Abstract
Background
Laboratory cue reactivity (CR) assessments are used to assess smokers' responses to cues. Likewise, EMA recording is used to characterize real-world response to cues. Understanding the relationship between CR and EMA responses addresses the ecological validity of CR.
Methods
In 190 daily smokers not currently quitting, craving and smoking responses to cues were assessed in laboratory CR and by real-world EMA recording. Separate CR sessions involved 5 smoking-relevant cues (smoking, alcohol, negative affect, positive affect, smoking prohibitions), and a neutral cue. Subjects used EMA to monitor smoking situations for 3 weeks, completing parallel situational assessments (presence of others smoking, alcohol consumption, negative affect, positive affect, and smoking prohibitions, plus current craving) in smoking and non-smoking occasions (averaging 70 and 60 occasions each). Analyses correlated CR craving and smoking cue responses with EMA craving and smoking correlations with similar cues.
Results
Although some cues did not show main effects on average craving or smoking, a wide range of individual differences in response to cues was apparent in both CR and EMA data, providing the necessary context to assess their relationship. Laboratory CR measures of cue response were not correlated with real-world cue responses assessed by EMA. The average correlation was 0.03; none exceeded 0.32. One of 40 correlations examined was significantly greater than 0.
Conclusions
Laboratory CR measures do not correlate with EMA-assessed craving or smoking in response to cues, suggesting that CR measures are not accurate predictors of how smokers react to relevant stimuli in the real world.
Keywords: smoking, craving, ecological momentary assessment, cue reactivity, ecological validity
1. INTRODUCTION
Smoking is motivated and maintained by nicotine (Benowitz, 2008), but smoking and craving are also influenced by situational stimuli (Kozlowski and Herman, 1984). Surveys reliably link smoking and craving to cues such as presence of other smokers and drinking alcohol (McKennell, 1970; Russell et al., 1974). Similarly, studies using electronic diaries show that people were more likely to smoke when seeing others smoking and when drinking, and less likely to smoke when smoking was prohibited (Shiffman et al. 2002, 2014b). The role of situational cues is particularly prominent in relapse (O'Connell and Martin, 1987; Shiffman et al., 1996). These situational linkages are often attributed to conditioned learning due to repeated pairing of smoking with environmental stimuli (Carter and Tiffany, 1999; Niaura et al., 1988). This leads to an emphasis on individual differences, since smokers are likely to differ in smoking patterns and thus in the stimuli they associate with smoking, and might also differ in conditionability (Mineka and Oehlberg, 2008). It also suggests that if individual cue associations could be identified, this might help to individually tailor treatment (Conklin et al., 2010; Drummond, 2000).
Ecological Momentary Assessment (EMA; Shiffman et al., 2008) methods, using electronic diaries to collect detailed real-time data, are increasingly being used to study the effects of cues in smokers' real-world settings. However, these methods, while they are considered ecologically valid, rely on smokers' naturalistic exposures to cues in their environment. In contrast, cue reactivity (CR) studies (Carter and Tiffany, 1999; Niaura et al., 1988) attempt to bring cue responses into the laboratory, where they can be manipulated and studied experimentally. As a consequence, CR studies are commonly used to assess the effects of cues on craving and smoking (as well as other drug use; Carter and Tiffany, 1999). However, the degree to which laboratory CR responses predict real-world responses to cues has not been studied. In this paper, we assess the associations between smokers' laboratory CR cue responses and their real-world cue responses, as assessed by EMA.
CR studies (Carter and Tiffany, 1999) expose smokers to stimuli thought to be associated with craving and/or smoking, such as cigarettes themselves, and compare these responses to those elicited by a neutral stimulus. Increases in craving or in the likelihood of smoking are seen as indicators of specific cue reactivity. Cues studied in this way have included cigarette-specific stimuli (termed ‘proximal cues’ by Conklin et al., 2008), and diverse cues such as positive or negative affect, alcohol cues, etc. (‘distal cues’; Conklin et al., 2008). CR effects have been observed both in abstinent smokers (McClernon et al., 2009; Tiffany et al., 2000) and minimally deprived smokers (Bailey et al., 2010), and reactivity seems similar across these conditions (Carter et al., 2009; Franklin et al., 2007). However, individual differences in CR are not consistently linked to smoking cessation outcomes (Perkins, 2012; Powell et al., 2010; Waters et al., 2004), causing some to question its relevance to cessation and treatment (Perkins, 2009).
A key question in the CR field is whether reactivity observed in laboratory settings actually reflects real-world responses to these cues. Such a link would be expected if CR responses reflect participants' condition history (Hogarth et al., 2010; Niaura et al., 1998), and necessary if CR responses are to be used to predict outcome (Perkins 2012) or to tailor treatment (Conklin, 2006; Drummond, 2000). However, to our knowledge no study has examined the degree to which CR measures predict how smokers react to stimuli in the real world. In this paper, we compare data from previously-published studies examining CR responses observed in the laboratory (Shiffman et al., 2013a) and real-world craving and smoking observed in response to analogous cues in EMA data on the same sample (Shiffman et al., 2014b).
EMA data comparing the presence of cues when subjects are and are not smoking provide estimates of the associations between stimuli and smoking (Paty et al., 1992), and can also be used to assess situational correlates of craving (Dunbar et al., 2010). These EMA data are essentially the real-world analogue of laboratory cue-reactivity measures, and thus an apt reference to assess the real-world generalizability of CR assessments.
The primary effects from both the CR and EMA studies have been reported in previous publications. The CR study (Shiffman et al., 2013a) assessed daily smokers' craving and smoking in response to a range of smoking-relevant cues. Craving was increased by exposure to smoking and alcohol cues, and decreased by positive affect; however, negative affect and smoking prohibition cues did not, on average, affect craving. As previously reported, both the probability of smoking and the amount smoked increased in proportion to craving, and in proportion to the observed increases in craving, though not in a cue-specific way (Shiffman et al., 2013a).
Similarly, the data from the EMA study also showed significant cue effects: smoking was associated with situational cues including others smoking and alcohol consumption, and inversely with smoking restrictions (Shiffman et al., 2014b). Negative affect was not associated with smoking, but positive affect showed curvilinear effects. Importantly, there was significant individual variation in response to cues, and when this was taken into account, cues, including affect, were very robust predictors of smoking. Knowing a smoker's affective state at any moment allowed one to predict whether s/he was smoking with 70% accuracy (Shiffman et al., 2015), again demonstrating the importance of individual differences even when average effects in the sample as a whole were not significant.
Our primary papers on the CR (Shiffman et al., 2013a) and EMA (Shiffman et al., 2014b) studies focused on the effects of cues in each paradigm. Here, we aim to directly compare the laboratory CR and real-world EMA responses observed to assess whether CR is an accurate proxy for real-world responses to cues. If so, one would expect to see that the magnitude of reactivity observed for each individual during CR exposure to correlate with that individual's responses in real-world observations. For example, if a particular smoker exhibits a high degree of craving reactivity to smoking cues during the CR sessions, one would similarly expect to see that person's craving be higher when such cues are present in real-world settings.
Note that such individual associations do not rest on there being a shared, common reaction across all smokers. Thus, for example, some smokers may be "negative affect smokers" and others not, with the result that the overall effect of negative mood on smoking is not significant across the whole sample of smokers. Nevertheless, one would expect that the particular individuals who demonstrate negative affect smoking in the CR assessment would show negative affect smoking in the EMA assessment. The validity of such individual assessments even in the absence of group-wide significant effects was illustrated in a previous EMA study. In that study, there was no overall significant relationship between negative mood and smoking (Shiffman et al, 2002). However, within this overall 'null' effect, there were substantial individual differences in the relationship between mood and smoking, and analyses showed that the individual associations predicted which individuals were most vulnerable to relapse (Shiffman et al., 2007). Accordingly, in addition to examining the cues that had group-wise significant effects in each paradigm, we also examine the correlation of individual differences in other cues as well.
For the data to be informative on individual differences, it needs to be demonstrated that each of the measures shows substantial between-subject variation; i.e., that some respond more strongly than others, or even that some respond in one direction (e.g., smoking more when upset) while others respond in the opposite direction (smoking less when upset). The potential for correlation across methods depends on such individual differences; if nearly everyone responds in the same way to a particular cue, individual differences – and correlations – would be minimized. Thus, we precede the analysis of correlations between CR and EMA data with an examination of between-person variation in cue associations within each method.
2. METHODS
2.1 Subjects
Subjects were 190 daily smokers who completed both the EMA assessment and the CR assessment (i.e., providing data on the neutral cue and one or more active cues). Volunteers had to be at least 21 years old, smoking 5–30 cigarettes per day, smoking for ≥3 years, at their current rate for ≥3 months, and not planning to quit within a month. Subjects in these analyses averaged 40.19 (±11.58) years old, on timeline follow-back reported smoking an average of 14.98 (±5.89) cigarettes per day, and with Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) scores averaging 5.18 (±2.03); 60% were female. By design, we oversampled African-American (AA) smokers to comprise 38% of the sample, and weighted observations to rebalance ethnic representation.
2.2 Procedures
2.2.1 Cue reactivity
CR procedures are described in detail in Shiffman et al. (2013a). Briefly, reactivity to five different cues (presented as sets of pictures) was assessed over separate sessions on different days: smoking cues (cigarettes, smoke), smoking-prohibition cues (no-smoking signs and settings), alcohol cues (drinks), negative affect, and positive affect (both via images from the International Affective Pictures System; Center for the Study of Emotion and Attention, 1999). Subjects were also tested on neutral cues (images matched to each of the ‘active’ cues – e.g., a person holding a pencil; non-alcoholic beverages). After a 30-minute abstinence period, a 3-minute acclimation period, and pre-cue craving assessment, smokers were exposed to cues for 3 minutes, after which craving was re-assessed, yielding values for craving change due to cue exposure (log-transformed to reduce skewness). Subjects were then provided with two cigarettes and permitted to smoke for 15 minutes, with cue display continuing. Subjects were informed in advance that cigarettes would be available (Wertz and Sayette, 2001). Craving was assessed using the brief 10-item Questionnaire on Smoking Urges (QSU; Cox et al., 2001), yielding scores for Appetitive and Distress-relief craving with a range of 1–49. Smoking was assessed by video observation, capturing whether the person smoked and how much they smoked (total puff time; Blank et al., 2009).
Cues consisted of 30 still images for each cue type, displayed on a video monitor for 6 seconds each during initial cue exposure, with the set redisplayed five times during the 15-minute smoking period. Photographic images have been reported to elicit greater cue reactivity than either imagery scripts or in vivo cues (Warthen and Tiffany, 2009; Wray et al., 2011). Cue images were drawn from validated sources and were pilot-tested (see Shiffman et al., 2013a). Order of cue presentation was randomized (Shiffman et al., 2013a), and order was controlled for in analyses.
2.2.2 EMA
EMA procedures are described in detail in Shiffman et al. (2014b). After completing CR procedures, subjects monitored their smoking for 3 weeks using an electronic diary (ED). Subjects were instructed to record every cigarette that they smoked during this period. The ED randomly sampled approximately 4–5 cigarettes a day for assessment. Additionally, ED “beeped” subjects at random approximately 3–4 times per day when not smoking (not within 15 minutes of smoking), and administered an identical assessment. Subjects completed an average of 70 smoking assessments, and 60 non-smoking (random) assessments over the course of monitoring.
Assessments captured craving and situational context, including mood. Craving and mood items were assessed on a 0–100 point Visual Analog Scale; 16 mood items were summarized by factor analysis and yielded factor scores for Negative Affect (NA) and Positive Affect (PA). This analysis focuses on situational variables corresponding to the cues tested in CR: smoking cues [seeing others smoking, yes/no]; alcohol [consuming alcohol, yes/no]; negative affect [continuous factor score]; positive affect [continuous factor score]; and smoking prohibitions [smoking forbidden, yes/no].
2.3 Analyses
We first present data on the between-subject variability in CR and EMA measures, by showing the distribution of responses to each of the cues in each assessment paradigm. For CR craving data, this consists of difference in the change in craving after presentation of each active cue, compared to the neutral cue. (We present data for QSU Appetitive Craving; distributions were similar for Distress-Relief Craving.) For CR smoking data, we present the difference in total puff time (see Shiffman et al., 2013a for details) following each cue compared to the neutral cue; in this display, subjects who did not smoke are counted as having 0 puff time. For the EMA data, we present the range of individual within-subject correlation coefficients, as described below.
The objective of the main analyses was to estimate how well smokers' craving and smoking responses to cues in laboratory CR assessments predicted their real-world reactions to similar cues, assessed via EMA. From the EMA data, we computed correlations reflecting the association of each situational cue with two end-points: craving and smoking. For each subject, we computed, across their EMA observations, the correlation between the relevant situational variables and craving ratings; since EMA contained both smoking and non-smoking (random) observations, we covaried the smoking status of the observation. This yielded, for each subject, a (partial) correlation coefficient between craving and each situational cue. We also computed for each subject and each cue, a correlation between the cue and smoking (i.e., contrasting smoking vs. non-smoking occasions). (Depending on the outcome, the coefficient was a Pearson correlation [both continuous], a point-biserial correlation [one dichotomous], or a Phi coefficient [both dichotomous]; all are valid indicators of the relevant associations.) Because some subjects had more observations than others, making their estimates more reliable, we weighted the data by the inverse of the standard error for the correlation. (This weighting had little effect: unweighted estimates were nearly identical.)
From the CR data, for each subject and each cue, we computed two measures of craving response (change in QSU Appetitive and Distress-relief craving following cue exposure). (Some CR studies covary baseline levels in analyses of difference scores [see Carter and Tiffany, 1999]; this was not relevant here, as correlations with baseline were very modest, averaging r < 0.10.) We also analyzed two measures of smoking, here distinguishing whether the subject smoked or not [scored as ‘1’ vs. ‘0’], and the total puff time [seconds] among those who smoked.
The analyses used an explicit two-step hierarchical approach. The estimated EMA correlations constituted the estimates from level 1 (within-subject analyses yielding subject-level estimates, weighted by their reliability). Between-subject regressions (level 2) were then executed predicting the EMA stimulus associations (correlations) from the laboratory cue reactivity indices. Each model also controlled for the subject’s response to the neutral cue (making responses cue-specific). The outcome was the standardized regression coefficient, essentially a correlation, indicating the magnitude of association between CR measures and EMA measures.
3. RESULTS
3.1 Individual variability in response
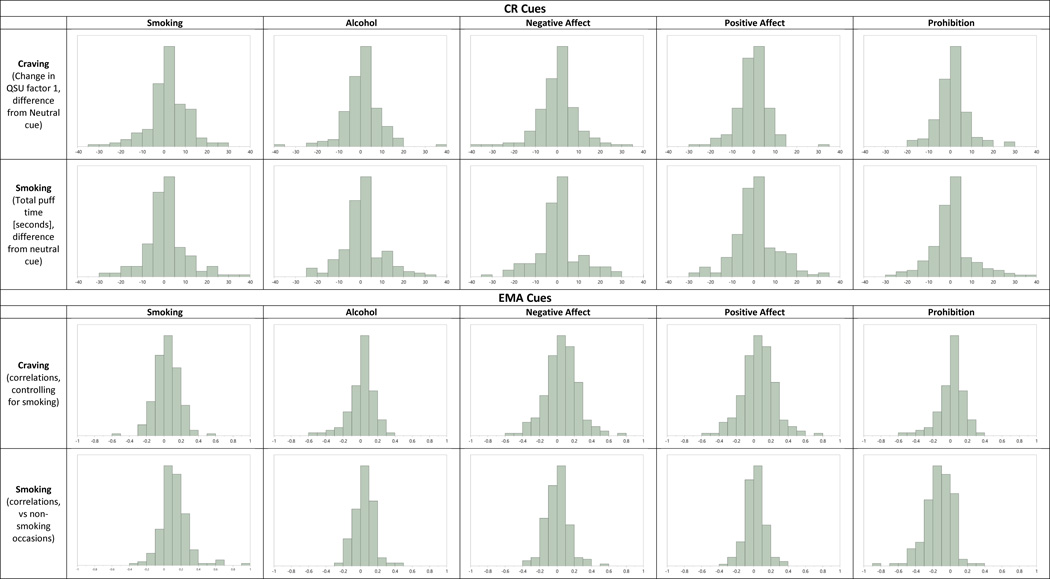
Figure 1 shows the distribution of associations between cues and craving and smoking for both CR and EMA. It can be seen that craving changes after each CR cue (vs neutral) demonstrated a wide range of individual differences, including both increases and decreases in craving, compared to neutral cue response. Similar dynamics held for total puff time following each cue (compared to neutral cue response). EMA data are graphed in Figure 1 as within-subject correlations between each cue and smoking or craving intensity (controlling for whether the subject was smoking or not). It can be seen that, for all cues, including those where there was a significant group-wide effect of cues on smoking (smoking cues, alcohol, positive affect, and smoking restrictions; Shiffman et al, 2014b), individuals varied substantially in how each cue affected their smoking, some showing positive associations and some showing negative associations. Thus, both the EMA and CR data displayed a range of individual differences in cue response, providing a context in which to observe correlations between the two methods.
Figure 1.
Distribution of smoker responses to cues, by method and cue. CR craving data (Shiffman et al, 2013a) are graphed as the difference between each cue and the neutral cue in post-cue vs pre-cue QSU Appetitive Craving, which was assessed on a 49-point scale. (Distress-relief craving, not shown, showed very similar distributions.) CR smoking data are graphed as the difference in total puff time (with no smoking represented as zero puff time) following each cue versus the neutral cue. EMA data (Shiffman et al., 2014b) are graphed as individual subject correlations between cues and rated contemporaneous EMA-reported craving (on a 0–100 scale, adjusting for whether the subject was smoking or not) or smoking (vs. not smoking). Negative and Positive Affect were represented by factor scores on composite affect scales; other cues were assessed as present or absent, based on EMA reports.
3.2 Correlations with EMA craving response
Table 1 shows the results of correlating EMA-assessed changes in craving with CR-assessed changes in craving (QSU Appetitive and Distress-relief craving) and smoking (0/1, and total puff time). For example, EMA-assessed changes in craving when others were seen smoking (labeled “smoking” in the table) correlated poorly (r = −0.04) with CR-measured changes in Appetitive craving in response to visual smoking cues. The correlation was 0 for Distress-relief craving, and −0.14 and 0.03, respectively, for whether the person smoked, and how long they puffed in the CR lab following exposure to smoking cues (vs. neutral cues). Overall, the correlations ranged from −0.21 to +0.35, averaging +0.03. None were significantly different from 0.
Table 1.
Cue reactivity outcomes predicting cue associations with EMA craving (controlling for smoking)
| Cue Reactivity Outcome | ||||
|---|---|---|---|---|
| Craving | Smoking | |||
| Cue | QSU Appetitive |
QSU Distress Relief |
Smoked (Y/N=1/0) |
Total puff-timea |
| Smokingb | −0.04 | 0.00 | −0.14 | 0.03 |
| Alcoholc | 0.18 | 0.18 | 0.23 | 0.35 |
| Negative affectd | 0.11 | −0.01 | 0.02 | 0.01 |
| Positive affecte | −0.08 | −0.07 | 0.00 | 0.15 |
| Prohibitionf | −0.08 | −0.06 | 0.09 | −0.21 |
Entries are standardized regression coefficients
p < 0.05 for test that coefficient differs from 0 (none differ from zero)
Among those who smoked
In CR, smoking cues; in EMA, whether others were smoking
In CR, alcohol cues; in EMA, whether respondent had consumed alcohol in the previous 15 minutes
In CR, a negative affect induction; in EMA, assessed by a multi-item factor score
In CR, a positive affect induction; in EMA, assessed by a multi-item factor score
In CR, smoking-prohibition cues; in EMA whether smoking was prohibited in the setting
3.3 Correlations with EMA smoking
Table 2 presents similar data, but focusing on cue associations with smoking in the EMA data. The correlations range from −0.20 to +0.32, averaging +0.03. Two correlations were significantly different from zero (both p< .025), but one of these was negative.
Table 2.
Cue reactivity outcomes predicting cue associations with EMA smoking
| Cue Reactivity Outcome | ||||
|---|---|---|---|---|
| Craving | Smoking | |||
| Cue | QSU Appetitive |
QSU Distress Relief |
Smoked (Y/N=1/0) |
Total puff-timea |
| Smokingb | 0.07 | 0.07 | 0.01 | −0.08 |
| Alcoholc | *0.26 | 0.14 | 0.02 | 0.32 |
| Negative affectd | 0.06 | 0.05 | *−0.20 | −0.09 |
| Positive affecte | 0.14 | −0.01 | 0.05 | 0.06 |
| Prohibitionf | −0.05 | −0.05 | 0.04 | −0.22 |
Entries are standardized regression coefficients
p < 0.025 for test that coefficient differs from 0
Among those who smoked
In CR, smoking cues; in EMA, whether others were smoking
In CR, alcohol cues; in EMA, whether respondent had consumed alcohol in the previous 15 minutes
In CR, a negative affect induction; in EMA, assessed by a multi-item factor score
In CR, a positive affect induction; in EMA, assessed by a multi-item factor score
In CR, smoking-prohibition cues; in EMA whether smoking was prohibited in the setting
Across both Tables 1 and 2 (EMA craving and EMA smoking), almost half the correlations (40%; 16/40) were negative.
4. DISCUSSION
For the first time, we tested whether craving and smoking observations from laboratory-based CR testing were actually related to corresponding measures of real-world craving and smoking, assessed via EMA, in the same sample. The results were highly consistent: There was no reliable relationship between CR measures and real-world behavior. The average correlation between the two was 0.03, and there was no robust evidence that the correlations were reliably greater than zero. (Two correlations were associated with p-values < .025, but one was negative.) Notably, the coefficients for the smoking cues, which are the most common cues evaluated in CR studies (Carter and Tiffany, 1999) and which showed significant group-wise effects on craving, were also near zero. Overall, the observed associations indicate that measures obtained via the CR paradigm do not reflect smokers’ reactions to similar stimuli in the real world.
Given the face validity of the CR laboratory methods, it is not clear why the correlations are so low. Laboratory stimuli do not resemble those encountered in the real world. Real-world cues are not pictures (or stories), but actual exposures and experiences, which may be more potent stimuli. Real-world cues are also complex natural contexts, rather than pure one-dimensional cues: when one encounters someone smoking, it may be a friend, with whom one has smoked before, who is smoking in a bar where one is drinking while socializing. Thus real-world cues often present an elaborate and integrated set of contextual cues that go beyond exposure to a single cue on its own (see Drummond, 2000).
The standardization of laboratory cues may also rob them of personal relevance; indeed, Conklin et al. (2010) report that individualized cues (subjects' photos of their own smoking settings) elicit stronger responses. Laboratory cues are also presented in an artificial context, in which smokers know they are being studied, and anticipate presentation of a cue. Having a cue imposed on one by the experimenter may elicit reactance, defensive counter-responses to the cues, which would not often occur in real life when smokers are not quitting. Also, since the laboratory context and the anticipation it elicits may themselves provoke craving, this setting may mask the effects of more specific cue exposures (Sayette et al., 2000).
Despite these limitations, CR studies have yielded findings indicating that smokers (and other drug users) react to smoking/drug cues (Carter and Tiffany, 1999), that such responses are reproducible (LaRowe et al., 2007), that some smokers react more strongly than others (Shiffman et al., 2003), and that these individual differences are sometimes (but not consistently) correlated with other variables of interest (Abrams et al., 1988; Powell et al., 2010; Waters et al., 2004; Watson et al., 2010). This has been seen not only for subjective measures of craving, but also for objective measures such as changes in regional brain activity (Engelmann et al., 2012; Janes et al., 2010; McClernon et al., 2009). This suggests that CR studies are measuring something, even if they are not representing real-world reaction to particular cues.
Even if CR responses are not correlated with real-world responses to cues, CR methods may nevertheless be useful. For example, Sayette and Tiffany (2013) have suggested that the methods are useful for provoking intense craving, even if it is not cue-specific, so that intense craving can be studied in the laboratory. Consistent with this, our CR analyses of provoked craving showed that craving prospectively predicted smoking behavior in the laboratory, even if it was not cue-specific (Shiffman et al., 2013a). Provoked craving paradigms can also assess the effects of medications on preventing or treating cue-provoked craving (Brandon et al., 2011; Hitsman et al., 2013; Niaura et al., 2005; Shiffman et al., 2003). Thus, CR studies may be useful, even if they do not relate to real-world patterns of smoking or craving responses to cues. Importantly, the results do, however, caution against using CR results as the basis for personalized treatment strategies.
The study was limited in several respects. We studied smokers who were only modestly deprived; responses might differ in deprived smokers or those engaged in a quit attempt. (However, we did not observe a strong influence of length of deprivation [Shiffman et al., 2013a], and previous studies have demonstrated similar CR effects during ad lib smoking and abstinence [Drobes and Tiffany, 1997; McClernon et al., 2005]). Ours was a volunteer sample, so may not represent all smokers. We used standardized still pictures as cues, and other cue modalities might better mirror real-world reactivity. Importantly, even when they were significant (for smoking, alcohol, and positive affect cues), our cue exposures yielded relatively small average responses, suggesting that the cues may have been weak. However, this in itself would not invalidate the assessment; indeed, a very strong cue that elicited strong responses from most subjects would minimize individual differences and variation, making it difficult to show correlation across methods. The fact that both CR and EMA responses covered a broad range should have created a favorable setting for observing concordance of individual differences between CR and EMA, so long as the variance is not simply due to unreliability of measurement. That the observed variance illustrated in Figure 1 is not just due to unreliability is demonstrated by the fact that individual differences in craving and craving change predicted subsequent smoking in the CR study (Shiffman et al., 2013a), as well as by the fact that both CR and EMA measures differentiated responses of daily and non-daily smokers (Shiffman et al., 2013b, 2014a, 2014b). More generally, Wray et al. (2014) and LaRowe et al.(2007) have demonstrated that CR measures are reliable over repeated assessments. Thus, variance in individual assessments can serve as an important predictor even when the average prediction is not significant, as illustrated in a prior study (Shiffman et al., 2007). However, some (Sayette and Tiffany, 2013) have argued that certain phenomena emerge only when craving is driven to a high level of intensity, which did not consistently occur here. Thus, it is possible that a CR study that provoked much stronger responses might predict real-world behavior.
Compliance with EMA data collection – both for recording each cigarette and for responding to every prompt – was imperfect, and it is possible that non-response biased the EMA estimates. However, compliance was generally quite high – subjects responded within 2 minutes to 88% of prompts, and previous analyses of biomarkers of smoking suggested that EMA subjects recorded smoking occasions in a timely way (Shiffman, 2009). Moreover, prior studies have shown that EMA-derived measures of smoking patterns can predict distal outcomes, and do so better than questionnaire data (Shiffman et al., 2007). Although reactivity is a concern in self-monitoring, studies have suggested that reactivity to EMA is minimal (Shiffman et al., 2008). However, it is possible that craving is subject to a more subtle form of reactivity, wherein asking about craving brings it into awareness in a way that would not occur in undisturbed ad libitum smoking (Tiffany, 1990). Of course, this would affect CR data as well (Sayette et al., 2000; Sayette and Tiffany, 2013), so should not necessarily reduce the CR-EMA correlations. We note also that the EMA data were based on numerous observations (averaging 130/subject), making the estimates of association highly reliable, whereas the CR data were based on a single observation for each cue (and one for the neutral cues). It is possible that assessing CR responses over many cue exposures would improve the reliability of the procedure. However, this is not how CR studies are typically conducted, and such assessments would raise issues of carry-over effects and habituation.
The study also benefited from notable strengths. It was based on a large sample (almost 4 times the typical sample size in CR studies; Heckman et al., 2013) that included a representative range of smoking rates. We tested multiple active cues, giving the CR data multiple opportunities to demonstrate validity. The analyses went beyond craving to include smoking, including quantitative measures of CR-induced smoking intensity. The EMA data were based on over 15,000 individual observations, and captured detailed real-world smoking patterns, without relying on subjects’ recall or their global impressions of smoking patterns. The EMA period covered three weeks, representing a robust sample of subjects’ smoking patterns.
The study’s findings raise questions about the validity of the CR paradigm as a model of individual differences in smokers’ – and perhaps other drug users’ – reactions to particular cues. More research examining the external validity of laboratory CR data is needed.
Highlights.
Smokers' responses to cues were assessed in a cue reactivity (CR) lab and via EMA
Analyses correlated craving and smoking in response to diverse cues in CR and EMA
CR cue responses were not correlated with real-world EMA responses to similar cues
Acknowledgements
The authors are grateful to Thomas Kirchner, and Deborah Scharf for help launching this study and for input on study design; to Anna Tsivina, Joe Stafura, Rachelle Gish, and Aileen Butera for their work conducting research sessions; to Chantele Mitchell-Miland for data management and preparation; and to Alexandra Cardy for assistance with manuscript preparation. We benefited from the comments of Michael Sayette and Kenneth Perkins on an earlier version of this manuscript.
Role of Funding Source
This work was supported by grant R01-DA020742 (PI: Shiffman) from the National Institutes of Health, National Institute on Drug Abuse. Additional support was provided by National Science Foundation Graduate Research Fellowship (Dunbar), National Center for Research Resources (KL2-RR024154-03; Tindle), and National Cancer Institute R25-CA057703-15 (Dunbar) and R01-CA141596-02 (Tindle).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author Shiffman designed the study and analysis. Authors Scholl, Dunbar, Tindle, and Ferguson participated in the development of the protocol. Author Scholl oversaw conduct of the study. Authors Li and Shiffman conducted the analysis. Author Shiffman drafted the paper and all authors contributed comment and have approved the final manuscript.
Conflict of Interest
Dr. Shiffman consults to and has an interest in eRT, which provides electronic diary services for clinical research.
REFERENCES
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav. Res. Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Bailey SR, Goedeker KC, Tiffany ST. The impact of cigarette deprivation and cigarette availability on cue-reactivity in smokers. Addiction. 2010;105:364–372. doi: 10.1111/j.1360-0443.2009.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob. Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl.) 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Generalized craving, self-report of arousal, and cue reactivity after brief abstinence. Nicotine Tob. Res. 2009;11:823–826. doi: 10.1093/ntr/ntp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. The Center for Research in Psychophysiology. Gainesville: University of Florida; 1999. International Affective Pictures System: Digitized photographs. [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp. Clin. Psychopharmacol. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environment on smokers' cue-reactivity. Exp. Clin. Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christensen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95:S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Dunbar MS, Scharf DM, Kirchner T, Shiffman S. Do smokers crave cigarettes in some smoking situations more than others? Situational correlates of craving when smoking. Nicotine Tob. Res. 2010;12:226–234. doi: 10.1093/ntr/ntp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heckman BW, Kovacs MA, Marquinez NS, Meltzer LR, Tsambarlis ME, Drobes DJ, Brandon TH. Influence of affective manipulations on cigarette craving: a meta-analysis. Addiction. 2013;108:2068–2078. doi: 10.1111/add.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Hogarth L, Tseng LJ, Teige JC, Shadel WG, DiBenedetti DB, Danto S, Lee TC, Price LH, Niaura R. Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug Alcohol Depend. 2013;130:135–141. doi: 10.1016/j.drugalcdep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Dickinsion A, Duka D. The associative basis of cue-elicited drug taking in humans. Psychopharmacology. 2010;208:337–351. doi: 10.1007/s00213-009-1735-9. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Herman CP. The interaction of psychosocial and biological determinants of tobacco use: more on the Boundary Model. J. Appl. Soc. Psychol. 1984;14:244–256. [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict. Behav. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl.) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKennell AC. Smoking motivation factors. Br. J. Soc. Clin. Psychol. 1970;9:8–22. doi: 10.1111/j.2044-8260.1970.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Niaura R, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J. Abnorm. Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Niaura R, Sayette M, Shiffman S, Glover ED, Nides M, Shelanski M, Shadel W, Koslo R, Robbins B, Sorrentino J. Comparative efficacy of rapid-release nicotine gum versus nicotine polacrilex gum in relieving smoking cue-provoked craving. Addiction. 2005;100:1720–1730. doi: 10.1111/j.1360-0443.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict. Behav. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J. Consult. Clin. Psychol. 1987;55:367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Paty JA, Kassel JD, Shiffman S. The importance of assessing base rates for clinical studies: an example of stimulus control of smoking. In: deVries MW, editor. The Experience of Psychopathology: Investigating Mental Disorders in their Natural Settings. New York: Cambridge University Press; 1992. pp. 347–352. [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Subjective reactivity to smoking cues as a predictor of quitting success. Nicotine Tob. Res. 2012;14:383–387. doi: 10.1093/ntr/ntr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl.) 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Russell MAH, Peto J, Patel UA. The classification of smoking by factorial structure of motives. J. R. Stat. Soc. 1974;137:313–346. [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura R, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108:1019–1025. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, Auriacombe M. Ecological momentary assessment in the investigation of craving and substance use in daily life: a systematic review. Drug Alcohol Depend. 2015;148:1–20. doi: 10.1016/j.drugalcdep.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line follow-Back, and Ecological Momentary Assessment. Health Psychol. 2009;28:519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by Ecological Momentary Assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: effects on craving and on smoking behavior. J. Abnorm. Psychol. 2013a;122:264–280. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Kirchner TR, Li X, Tindle HA, Anderson SJ, Scholl SM, Ferguson SG. Cue reactivity in non-daily smokers: effects on craving and on smoking behavior. Psychopharmacology. 2013b;226:321–333. doi: 10.1007/s00213-012-2909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Craving in intermittent and daily smokers during ad libitum smoking. Nicotine Tob. Res. 2014a;16:1063–1069. doi: 10.1093/ntr/ntu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One. 2014b;9:e89911. doi: 10.1371/journal.pone.0089911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Ferguson S. Stimulus control in intermittent and daily smokers. Psychol. Addict. Behav. 2015 doi: 10.1037/adb0000052. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J. Abnorm. Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: within-subjects analyses of real-time reports. J. Consult. Clin. Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niarua R, Khayrallah MA, Jorenby DE, Ryan CF, Ferguson CL. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. 2003;166:343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug use behavior: role of automatic and non-automatic processes. Psychol. Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J. Consult. Clin. Psychol. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Warthen M, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using Ecological Momentary Assessment. Exp. Clin. Psychopharmacol. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue provoked craving and nicotine replacement therapy in smoking cessation. J. Consult. Clin. Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Watson N, Carpenter M, Saladin M, Gray K, Upadhyaya H. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict. Behav. 2010;35:673–677. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol. Addict. Behav. 2001;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wray JM, Gass JC, Tiffany ST. The magnitude and reliability of cue-specific craving in nondependent smokers. Drug Alcohol Depend. 2014;134:304–308. doi: 10.1016/j.drugalcdep.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Wray JM, Godleski SA, Tiffany ST. Cue-reactivity in the natural environment of cigarette smokers: the impact of photographic and in vivo smoking stimuli. Psychol. Addict. Behav. 2011;25:733–737. doi: 10.1037/a0023687. [DOI] [PMC free article] [PubMed] [Google Scholar]