Abstract
The quality of platelets decreases over storage time, shortening their shelf life and potentially worsening transfusion outcomes. The changes in mitochondrial function associated with platelet storage are poorly defined and to address this we measured platelet bioenergetics in freshly isolated and stored platelets. We demonstrate the hypotonic stress test stimulates both glycolysis and oxidative phosphorylation and the stored platelets showed a decreased recovery to this stress. We found no change in aggregability between the freshly isolated and stored platelets. Bioenergetic parameters were changed including increased proton leak and decreased basal respiration and this was reflected in a lower bioenergetic health index (BHI). Mitochondrial electron transport, measured in permeabilized platelets, showed only minor changes which are unlikely to have a significant impact on platelet function. There were no changes in basal glycolysis between the fresh and stored platelets, however, glycolytic rate was increased in stored platelets when mitochondrial ATP production was inhibited. The increase in proton leak was attenuated by the addition of albumin, suggesting that free fatty acids could play a role in increasing proton leak and decreasing mitochondrial function. In summary, platelet storage causes a modest decrease in oxidative phosphorylation driven by an increase in mitochondrial proton leak, which contributes to the decreased recovery to hypotonic stress.
Keywords: Platelet Storage Lesion, Bioenergetics, Proton Leak, Aggregation, Hypotonic Stress Response
INTRODUCTION
Platelet transfusions are an important clinical treatment for patients undergoing chemotherapy, radiation, after surgery or trauma, and for patients with inherited platelet disorders such as Bernard-Soulier Syndrome and idiopathic thrombocytopenic purpura [1, 2]. Platelets from healthy human volunteers which are collected and stored in the blood bank undergo a progressive decline in function and quality during storage, which is generally characterized as the platelet storage lesion [3]. Key features of the platelet storage lesion include change in morphology, decreased aggregation, increased glycolytic rate, decreased plasma pH, decreased mitochondrial function, increased expression of activation markers, and a decreased hypotonic stress response [4–8]. Successful platelet transfusion requires an increment in platelet count of 30,000–50,000/microliter per unit transfused, accompanied by normal function in primary hemostasis [9]. Due to the platelet storage lesion and the potential for bacterial contamination, hospitals discard platelets past day 5 of collection, leading to shortages and economic loss [10]. In this manuscript, we will define the bioenergetic changes of stored platelets, and the mechanisms underlying these changes.
The processes of platelet activation and aggregation require both glycolysis and oxidative phosphorylation, raising the question whether the metabolic impairments of the storage lesion impact on platelet function. It has been reported that ATP, ADP and AMP levels decrease during storage suggesting a deficiency in glycolysis and/or oxidative phosphorylation [11]. A number of studies have reported decrease in glucose levels, and an increase in lactate, in the platelet storage bags, suggesting an active glycolytic pathway and possibily inhibition of mitochondrial function [12–14]. Since this increase in glycolytic rate occurs despite an increase in fatty acids, which we would expect could support mitochondrial function, it also suggests a possible mitochondrial defect. Indeed, a decrease in mitochondrial membrane potential has been reported over storage time, but studies regarding mitochondrial respiratory activities are conflicting. Some reports have shown that the capacity to consume oxygen over 7 days of storage was not altered, as measured by blood gas analyzer or Clark oxygen electrodes [12, 15]. Other studies have demonstrated an almost complete loss of mitochondrial function. For example, one study reported a decrease of basal respiration of approximately 60% at day 8 whereas others report an almost complete loss of function at the second day which contrasts with other reports that show essentially little or no change [8, 16, 17]. Typically, these studies use very small sample sizes and varying protocols for platelet storage which are different from those used in transfusion medicine [8, 16, 18]. In addition, isolation of mitochondria from the platelets to analyze complex activities may lead to damage to the organelle which can be avoided by using cellular bioenergetic analysis [19]. These factors could partially explain the inconsistency of these data. Since both glycolysis and oxidative phosphorylation play an important role in platelet function [20, 21] it is important to resolve these issues so effective strategies can be developed to improve the performance of stored platelets following transfusion.
In order to better understand the metabolic and functional changes during storage, 38 platelet concentrates, between ages day 6–9, were obtained from the blood bank, and their mitochondrial and glycolytic function were measured using the Seahorse Extracellular flux analyzer, and compared to freshly isolated platelets from healthy donors. We found that aggregation following thrombin stimulation was not significantly different in the stored platelets, but recovery after the hypotonic stress response was impaired in the stored platelets. Additionally, stored platelets showed a decrease in basal, ATP linked OCR, and an increase in proton leak and reserve capacity. We also observed that the changes in bioenergetics in the stored platelets were primarily due to an increase in proton leak which we ascribed to the uncoupling effect of fatty acids. In support of this hypothesis, addition of human serum albumin (HSA) to stored platelets attenuated the increase in proton leak, suggesting that it could be used as a possible additive to preserve mitochondrial function in stored platelets.
Methods
Isolation of Platelets
Platelets were isolated from freshly drawn healthy human blood and platelet concentrates obtained from the blood bank as previously described [21, 22]. In brief, the platelet rich plasma was centrifuged at 1500 g and washed with PBS containing PGI2 (1 µg/ml) before counting platelet number by turbidimetry. Institutional Review Board approval (Protocol #X110718014) was obtained from the University of Alabama at Birmingham for the isolation and use of platelets in these experiments. In the blood bank, there are a minimum of 3×1011 platelets per bag in approximately 300 ml of plasma containing acid citrate dextrose (ACD) [2]. As a result, there are more platelets per volume of plasma in the storage bags (100×107/ml) than in the circulation (150–400×106/ml).
2.2 Aggregation and Hypotonic Stress Response
2.3 Platelet aggregation was monitored by light transmittance at 405 nm after addition of thrombin (0.5 U/ml) [23]. Platelet hypotonic stress response measurements were modified and performed as previously described [24]. Briefly, 40×106 platelets/ml were suspended in 100 µl XF assay buffer and added to a 96-well microtiter plate. Next, 100 µl water was added as the hypotonic challenge, and change in light transmission was measured at 405 nm. Immediately following the hypotonic challenge platelets swell, and subsequently return to their original shape and size.
2.4 Mitochondrial measurements
Cellular bioenergetics were performed on the Seahorse Extracellular Flux (XF) Analyzer as described previously [21, 22]. In brief, 1 × 107 platelets were plated on Cell-Tak coated 96 well format XF plates in XF DMEM media (DMEM with 1 mM pyruvate, 5.5 mM D-glucose, 4 mM L-glutamine, pH 7.4). The mitochondrial stress test was performed by measuring the basal oxygen consumption rate (OCR) followed by sequential injection of oligomycin (1 µg/ml), FCCP (0.6 µM) and antimycin A (10 µM). Extracellular acidification (ECAR) was simultaneously measured. The mitochondrial permeabilization assay was modified and performed as previously described [25]. Briefly, the platelets were plated in MAS buffer (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EDTA, pH 7.2). To measure complex I and complex II respiratory activity, saponin (60 µg/ml), pyruvate (5 mM), malate (2.5 mM), succinate (10 mM) and ADP (10 mM) or FCCP (0.6 µM) were injected. Then rotenone (1 µM) was injected to assess complex I linked respiration, followed by injection of antimycin A to measure complex II linked respiration. In order to measure state 3 and 4 respiration with complex IV substrates, saponin (60 µg/ml), N,N,N',N'-Tetramethyl-p-Phenylenediamine (TMPD 0.5 mM), ascorbate (2 mM) were injected followed by oligomycin (1 µg/ml) then antimycin A (10 µM).
2.5 Statistical Analysis
Freshly isolated platelets from 35 adult volunteers and stored platelets from 38 bags ranging from day 6–9 of storage were used for the experiments presented here. Statistical significance was calculated using a 2 tailed student t-test, and p<0.05 was considered significant.
3. RESULTS
3.1 Aggregation and Hypotonic Stress Response of Freshly Isolated and Stored Platelets
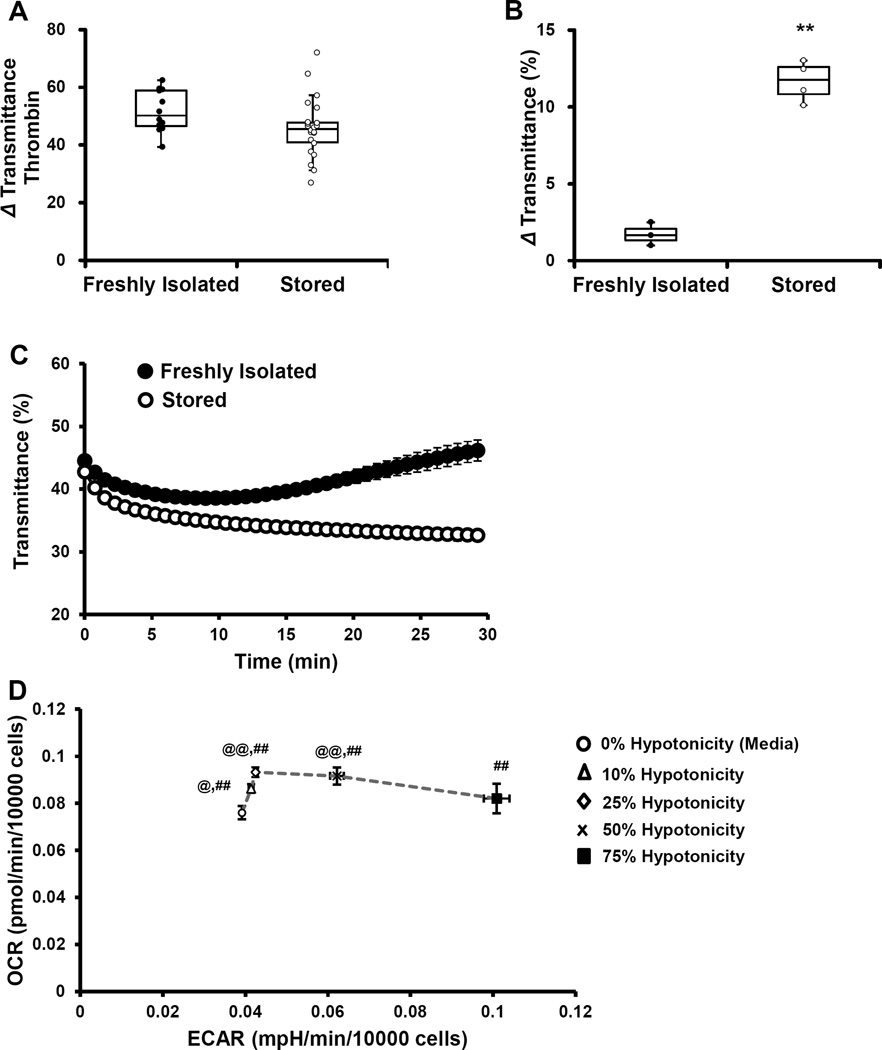
No significant difference in the thrombin-dependent (0.5 U/ml) aggregation of freshly isolated platelets or stored platelets was found (Figure 1A). The hypotonic stress response was used as an additional measure of platelet function, which is also an indicator of platelet survival after transfusion in vivo [26]. For this experiment, an equal volume of water (100 µl) was added to a platelet suspension in XF-DMEM, after which light transmittance was monitored. In both types of platelets, the light transmittance rapidly decreased after the addition of water, due to swelling of the platelets (Figure 1B). In the freshly isolated platelets, there was recovery, as evidenced by change in light transmittance which gradually increased and returned to baseline levels. On the other hand, in the stored platelets, the light transmittance continued to decrease, suggesting the platelets continued to swell and were unable to restore their former morphology (Figure 1B and C).
Figure 1. Aggregation and hypotonic stress response of freshly isolated and stored platelets.
Platelets from fresh blood or storage bags were isolated, followed by evaluation of (A) aggregation using thrombin (0.5 U/ml) and (B) hypotonic stress response using equal volume of water. (C) Change in light transmittance after hypotonic stress response assay and (D) OCR vs. ECAR plot of platelets exposed to different hypotonic stress levels (0–75%) from one healthy volunteer donor. Extent of aggregation is expressed as change in light transmittance after thrombin (0.5 U/ml). HSR represented as change in transmittance after addition of water. Aggregation and HSR data graphed as box plots with lower 25th percentile, median, upper 75th percentile, and whiskers drawn at 1.5 × interquartile range. Data expressed as mean±SEM. Aggregation - 12 freshly isolated platelets and 22 stored platelets; HSR - 3–4 donors. n=3 replicates per sample. **p<0.01, different from freshly isolated. %%p<0.01, %p<0.05, OCR different from 0% water. ##p<0.01, ECAR different from 0% water.
In order to determine the impact of the hypotonic stress response on bioenergetics, both basal OCR and ECAR were measured for different levels of hypotonicity. For this assay, freshly isolated platelets were supplemented with increasing amounts of water (0–75%), 30 minutes prior to the measurement of basal OCR and ECAR. The data obtained was plotted as oxygen consumption rate (OCR) vs. extracellular acidification rate (ECAR) energy diagram (Figure 1D). Even at 10% hypotonic stress, there was a significant increase in both OCR and ECAR consistent with increased energy demand. As the amount of water increased, OCR also increased until it reached a plateau. However, with 75% water, the increase in OCR was attenuated and not different from control platelets (Figure 1D). These data are consistent with the activation of both glycolysis and oxidative phosphorylation during the hypotonic stress test and suggest that bioenergetics could be impaired in the stored platelets.
3.2 Cellular Bioenergetics for Freshly Isolated and Stored Platelets
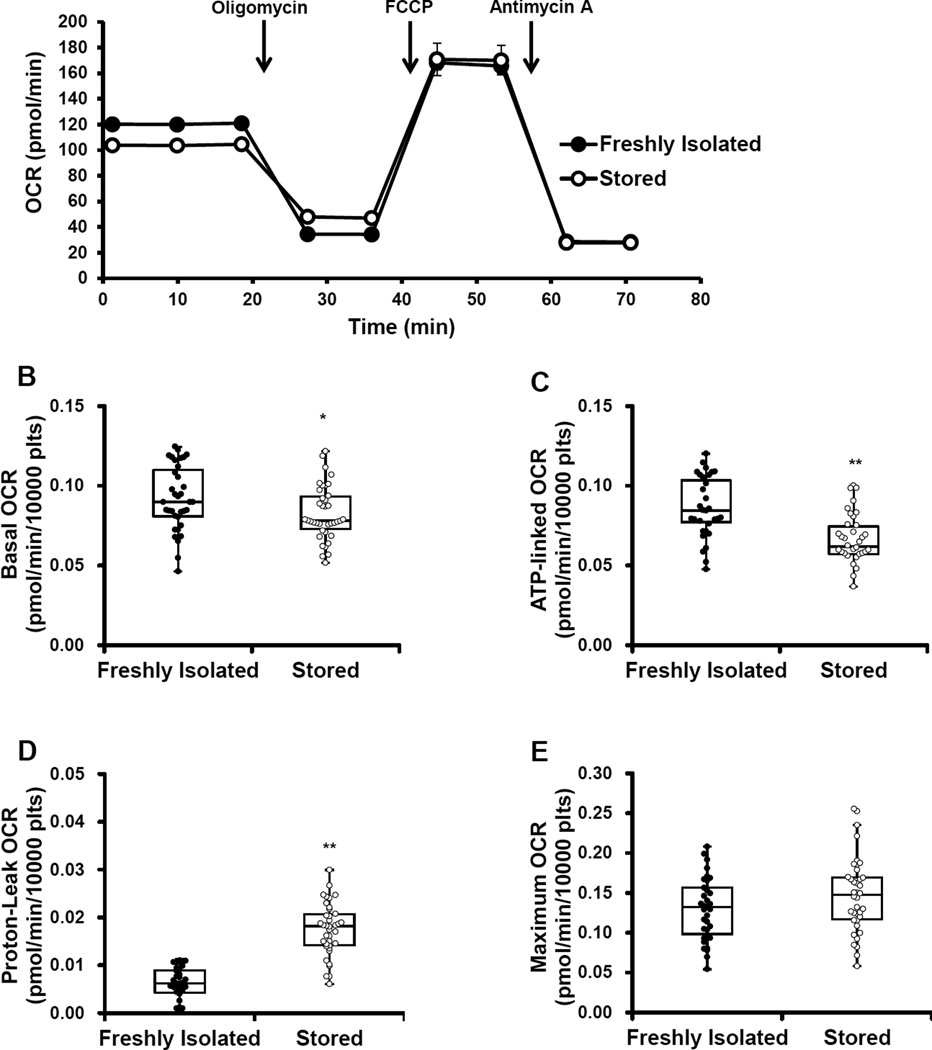
Next a mitochondrial stress test was performed on platelets by first, establishing a basal OCR, which was 10% lower in the stored compared to the freshly isolated platelets (Figure 2A and B). Next, oligomycin (1 µg/ml) was injected to inhibit the mitochondrial ATP synthase, which resulted in the expected decrease in OCR. Oligomycin-dependent decrease in OCR was greater in the freshly isolated platelets compared stored (Figure 2A). Next FCCP (0.6 µM) was injected to uncouple the mitochondria and elicit maximal cellular respiration. FCCP increased maximal respiration to the same extent in both the freshly isolated and stored platelets (Figure 2A and E). Finally, antimycin A (10 µM) was injected to inhibit all mitochondrial oxygen consumption and measure non-mitochondrial OCR, which decreased OCR to the same extent in both groups of platelets (Figure 2A and G). The indices of ATP-linked, proton leak, and reserve capacity were calculated from the bioenergetic profiles. ATP-linked OCR, a measure of oxygen consumption linked to ATP production, was calculated by subtracting the OCR after oligomycin injection from the basal OCR. ATP-linked respiration significantly decreased by approximately 23% in the stored platelets compared to the freshly isolated (Figure 2 C). A measure of oxygen consumption not coupled to ATP production is proton leak, which was calculated by subtracting the rate after antimycin A injection from the rate after oligomycin injection. Proton leak in the stored platelets significantly increased by 190% compared to the freshly isolated platelets, consistent with mitochondrial uncoupling increasing in the stored platelets (Figure 2D). Reserve capacity, which is a measure of how much the platelet can increase its ATP output in response to demand, was calculated by subtracting basal OCR from the rate after FCCP addition. Reserve capacity was significantly higher in the stored platelets compared to the freshly isolated platelets (Figure 2F). Integrating these indices of mitochondrial function into one value, we have used the Bioenergetic Health Index (BHI) calculated using the formula:
BHI was 52% lower in the stored platelets compared to the freshly isolated platelets (Figure 2H).
Figure 2. Mitochondrial function of freshly isolated and stored platelets.
Freshly isolated and stored platelets (10×106/well) were plated on Cell-Tak coated plates and mitochondrial stress test was performed by establishing basal OCR followed by sequential injection of 1 µg/ml oligomycin, 0.6 µM FCCP and 10 µM antimycin A. (A) Representative OCR traces of mitochondrial stress test. Indices of mitochondrial function – (B) Basal, (C) ATP-linked (AL), (D) Proton leak (Pl), (E) Maximal (Max), (F) Reserve capacity (RC), (G) Non-mitochondrial (NM) OCR and (H) BHI [(ALxRC)/(PLxNM)] were calculated. The indices are represented in a box plot with lower 25th percentile, median, upper 75th percentile, and whiskers drawn at 1.5 × interquartile range. n = 35 individual donors for the freshly isolated and n = 38 individual bags for the stored. *p<0.05, **p <0.01, different from freshly isolated.
3.3 Mitochondrial Respiration in Freshly Isolated and Stored Platelets
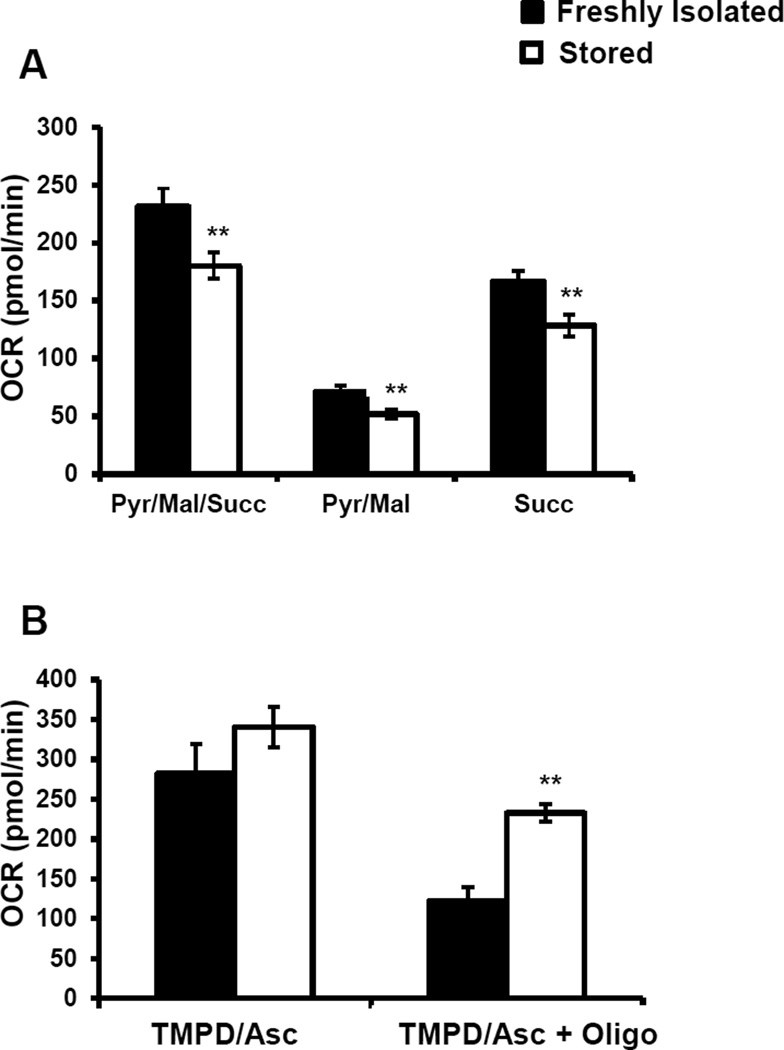
The decrease in basal and ATP-linked respiration could be due to a decrease in the activities of the mitochondrial electron transport chain complexes, hence complex I and II mediated respiration was determined by permeabilizing the plasma membrane and supplementing the mitochondria with excess amounts of substrates and ADP. Saponin was used to permeabilize the plasma membrane along with the addition of pyruvate, malate (complex I associated substrates), succinate (complex II associated substrate) and ADP (Figure 3A). This caused a stimulation of complex I and II dependent respiration in both the freshly isolated and stored platelets, but this increase was significantly lower in the stored platelets (Figure 3A). Next, rotenone was injected to inhibit complex I mediated respiration, which decreased OCR in both groups of platelets (Figure 3 A). Finally, antimycin A was injected to inhibit all mitochondrial respiration, which as expected decreased OCR in both fresh and stored platelets (Figure 3A). Complex I or pyruvate/malate contribution to respiration was calculated by subtracting the rate after rotenone injection from the rate after saponin and substrate injection. These data showed an approximately 27% decrease in complex I mediated respiration in stored platelets compared to control (Figure 3A). Complex II or succinate linked respiration was calculated by subtracting the rate after antimycin A injection from the rate after rotenone injection, which showed a 23% decreased in complex II mediated respiration in the stored platelets (Figure 3 A).
Figure 3. Mitochondrial respiration in freshly isolated and stored platelets.
Freshly isolated and stored platelets were plated on Cell-Tak coated plates in MAS buffer, and the permeabilization assay was performed. First basal OCR was established, then saponin (60 µg/ml), pyruvate (5 mM), malate (2.5 mM) and ADP (1 mM) were injected, followed by rotenone (10 µM), then antimycin A (10 µM). (A) Complex I + Complex II-dependent respiration (C) Complex IV-dependent respiration was determined. Saponin (60 µg/ml), TMPD (0.5 mM), ascorbate (2 mM), ADP (1 mM) were injected followed by sequential injections of oligomycin (1 µg/ml) and antimycin (10 µM) + azide (20 mM). Data expressed as mean±SEM, n = 3–5 individual donors for the freshly isolated and n = 3–7 individual donors for the stored platelets. **p<0.01, different from freshly isolated.
Next, the activity of complex IV was tested by permeabilizing the mitochondria and providing Tetramethyl-p-Phenylenediamine (TMPD) as an electron donor to cytochrome c, along with ADP to stimulate respiration. Ascorbate (Asc) was added to reduce oxidized TMPD to regenerate its electron donation potential. There was no difference in the TMPD/Asc stimulated respiration between the freshly isolated and stored platelets (Figure 3B). Next oligomycin was added to inhibit ATP synthase, followed by injection of antimycin A + azide. The TMPD/Asc + oligomycin OCR was calculated by subtracting the rate after antimycin A + azide from the rate after oligomycin injection. This value is a measure of proton leak or uncoupling, which was approximately 90% higher OCR in the stored platelets compared to the freshly isolated platelets (Figure 3B).
3.4 Extracellular Acidification in Freshly Isolated and Stored Platelets
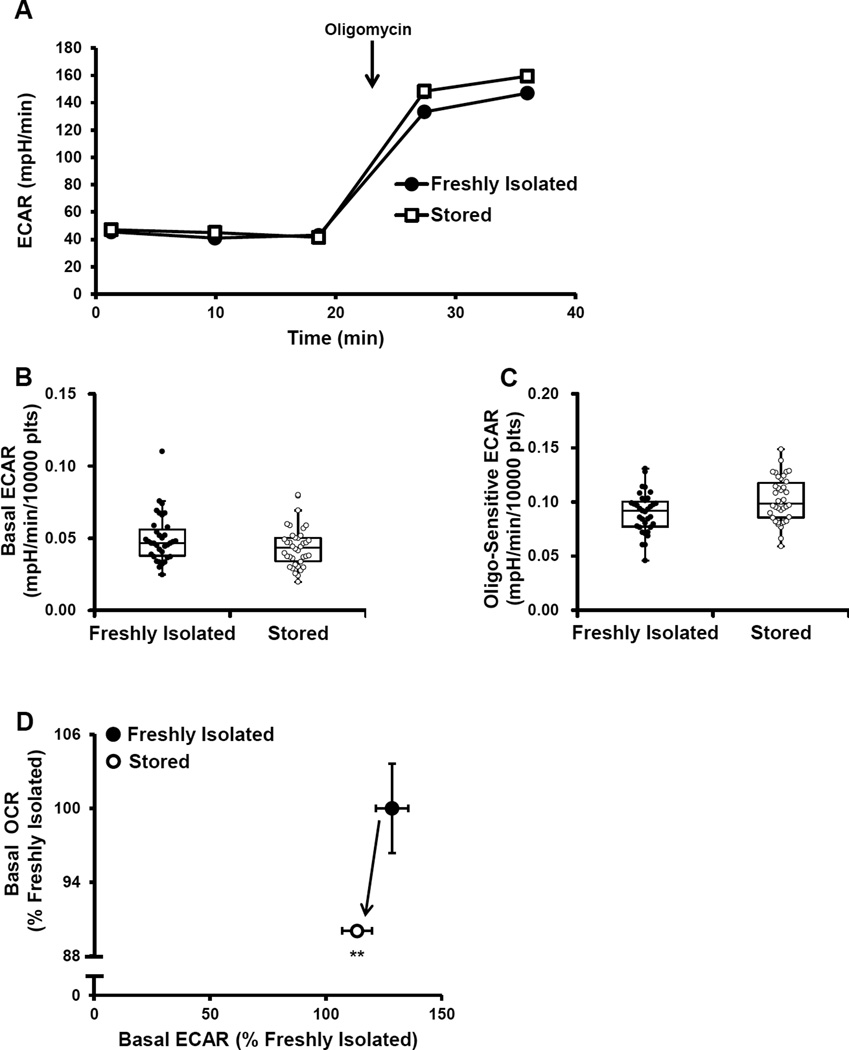
ECAR was measured simultaneously as OCR in both freshly isolated and stored platelets as described in Figure 2A. We have previously shown that over 90% of the ECAR can be ascribed to glycolysis in platelets [21]. Basal ECAR was not significantly different between either group of platelets (Figure 4A,B). Oligomycin sensitive ECAR was calculated by subtracting the rate after oligomycin injection from basal ECAR. Oligomycin sensitive ECAR was approximately 13% higher in the stored compared to the freshly isolated platelets, consistent with a higher glycolytic capacity in stored platelets (Figure 4A).
Figure 4. Glycolytic function of freshly isolated and stored platelets.
Freshly isolated and stored platelets were plated on Cell-Tak coated plates and basal ECAR was established followed by the injection of 1 µg/ml oligomycin (A) Representative ECAR traces of fresh and stored platelets. Indices of glycolytic function – (B) Basal and (C) Oligomycin sensitive ECAR were calculated. (D) Basal OCR vs Basal ECAR plot of both freshly isolated and stored platelets, plotted as a percentage of freshly isolated. The indices are represented in a box plot with lower 25th percentile, median, upper 75th percentile, and whiskers drawn at 1.5 × interquartile range. n = 35 individual donors for the freshly isolated and n = 38 individual donors for the stored. *p<0.05, **p<0.01, different from freshly isolated.
To further demonstrate the relationship between mitochondrial function and glycolysis, an OCR vs ECAR plot was utilized. The plot showed higher OCR in the freshly isolated platelets, and no change in the basal level of glycolysis (Figure 4 C), indicating that at a resting state there is a lower mitochondrial contribution to ATP generation in stored platelets, but no apparent loss in glycolytic capacity.
3.5 Mechanism of Increased Proton Leak in Platelets
Previous literature has shown that free fatty acids have a mitochondrial uncoupling effect in hepatocytes, rat liver, heart and skeletal muscle mitochondria, which can be attenuated by the addition of albumin [27–32]. To investigate the mechanism of increased proton leak in stored platelets, human serum albumin (HSA) was injected to determine its effect on bioenergetics. The bioenergetic assay was modified and performed as shown in Figure 2A, with the addition of HSA (150 µM) after oligomycin injection and followed for 32 minutes. In the freshly isolated platelets, basal OCR was established, after which oligomycin followed by HSA were injected which caused a small but non-significant decrease in OCR. Following HSA injection, FCCP and antimycin A were injected sequentially (Figure 5A). Since albumin can bind FCCP, it was titrated to give a similar maximal response as the control group. A higher dose of FCCP (3.5 µM) was used in all experiments with HSA injections. The proton leak remaining after HSA injection was calculated by subtracting the rate after HSA injection from the rate after antimycin injection, which showed no difference in proton leak before and after HSA injection (Figure 5B). Maximal, reserve capacity and non-mitochondrial OCR were not changed after HSA injection compared to the control. Similar experiments were also performed with stored platelets. After the injection of HSA, there was a large decrease in OCR in the HSA treated group compared to the control (Figure 5C). The proton leak after HSA injection was approximately 80% lower compared to the control (Figure 5D). There were no changes in maximal, reserve capacity or non-mitochondrial observed after HSA treatment compared to the control platelets (Figure 5 D).
Figure 5. Effect of HSA on freshly isolated and stored platelet mitochondrial function.
Freshly isolated and stored platelets were plated on Cell-Tak coated plates and a modified mitochondrial stress test was performed with measurement of basal, followed by injection of oligomycin (1 µg/ml), HSA (150 µM), FCCP (0.6 µM for controls and 3.5 µM for HSA group) and antimycin A (10 µM). Representative bioenergetic traces of (A) freshly isolated and (C) stored platelets. Indices of bioenergetics including proton leak HSA (rate after HSA injection – rate after antimycin A injection) were calculated for (B) fresh and (D) stored platelets. Data expressed as mean±SEM, n = 3 individual donors for the freshly isolated and stored platelets. **p<0.01, different from freshly isolated.
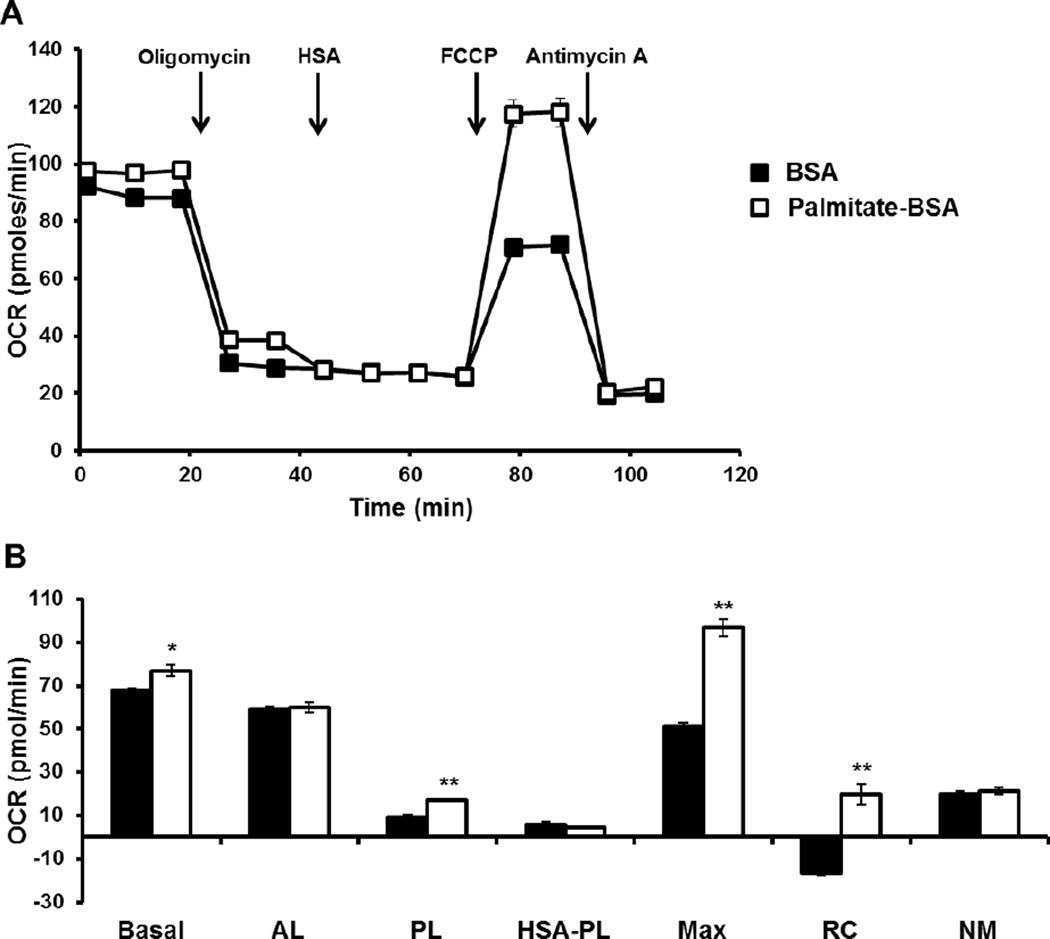
These data suggest that fatty acids are contributing to the proton leak in the stored platelets. To confirm this hypothesis, platelets were pre-treated with either BSA alone or palmitate conjugated to BSA (200 µM) prior to performing the bioenergetic assay as described in (Figure 5A). Addition of palmitate-BSA increased basal OCR, which we have shown previously, and was ascribed to an increase in substrate supply causing increased oxidative phosphorylation [21]. Importantly, the oligomycin-sensitive OCR was lower in the palmitate-BSA treated group compared to the BSA control (Figure 6 A). Next, excess HSA (150 µM) not conjugated with fatty acid was injected, which decreased the proton leak only in the palmitate-BSA group (Figure 6A).
Figure 6. Palmitate increases proton leak which can be inhibited by HSA.
Freshly isolated and stored platelets were plated on Cell-Tak coated plates and pre-treated with either BSA or palmitate-BSA (200 µM). (A) Bioenergetic profile was established with first a basal rate, then sequential injection of oligomycin (1 µg/ml), HSA (150 µM), FCCP (0.6 µM for controls and 3.5 µM for HSA group) and antimycin A (10 µM). (B) Indices of bioenergetics including proton leak after HSA injection (rate after HAS injection – rate after antimycin A injection) were calculated. Data expressed as mean±SEM, from one representative donor, n = 3–5 replicates per sample. *p<0.05, **p<0.01, different from freshly isolated.
Addition of FCCP increased maximal respiration to a greater extent in the palmitate-BSA group (Figure 6 A). Finally, addition of antimycin A decreases OCR to the same level in both the palmitate-BSA and BSA group (Figure 6 A). Calculation of proton leak revealed an approximately 90% increase in proton leak in the palmitate-BSA treated group, and a 75% decrease in the proton leak when HSA was added, bringing both the control and HSA treated groups to the same level of proton leak (Figure 6B). Reserve capacity was higher in the HSA treated platelets compared to the control (Figure 6B). Based on these results, it is evident that fatty acids contribute to uncoupling in stored platelets which can be attenuated by the addition of albumin.
4. DISCUSSION
Platelet transfusion therapy is an essential and lifesaving treatment for trauma and other conditions causing thrombocytopenia [1, 2]. However, the storage of donor platelets for transfusions is problematic because of the decline in platelet quality over storage time, collectively known as the storage lesion [4–7, 10]. The storage lesion combined with risk of bacterial contamination due to the need to incubate the platelet units at room temperature, leads to a short shelf-life of 5 days for banked platelets [10]. The platelet storage lesion is associated with metabolic changes which could affect the quality and function of the platelets in vivo, post-transfusion [12, 15–17]. The increase in glycolysis during storage accelerates plasma acidification, and the decrease in mitochondrial oxidative phosphorylation could have important effects on the ability of platelets to activate and aggregate [21, 33–39]. Previous studies on the metabolic characteristics of the storage lesion have described different levels of mitochondrial dysfunction, and the focus of this study was to accurately define the metabolic changes. Here, we utilized a non-invasive Extracellular Flux analysis methodology to simultaneously monitor both mitochondrial function and glycolysis in stored platelets after day 5 of storage compared to freshly isolated platelets.
The functional ability of freshly isolated and stored platelets were compared by measuring aggregation and hypotonic stress responses. We found that in the stored platelets the variability in aggregation was considerably greater than those which were freshly isolated suggesting a greater severity in function among the stored samples but this did not reach significance. As reported previously the stored platelets could not recover during the hypotonic stress and this test is energetically demanding for the platelet (Figure 1). In support of these data, studies using glycolytic inhibitors, and the non-specific inhibitor of mitochondrial oxidative phosphorylation, furosemide, reported a decrease in recovery after swelling, suggesting that the hypotonic stress response is an energetically demanding process [40, 41]. This predicted increased demand for glycolysis and oxidative phosphorylation is demonstrated in Figure 1D which shows an almost 3 fold stimulation of ECAR as the severity of the hypotonic stress increases. Interestingly, it has been shown that recovery after the hypotonic stress response is a positive indicator of platelet survival and recovery in vivo.
Analysis of bioenergetics showed a decrease in basal and ATP-linked OCR, and increase in proton leak and reserve capacity in the stored platelets compared to those which were freshly isolated (Figure 2). The basal respiration represents the oxygen consumption by the mitochondria required to provide the ATP necessary for the metabolic demands of the platelet. The fact that basal respiration is lower in the stored platelets than in the control could be due to a decrease in ATP demand by the platelet or partial inhibition of the mitochondrial ATP synthase. Interestingly, the platelet mitochondrial ATP synthase has been reported to be inhibited in patients with sickle cell disease [42]. Further, analysis of state 3 respiration with complex I, II substrates and ADP by permeabilizing the plasma membrane revealed a 22% decrease in respiration in the stored platelets (Figure 3A). In marked contrast to other studies that have shown no changes in mitochondrial respiration, or complete loss of mitochondrial function, we demonstrated a modest decrease in individual parameters of cellular platelet bioenergetics, associated with storage. In the intact platelet the substrates for oxidative phosphorylation are supplied by the sum of the metabolic pathways capable of providing substrates for mitochondrial respiration [19]. For this reason maximal respiration in the intact platelet can be limited by the endogenous substrate supply and likely explains why the inhibition of mitochondrial electron transport at complex I and II does not result in a decrease maximal respiration. These data also suggest that such minor changes in the activity of the mitochondrial electron transport chain are unlikely to have a significant impact on platelet function.
Importantly, the individual respiratory components from the mitochondrial stress test are interactive and contribute to the overall bioenergetic health of the platelet population [43]. Integrating all the indices of mitochondrial function in the BHI equation, it is evident that the overall bioenergetic health of the stored platelets is significantly lower than the freshly isolated platelets suggesting that this parameter could be used as a screen of platelet bioenergetic function (Figure 2H). These data would suggest that the stored platelet would be more susceptible to oxidative stress [19]. Additionally, there were no differences in basal glycolysis, but upon inhibition of mitochondrial function, glycolytic rate was significantly enhanced in the stored compared to the freshly isolated platelet (Figure 4). This data demonstrates that stored platelets have an enhanced capacity to augment glycolysis when mitochondria ATP synthesis is inhibited. Previous studies have shown an increase in lactate, during storage [7]. The data shown in Figure 4D shows no significant changes in basal ECAR which suggests that the accumulation of lactate in the platelet bag is a consequence of normal accumulation during basal metabolism.
Interestingly, the most striking change in bioenergetics observed was an increase in proton leak in the stored platelets (Figure 2D and 3B) which was inhibited by albumin (Figure 5A and B). Earlier studies have shown that free fatty acids can act as mitochondrial uncouplers, and addition of albumin attenuates this effect. Notably, it has also been shown that plasma free fatty acids increase during platelet storage [44]. In support of this concept, we show that addition of palmitate increases proton leak, which was decreased with the addition of albumin (Figure 6). Mitochondrial uncoupling has been described as an adaptation to cold stress to increase thermogenesis, and free fatty acids can induce this thermogenesis by its action on uncoupling proteins and adenine nucleotide translocator (ANT) [31, 45–49]. In the blood bank, platelets are stored at room temperature inducing a cold stress, which along with the increase in plasma free fatty acid could contribute to thermogenesis by uncoupling of the mitochondria.
In summary, we have shown an approximately 10% decrease in basal mitochondrial respiration and a substantial increase in proton leak or mitochondrial uncoupling in platelets after the end of the storage period of 5 days. This increase in proton leak was attenuated by albumin, indicating that free fatty acids could be causing the uncoupling effect. These data suggest that albumin could be explored as a novel additive for platelet storage, in order to halt the increase in proton leak and increase platelet survival, recovery and function in the patient after transfusion.
HIGHLIGHTS.
The functional and bioenergetic differences between freshly isolated and stored platelets were determined.
Decreased recovery after hypotonic stress in stored platelets compared to freshly isolated was shown.
Decreased bioenergetic parameters including basal, ATP-linked OCR, increased proton leak and reserve capacity were observed in stored platelets.
Increased proton leak in stored platelets was dependent on fatty acids.
ACKNOWLEDGEMNTS
This work was supported by American Heart Association 13PRE16390001 (SR), and the O’Brien Center P30 DK079337 (VDU).
CONFLICT OF INTEREST DISCLOSURE
Victor Darley-Usmar receives project support from Seahorse Bioscience but it is not related to the data included in this manuscript.
Abbreviations
- AA
antimycin A
- ACD
acid citrate dextrose
- Asc
Ascorbate
- BHI
Bioenergetic Health Index
- ECAR
extracellular acidification rate
- HSA
human serum albumin
- HSR
hypotonic stress response
- TMPD
Tetramethyl-p-Phenylenediamine
- XF
Extracellular Flux
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. The Journal of clinical investigation. 1971;50:370–377. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfusion medicine reviews. 1997;11:130–144. doi: 10.1053/tm.1997.0110130. [DOI] [PubMed] [Google Scholar]
- 3.Plaza EM, Lozano ML, Guiu IS, Egea JM, Vicente V, De Teran LC, Rivera J. Evaluation of platelet function during extended storage in additive solution, prepared in a new container that allows manual buffy-coat platelet pooling and leucoreduction in the same system. Blood transfusion = Trasfusione del sangue. 2012;10:480–489. doi: 10.2450/2012.0112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastava M. The platelet storage lesion. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2009;41:105–113. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30:475–487. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Sjovall F, Ehinger JK, Marelsson SE, Morota S, Frostner EA, Uchino H, Lundgren J, Arnbjornsson E, Hansson MJ, Fellman V, Elmer E. Mitochondrial respiration in human viable platelets--methodology and influence of gender, age and storage. Mitochondrion. 2013;13:7–14. doi: 10.1016/j.mito.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 7.McCullough JJ. Transfusion medicine. 3rd ed. West Sussex, UK: Wiley-Blackwell, Chichester; 2011. [Google Scholar]
- 8.Toner RW, Pizzi L, Leas B, Ballas SK, Quigley A, Goldfarb NI. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Applied health economics and health policy. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Rao GH, Escolar G, White JG. Biochemistry, physiology, and function of platelets stored as concentrates. Transfusion. 1993;33:766–778. doi: 10.1046/j.1537-2995.1993.33994025028.x. [DOI] [PubMed] [Google Scholar]
- 10.Kilkson H, Holme S, Murphy S. Platelet metabolism during storage of platelet concentrates at 22 degrees C. Blood. 1984;64:406–414. [PubMed] [Google Scholar]
- 11.Murphy S. Platelet storage for transfusion. Beitr Infusionther Klin Ernahr. 1986;15:93–106. [PubMed] [Google Scholar]
- 12.Murphy S, Gardner FH. Platelet storage at 22 degrees C: role of gas transport across plastic containers in maintenance of viability. Blood. 1975;46:209–218. [PubMed] [Google Scholar]
- 13.Dumont LJ, VandenBroeke T. Seven-day storage of apheresis platelets: report of an in vitro study. Transfusion. 2003;43:143–150. doi: 10.1046/j.1537-2995.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 14.Perales Villarroel JP, Figueredo R, Guan Y, Tomaiuolo M, Karamercan MA, Welsh J, Selak MA, Becker LB, Sims C. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. J Surg Res. 2013;184:422–429. doi: 10.1016/j.jss.2013.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picker SM, Schneider V, Oustianskaia L, Gathof BS. Cell viability during platelet storage in correlation to cellular metabolism after different pathogen reduction technologies. Transfusion. 2009;49:2311–2318. doi: 10.1111/j.1537-2995.2009.02316.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Tanaka S, Hori Y, Hirayama F, Sato EF, Inoue M. Role of mitochondria in the maintenance of platelet function during in vitro storage. Transfus Med. 2011;21:166–174. doi: 10.1111/j.1365-3148.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biological chemistry. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox biology. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi S, Chacko B, Sawada H, Kramer PA, Johnson MS, Benavides GA, O'Donnell V, Marques MB, Darley-Usmar VM. Metabolic plasticity in resting and thrombin activated platelets. PloS one. 2015;10:e0123597. doi: 10.1371/journal.pone.0123597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Laboratory investigation; a journal of technical methods and pathology. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O'Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AA. Aabb, Platelet transfusion: a clinical practice guideline from the AABB. Annals of internal medicine. 2015;162:205–213. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 22.Bednar B, Condra C, Gould RJ, Connolly TM. Platelet aggregation monitored in a 96 well microplate reader is useful for evaluation of platelet agonists and antagonists. Thrombosis research. 1995;77:453–463. doi: 10.1016/0049-3848(95)93881-y. [DOI] [PubMed] [Google Scholar]
- 23.Farrugia A, Hughes C, Douglas S, Neal M, James J. Microtitre plate measurement of platelet response to hypotonic stress. Journal of clinical pathology. 1989;42:1298–1301. doi: 10.1136/jcp.42.12.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc. 2014;9:421–438. doi: 10.1038/nprot.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy S, Rebulla P, Bertolini F, Holme S, Moroff G, Snyder E, Stromberg R. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion. Transfusion medicine reviews. 1994;8:29–36. doi: 10.1016/s0887-7963(94)70095-x. [DOI] [PubMed] [Google Scholar]
- 26.Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP, Ferrick D, Singal AK, Ballinger SW, Bailey SM, Hardy RW, Zhang J, Zhi D, Darley-Usmar VM. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojtczak L. Effect of long-chain fatty acids and acyl-CoA on mitochondrial permeability, transport, and energy-coupling processes. Journal of bioenergetics and biomembranes. 1976;8:293–311. doi: 10.1007/BF00765158. [DOI] [PubMed] [Google Scholar]
- 28.Borst P, Loos JA, Christ EJ, Slater EC. Uncoupling activity of long-chain fatty acids. Biochimica et biophysica acta. 1962;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- 29.Chan SH, Higgins E., Jr Uncoupling activity of endogenous free fatty acids in rat liver mitochondria. Can J Biochem. 1978;56:111–116. doi: 10.1139/o78-018. [DOI] [PubMed] [Google Scholar]
- 30.Saviani EE, Martins IS. Fatty acid-mediated uncoupling of potato tuber mitochondria. Biochemistry and molecular biology international. 1998;44:833–839. doi: 10.1080/15216549800201882. [DOI] [PubMed] [Google Scholar]
- 31.Brustovetsky NN, Egorova MV, Gnutov D, Gogvadze VG, Mokhova EN, Skulachev VP. Thermoregulatory, carboxyatractylate-sensitive uncoupling in heart and skeletal muscle mitochondria of the ground squirrel correlates with the level of free fatty acids. FEBS letters. 1992;305:15–17. doi: 10.1016/0014-5793(92)80645-w. [DOI] [PubMed] [Google Scholar]
- 32.Soboll S, Seitz HJ, Sies H, Ziegler B, Scholz R. Effect of long-chain fatty acyl-CoA on mitochondrial and cytosolic ATP/ADP ratios in the intact liver cell. The Biochemical journal. 1984;220:371–376. doi: 10.1042/bj2200371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham A, Wang J. Bernard-Soulier syndrome: an inherited platelet disorder. Archives of pathology & laboratory medicine. 2007;131:1834–1836. doi: 10.5858/2007-131-1834-BSAIPD. [DOI] [PubMed] [Google Scholar]
- 34.Holmsen H, Setkowsky CA, Day HJ. Effects of antimycin and 2-deoxyglucose on adenine nucleotides in human platelets. Role of metabolic adenosine triphosphate in primary aggregation, secondary aggregation and shape change of platetets. The Biochemical journal. 1974;144:385–396. doi: 10.1042/bj1440385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barile CJ, Herrmann PC, Tyvoll DA, Collman JP, Decreau RA, Bull BS. Inhibiting platelet-stimulated blood coagulation by inhibition of mitochondrial respiration. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2539–2543. doi: 10.1073/pnas.1120645109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusak T, Tomasiak M, Ciborowski M. Peroxynitrite can affect platelet responses by inhibiting energy production. Acta biochimica Polonica. 2006;53:769–776. [PubMed] [Google Scholar]
- 37.Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 38.Misselwitz F, Leytin VL, Repin VS. Effect of metabolic inhibitors on platelet attachment, spreading and aggregation on collagen-coated surfaces. Thrombosis research. 1987;46:233–240. doi: 10.1016/0049-3848(87)90285-4. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhry AA, Sagone AL, Jr, Metz EN, Balcerzak SP. Relationship of glucose oxidation to aggregation of human platelets. Blood. 1973;41:249–258. [PubMed] [Google Scholar]
- 40.Akkerman JW, Holmsen H. Interrelationships among platelet responses: studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood. 1981;57:956–966. [PubMed] [Google Scholar]
- 41.Kim BK, Baldini MG. The platelet response to hypotonic shock. Its value as an indicator of platelet viability after storage. Transfusion. 1974;14:130–138. doi: 10.1111/j.1537-2995.1974.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Conde J, Merino J, Golpe JA, Benet I. [Platelet anti-aggregating activity of furosemide (author's transl)] Revista espanola de fisiologia. 1976;32:269–274. [PubMed] [Google Scholar]
- 43.Patruno R, Arpaia N, Gadaleta CD, Passantino L, Zizzo N, Misino A, Lucarelli NM, Catino A, Valerio P, Ribatti D, Ranieri G. VEGF concentration from plasma-activated platelets rich correlates with microvascular density and grading in canine mast cell tumour spontaneous model. J Cell Mol Med. 2009;13:555–561. doi: 10.1111/j.1582-4934.2008.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, Novelli EM, Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123:2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cesar J, DiMinno G, Alam I, Silver M, Murphy S. Plasma free fatty acid metabolism during storage of platelet concentrates for transfusion. Transfusion. 1987;27:434–437. doi: 10.1046/j.1537-2995.1987.27587320540.x. [DOI] [PubMed] [Google Scholar]
- 46.Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PloS one. 2008;3:e1777. doi: 10.1371/journal.pone.0001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y. Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS letters. 2002;529:313–318. doi: 10.1016/s0014-5793(02)03395-1. [DOI] [PubMed] [Google Scholar]
- 48.Andreyev A, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Tsofina LM, Volkov NI, Vygodina TV. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. European journal of biochemistry / FEBS. 1989;182:585–592. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
- 49.Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochimica et biophysica acta. 1993;1183:41–57. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 50.Jezek P, Engstova H, Zackova M, Vercesi AE, Costa AD, Arruda P, Garlid KD. Fatty acid cycling mechanism and mitochondrial uncoupling proteins. Biochimica et biophysica acta. 1998;1365:319–327. doi: 10.1016/s0005-2728(98)00084-x. [DOI] [PubMed] [Google Scholar]