguth and Barr, 1998). All tests were administered by the local school staff psychologists blinded to all other findings in this study.
2.4 Maternal Interviews
Also in Tier III, all available mothers of the children with preliminary diagnoses of FASD or deferred and the mothers of control candidates who signed a second, separate consent form, were interviewed in person with a maternal risk factor questionnaire. One hundred and twenty-three (123) mothers were interviewed: 65.4% of all mothers of the FAS or PFAS cases, and 56.9% of the mothers of the randomly-selected, normal controls completed interviews. All participants received gift cards to a local store as an incentive.
The questionnaire was developed specifically for FASD epidemiology studies. Retrospective data collection reconstructs maternal behavior and traits before, during, and after gestation of the index child. While a substantial focus is on alcohol use during the index pregnancy, questions also include items on distal risk variables: childbearing history; drinking history and drinking by quantity, frequency, and timing; marital status; socioeconomic status (SES); and nutrition. To establish rapport, nonthreatening questions are asked first, and the interview moves to information on health and childbearing. Next, current drinking is explored via dietary intake and a 1-week, day-by-day log, as alcohol consumption accuracy is facilitated by this format, especially in the context of dietary questions (King, 1994). Multiple measures of alcohol use before, during, and after pregnancy are asked, paying special attention to alcohol brands and containers used (vessels measurement) to calibrate amounts consumed (Kaskutas and Graves, 2000, 2001). Alcohol is calibrated in standard US units where one drink equals: 340 ml can/bottle of beer (5% ethanol), 120 ml of wine (11% ethanol), or 44 ml of distilled spirits (43% ethanol). Questions on current drinking are used as benchmarks to establish the method of reporting and for refreshing recall before the timeline follow-back questions begin (Sobell et al., 1988, 2001). Then gestational drinking is queried. Although some studies indicate that prospective (prenatal) reports of one’s drinking are valid predictors of neurobehavioral outcomes (Jacobson et al., 2002), other studies indicate that retrospective reports of gestational alcohol use are accurate for a variety of other topics (Alvik, 2006; Czarnecki et al., 1990; Hannigan et al., 2010). All interviews were conducted by experienced interviewers, predominantly nurses employed by the city/county health department and subcontracted by the project.
2.5 Data Analysis
Data processing utilized EPI INFO (Dean et al., 1994) and SPSS (IBM, 2011) to compare groups via two-tailed statistical tests of significance with Bonferroni adjustments of alpha levels in the first two tables to account for possible familywise error among certain variables (Tabachnick and Fidell, 2013). Comparing three groups on continuous variables, one-way Analysis of Variance (ANOVA) was used, with Welch adjustment when homogeneity of variance was significantly violated at alpha = .01. Post-hoc analyses tested for significant differences between each of the three groups via Dunnett Pairwise comparison (C) tests (which compensate for highly different sample sizes and heterogeneity of variance among groups) at an alpha level of .05. Due to the exploratory nature of this inquiry into maternal risk factors in a general U.S. population, alpha level of .05 (two-tailed) was used to interpret significance for all maternal risk comparisons in Table 3.
TABLE 3.
Maternity History and Substance Use by Mothers of Randomly-selected Control Children Compared to Mothers of Children with FASD: Rocky Mountain Region City, USA
| Variable | Mothers of Randomly-selected Control Children (n = 106) | Mothers of Children with FASD (n = 17) | Significance P |
|---|---|---|---|
|
Demographic and Physical variables
| |||
| Educational attainment | |||
| High School diploma or less | 7.9 | 38.9 | |
| Some college or 2 year degree | 22.5 | 16.7 | |
| Bachelors/Graduate School | 69.7 | 44.4 | .002a |
|
| |||
| Annual household income at time of interview | 55436 (45118) | 45825 (27139) | .560b |
|
| |||
| Annual household income during pregnancy | 54563 (54148) | 37114 (26023) | .407b |
|
| |||
| Age during index pregnancy - Mean (SD) | 28.3 (6.06) | 25.8 (5.76) | .120 b |
|
| |||
| Height (inches) – Mean (SD) | 64.9 (3.1) | 63.3 (2.9) | .049b |
|
| |||
| Weight (lbs) – Mean (SD) | 163.2 (38.4) | 160.8 (39.5) | .816b |
|
| |||
| BMI – Mean (SD) | 27.3 (6.1) | 28.5 (7.7) | .461b |
|
| |||
| Gravidity - Mean (SD) | 3.3 (2.11) | 4.4 (1.73) | .060b |
|
| |||
| Parity -Mean (SD) | 2.8 (1.53) | 3.2 (1.76) | .317b |
|
| |||
| Age during index pregnancy - Mean (SD) | 28.3 (6.06) | 25.8 (5.76) | .120 b |
|
| |||
| Birth order of index child - Mean (SD) | 2.0 (1.49) of 2.7 (1.51) |
2.5 (1.61) of 3.3 (1.89) |
.195b .222b |
|
| |||
| Number of weeks before woman realized her pregnancy -Mean (SD) | 5.8 (3.17) | 8.6 (8.17) | .012b |
|
| |||
| First sought prenatal care | |||
| 1st trimester | 96.2 | 82.4 | |
| After 1st trimester | 3.8 | 17.6 | .055c |
|
| |||
| Took vitamins during pregnancy (% Yes) | 98.1 | 87.5 | .083c |
|
| |||
| Sexually Transmitted Infection during pregnancy (% Yes) | 0.9 | 11.8 | .050c |
|
| |||
| Experienced Post-Partum depression (% Yes) | 22.7 | 46.7 | .048b |
|
| |||
| Maternal Alcohol and Drug Use | |||
|
| |||
| Drank in lifetime (% Yes) | 95.2 | 100.0 | .345a |
|
| |||
| Current drinker (in last year) (% Yes) | 78.6 | 68.8 | .379a |
|
| |||
| Among current drinkers, drank in week prior (% Yes) | 54.3 | 36.4 | .286a |
|
| |||
| Age first tried alcohol – Mean (SD) | 16.6 (3.24) | 15.4 (2.98) | .170b |
|
| |||
| Age began drinking regularly – Mean (SD) | 19.8 (5.06) | 17.8 (2.91) | .138b |
|
| |||
| Drank in 3 months before index pregnancy (% Yes) | 57.6 | 76.0 | .516a |
|
| |||
| Drank during index pregnancy (% Yes) | 39.4 | 75.0 | .003d |
|
| |||
| Among those who drank during pregnancy, usual drinks consumed on typical drinking day during 1st trimester– Mean (SD) | 3.2 (2.38) | 5.3 (0.58) | .146b |
|
| |||
| Among those who drank during pregnancy, usual drinks consumed on typical drinking day during 2nd trimester– Mean (SD) | 0.0 (0.00)* | 2.0 (0.00)* | 1.00b |
|
| |||
| Among those who drank during pregnancy, usual drinks consumed on typical drinking day during 3rd trimester - Mean (SD) | 1.0 (0.00)* | 1.0 (0.00)* | 1.00b |
|
| |||
| Binged (3+) during index pregnancy (% Yes) | 38.5 | 100.0 | .054a |
|
| |||
| Used meth in lifetime (% Yes) | 4.2 | 23.5 | .008a |
|
| |||
| Use painkillers without a prescription in lifetime (% Yes) | 5.6 | 18.8 | .078a |
|
| |||
| Marijuana use during pregnancy (% Yes) | 0.0 | 23.5 | .001c |
|
| |||
| Meth use during pregnancy (% Yes) | 1.4 | 11.8 | .092c |
X2 test.
t- test.
Fisher’s exact
z-test of proportions
Only one mother reported drinking during the 2nd and 2 during the 3rd trimester.
3. RESULTS
3.1 Child Physical Growth Traits and Dysmorphology
Table 1 summarizes demographic and physical features of all consented children (column 1), randomly-selected controls (column 2, n = 190), and children with final diagnoses of PFAS (n = 19), and FAS (n = 7). Values for the common measures of the randomly-selected control sample and those of consented children are similar; randomly-selected children are well within overall growth parameters. The children of the diagnostic categories did not differ significantly on sex or race/ethnicity. However, the FAS and PFAS children differed significantly from the randomly-selected normal controls on age, height, weight, and body mass index (BMI) centile, and height and weight differences were expected by definition. Bonferroni corrected values (p<.005) are not appropriate for these particular variables as they represent required diagnostic criteria. Also as planned and required by definition, averages for each of the key FAS clinical markers, FAS, PFAS, and controls differed significantly: OFC, palpebral fissure length (PFL), smooth philtrum, and narrow vermilion border. For other minor anomalies that are frequently found in children with FAS or PFAS, flat nasal bridge, heart murmur, hypertrichosis, and maxillary and mandibular arcs differed significantly across groups with either standard alpha levels (p<.05) or the Bonferroni corrected alpha level (p<.0063). In addition, clinical assessments identified hypoplastic midface, clinodactyly, and camptodactyly as approaching statistical significance between groups. Dunnett C analyses indicated that height and weight differed significantly among all three groups (FAS from PFAS, FAS from controls, and PFAS from controls), OFC and PFL differ between both FAS and controls and PFAS and controls, and maxillary arc differs significantly between FAS and PFAS and FAS and controls. Total dysmorphology scores differ significantly across groups, and while it does not differ in an individual Dunnett comparison between FAS and PFAS, it is highest for the children with FAS (mean = 13.3, SD = 4.7), and then PFAS (mean = 11.3, SD = 3.9). Children with FAS met standard clinical criteria and exceeded it in many cases as they are the most dysmorphic and growth deficient, followed by children with PFAS.
TABLE 1.
Demographic and Growth Parameters for all 1st Grade Children, Children with FAS, Partial FAS, and Randomly-selected Controls: Rocky Mountain Region City, USA
| Physical variable | All 1st Grade Childrena (N = 1278) | Randomly Selected Control Children (n =190) | Children with Partial FAS (n = 19) | Children with FAS (n = 7) | P |
|---|---|---|---|---|---|
| Demographic, Growth, and Cardinal FASD variables* | |||||
|
| |||||
| Sex (%) | |||||
| Males | 54.5 | 64.2 | 63.2 | 28.6 | |
| Females | 45.5 | 35.8 | 36.8 | 71.4 | (0.159)b |
|
| |||||
| Race/Ethnicity (%) | |||||
| White (Non-Hispanic) | 86.6 | 85.8 | 77.8 | 85.7 | |
| Hispanic | 2.6 | 2.3 | 11.1 | 0.0 | |
| Black, African American | 1.5 | 2.8 | 0.0 | 0.0 | |
| Asian or Pacific Islander | 0.7 | 1.1 | 0.0 | 0.0 | |
| American Indian/Alaska Native | 6.6 | 5.1 | 5.6 | 14.3 | |
| Mixed | 1.7 | 2.8 | 0.0 | 0.0 | |
| Other | 0.4 | 0.0 | 5.6 | 0.0 | (0.131)b |
|
| |||||
| Age (months) - Mean (SD) | 84.3 (4.52) | 84.5 (4.42) | 89.4 (6.40) | 85.6 (5.77) | <.001c,3 |
|
| |||||
| Height (cm) - Mean (SD) | 121.8 (5.77) | 122.8(5.53) | 113.0 (4.55) | 119.5 (6.51) | <.001c,1,2,3 |
|
| |||||
| Weight (kg) - Mean (SD) | 25.6 (5.89) | 26.8 (6.50) | 18.8 (2.60) | 26.6 (8.85) | .008c,1,2,3 |
|
| |||||
| Child’s BMI - Mean (SD) | 16.8 (2.51) | 17.3 (2.78) | 16.4 (2.60) | 15.2 (1.38) | .049c |
|
| |||||
| BMI centile - Mean (SD) | 63.1 (26.42) | 69.4 (23.44) | 54.4 (24.00) | 40.0 (30.67) | <.001c,3 |
|
| |||||
| Occipital Circumference (cm) - Mean (SD) | 52.3 (2.01) | 52.6 (1.49) | 51.3 (1.40) | 49.7 (1.45) | <.001c,2,3 |
|
| |||||
| Palpebral Fissure Length (cm) - Mean (SD) | - | 2.5 (0.11) | 2.4 (0.14) | 2.3 (0.10) | <.001c,2,3 |
|
| |||||
| Smooth Philtrum (%) | - | 22.1 | 89.5 | 85.7 | <.001b |
|
| |||||
| Narrow Vermilion Border (%) | - | 24.2 | 68.4 | 85.7 | <.001b |
|
| |||||
| Other minor anomalies** | |||||
|
| |||||
| Hypoplastic Midface (%) | - | 24.2 | 38.9 | 57.1 | (.071)b |
|
| |||||
| Flat Nasal Bridge (%) | - | 1.1 | 15.8 | 14.3 | <.001b |
|
| |||||
| Heart Murmur (%) | - | 1.1 | 0.0 | 14.3 | 0.012b |
|
| |||||
| Clinodactyly (%) | - | 28.9 | 52.6 | 14.3 | (0.065)b |
|
| |||||
| Camptodactyly (%) | - | 5.8 | 10.5 | 28.6 | .054b |
|
| |||||
| Hypertrichosis (%) | - | 1.6 | 0.0 | 14.3 | 0.041b |
|
| |||||
| Maxillary Arc – Mean (SD) | - | 24.9 (1.1) | 24.5 (1.1) | 24.5 (1.1) | .005c1,2 |
|
| |||||
| Mandibular Arc – Mean (SD) | - | 25.9 (1.3) | 25.4 (1.1) | 25.4 (1.1) | .029c2 |
|
| |||||
| Dysmorphology Score - Mean (SD) | - | 4.1 (3.18) | 11.3 (3.92) | 13.3 (4.65) | <.001c2,3 |
The “All Children” group is not included in any of the Table 1 statistical test analyses. The 1,278 children represent all who had consent to participate in the study.
X2 test of data comparing children with FAS, Partial FAS, and controls.
ANOVA of data comparing children with FAS, Partial FAS, and controls.
Bonferoni corrected alpha level for section 1 is <.005
Bonferoni corrected alpha for section 2 is <.0063
Significant Dunnett C post-hoc difference (p<.05) between:
FAS & PFAS;
FAS & Controls;
PFAS & Controls
3.2 Cognitive and Behavioral Indicators of FASD
Scores on neurodevelopment tests (Table 2 and Figure 3) are compared among children of the three diagnostic groups. Mean I.Q. scores were similar for FAS and PFAS groups and significantly lower than those for controls with both the ANOVA values and Dunnett C comparisons. Overall the means of 12 of the developmental and behavioral variables were statistically significant among groups with alpha=.05 and 11 of 13 with Bonferroni corrected values. Pairwise analyses revealed that the traits that most distinguish FAS and PFAS groups from controls were reading and communication, as both FAS and PFAS groups performed significantly worse than controls. Children with PFAS had the most documented behavioral problems, and total behavioral problems did not differ between the FAS and control children.
TABLE 2.
Developmental and Behavioral Indicators+,^ of Randomly-Selected Control Children Compared to Children with PFAS and FAS: Rocky Mountain Region City, USA
| Child Variables | Randomly Selected Control Children (n= 186) | Children with PFAS (n = 19) | Children with FAS (n = 7) | Significance P |
|---|---|---|---|---|
|
Developmental Traits*
| ||||
| Verbal IQa - Mean (SD) | 108.1 (14.14) | 90.2 (15.78) | 84.3 (10.19) | <.0012,3 |
| Non-verbal IQa - Mean (SD) | 104.5 (16.84) | 89.0(11.98) | 92.6 (9.13) | <.0012,3 |
| Full Scale IQa - Mean (SD) | 107.2 (13.85) | 88.3 (13.14) | 87.0 (8.25) | <.0012,3 |
| Digit Spanb - Mean (SD) | 10.3 (2.91) | 7.5 (3.22) | 8.3 (2.22) | <.0013 |
| Codingb - Mean (SD) | 11.3 (3.44) | 9.6 (3.92) | 12.3 (3.73) | (.097) |
| Mazesb - Mean (SD) | 10.8 (3.60) | 8.4 (4.6) | 9.1 (2.27) | .015 |
| Readingc - Mean (SD) | 111.8 (14.26) | 92.6 (17.53) | 99.9 (8.92) | <.0012,3 |
| Spellingc - Mean (SD) | 108.4 (13.31) | 90.4 (16.45) | 102.4 (13.87) | <.0013 |
| Arithmeticc - Mean (SD) | 107.8 (15.81) | 84.8 (14.84) | 89.1 (21.12) | <.0013 |
|
| ||||
|
Behavioral Traits**
| ||||
| Communicationd - Mean (SD) | 106.1 (16.42) | 85.3 (14.23) | 94.7 (9.27) | <.0012,3 |
| Daily Living Skillsd - Mean (SD) | 107.6 (16.39) | 90.8 (18.25) | 99.4 (11.79) | <.0013 |
| Socializationd - Mean (SD) | 104.5 (15.40) | 92.3 (14.43) | 99.1 (10.30) | .0033 |
| Composited - Mean (SD) | 107.2 (17.41) | 86.9 (14.44) | 97.0 (8.81) | <.0013 |
| Behaviore - Mean (SD) | 6.0 (5.94) | 15.3 (9.55) | 7.9 (6.57) | <.0013 |
All scores standardized for age of child at time of testing.
ANOVA of data comparing randomly-selected control children and children with Partial FAS, and FAS. NS = Not statistically significant.
Wechsler Abbreviated Scale of Intelligence - (WASI). (Mean score of 100 with Standard Deviation of 15).
Wechsler Intelligence Scale for Children 3rd edition coding, mazes and digit span subtests - (WISC). (Score range: maximum = 20, minimum = 0).
Wide Range Achievement Test Revision 3 - (WRAT). (Mean score = 100 with a Standard Deviation of 15).
Vineland Adaptive Behavior Scales (Classroom Edition). (Mean score = 100 with a Standard Deviation of 15).
Personal Behavior Checklist (PBCL-36). Higher scores indicate more behavioral problems.
Bonferoni corrected alpha for section developmental traits = <.005;
Bonferoni corrected alpha for behavioral traits = <.01
Significant Dunnett C post-hoc difference (p<.05) between:
FAS & PFAS;
FAS & Controls;
PFAS & Controls
Figure 3.
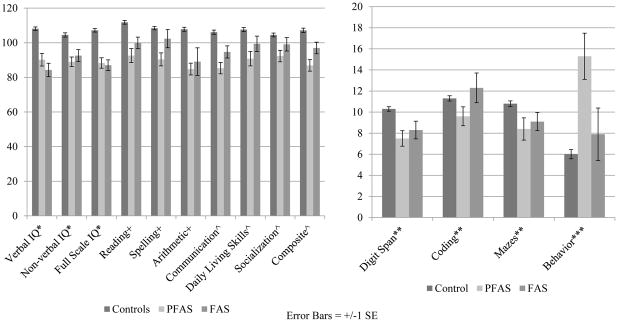
Comparison of Developmental Tests and Behavioral Indicators of Children with FAS, PFAS, and Normal Controls: Rocky Mountain Region City, USA.
#All scores standardized for age of child at time of testing.
*Wechsler Abbreviated Scale of Intelligence - (WASI). (Mean score of 100 with Standard Deviation of 15).
+Wide Range Achievement Test Revision 3 - (WRAT). (Mean score = 100 with a Standard Deviation of 15).
^d. Vineland Adaptive Behavior Scales (Classroom Edition). (Mean score = 100 with a Standard Deviation of 15).
**Wechsler Intelligence Scale for Children 3rd edition coding, mazes and digit span subtests - (WISC). (Score range: 0–20).
**Personal Behavior Checklist (PBCL-36). Higher scores indicate more behavioral problems.
3.3 Variables of Maternal Risk for FASD
Maternal risk variables for the combined FASD diagnostic groups and randomly-selected controls are in Table 3. As the maternal questionnaire cast a broad net in a small sample in a US community, many variable comparisons did not reach statistical significance among groups. Mothers of children with FAS or PFAS were significantly more likely to have reported drinking during the index pregnancy than mothers of normal controls (75% vs 39%). They also have significantly lower educational attainment levels than controls, are shorter in stature, and report finding out that they were pregnant significantly later in the index pregnancy (8.6 weeks, SD = 8.2) than controls (5.8, SD = 3.2), have had a sexually transmitted disease sometime in their life, and were more likely to report post-partum depression. Approaching significance at alpha = .05 were: higher gravidity, a lower percentage seeking prenatal care in the first trimester, and a lower use of prenatal vitamins for the mothers of children with a FASD. Mothers of children with FASD were significantly more likely to report using marijuana in addition to alcohol during pregnancy. Approaching significance were reports of binge drinking during the index pregnancy among mothers of children with FASD (100% vs. 38.5%, p=.054) and use of methamphetamines and painkillers without a prescription. Even though more mothers of children with FASD reported drinking during the index pregnancy, there were no significant differences between the groups on any current drinking measures (not shown in Table 3). Overall, alcohol use during early life and during the index pregnancy was reported to be higher for mothers of children with FASD: drinking regularly at an earlier age, consuming alcohol three months prior to pregnancy, and drinks per drinking day during the first and second trimesters of the index pregnancy.
3.4 Prevalence of FASD Estimated by Three Techniques
Final diagnosis numbers and rates for the children are presented in Table 4, left section. Seven children were diagnosed with FAS and 19 with PFAS. With the first estimation technique, two denominators were used: children enrolled in 1st grade classes at all schools (n=2377), and the number with consent to participate in this study (n=1278). The assumption was that oversampling small children provided the highest probability of including most of the children with FAS or PFAS. The rate of FAS with this technique is between 2.9 and 5.5 per 1,000. PFAS is 7.9 to 14.9 per 1,000 (see Table 4).
TABLE 4.
Rocky Mountain Region Pilot Studies of FAS & PFAS prevalence: combined data from three samples (rate per 1,000)
| Diagnosis | Oversample of children ≤ 10th centile on height, weight, or OFC | Randomly-Selected Children Only (n=194) | Combined rate from consented sample (n=1278) and estimated cases in the non-consented sample (n=1099) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) n | Rate per all children in 1st grade (n=2377)a | Rate for all children with consent for study (n=1278)b | Mid-point | n | Proportion of FASD cases in random sample | (b) Estimated case in non-consented population (n=1099) | Rate of FASD cases from random samplec | 95% Confidence Intervals | (a + b) Total Estimated Cases n | Estimated rate for all enrolled studentsd | 95% Confidence Intervals | |
| FAS | 7 | 2.9 | 5.5 | 4.2 | 2 | .0103 | +11 | 10.3 | 0 to 24.5 | 18 | 7.5 | 4.1 to 11.1 |
| PFAS | 19 | 7.9 | 14.9 | 11.4 | 4 | .0206 | +23 | 20.6 | .62 to 40.6 | 42 | 17.7 | 12.4 to 22.9 |
| FAS & PFAS combined | 26 | 10.9 | 20.3 | 15.6 | 6 | .0309 | +34 | 30.9 | 6.6 to 55.3 | 60 | 25.2 | 18.9 to 31.5 |
Rate per 1,000 children based on the entire enrollment in 1st grade classes, denominator = 2377.
Rate per 1,000 children based on the sample screened, denominator = 1278.
Rate per 1,000 children based on the randomly-selected children only, denominator = 190.
Rate per 1,000 children calculated from FASD cases diagnosed in consented sample added to the estimated cases in the non-consented sample utilizing the proportional diagnostic distribution of FASD cases from the randomly-selected children. Denominator is 2377.
A second rate was calculated from the 6 cases of FASD (2 FAS and 4 PFAS) found within the 194 children who entered the study via random selection (Table 4, middle columns). The estimated rates from this sample are: 10.3 FAS cases per 1,000 (95% CI = 0 to 24.5) and 20.6 cases of PFAS per 1,000 (95% CI = .62 to 40.6). The combined FAS and PFAS rate is 30.9 per 1,000 (95% CI = 6.6 to 55.3).
The third rate (Table 4, far right columns) was calculated from the number of cases which would likely have been found in the 1,099 unconsented children. Projecting the proportions of FAS (.0103) and PFAS (.0206) diagnoses found in the random sample (technique 2) to estimate the number of cases among the unconsented children (n = 1099) and adding them (FAS = 11 estimated cases, PFAS = 23 estimated cases) to the cases diagnosed in the consented population, technique 3 yields a FAS rate of 7.5, PFAS of 17.7, and a combined rate of 25.2 per 1,000 (95% CI=18.9 to 31.5) or 2.5% (Table 4, section 3).
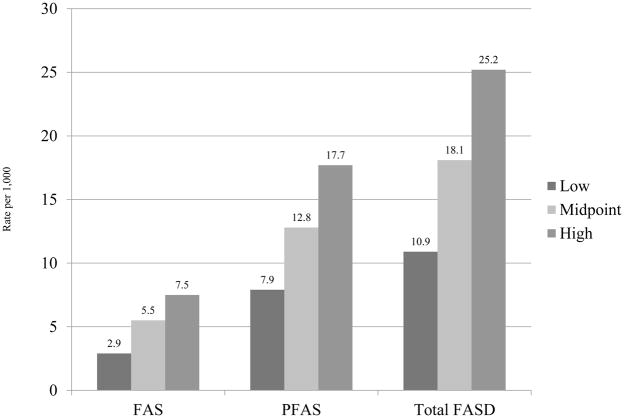
From the above estimation techniques, we believe that the most accurate and empirically-based rates for this community are: FAS 2.9 to 7.5 per 1,000 and PFAS 7.9 to 17.7 per 1,000. The combined rate of FAS and PFAS is 10.9 to 25.2 per 1,000 or 1.1% to 2.5% (Figure 4).
Figure 4.
Final Prevalence of FAS, PFAS, and the Two Diagnoses Combined among First Grade Children in a Rocky Mountain Region City
4. DISCUSSION
Active case ascertainment methods for identifying children with lagging growth and development and significant dysmorphia associated with FAS and PFAS worked well in this population. Non-reporting or underreporting of maternal drinking, however was a greater challenge in this US community than encountered in South Africa, but comparable to the challenges in Italy and other first world countries (Alvik et al., 2006, 2005; Ceccanti et al., 2014; Garcia-Algar et al., 2008; Manich, et al., 2012; Montag et al., 2015; Morini et al., 2013; Wurst et al., 2008). Nevertheless, the overall profile established for mothers of the children with FASD is a consistent one of consistently higher risk for mothers of FASD than for mothers of normal controls. For the diagnostic process, both the child and maternal information proved to be informative and vital, and we have found many similarities to other populations in this RMRC sample. The early experience within the RMRC, and our findings overall led us to explore opportunities for larger and more comprehensive in-school studies in other US communities.
4.1 Findings Common to Other US Studies
A similar study which we have completed in a representative Midwestern City produced similar prevalence and traits: the FAS rate was 6 to 9 children per 1,000; PFAS was 11.3 to 17.2 per 1,000; and the FAS and PFAS combined rate was 17.2 to 26.1 per 1,000 or 1.7 to 2.6% (May et al., 2014). The prevalence of FAS in these two studies is also comparable to, but somewhat higher than the three other US in-school studies cited in the introduction (Burd et al., 1999; Clarren et al., 2001; Poitra et al., 2003). Furthermore, the child and maternal risk traits in the Midwestern City are consistent with those in the RMRC study. In addition to the cardinal dysmorphic facial features of FAS and PFAS dictated by IOM criteria, a substantial number of minor anomalies that discriminate children with FASD from controls and are consistent between studies are reflected in the total dysmorphology score. The following minor anomalies haven proven to significantly distinguish children with FASD from normal children in our two US studies and in other, clinic-based dysmorphology studies in the US: diminished maxillary and mandibular arcs (other subtle measures of midface underdevelopment), and clinodactyly and camptodactyly of the fingers (Feldman et al., 2011, 2012; Jones et al., 2010).
There are also some common maternal risk factors in the two US studies: later recognition of pregnancy and fewer prenatal visits in the index pregnancy, substantial drinking reported during the 3 months prior to pregnancy, admission of drinking in 2 of 3 trimesters, and reports of bingeing at some time in adult life.
4.2 Maternal Risk Factors and Recruitment Issues
In both US community prevalence studies to date it was difficult to recruit mothers and to obtain what were believed to be accurate reports of prenatal drinking, let alone accuracy of quantity, frequency, and timing (QFT) of drinking. Drinking results, when compared across mothers of FASD and control groups, were often not statistically significant for the US samples, but the profile of maternal risk factors was consistently patterned in the directions predicted by similar, maternal risk studies elsewhere (Ceccanti et al., 2014; May et al., 2005, 2008, 2013a; Viljoen et al. 2002). Maternal drinking studies in more candid populations such as among South African mothers in small towns, have proven to be far more discriminating among diagnostic groups and have produced more realistic reports of drinking QFT than encountered in US studies thus far (May et al., 2013b). Knowing that prenatal drinking is potentially damaging for the fetus, and the stigma that this knowledge produces in well-educated populations, suppresses the willingness among women to be forthcoming about alcohol consumption during pregnancy. It has proven to be more difficult to engage US mothers successfully and therefore, to assess proximal risk. QFT drinking measures did not discriminate the groups as well as in South Africa, but did so in a manner comparable to previous studies in Italy (Ceccanti et al., 2014). While the overall trajectory of reported drinking was in the direction predicting greater risk among the mothers of children with a FASD diagnosis, only some measures were significantly significant or approached significance. Many studies indicate, especially those that compare self-reported usage of alcohol to biomarker evidence, that American and European women underreport or deny drinking, limiting the opportunity to determine actual alcohol exposure levels to the fetus (Morini et al., 2013; Wurst et al., 2008).
The less sensitive, less guilt-inducing, distal risk variables such as childbearing history, education, and demographic measures are more accurately reported than are drinking variables. However, in a well-nourished, middle SES, stable population, these distal variables prove to be less influential in creating risk for FASD than in low-SES, socially and nutritionally challenged populations. Maternal risk factors such as high gravidity, low BMI, poverty, and depression that are statistically significant in the less economically developed, binge drinking subpopulations of South Africa, were less discriminating in this US population and in Italy (Ceccanti et al., 2014). Abel and Hannigan (1995) referred to these distal co-factors of risk as permissive and provocative variables, for in the presence of the teratogenic agent (alcohol), they increase vulnerability of the fetus to the toxic effects of the agent. In South Africa they are quite influential, but in this US and Italian studies, less so.
4.3 Strengths and Weaknesses of the Study
The foremost strengths of this study were the active case ascertainment methods employed by using a multidisciplinary team of experts in assessing three samples of a representative school population. A second strength of the study was a high degree of local interest and engagement. A sage nurse for the city/county health department asked to have the study initiated in this community. After many meetings, discussions, and planning, the study was approved and supported enthusiastically. Third, school psychologists were pleased to have the opportunity to provide more cognitive and behavioral testing and developmental advice than would otherwise have been possible. Fourth, the administrators of the school system were grateful to have detailed aggregate growth and development data for their children to assist in monitoring growth, development, and BMI over the years.
There were also weaknesses. First, funding and time resources were limited, and field staffing was minimal. More staff would have helped complete more maternal interviews. Second, time only permitted two distributions of consent forms and limited follow-up in the first two samples. Even though the overall consent to participate was quite acceptable at 53.8%, recruitment in the third sample was more effective and approached 70%. Third, because of lack of time and monetary resources, over-sampling of the smaller children was limited to those who were ≤ 10th centile on height, weight, and head circumference rather than ≤ 25th as we would have liked. The latter cut-off might have recruited more children with PFAS and allowed for exploration of the diagnosis of alcohol-related neurodevelopmental disorder (ARND). Limited resources and the evolutionary state of ARND diagnostic criteria at this time, necessitated limiting the study to FAS and PFAS. Finally, we could only provide limited follow-up testing and other services for a few of the 10 equivocal cases who remained in the “deferred” category after the case conference. Further follow-up may have resulted in more cases of FASD and would have further benefitted the community and families.
Active case ascertainment methods and a comprehensive diagnostic process determined the prevalence and characteristics of FAS and PFAS in three first grade cohorts. Because of the rigor of the approach, the utilization of highly experienced clinical experts, and the characteristics of the population of this city, the prevalence of FAS and PFAS is greater in this US community than previously accepted estimates of FASD would dictate. Overall, the rate of FAS is 2.9 to 7.5 per 1,000 and PFAS is 7.9 to 17.7 per 1,000. Previously popular rates of FAS were between 0.5 and 3.0 per 1,000 (CDC, 1995, 1997; Stratton et al., 1996). Not only is the rate of FAS higher in this community, but the combined rate of FAS and PFAS is higher (1.1 to 2.5%) than the long-standing, previous estimate for total FASD of 1% (Sampson et al., 1997).
Highlights.
Fetal alcohol spectrum disorders (FASD) were studied in a United States community.
Physical and neurobehavioral traits of children with FASD are presented.
Maternal risk factors for FASD in this United States population are presented.
The rate of fetal alcohol syndrome (FAS) is 2.9 to 7.5 per 1,000 children.
Total rate of FAS and partial FAS is higher than previous estimates (1.1 to 2.5%).
Acknowledgments
Role of the funding source
The protocols and consent forms used in the study were approved by the IRB of the University of New Mexico. This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA) RO1 AA09440 and RO1/U01 AA11685. The authors have no conflict of interest to declare.
We gratefully thank our colleagues who assisted in the study, especially the Rocky Mountain City/County health officials, the Trustees and Administrators of the public and private education systems, primary school principals, school secretaries, psychologists, teachers, and others who graciously received us and facilitated the study. Craig Sivak and Mary Kay Burns also preformed maternal interviews, clinical coordination, and reserved transportation and drove the Green Breeze. We further acknowledge our appreciation of Dr. Suzanne Dixon, who followed up on some of the outstanding special developmental and medical needs of any children as identified in the study.
Co-author Jason Blankenship, who performed much of the initial data analysis and some text writing, passed away when the first draft of the manuscript was being completed.
Footnotes
Contributors
Philip May was the principle investigator (PI), and along with assistance from Julie Hasken, was the major writer and final editor of all drafts. Jason Blankenship, Wendy Kalberg, David Buckley, Jan Gossage, and Marita Brooks all played major roles in data analysis and preparation of text for the methods and results sections. Luther Robinson, Melanie Manning, and Gene Hoyme generated all dysmorphology data and made final diagnoses of the children in the field. Carol Keaster, Rosemary Bozeman, and Joelene Goodover carried out and oversaw all study and data collection activities in the community including: recruitment of subjects, incentive payments, maternal interviews, and cognitive and behavioral testing. Each author read, edited, contributed to, and approved various drafts of the manuscript.
Conflict of interests
None of the authors have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Philip A. May, Email: philip_may@unc.edu.
Jason Blankenship, Email: jblanken@unm.edu.
Wendy O. Kalberg, Email: wkalberg@unm.edu.
David Buckley, Email: dbuckely@unm.edu.
Marita Brooks, Email: maritab@unm.edu.
Julie Hasken, Email: julie_hasken@unc.edu.
J. Phillip Gossage, Email: jgossage@unm.edu.
Luther K. Robinson, Email: lrobinson@upa.chob.edu.
Melanie Manning, Email: mmanning@stanford.edu.
H. Eugene Hoyme, Email: Gene.Hoyme@sanfordhealth.org.
References
- Abel EL. Fetal Alcohol Abuse Syndrome. Plenum Press; New York: 1998. [Google Scholar]
- Abel EL, Sokol RJ. Incidence of Fetal Alcohol Syndrome and economic impact of FAS related anomalies. Drug Alcohol Depend. 1987;19:51–70. doi: 10.1016/0376-8716(87)90087-1. [DOI] [PubMed] [Google Scholar]
- Abel EL, Sokol RJ. A revised conservative estimate of the incidence of FAS and its economic impact. Alcohol Clin Exp Res. 1991;15:514–524. doi: 10.1111/j.1530-0277.1991.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Alvik A, Haldorsen T, Lindemann R. Consistency of reported alcohol use by pregnant women: anonymous versus confidential questionnaires with item nonresponse differences. Alcohol Clin Exp Res. 2005;29:1444–1449. doi: 10.1097/01.alc.0000175014.31463.9a. [DOI] [PubMed] [Google Scholar]
- Alvik A, Haldoresen T, Groholt B, Lindeman R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Burd L, Cox C, Poitra B, Wentz T, Ebertowski M, Martsolf JT, Kerbeshian J, Klug MG. The FAS Screen: a rapid screening tool for fetal alcohol syndrome. Addict Biol. 1999;4:329–336. doi: 10.1080/13556219971542. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Fiorentino D, Coriale G, Kalberg WO, Buckley D, Hoyme HE, Gossage JP, Robinson LK, Manning M, Romeo M, Hasken JM, Tabachnick B, Blankenship J, May PA. Maternal risk factors for fetal alcohol spectrum disorders in a province in Italy. Drug Alcohol Depend. 2014;145:201–208. doi: 10.1016/j.drugalcdep.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in fosters and adopted children with prenatal alcohol exposure. Pediatrics. 2015 doi: 10.1542/peds.2014-2171. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderso M, Fineman RM. Screening for Fetal Alcohol Syndrome in primary schools: a feasibility study. Teratology. 2001;63:3–10. doi: 10.1002/1096-9926(200101)63:1<3::AID-TERA1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Surveillance for fetal alcohol syndrome using multiple sources—Atlanta, Georgia, 1981–1989. MMWR. 1997;46:1118–20. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Update: trends in fetal alcohol syndrome—United States, 1979–1993. MMWR. 1995;44:249–251. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital Signs: binge drinking among women and high school gifts – United States, 2011. MMWR. 2013;62:9–13. [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D. Five -year reliability of self- reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Dean AG, Dean JA, Coulambier D, Brendel KA, Smith DC, Burton AH, Dickers RC, Sullivan K, Faglen RF, Arnir RG. Epi Info, Version 6: A word Processing Data Base, And Statistical Program For Epidemiology In Microcomputers. Centers for Disease Control and Prevention; Atlanta, Georgia: 1994. [Google Scholar]
- Feldman HS, Jones KL, Lindsay S, Slymen D, Klonoff-Cohen H, Kao K, Rao S, Chambers C. Patterns of prenatal alcohol exposure and associated non-characteristics minor structural malformations: a prospective study. Am J Med Genet A. 2011;155A:2949–2955. doi: 10.1002/ajmg.a.34276. [DOI] [PubMed] [Google Scholar]
- Feldman HS, Jones KL, Lindsay S, Slymen D, Klonoff-Cohen H, Kao K, Rao S, Chambers C. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36:670–676. doi: 10.1111/j.1530-0277.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- Fox DJ, Pettygrove S, Cunniff C, O’Leary LA, Gilboa SM, Vertrand J, Druschel CM, Breen A, Robinson L, Ortiz L, Frias JL, Ruttenber M, Klumb D, Meaney FJ. Fetal alcohol syndrome among children aged 7–9 – Arizona, Colorado, and New York, 2010. MMWR. 2015;64:54–57. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Algar O, Kulaga V, Gareri J, Koren G, Vall O, Zuccaro P, Pacifici R, Pichini S. Alarming prevalence of fetal alcohol exposure in a Mediterranean city. Ther Drug Monit. 2008;30:249–254. doi: 10.1097/FTD.0b013e31816a8657. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller J, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. SPSS Statistics for Windows. Version 20.0. Armonk, NY: 2011. [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcomes. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, Del Campo M, Manning MA, Prewitt LM, Chambers CD. Fetal alcohol spectrum disorders: extending the range of structural defects. Am J Med Genet A. 2010;152A:2731–2735. doi: 10.1002/ajmg.a.33675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Pub Health. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee RA, Yi H. Surveillance Report #92: Apparent Per Capita Alcohol Consumption: National, State, And Regional Trends, 1977–2009. National Institute on Alcohol Abuse and Alcoholism; Rockville: 2011. [Google Scholar]
- Kaskutas LA, Graves K. An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse. 2000;12:67–78. doi: 10.1016/s0899-3289(00)00042-0. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- Manich A, Velasco M, Joya X, Garcia-Lara NR, Pichini S, Vall O, Garcia-Algar O. Validity of a maternal alcohol consumption questionnaire in detecting prenatal exposure. An Pediatr (Barc) 2012;76:324–328. doi: 10.1016/j.anpedi.2011.09.016. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke LE, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson LK, Viljoen D. The epidemiology of Fetal Alcohol Syndrome in a South African community in the Western Cape Province. Am J Pub Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke LE, Gossage JP, Snell C, Hendricks L, Croxford J, Marais AS, Viljoen DL. Maternal risk factors for Fetal Alcohol Syndrome in the Western Cape Province of South Africa: a population-based study. Am J Pub Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg WO, Hoyme HE, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. The epidemiology of FASD in a province in Italy: prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams C, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Hoyme HE, Aragon AS, Buckley D, Stellavato C, Gossage JP, Robinson LK, Jones KL, Manning M, Ceccanti M. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: new estimated rates are higher than previous estimates. Int J Env Res Pub Health. 2011;8:2331–2351. doi: 10.3390/ijerph8062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, de Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013a;37:818–30. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Jourbert B, Cloete M, Barnard R, de Vries MM, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013b;133:502–5012. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag AC, Brodine SK, Alcarz JE, Clapp JD, Allison MA, Calac DJ, Hull AD, Gorman JR, Jones KL, Chambers CD. Preventing alcohol-exposed pregnancy among an American Indian/Alaska Native Population: effect of a screening, brief intervention, and referral to treatment intervention. Alcohol Clin Exp Res. 2015;39:126–135. doi: 10.1111/acer.12607. [DOI] [PubMed] [Google Scholar]
- Morini L, Marchei E, Tarani L, Trivelli M, Rapisardi G, Elicio MR, Ramis J, Garcia-Algar O, Memo L, Pacifici R, Groppi A, Danesino P, Pichini S. Testing ethylglucuronide in maternal hair and nails for the assessment of fetal exposure to alcohol: comparison with meconium testing. Ther Drug Monit. 2013;35:402–407. doi: 10.1097/FTD.0b013e318283f719. [DOI] [PubMed] [Google Scholar]
- Poitra BA, Marion S, Dionne M, Wilkie E, Dauphinais P, Wilkie-Pepion M, Martsolk JT, Klug MG, Burd L. A school-based screening program for fetal alcohol syndrome. Neurotoxicol Teratrol. 2003;25:725–729. doi: 10.1016/j.ntt.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales – Second Edition. Pearson Assessment; San Antonio, TX: 2006. [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. Fetal Alcohol Syndrome Diagnosis, Epidemiology, Prevention, And Treatment. Institute of Medicine. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Streissguth AP, Barr HM. Personal Behavior Checklist (PCBL - 36) Seattle, WA: 1998. [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinker’s reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6. Pearson; Boston: 2013. [Google Scholar]
- Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, Viljoen D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S Afr Med J. 2008;98:877–882. [PubMed] [Google Scholar]
- U.S. Census Bureau. [11 February 2015];State and County QuickFacts. 2015a Available from: < http://quickfacts.census.gov/qfd/states/00000.html>.
- United Health Foundation. [11 February 2015];America’s Health Rankings. 2014 Available from: < http://www.americashealthrankings.org>.
- Viljoen DL, Gossage JP, Adnams C, Jones KL, Robinson LK, HE, Snell C, Khaole N, Asante KK, Findlay R, Quinton B, Brooke LE, May PA. Fetal Alcohol Syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen DL, Croxford J, Gossage JP, May PA. Characteristics of mothers of children with Fetal Alcohol Syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Abbreviated Scale of Intelligence. Pearson Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Western City-County Health Department. The Health of Western County. 2011. [Google Scholar]
- Western State Department of Public Health and Human Service. 2013 State Behavioral Risk Factor Surveillance System. 2014. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test. 3. Psychological Assessment Resources, Inc; Lutz, FL: 1993. [Google Scholar]
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT—a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198:407.e1–407.e5. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]