Abstract
In this study, autoantibody responses to annexin A2 were found in 11–15% of 278 patients with Lyme disease, including in those with erythema migrans (EM), an early sign of the illness, and in those with antibiotic-responsive or antibiotic-refractory Lyme arthritis (LA), a late disease manifestation. In contrast, robust T cell reactivity to annexin A2 peptides was found only in patients with responsive or refractory LA. In LA patients, annexin A2 protein levels, which were higher in the refractory group, correlated with annexin A2 antibody levels in sera and synovial fluid. In addition, in patients with antibiotic-refractory LA who had anti-annexin A2 antibodies, synovial tissue had intense staining for annexin A2 protein, greater synovial fibroblast proliferation and more tissue fibrosis. Thus, a subset of LA patients had T and B cell responses to annexin A2, and in the refractory group, annexin A2 autoantibodies were associated with specific pathologic findings.
Keywords: Annexin A2, Autoantigen, Lyme arthritis, Lyme disease, Borrelia burgdorferi
1. Introduction
Lyme disease, which is caused by the tick-borne spirochete Borrerlia burgdorferi, usually begins with an expanding skin lesion, erythema migrans (EM), that occurs at the site of the tick bite [1]. Months later, untreated patients commonly develop Lyme arthritis (LA) [2]. As the infection progresses, patients develop antibody responses to an increasing array of spirochetal proteins and glycolipids [3, 4]. Although most LA patients respond to antibiotic therapy, called antibiotic-responsive LA, a small percentage of patients have persistent synovitis for months or years after 2–3 months of oral and intravenous antibiotic therapy, termed antibiotic-refractory LA [5, 6]. Excessive inflammation, immune dysregulation, and Infection-induced autoimmunity are thought to play a role in this outcome [7].
Identification of relevant autoantigens has been challenging in any disease, but newer discovery-based methods offer innovative approaches to this problem. One method is the screening of serum samples for autoantibodies by protein array [8–10] or by determination of the antibody repertoire in plasmablasts [11]. More recently, we have developed an approach in which patients’ inflamed synovial tissue is used as a source to isolate and identify in vivo HLA-DR-presented peptides (T cell epitopes) using tandem mass spectrometry (LC-MS/MS) [12]. T and B cell responses to identified peptides or their source proteins are then determined using patient samples.
With this approach, we previously identifed two novel autoantigens in Lyme disease, endothelial cell growth factor (ECGF) and apolipoprotein B-100 (apoB-100) [13, 14]. With each autoantigen, autoantibodies were found in about 10% of patients with EM, and T and B cell responses were present in 15–30% of patients with LA. In patients with antibiotic-refractory LA, autoantibodies to ECGF correlated with the histologic finding of obliterative microvascular lesions in synovial tissue [15], whereas autoantibodies to apo-B-100 correlated with higher numbers and activation of endothelial cells in the tissue and greater synovial fibroblast proliferation [14]. These correlations suggested that autoimmune responses to ECGF or apoB-100 may have specific pathologic consequences, relating primarily to synovial microvasculature.
We employed the same methodology to identify disease-associated autoantigens in rheumatoid arthritis (RA) [12, 16, 17]. In the first patient tested (RA1), an immunogenic HLA-DR-presented peptide derived from annexin A2 was identified from her synovial tissue. Annexin A2 is a known autoantigen in several rheumatic diseases, particularly in the anti-phospholipid syndrome (APS) and in lupus-associated APS, but also in RA [18–20]. Consistent with a previous report [20], 14% of our RA patients had autoantibody responses to annexin A2, providing “proof-of-concept” for this approach of autoantigen identification. In addition, when we tested serum samples from patients with other forms of arthritis, we learned that annexin A2 was also an autoantigen in a subset of patients with Lyme disease, which was not previously known. Thus, in the current report, we assessed T and B cell responses to annexin A2 and associated synovial pathology in patients with Lyme disease and in control subjects.
2. Patients and methods
2.1 Patients and control subjects
The study “Immunity in Lyme Arthritis” was approved by the Human Investigations Committees at Tufts Medical Center from 1988–2002 and at MGH from 2002–2014. In addition, the study “Diagnosis and Pathogenesis of Early Lyme Disease” was approved by the Committee at Tufts Medical Center from 1998–2001. All 278 patients whose samples were used in the current study met the Centers for Disease Control and Prevention (CDC) criteria for Lyme disease [21]. All patients with erythema migrans (EM) had culture and/or serologic evidence of the infection; serum samples and PBMC were collected from these patients. Patients with LA were categorized as having antibiotic-responsive or antibiotic-refractory LA, as previously defined [6]. Specimens collected from these patients included serum samples, PBMC, and if available, synovial fluid (SF). In patients who underwent synovectomies, synovial tissue was also obtained. As a control group for EM patients, serum samples were tested from 13 patients with influenza, another acute infection; and as comparison groups for LA patients, serum samples were assayed from 91 patients with RA, 24 patients with spondyloarthropathies, and 5 patients with osteoarthritis. Additionally, serum samples and PBMC were collected from 10 healthy hospital personnel who did not have a history of LA, and serum samples were obtained from 42 healthy blood bank donors. All case and control subjects gave written informed consent.
2.2 Enzyme-linked immunospot (ELISpot) T cell assay
T cell reactivity was determined to 4 annexin A2 peptides. The 4 peptides included the immunogenic HLA-DR-presented peptide identified from the synovial tissue of patient RA1, and 3 additional promiscuous annexin A2 peptides that were each predicted to bind >16 HLA-DR molecules. The peptides were synthesized and HPLC-purified in the MGH Core Facility. The sequences of the peptides were: 50GVDEVTIVNILTNRSNAQR68, 97TVILGLLKTPAQYDA111, 164SGDFRKLMVALAKGRRA180 and 285DKVLIRIMVSRSEVD299; the first 3 sequences were the promiscuous peptides and the last sequence was identified from patient RA1. Because cell numbers were limited, the 4 peptides (1 μM) were pooled for stimulation of patients’ PBMC in duplicate wells, using an IFN-γ ELISpotplus kit (MabTech); PHA (phytohemagglutinin) was the positive control and no antigen was the negative control. After 5 days, cells were transferred to ELISpot plates coated with IFN-γ antibodies, and incubated overnight. Images of wells were captured using ImmunoSpot series 3B analyzer, and spots were counted using ImmunoSpot software.
2.3 ELISA for serum IgG anti-annexin A2 antibodies
ELISA plates were coated with 1 μg/ml of recombinant human annexin A2 (Novoprotein, Summit, NJ) overnight at 4°C. Subsequent incubations and washes were performed at room temperature. After washing with phosphate buffered saline with 0.05% Tween-20 (PBST), the plates were blocked with blocking buffer (5% nonfat dry milk in PBST) for one hour. Afterwards, 100 μl of each patient’s serum sample (diluted 40-fold) was added in duplicate wells for 1.5 hours, followed by horseradish-peroxidase (HRP)-conjugated goat anti-human IgG (sc-2453, Santa Cruz Biotech), and then TMB substrate (BD, San Diego, CA). For generation of a standard curve, serial dilutions of rabbit polyclonal antibody against annexin A2 (sc-9061, Santa Cruz Biotech) were included with each assay.
2.4 Quantification of annexin A2 protein levels in serum and synovial fluid
ELISA plates were coated with 2 μg/ml capture antibody (sc-1924, Santa Cruz Biotech.) overnight at 4°C. Plates were then washed with PBST and blocked with 1% BSA PBST for one hour. Afterwards, serum or synovial fluid samples (diluted 1:10 in PBST) were added in duplicate and incubated at 37°C for one hour. Next, detection antibody (5 μg/ml) (sc-9061, Santa Cruz Biotech.) was added for 2 hours. The plates were then incubated with goat anti-rabbit IgG-HRP (sc-2030, Santa Cruz Biotech), followed by TMB substrate. For inter-plate standardization, 2 control samples were included on each plate.
2.5 Immunohistochemistry
Fresh frozen synovial tissue samples were available from 9 LA patients. Staining for annexin A2 was done as previously described for ECGF [15]. Briefly, after blocking, the sections were incubated with rabbit polyclonal antibody against annexin A2 (sc-906, Santa Cruz Biotech) at 4°C overnight. For negative controls, non-specific rabbit IgG was used. The following day, the sections were incubated with biotinylated anti-rabbit secondary antibody, peroxidase-streptavidin, and then, diaminobenzidine substrate. The slides were counterstained with Mayer’s hemotoxylin and mounted with permount. Microscopic images were obtained with a Nikon eclipse ME6000 microscope.
For grading, two independent observers (AP and ACS), who were not aware of the annexin A2 antibody titers, ranked the degree of annexin A2 staining of the tissue on a scale of 1 to 9, with 9 being the highest ranked patient. Staining and ranking of slides for other histologic features of synovial tissue, which were made previously by two independent observers [15], were stratified here according to positive or negative anti-annexin A2 values.
2.6 Statistical analysis
Categorical data were analyzed by chi-square test or Fisher’s exact test, and quantitative data were analyzed using unpaired t test with Welch correction. Correlations were analyzed using Pearson correlation coefficients. All analyses were performed using GraphPad Prism 6. All P values were two-tailed. P values less than or equal to 0.05 were considered statistically significant.
3. Results
3.1 T and B cell responses to annexin A2 in patients with Lyme disease and control subjects
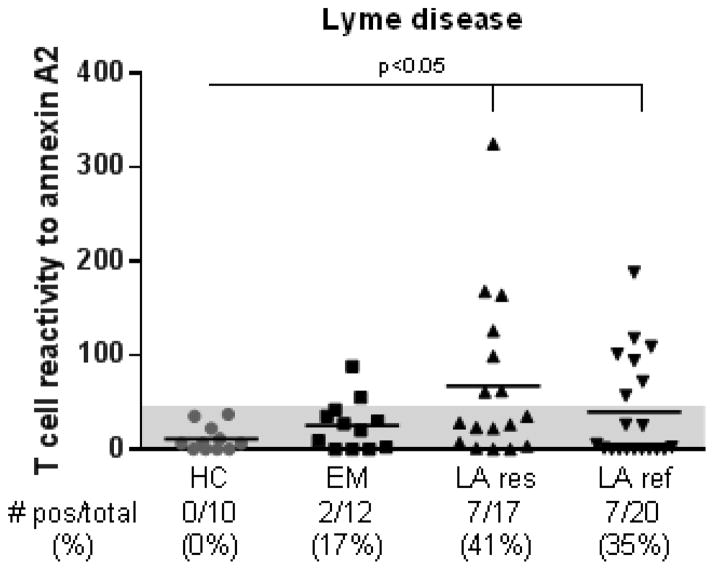
Early in the illness, 2 of 12 patients (17%) with erythema migrans (EM), the initial skin lesion of the infection, had low-level T cell reactivity with annexin A2 peptides (Fig. 1). In contrast, T cells from 7 of 17 patients (41%) with antibiotic-responsive LA and 7 of 20 patients (35%) with antibiotic-refractory LA secreted levels of IFN-γ that were >3 SD above the mean value of 10 healthy control subjects (in both instances, P<0.05). Thus, robust T cell reactivity to annexin A2 peptides was only found late in the illness in patients with LA.
Fig. 1.
T cell reactivity against annexin A2 peptides in Lyme disease patients and healthy control subjects. PBMCs from patients with erythema migrans (EM), antibiotic-responsive Lyme arthritis (LA res), antibiotic-refractory Lyme arthritis (LA ref) and healthy controls (HC) subjects were incubated with a set of 4 annexin A2 peptides (1 μM each), phytohemagglutinin (positive control), or no peptide (negative control), and assayed for IFN-γ secretion using ELISpot assay. A positive response was defined as >3 standard deviation (SD) above the mean of the HC subjects (area above the shaded region). Each symbol indicates the value in an individual subject. The horizontal lines represent the mean values of each group. The P-value for the corresponding statistical comparisons are indicated.
When serum samples were examined for autoantibody responses, 16 of 104 patients (15%) with EM, 9 of 85 patients (11%) with antibiotic-responsive LA, and 11 of 89 patients (12%) with antibiotic-refractory LA had anti-annexin A2 antibody responses that were >3 SD above the mean value in 52 HC (P<0.0001, P=0.002 and P<0.0001, respectively) (Fig. 2). Similarly, 13 of 91 RA patients (14%) had anti-annexin A2 antibody levels that were significantly higher than HC (P=0.002). In contrast, none of 13 patients with influenza; none of 24 patients with seronegative spondyloarthropathies (SpA), including 15 with psoriatic arthritis, 7 with ankylosing spondylitis, and 2 with reactive arthritis; and none of 5 patients with osteoarthritis had positive responses. Thus, the SpA group differed significantly from both the responsive and refractory LA groups (for both comparisons, P<0.0001) and from the RA group (P=0.0003).
Fig. 2.
Determination of IgG anti-annexin A2 antibody levels as assessed by ELISA. Plates were coated with recombinant human annexin-A2 and incubated with serum from patients or control subjects. All serum samples were tested in duplicates. Positivity was defined as >3 SD above the mean value of healthy control (HC) subjects (area above the shaded region). Symbols represent values in individual patients and horizontal lines show mean values. Only significant P values are shown. HC = healthy control; Flu = influenza; EM = erythema migrans; LA res = antibiotic-responsive Lyme arthritis; LA ref = antibiotic-refractory Lyme arthritis; RA = rheumatoid arthritis; SpA = spondyloarthropathies, OA = osteoarthritis.
Patients in whom antibody testing was performed included the subset of 37 LA patients in whom T cell testing was done. When concordance was assessed between T cell (ELISpot) and B cell (ELISA) assays, 2 (5%) had concordant positive responses, and 22 (59%) had concordant negative responses.
Among 51 patients with antibiotic-refractory LA in whom sera were available to measure antibodies to annexin A2, ECGF and apoB-100 (the previously identified autoantigens in Lyme disease), none had responses to 3 of these proteins, 2 had responses to two antigens (4%) and 20 (39%) had reactivity with one of these autoantigens. Among the 50 patients with antibiotic-responsive LA, one (2%) had responses to two of the proteins, and 13 (26%) had reactivity with one of the proteins. Thus, annexin A2, ECGF and apoB-100 autoantibodies occurred primarily in different subgroups of LA patients.
3.2 Annexin A2 protein in serum, synovial fluid, or synovial tissue
For annexin A2 to become a target of host immune responses, one would predict that the protein would be present in high concentrations in patients’ inflamed synovial tissue, SF, and perhaps serum. Therefore, in patients in whom enough serum or SF remained, or in whom synovial tissue was available, annexin A2 protein levels or staining were assessed.
Compared with HC, 11 of the 30 patients (37%) with antibiotic-refractory LA had elevated levels of annexin A2 in serum (P=0.0008), and 5 (17%) had increased levels in SF (P=0.009) (Fig. 3); serum levels tended to be lower in the responsive group (P=0.1). Similar to refractory LA patients, 16 of 63 RA patients (23%) had elevated serum levels of annexin A2 protein. In contrast, annexin A2 levels were not elevated in patients with EM, influenza, or SpA. Thus, patients with antibiotic-refractory LA or RA often had elevated annexin A2 levels, but those with SpA did not (for each comparison, P<0.0001).
Fig. 3.
Determination of serum or synovial fluid (SF) annexin-A2 protein levels as assessed by sandwich ELISA. Positivity was defined as >3 SD above the mean value of healthy control (HC) subjects (area above the shaded region). Symbols represent values in individual patients and horizontal lines show mean values. Only significant P values are shown. HC = healthy control; Flu = influenza; EM = erythema migrans; LA res = antibiotic-responsive Lyme arthritis; LA ref = antibiotic-refractory Lyme arthritis; RA = rheumatoid arthritis; SpA = spondyloarthropathies, OA = osteoarthritis.
When the levels of annexin A2 protein in serum were correlated with serum anti-annexin A2 antibody titers, a positive correlation was found in LA patients both in serum and SF (P=0.0001 and P<0.0001, respectively) (Fig. 4A and B). Similarly, when the degree of annexin A2 protein staining in 9 synovial tissue samples from antibiotic-refractory LA patients was assessed using immunohistologic techniques, the ranking for annexin A2 protein (from highest to lowest) correlated strongly with anti-annexin A2 antibody titers (P=0.006) (Fig. 4C). Similarly, RA patients also had a positive correlation between serum annexin A2 protein levels and anti-annexin A2 antibody levels (P=0.004) (Fig. 4D), but no correlation was observed in patients with SpA (Fig. 4E) or in HC (Fig 4F). Thus, in LA patients, the greater the levels of annexin A2 protein in serum, SF or synovial tissue, the higher the serum anti-annexin A2 antibody titer.
Fig. 4.
Correlations between annexin A2 antibody titers in serum and annexin A2 protein levels in serum, synovial fluid or synovial tissue of patients with Lyme arthritis, rheumatoid arthritis, spondyloarthropathies, or healthy control subjects. Annexin A2 antibody titers correlation with serum annexin A2 protein levels (A) or synovial fluid annexin A2 protein levels (B) in Lyme arthritis patients. Correlation between annexin A2 antibody titers and synovial tissue annexin A2 protein levels as determined by immunohistochemistry (IHC) in patients with Lyme arthritis (C). Correlation between annexin A2 antibody titers and serum annexin A2 protein levels in patients with rheumatoid arthritis (D), spondyloarthropathies (E), or healthy control subjects (F). Ab = antibody.
3.3 Histologic findings analyzed according to annexin A2 antibody responses
Of the 9 patients with LA in whom synovial tissue was available, 5 (55%) had positive results for annexin A2 antibodies and 4 (45%) had negative results. When histologic findings were stratified according to annexin A2 antibody responses, the 5 patients with positive responses had more intense staining for annexin A2 protein (P=0.004), greater synovial fibroblast proliferation (P=0.04), and a trend toward greater fibrosis (P=0.1) than the 4 patients with negative antibody responses (Fig. 5).
Fig. 5.

Histologic findings in synovial tissue stratified by annexin A2 antibody (Ab) responses. The samples analyzed were from 9 patients with antibiotic-refractory Lyme arthritis: 4 had negative annexin A2 Ab responses and 5 had positive responses. The slides were stained with hematoxylin and eosin, or trichrome (for fibrosis) (A), or with antibodies to cell surface markers or to annexin A2 (B). Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median values. Lines outside the boxes represent the 5th and the 95th percentiles. The P-value for the corresponding statistical comparisons is indicated.
Figure 6 demonstrates these findings in one patient with positive anti-annexin A2 antibody responses compared with one patient with negative responses. In patient 1, who had a positive annexin A2 antibody response, synovial tissue showed intense staining for annexin A2 protein along with apparent staining of synovial fibroblasts. In addition, synovial tissue from this patient showed intense fibrosis, as demonstrated by the blue color in trichrome staining. In contrast, in patient 2, who had a negative annexin A2 antibody response, no staining for annexin A2 protein was seen in the tissue. Although synovial fibroblasts were apparent, their numbers appeared to be lower, and minimal fibrosis was seen in the tissue.
Fig. 6.

Findings in synovial tissue pertaining to annexin A2 protein expression, synovial fibroblasts, and fibrosis in 2 representative LA patients who did or did not have anti-annexin A2 antibodies. The sections were stained with rabbit polyclonal antibody against annexin A2, rabbit polyclonal antibody against vimentin (fibroblast), or trichrome (fibrosis). Low-power and high-power views are shown. In the upper panels, the tissue in patient 1 (Pt 1), who had anti-annexin A2 antibodies, showed marked annexin A2 expression, dense synovial fibroblasts, and intense fibrosis. In the lower panels, the tissue in patient 2 (Pt 2), who did not have anti-annexin A2 antibodies, showed minimal annexin A2 expression, less dense synovial fibroblasts, and minimal fibrosis. Ab = antibody.
4. Discussion
In this study, high levels of annexin A2, a widely expressed, calcium-dependent, phospholipid-binding protein [22, 23], were found in the serum, SF, and synovial tissue of a subset of patients with LA, particularly in those with antibiotic-refractory LA. In these patients, annexin A2 served as a target for T and B cell responses. In patients with antibiotic-refractory LA who had anti-annexin A2 antibodies, synovial tissue had intense staining for annexin A2 protein, greater synovial fibroblast proliferation and more tissue fibrosis, implying that this autoantibody response may have specific pathologic consequences.
Annexin A2, which is expressed on a number of cell types, including monocytes and endothelial cells, has a high affinity for phospholipids [24] and is involved in the maintenance of cell membranes and the cytoskeleton [22]. Annexin A2 on endothelial cells acts as a receptor for both plasminogen and tissue plasminogen activator [25], thereby playing an important role in cell surface fibrinolysis. However, this process may be impaired by anti-annexin A2 antibodies, which can activate endothelial cells, leading to loss of their anti-coagulant phenotype [18]. This is a particular problem in several autoimmune rheumatic diseases [24]. In one study, anti-annexin A2 autoantibodies were found in 22% of patients with the anti-phospholipid syndrome (APS) and in 26% of patients with lupus-associated APS [26].
In addition, high levels of annexin A2 have been reported in several immune-mediated diseases in which they enhance fibroblast proliferation and fibrosis in a target tissue. For example, annexin A2 has been associated with fibroblast proliferation in idiopathic pulmonary fibrosis [27] and in immune liver fibrosis [28]. In murine collagen-induced arthritis, annexin A2 promoted migration and invasion of fibroblast synoviocytes into cartilage, leading to cartilage destruction. Conversely, down-regulation of annexin A2 expression alleviated joint damage [29]. Moreover, annexin A2 levels in synovial tissue were higher in RA patients than in OA patients [29].
Consistent with these findings, our patients with antibiotic-refractory LA who had anti-annexin A2 antibodies had significantly greater expression of annexin A2 protein, greater synovial fibroblast proliferation, and a trend toward greater tissue fibrosis. In contrast, although the numbers of SpA patients that we tested was relatively small (N=24), they did not have high annexin A2 protein levels or autoantibody responses to the protein. Thus, high levels of annexin A2 protein and antibodies appear not to be features of all forms of chronic inflammatory arthritis.
To explain how annexin A2 may become immunogenic in Lyme disease, we postulate the following sequence of events. First, as was shown with both ECGF and apoB-100 [13, 14], autoantibodies to annexin A2 typically develop early in the illness in patients with erythema migrans, months before the onset of arthritis. B. burgdorferi spirochetes bind plasminogen and its activator, which facilitate spreading of the organism in the tick and in the host [30, 31]. Since annexin A2 binds both plasminogen and tissue plasminogen activator, the spirochete may also bind annexin A2, thereby acting as a conduit for phagocytosis of these human proteins. The development of these autoantibodies, which result from close interaction between the spirochete and host, would constitute the “first hit” in the autoimmune process, though this early response does not appear to be pathogenic.
Later in the disease, in LA patients, the inflammatory microenvironment of the joint may lead to increased expression of annexin A2, particularly in patients with antibiotic-refractory LA, resulting in greater HLA-DR presentation of annexin A2 peptides, and the development of both T and B cell responses to the protein. These events would constitute the “second immunologic hit”. In support of this idea, the strong correlation between annexin A2 protein levels and anti-annexin A2 antibody values suggests that abundance of the protein is a factor in antigenicity. Although T and B cell responses to annexin A2 are found in antibiotic-responsive and antibiotic-refractory LA, refractory patients have significantly higher levels of inflammatory cytokines in joints, including IFN-γ and TNF-α [32, 33]. These cytokines are known to alter the immunoregulatory set point of T cells [34, 35], which may lead to immune dysregulation [36, 37]. Moreover, high levels of annexin A2 protein in the refractory group may stimulate synovial fibroblast proliferation, an important cell in the secretion of pro-inflammatory cytokines [38], and annexin A2 protein-antibody complexes may further accentuate the inflammatory response. This model suggests that the pathogenicity of the anti-annexin A2 immune response is context dependent, occurring primarily within an intense inflammatory microenvironment in joints.
In patients with antibiotic-refractory LA, synovitis resolves within months to several years after antibiotic treatment. In these patients, we postulate that the “danger” signals provided by live spirochetes to innate immune cells are no longer present. Without these adjuvant-like signals, the adaptive immune response to autoantigens eventually regains homeostasis, and the arthritis resolves.
In summary, T and B cell responses occur to annexin A2 in a subset of patients with LA. Early in the illness, in patients with EM, close interactions between the spirochete and the host may be an initial factor in the development of autoantibody responses to annexin A2. Later in the disease, in patients with LA, marked abundance of the protein in joints may lead to antigen presentation by HLA-DR molecules, and the development of T and B cell responses to the protein. In patients with antibiotic-refractory LA, the intense inflammatory microenvironment in joints may allow this immune response to become pathogenic.
Highlights.
A subgroup with Lyme arthritis had T and B cell responses to annexin A2.
Annexin A2 antibodies found in erythema migrans prior to arthritis.
Annexin A2 protein levels correlated with annexin A2 autoantibody titers.
Annexin A2 protein levels were highest in refractory arthritis.
Annexin A2 antibodies correlated with greater synovial fibroblasts and fibrosis.
Acknowledgments
This work was supported by NIH grant (RO1 AI-101175), the English, Bonter. Mitchell Foundation, the Ounsworth-Fitzgerald Foundation, the Eshe Fund, and the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital. Dr. Arvikar was supported by the NIH (training grant AR-007258), and Dr. Strle was supported by the NIH (grant K01-AR-062098).
The authors thank Drs. Diana Londono and Diego Cadavid for help in reading histologic features of synovial tissue in patients with LA; Drs. John Aversa and Dennis Burke for help in obtaining synovial tissue from patients with LA, respectively; Drs. Nitin Damle, Vijay Sikand, Greg Emkey, Ryan Antolini, and Deborah Collier for help in collecting samples from LA and RA patients; Dr. Stephanie Yost for providing serum samples from patients with influenza; Dr. John Branda for providing serum samples from healthy blood donors, and Ms. Gail McHugh for laboratory assistance.
Abbreviations
- apoB-100
apolipoprotein B-100
- APS
anti-phospholipid syndrome
- CDC
Centers for Disease Control and Prevention
- ECGF
endothelial cell growth factor
- EM
erythema migrans
- HC
healthy control
- LA
Lyme arthritis
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- PBMC
peripheral blood mononuclear cells
- PBST
phosphate buffered saline with 0.05% Tween-20
- RA
rheumatoid arthritis
- SD
standard deviation
- SFU
spot forming units
Footnotes
This work was presented in part at the annual meeting of the American College of Rheumatology in Boston 2014 [16].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47:188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KL, Seward RJ, Ben-Menachem G, Glickstein LJ, Costello CE, Steere AC. Strong IgG antibody responses to Borrelia burgdorferi glycolipids in patients with Lyme arthritis, a late manifestation of the infection. Clin Immunol. 2009;132:93–102. doi: 10.1016/j.clim.2009.03.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheum. 2014;66:2313–23. doi: 10.1002/art.38756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–52. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 8.Sokolove J, Lindstrom TM, Robinson WH. Development and deployment of antigen arrays for investigation of B-cell fine specificity in autoimmune disease. Front Biosci (Elite Ed) 2012;4:320–30. doi: 10.2741/379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52:2645–55. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 10.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 11.Tan YC, Kongpachith S, Blum LK, Ju CH, Lahey LJ, Lu DR, et al. Barcode-enabled sequencing of plasmablast antibody repertoires in rheumatoid arthritis. Arthritis Rheum. 2014;66:2706–15. doi: 10.1002/art.38754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seward RJ, Drouin EE, Steere AC, Costello CE. Peptides presented by HLA-DR molecules in synovia of patients with rheumatoid arthritis or antibiotic-refractory Lyme arthritis. Mol Cell Proteomics. 2011;10:M110 002477. doi: 10.1074/mcp.M110.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouin EE, Seward RJ, Strle K, McHugh G, Katchar K, Londono D, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65:186–96. doi: 10.1002/art.37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley JT, Drouin EE, Pianta A, Strle K, Wang Q, Costello CE, et al. A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J Infect Dis. 2015 doi: 10.1093/infdis/jiv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Londono D, Cadavid D, Drouin EE, Strle K, McHugh G, Aversa JM, et al. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2014;66:2124–33. doi: 10.1002/art.38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pianta A, Drouin EE, Arvikar S, Costello CE, Steere AC. Identification of annexin A-2 as an autoantigen in rheumatoid arthritis and in Lyme arthritis. Arthritis Rheum. 2014;66:S437. [Google Scholar]
- 17.Pianta A, Drouin EE, Wang Q, Arvikar S, Costello CE, Steere AC. Identificatiom of N-acetylglucosamine-6-sulfatase and filamin A as novel targets of autoimmune T and B cell responses in rheumatoid arthritis. Ann Rheum Dis. 2015;74(Suppl2):112. [Google Scholar]
- 18.Cockrell E, Espinola RG, McCrae KR. Annexin A2: biology and relevance to the antiphospholipid syndrome. Lupus. 2008;17:943–51. doi: 10.1177/0961203308095329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ao W, Zheng H, Chen XW, Shen Y, Yang CD. Anti-annexin II antibody is associated with thrombosis and/or pregnancy morbidity in antiphospholipid syndrome and systemic lupus erythematosus with thrombosis. Rheumatol Int. 2011;31:865–9. doi: 10.1007/s00296-010-1379-4. [DOI] [PubMed] [Google Scholar]
- 20.Salle V, Maziere JC, Smail A, Cevallos R, Maziere C, Fuentes V, et al. Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–7. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 21.Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep. 1990;39:1–43. [PubMed] [Google Scholar]
- 22.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 23.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–61. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 24.Iaccarino L, Ghirardello A, Canova M, Zen M, Bettio S, Nalotto L, et al. Anti-annexins autoantibodies: their role as biomarkers of autoimmune diseases. Autoimmun Rev. 2011;10:553–8. doi: 10.1016/j.autrev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Semin Thromb Hemost. 2013;39:338–46. doi: 10.1055/s-0033-1334143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, del Cravioto MC, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–82. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuliga MYX, Langenbach S, Harris T, Stewart AG. Extracellular annexin A2 mediates lung fibroblast cytokine production and proliferation: a potential role in pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:A2031. [Google Scholar]
- 28.Zhang L, Peng X, Zhang Z, Feng Y, Jia X, Shi Y, et al. Subcellular proteome analysis unraveled annexin A2 related to immune liver fibrosis. J Cell Biochem. 2010;110:219–28. doi: 10.1002/jcb.22529. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W, Zhang C, Shi M, Zhang J, Li M, Xue X, et al. The discoidin domain receptor 2/annexin A2/matrix metalloproteinase 13 loop promotes joint destruction in arthritis through promoting migration and invasion of fibroblast-like synoviocytes. Arthritis Rheum. 2014;66:2355–67. doi: 10.1002/art.38696. [DOI] [PubMed] [Google Scholar]
- 30.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, Benach JL. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–9. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 31.Hu LT, Perides G, Noring R, Klempner MS. Binding of human plasminogen to Borrelia burgdorferi. Infect Immun. 1995;63:3491–6. doi: 10.1128/iai.63.9.3491-3496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–35. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 33.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–8. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–5. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, et al. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010;62:2127–37. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vudattu NK, Strle K, Steere AC, Drouin EE. Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2013;65:1643–53. doi: 10.1002/art.37910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]