Abstract
In this observational cohort study, we examined the prognostic value of growth and differentiation factor 15 (GDF15) in indicating and monitoring general mitochondrial disease severity and progression in adult carriers of the m.3243A>G mutation.
Ninety-seven adult carriers of the m.3243A>G mutation were included in this study. The Newcastle mitochondrial disease adult scale was used for rating mitochondrial disease severity. In parallel, blood was drawn for GDF15 analysis by ELISA. Forty-nine carriers were included in a follow-up study. In a small subset of subjects of whom an echocardiogram was available from general patient care, myocardial deformation was assessed using two-dimensional speckle-tracking strain analysis.
A moderate positive correlation was found between the concentration of GDF15 and disease severity (r = 0.59; p < 0.001). The concentration of serum GDF15 was higher in m.3243A>G carriers with diabetes mellitus, cardiomyopathy, and renal abnormalities. After a 2-year follow-up, no significant correlation was found between the change in disease severity and the change in the concentration of GDF15 or between the GDF15 level at the first assessment and the change in disease severity. In the subcohort of patients of whom an echocardiogram was available, the concentration of GDF15 correlated moderately to longitudinal global strain (r = 0.55; p = 0.006; n = 23) but not to circumferential or radial strain.
Our results indicate that serum GDF15 is not a strong surrogate marker for general mitochondrial disease severity. Its value in indicating myocardial deformation should be confirmed in a prospective longitudinal study.
Introduction
One of the key aspects of improving the quality of clinical trials is the identification of biomarkers that are indicative of clinically relevant outcome (Pfeffer et al. 2013). The perfect biomarker, correlating closely to clinical disease severity, would make the follow-up of patients easier, cheaper, and less invasive, both in clinical trials and in regular patient care (Mayeux 2004). Moreover, since functional measures are subject to bias (including patient factors influencing performance, report bias, and inter- and intrarater variability), measuring more objective disturbances of physiology may seem more reliable. Several tests have been used to indirectly measure the disturbed mitochondrial energy metabolism in patients with mitochondrial disease, including the determination of lactic acid concentration in the brain by magnetic resonance spectroscopy or serum and serum fibroblast growth factor 21 (FGF21) (Suomalainen 2011). Although both lactic acid and FGF21 seemed to correlate to disease severity, the concentration during follow-up did not correlate to disease progression (Koene et al. 2014; Lee et al. 2010).
A recent study reported growth and differentiation factor 15 (GDF15) as a potential new diagnostic biomarker for mitochondrial disease (Kalko et al. 2014). GDF15 was already known as a quite nonspecific biomarker for cancer, as well as cardiac, pulmonary, renal, and gynecological disease (Izumiya et al. 2014; Kempf and Wollert 2013; Trovik et al. 2014; Yang et al. 2014; Breit et al. 2012; Montoro-Garcia et al. 2012). However, the concentrations reported in these disorders are within the 1.000–7.000 pg/mL range (Dominguez-Rodriguez et al. 2014; Ho et al. 2013; Izumiya et al. 2014; Montoro-Garcia et al. 2012; Trovik et al. 2014), whereas concentrations as high as 85.252 pg/mL were reported in patients with mitochondrial disease (Kalko et al. 2014). A child with the m.3243A>G mutation, the most commonly observed mutation leading to mitochondrial disease, was reported to have a concentration of 6.999 pg/mL (reference value, 380 pg/mL (95%CI, 59–701 pg/mL)).
To evaluate the value of GDF15 as a surrogate marker for disease severity and disease progression, we examined GDF15 in a large cohort of adult carriers of the m.3243A>G mutation. Since GDF15 was previously reported as a biomarker for symptoms associated with the m.3243A>G mutation, such as cardiomyopathy (Hollingsworth et al. 2012; Xu et al. 2011), diabetes mellitus (Dominguez-Rodriguez et al. 2014), and renal failure (Emma et al. 2012; Ho et al. 2013), we assessed these symptoms and organ functions in more detail.
Methods
Patients
We determined the serum GDF15 concentration in adult carriers of the m.3243A>G mutation included in our “national inventory of patients with the m.3243A>G mutation” study. In each subject, the heteroplasmy percentage in buccal mucosa cells, urinary epithelial cells (UEC), and leukocytes was determined using pyrosequencing (de Laat et al. 2012). A heteroplasmy percentage ≥5% can be detected using this technique. Subjects with a detectable heteroplasmy percentage in either buccal mucosa cells, leukocytes, or UEC were considered to be carriers of the mutation. In this national inventory, clinical disease severity is monitored approximately 2-yearly in both symptomatic and asymptomatic subjects carrying the m.3243A>G mutation (de Laat et al. 2012). Clinical disease severity is rated using the Newcastle mitochondrial disease adult scale (NMDAS), a multidimensional clinical scale encompassing current function (patient’s opinion), system-specific involvement (assessment of multisystem disease), and current clinical assessment (physical examination) (Schaefer et al. 2006). Carriers were rated as having asymptomatic (NMDAS = 0); mild (NMDAS = 1–5), moderate (NMDAS = 6–20), or severe (NMDAS >20) mitochondrial disease (cutoff values based on expert opinion). Seventy-six carriers were included in the follow-up study, and serum of 50 of these carriers was available for analysis (see Fig. 1 for a flowchart). For a more detailed description of the methods, we refer to our previous study on fibroblast growth factor 21 (FGF21) concentrations in this population (Koene et al. 2014). Patients with cancer and pregnant women were excluded since GDF15 is a known biomarker for these conditions. Since cardiomyopathy, diabetes mellitus, and renal failure – for which GDF15 is also a biomarker – are highly prevalent in carriers of the m.3243A>G mutation (de Laat et al. 2012), we also evaluated the influence of these conditions on the GDF15 concentration. Microalbuminuria was defined as an albumin-to-creatinine ratio of >2.0 g/mol for men and >2.5 g/mol for women, measured in a spot sample of urine. Decreased creatinine clearance was defined as a glomerular filtration rate <60 mL/min/1.73 m2. Carriers were classified as having decreased creatinine clearance only, microalbuminuria only, both, or neither. The presence and severity of diabetes mellitus (DM) follows from the NMDAS (the presence of DM was rated as DM requiring diet or medication). The measurement of myocardial strain is explained in more detail later in this section.
Fig. 1.
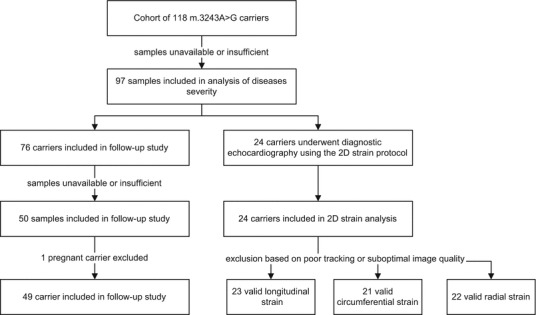
Flowchart of the study cohort
Thirty noncarrier family members were included as a nuclear genetic and environmental matched reference population. The maternal relatives who showed no signs of diabetes mellitus, renal disease, or cardiac disease were included in the study. In these subjects, heteroplasmy percentages ≤4% (the assay’s detection limit) in UEC, leukocytes, and/or buccal mucosa cells were established.
GDF15
All samples were measured in duplicate, following the instructions of the manufacturer (R&D Biosystems, Minneapolis, USA). The inter-assay and intra-assay variability for high and low values was determined based on high and low control samples, respectively. The functional sensitivity determined at 20% covariance (CV) was 9.8 pg/mL. At a level of 1,695 pg/mL, the within-assay CV was 3.5% and the between-assay CV was 5.7%. At a level of 729 pg/mL, the within-assay CV was 3.6% and the between-assay CV was 2.2%. Samples with an initial intra-assay covariance (CV) >15% were repeated. The two values obtained from the duplicated measurements were averaged, and the mean of these two measurements was used for further analysis. Samples with a concentration higher than the highest standard value were diluted and analysis was repeated. According to the kit’s manufacturer, the assay has no cross-reactivity with human GDF9 and GDF11. Age- and gender-based reference values for serum GDF15 were adopted from the Framingham Offspring Study (Ho et al. 2012) (elevated GDF15 concentration is above the 97.5th percentile matched for age and gender).
Medical Ethical Approval
This study (“national inventory of patients with the m.3243A>G mutation”) was approved by the regional Medical Research Ethics Committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 (World Medical Association 2013). Informed consent was obtained from all patients before being included in the study.
Myocardial Strain Measurement
As part of general patient care, carriers of the m.3243A>G mutation regularly undergo diagnostic echocardiography. Myocardial deformation was only assessed only if an echocardiogram was performed less than 1 year from an available GDF15 sampling. The myocardial strain, a measure for the deformation of the myocardium throughout the cardiac cycle, was determined using two-dimensional speckle-tracking strain analysis in accordance with a previously published protocol (Bulten et al. 2014). Since echocardiography was done as part of general patient care and 2D strain measurement can only be performed when the images are obtained following a specific protocol, only a subset of carriers was included in this part of the study.
Strain values are dimensionless and are expressed in percentages. Global longitudinal left ventricular myocardial strain was calculated by averaging the six segments of the 4-chamber long-axis view. Global radial and circumferential myocardial strain was calculated by averaging the six segments of the mid-cavity short-axis view (at the level of the papillary muscles). If less than four out of six segments showed valid tracking of the myocardium (e.g., because of regional inferior image quality or poor tracking by the software), the strain measurement was excluded from our analyses.
Statistics
The absolute difference between two parameters was calculated by subtracting the first measurement from the second measurement. All parameters were assessed for (log)normality. To prevent non-real values for zero values of the NMDAS including its subdomains and symptom-specific items, all values were increased by 1 prior to elog transformation. The changes in GDF15 concentration and NMDAS score were increased by 3,000 and 10, respectively. Variables with a (log)normal distribution were compared using parametric tests, and the mean and 95% confidence intervals are reported. Variables that deviated strongly from a (log)normal distribution were analyzed by performing a nonparametric test, and the median and interquartile ranges are reported. In case a high number of tests were performed (5 or more), critical p-values were adjusted using the Bonferroni method (i.e., critical p = 0.05/n where n = number of tests). Correlation coefficients were interpreted in accordance with the guidelines provided at the BMJ website (http://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression; consulted 31-Jul-2014). Thus, a correlation coefficient (r) of 0.80–1.0 is considered a very strong relationship; r = 0.60–0.79 is considered a strong relationship; r = 0.40–0.59 is considered a moderate relationship; r = 0.20–0.39 is considered a weak relationship; and r = 0.00–0.19 is considered a very weak or no relationship.
Several covariates for GDF15 are known from literature (Ho et al. 2012), including age, presence of diabetes mellitus (DM), smoking (covariates with higher estimated coefficient than 0.1), and renal failure. These, together with other possible clinical covariates (gender, body mass index (BMI), heteroplasmy percentage in urinary epithelial cells (UEC) and leukocytes, and disease severity (NMDAS)), were included as candidate predictors for GDF15. Gender, age, BMI, heteroplasmy percentage in UEC and leukocytes, and the concentration of GDF15 and FGF21 were considered candidate predictors for disease severity (NMDAS score). The influence of nominal and ordinal candidate predictors was determined by comparing between groups; the influence of continuous data was evaluated in a bilinear regression analysis. The influence of cardiomyopathy was studied in more detail in a subgroup of carriers. For the correlation between the concentration of GDF15 and strain measurements, only echocardiography examinations performed no more than 1 year from sampling were analyzed. Forward and backward iterative multivariate linear regression models were used to determine the influence of covariates and to determine the contribution of GDF15 in predicting or monitoring the disease course. Possible candidate predictors were only included for iterative multivariate modeling if they correlated to the dependent variable during univariate correlation analysis (p < 0.1). Standardized regression coefficients (β) are presented for each variable.
Because 38% of the variance in the concentration of GDF15 is genetically determined (Ho et al. 2012), we performed two additional analyses to correct for the effect of kinship in our analyses. First, we performed a separate analysis that included only the most severely affected patient in each family (in case two family members had the same NMDAS score, we included the youngest person with that score, assuming a relatively more severe disease in this person as age-related complaints are also included in the NMDAS). Secondly, we used generalized estimating equation models (working correlation structure: independence, with robust standard error for correction) to confirm the contribution of covariates and candidate predictors found by linear regression models, corrected for kinship.
Because genetic factors between family members are not likely to influence (intraindividual) changes in GDF15 levels during follow-up, we only used linear regression models to determine the influence of covariates on the change in disease severity and the concentration of GDF15 (longitudinal study). The (absolute) change in disease severity (i.e., NMDAS score) was used as a possible candidate predictor for the change in GDF15 concentration. Both the change in the concentration of GDF15 and the change in the concentration of FGF21 were included as candidate predictors for the change in disease severity (i.e., NMDAS score).
All analyses were performed using IBM’s SPSS statistics software packages, version 20.0.0.1.
Results
Patient Characteristics
We initially included 118 adult subjects in our national inventory study. For a variety of reasons (e.g., the samples were not available or had too small remaining volume for measuring GDF15), we were unable to determine the GDF15 levels in 21 of these subjects. No data were excluded because of high intra-assay covariance of GDF15 assessment. Thus, our final cohort included 97 adult carriers of the m.3243A>G mutation (Fig. 1). The m.3243A>G mutation is not associated with a higher prevalence of cancer, and none of our subjects was known to suffer from any form of cancer at the time of the study. One patient did have a history of acute myeloid leukemia (in remission for 8 years before samples were taken; [GDF15] 731 (baseline) and 1926 (follow-up) pg/mL). One pregnant woman was excluded ([GDF15] 43,304 pg/mL) from the follow-up cohort (Moore et al. 2000). For patient characteristics, we refer to Tables 1 and 2. Renal function at the time of the sampling (spread, 6 months) was known in 86 patients (89% of total). Seventy-one percent of the total cohort had normal renal function, 17% had microalbuminuria only, 2% had decreased creatinine clearance only, and 9% suffered from both. Three patients had had a renal transplant, two of which had moderate transplant function (GFR 24 and 50 mL/min/1.73 m2) and one had normal transplant function (GFR >75 mL/min/1.73 m2). The m.3243A>G carriers came from 41 distinct families (median, two subjects per family; range, 1–10 subjects per family). This cohort of adult carriers contained two asymptomatic patients (2%), 18 patients with mild mitochondrial disease (19%), 46 patients with moderate mitochondrial disease (47%), and 31 patients with severe mitochondrial disease (32%). Because sufficient material was not always available, heteroplasmy percentage in UEC is absent in two subjects; heteroplasmy percentage in leukocytes is absent for another two patients. In these four subjects, heteroplasmy levels ≥5% were established in the other available tissue (buccal mucosa cells, leukocytes, or UEC).
Table 1.
Characteristics of the cohort of all adult m.3243A>G carriers at baseline and the follow-up cohort
| All carriers | Follow-up (49 carriers) | Difference at baseline | Difference from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Central value | Spread | Range | n | Central value | Spread | Range | n | p | p | ||
| Gender | % female | 71 | 97 | 65 | 0.20 | ||||||
| Age (years) | Mean, 95%CI | 45 | 17–72 | 18–81 | 97 | 45a | 18–64 | 22–64 | 49 | 0.65 | |
| BMI (kg/m2) | Median, IQR | 22.8 | 20.7–26.0 | 16.7–40.9 | 97 | 23.6a | 17.6–33.2 | 17.6–33.1 | 49 | 0.96 | |
| Smoking (current) | % yes | 22 | 84 | 25 | 48 | 1.00 | |||||
| Diabetes mellitus (prevalence) | % yes | 39 | 97 | 37a | 49 | 0.62 | |||||
| Heteroplasmy percentage, leukocytes (%) | Median, IQR | 17 | 8–26 | 2–49 | 95 | 19a,b | 4–41 | 2–49 | 47 | 0.56 | |
| Heteroplasmy percentage, UEC (%) | Median, IQR | 45 | 27–69 | 4–96 | 95 | 52a,b | 7–91 | 5–96 | 47 | 0.1 | |
| NMDAS score | Mean, 95%CI | 12c | 1–67 | 0–56 | 97 | 14c | 2–47 | 1–92 | 49 | 0.81 | 0.15 |
| Domain 1 | Median, IQR | 4 | 1–9 | 0–23 | 97 | 6b,c | 1–21 | 0–38 | 49 | 0.85 | 0.001 |
| Domain 2 | Median, IQR | 5 | 3–9 | 0–22 | 97 | 5b,c | 0–16 | 0–31 | 49 | 0.81 | 0.48 |
| Domain 3 | Median, IQR | 3 | 1–6 | 0–20 | 97 | 3b,c | 0–14 | 0–23 | 49 | 0.87 | 0.96 |
| Myopathy score | Median, IQR | 2 | 1–4 | 0–11 | 97 | 2b,c | 0–12 | 0–14 | 49 | 0.96 | 0.11 |
| Encephalopathy score | Median, IQR | 2 | 0–3 | 0–12 | 97 | 1b,c | 0–5 | 0–17 | 49 | 0.98 | 0.38 |
| Diabetes mellitus (severity) | Median, IQR | 0 | 0–5 | 0–5 | 97 | 1b,c | 0–3 | 0–5 | 49 | 0.37 | 0.002 |
| Cardiomyopathy (severity) | Median, IQR | 0 | 0–1 | 0–5 | 97 | 0 | 0–5 | 0–5 | 49 | 0.59 | 0.001 |
| Stroke-like episodes (severity) | Median, IQR | 0 | 0–0 | 0–5 | 97 | 0 | 0–0 | 0–5 | 49 | 0.98 | 0.58 |
| QoL mental | Median, IQR | 48 | 41–55 | 25–66 | 97 | 45 | 39–55 | 14–62 | 47 | 0.11 | 0.63 |
| QoL physical | Median, IQR | 41 | 33–51 | 17–61 | 97 | 41 | 35–49 | 21–64 | 47 | 0.29 | 0.36 |
| [GDF15] (pg/mL) | Mean, 95%CI | 1,525c | 411–5,691 | 333–7,421 | 97 | 1,484d | 1,072–2,577 | 370–7,359 | 49 | 0.66 | 0.22 |
| [FGF21] (pg/mL) | Median, IQR | 263 | 142–534 | 3–1,491 | 93 | 278 | 117–473 | 56–1,776 | 24 | 0.96 | 0.27 |
Characteristics for all adult carriers (n = 97) at baseline and for the patients within the follow-up cohort (n = 49) at follow-up. P-values for the difference at baseline between the follow-up cohort and all carriers were calculated (difference at baseline; χ-square test (2 sided); Fisher’s exact test (2 sided), Mann–Whitney U test; independent sample t-test). Also, p-values for the difference between the first and the second measurement within the follow-up cohort are shown (difference from baseline; Wilcoxon signed-rank test, paired t-test). The presence of diabetes mellitus was obtained from the NMDAS scale (score on diabetes mellitus item 3)
BMI body mass index, 95%CI 95% confidence interval, Domain 1 current function, Domain 2 system-specific involvement, Domain 3 current clinical assessment, Encephalopathy score sum of the encephalopathic symptoms of the NMDAS (psychiatric symptoms, migraine, seizure, stroke-like episodes, encephalopathy, and cognition), GDF15 growth and differentiation factor 15, IQR interquartile range, Myopathy score sum of the myopathic symptoms of the NMDAS (exercise intolerance, respiratory muscle weakness, ptosis, external ophthalmoplegia, and myopathy), n number of carriers of which data were available at that specific time point, NMDAS Newcastle mitochondrial disease adult scale, UEC urinary epithelial cells
aAt baseline
bMean and 95%CI instead of median and IQR are given
cLognormal distribution
dMedian and IQR instead of mean and 95%CI are given
Table 2.
Characteristics of the cohort of all adult m.3243A>G carriers and their family-matched controls
| All carriers (n = 97) | Family-matched controls (n = 30) | Difference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Central value | Spread | Range | n | Central value | Spread | Range | n | p | ||
| Gender | % female | 71 | 97 | 73 | 30 | 1.00 | ||||
| Age (years) | Mean, 95%CI | 45 | 17–72 | 18–81 | 97 | 41 | 20–68 | 18–68 | 30 | 0.518 |
| BMI (kg/m2) | Median, IQR | 22.8 | 20.7–26.0 | 16.7–40.9 | 97 | 24.0a | 19.7–33.0 | 19–37 | 30 | 0.004 |
| Smoking (current) | % yes | 22 | 84 | 8 | 13 | 0.286 | ||||
| Diabetes mellitus (prevalence) | % yes | 39 | 97 | 7 | 30 | 0.001 | ||||
| Heteroplasmy percentage, leukocytes (%) | Median, IQR | 17 | 8–26 | 2–49 | 95 | 1 | 0–3 | 0–3 | 30 | <0.001 |
| Heteroplasmy percentage, UEC (%) | Median, IQR | 45 | 27–69 | 4–96 | 95 | 1 | 0–2 | 0–2 | 29 | <0.001 |
| NMDAS score | Mean, 95%CI | 12a | 1–67 | 0–56 | 97 | 3a | 0–17 | 0–22 | 30 | <0.001 |
| QoL mental | Median, IQR | 48 | 41–55 | 25–66 | 97 | 49 | 33–64 | 31–65 | 30 | 0.314 |
| QoL physical | Median, IQR | 41 | 33–51 | 17–61 | 97 | 51 | 43–56 | 33–64 | l | <0.001 |
| [GDF15] (pg/mL) | Mean, 95%CI | 1,525a | 411–5,691 | 333–7,421 | 97 | 490a | 272–1,616 | 236–1,687 | 30 | <0.001 |
| [FGF21] (pg/mL) | Median, IQR | 263 | 142–534 | 3–1,491 | 93 | 13 | 0–444 | 3–1,072 | 25 | <0.001 |
Characteristics for all adult carriers (n = 97) at baseline and for the family-matched controls (n = 30). P-values for the difference at baseline between the family-matched controls and all carriers were calculated (difference at baseline; χ-square test (2 sided); Fisher’s exact test (2 sided), Mann–Whitney U test; independent sample t-test). The presence of diabetes mellitus was obtained from the NMDAS scale (score on diabetes mellitus item 3)
BMI body mass index, 95%CI 95% confidence interval, GDF15 growth and differentiation factor 15, IQR interquartile range, n number of carriers/controls of which data were available, NMDAS Newcastle mitochondrial disease adult scale, UEC urinary epithelial cells
aLognormal distribution
The Value of GDF15 as an Indicator of Clinical Disease Severity
Fifty-one carriers (53%) had an elevated concentration of GDF15 (i.e., higher than the 97.5th percentile of healthy age- and sex-matched controls) compared to the age- and gender-matched reference population from literature (Ho et al. 2012). Carriers with an elevated concentration of GDF15 had higher NMDAS scores compared to carriers with normal concentrations of GDF15 (2,440 pg/ml (95%CI, 1,097–5,431 pg/mL) versus 902 pg/mL (95%CI, 381–2,131 pg/mL); p = < 0.001; independent sample t-test). The correlation between the concentration of GDF15 and total NMDAS score in the cohort of m.3243A>G carriers at baseline was r = 0.59 (p < 0.001; n = 97; Pearson’s correlation coefficient) (see Fig. 2). This correlation coefficient is not significantly higher (p = 0.20; Fisher r-to-z transformation) than the correlation between NMDAS score and FGF21 (r = 0.45; p < 0.001; n = 93; Spearman’s correlation coefficient) in this cohort); 95 out of 99 patients (96%) of the current cohort are the same as in the study of FGF21 (Koene et al. 2014). No significant correlation was found between the heteroplasmy percentage in UEC and the NMDAS score nor between the heteroplasmy percentage in leukocytes and the NMDAS score. Among the 66 patients with asymptomatic, mild, or moderate disease, the correlation coefficient between the total NMDAS score and GDF15 was 0.54 (p < 0.001; Spearman’s correlation coefficient); among the 31 patients with severe disease, we found no significant correlation between the total NMDAS score and the concentration of GDF15 (Pearson’s correlation coefficient). The correlation between the heteroplasmy level in UEC and GDF15 was 0.30 (p = 0.003; n = 95; Spearman’s rank coefficient). The correlation between the heteroplasmy level in leukocytes and GDF15 was 0.22 (p = 0.031; n = 95; Spearman’s rank coefficient).
Fig. 2.
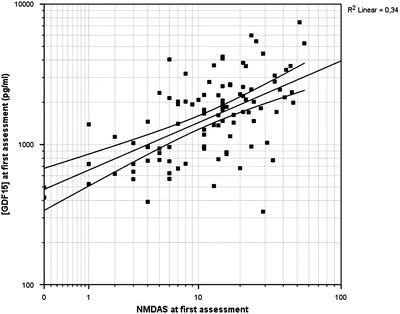
Correlation between the concentration of GDF15 and disease severity in adult m.3243A>G carriers. The correlation between the concentration of GDF15 and the total NMDAS score (disease severity) is r = 0.59 (p < 0.001). Scales are loglinear
Since the 97 adult carriers came from 41 families, we minimized the role of kinship in these associations by analyzing only the most severely affected patient in each of the 41 families. The correlation coefficient between the total NMDAS score and GDF15 concentration in this cohort was r = 0.46 (p = 0.003; Pearson’s correlation coefficient) and was similar (p = 0.58; Fisher r-to-z transformation) compared to the full cohort. During iterative multivariate linear modeling, the following parameters were found to be independent predictors for disease severity: concentration of GDF15, age, concentration of FGF21, and heteroplasmy percentage in UEC (β(GDF15) = 0.38 (p < 0.001); β(age) = 0.32 (p < 0.001); β(heteroplasmy UEC) = 0.24 (p = 0.005); β(FGF21) = 0.21 (p = 0.033; forward multilinear regression modeling)). When GDF15 and FGF21 were included as the only independent predictors for disease severity in a linear regression model, only GDF15 was included (β(GDF15) = 0.59 (p < 0.001); forward multilinear regression modeling). We found a moderate correlation between the concentration of GDF15 and the concentration of FGF21 (r = 0.54; p > 0.001; n = 93; Spearman’s correlation coefficient).
Covariates for the Concentration of GDF15
The concentration of GDF15 was not higher in females compared to males (p = 0.38; general linear model). The concentration of GDF15 was not higher among smokers compared to nonsmokers (p = 0.70; n = 84; general linear model). Patients without microalbuminuria or decreased creatinine clearance had lower concentrations of GDF15 compared to patients with microalbuminuria only or both microalbuminuria or decreased creatinine clearance (p = 0.031 and 0.002, respectively; Tukey HSD), but not compared to patients with decreased creatinine clearance only (p = 0.24; Tukey HSD). The subgroups with renal abnormalities were comparable with respect to GDF15 concentrations (Tukey HSD). (see Fig. 3). The concentration of GDF15 was significantly higher in patients with any kind of renal abnormalities compared to those without renal abnormalities (2,515 pg/mL (95%CI, 699–9,045 pg/mL) versus 1,261 pg/mL (95%CI, 373–4,264 pg/mL; n = 61); p < 0.001; independent samples t-test). Carriers with microalbuminuria had higher concentrations of GDF15 compared to those without microalbuminuria (2,574 pg/mL (IQR, 1,937–4,069 pg/mL; n = 25) versus 1,406 pg/mL (IQR, 781–1,973 pg/mL); p < 0.001; Mann-Whitney U test). Carriers with decreased creatinine clearance had higher concentrations of GDF15 compared to carriers with normal creatinine clearance including those with microalbuminuria only (3,418 pg/mL (IQR, 2,463–4,388 pg/mL; n = 12) versus 1,471 pg/mL (IQR, 867–2,105 pg/mL); Mann-Whitney U test; p < 0.001).
Fig. 3.
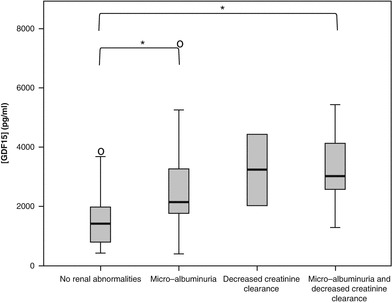
GDF15 concentrations in patients with and without renal abnormalities, including decreased creatinine clearance and microalbuminuria. Significant differences between groups are flagged with an asterisk
Carriers with DM had higher concentrations of GDF15 compared to carriers without DM (1,958 pg/mL (95%CI, 579–6,620 pg/mL) versus 1,299 pg/mL (95%CI, 345–4,902 pg/mL); p = 0.003; independent samples t-test). (see Fig. 4). We found significantly higher serum GDF15 concentrations in patients with cardiomyopathy according to the NMDAS (including patients with asymptomatic ECG changes; NMDAS cardiomyopathy ≥ 1) compared to carriers without cardiomyopathy (2,574 pg/mL (998–6,638) versus 1,371 pg/mL (381–4,937); p < 0.001; independent samples t-test).
Fig. 4.
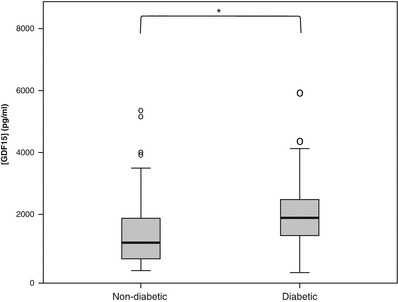
GDF15 concentrations in patients with and without diabetes mellitus. Significant differences are flagged with an asterisk
Univariate regression analysis showed that the total NMDAS score was the only significant contributor to the concentration of GDF15 (β(NMDAS) = 0.59; p < 0.001). In our cohort, we found no significant contribution of age and BMI to the concentration of GDF15. Generalized estimating equations confirmed the predictive value of disease severity as a significant contributor to the concentration of GDF15, after correcting for kinship clustering (p < 0.001; generalized estimating equations).
The Value of GDF15 in Predicting Clinical Disease Progression
Approximately 2 years after the initial assessment, the GDF15 concentration and disease severity (NMDAS score) were measured again in 76 carriers from the initial cohort of 97 carriers. Of these 76 carriers, 50 samples were available (see Fig. 1). One carrier included in the follow-up group was excluded because of pregnancy. The remaining 49 carriers in the follow-up study were similar compared to the total cohort with respect to (distribution of) gender, age, BMI, GDF15 concentration, presence and severity of DM, cardiomyopathy, stroke-like episodes, myopathy and encephalopathy, heteroplasmy percentage in UEC, heteroplasmy percentage in leukocytes, and NMDAS total and subdomain scores (independent Mann-Whitney U test and 2-sided chi-square test). The characteristics of the entire cohort of m.3243A>G carriers at baseline and the follow-up cohort are summarized in Tables 1 and 2. In this follow-up cohort, we found no significant correlation between the change in the NMDAS score (i.e., the change in NMDAS score between the first and second follow-up visit) and the change in the concentration of GDF15 (i.e., the change in GDF15 concentration between the first and the second follow-up visit) (r = 0.006; p = 0.97; n = 49; Spearman’s correlation coefficient). Moreover, no significant correlation was found between GDF15 concentration at the first visit and change in the NMDAS (disease progression) during follow-up (r = −0.19; p = 0.18; n = 49; Spearman’s correlation coefficient). Linear regression also revealed that the change in total disease severity did not contribute significantly to the change in the GDF15 concentration (p = 0.72; univariate linear regression). A linear regression model revealed that the change in FGF21 concentration, but not the change in GDF15 concentration contributed significantly to the change in disease severity (i.e., NMDAS score; β(FGF21) = 0.45 (p = 0.03; n = 24); forward multilinear regression modeling). There was no correlation between the change in the concentration of GDF15 and the change in the concentration of FGF21 (r = −0.047; p = 0.85; n = 18; Pearson’s correlation coefficient).
Myocardial Strain
Twenty-four subjects underwent echocardiographies as part of clinical care within 1 year of GDF15 sampling. These subjects were similar compared to the whole group with respect to gender, age, BMI, and total NMDAS score and cardiac and DM subscores (independent Mann-Whitney U test and 2-sided chi-square test; see Tables 3 and 4). Qualitative descriptions of gross echocardiography findings include (not mutually exclusive): left ventricle (LV) hypertrophy (n = 8), LV systolic dysfunction (n = 3), LV diastolic dysfunction (n = 8), mild aortic regurgitation (n = 2), and mild mitral regurgitation (n = 1). Ten carriers had normal gross echocardiography findings.
Table 3.
Patient characteristics of the cohort of all adult m.3243A>G carriers of which myocardial strain was reported
| Cardiac 2D strain cohort (24 carriers) | Difference from all carriers | |||||
|---|---|---|---|---|---|---|
| Central value | Spread | Range | n | p | ||
| Gender | % female | 75 | 24 | 0.80 | ||
| Age (years) | Mean, 95%CI | 44 | 25–63 | 24–64 | 24 | 0.80 |
| BMI (kg/m2) | Median, IQR | 22.3 | 21–26 | 18.3–35.8 | 24 | 0.91 |
| Smoking (current) | % yes | 22 | 23 | 0.78 | ||
| Diabetes mellitus (prevalence) | % yes | 45 | 24 | 0.48 | ||
| Heteroplasmy percentage, leukocytes (%) | Mean, 95%CI | 18a | 7–50 | 7–49 | 23 | 0.22 |
| Heteroplasmy percentage, UEC (%) | Mean, 95%CI | 56 | 24–93 | 23–96 | 24 | 0.078 |
| NMDAS score | Mean, 95%CI | 20 | 1–54 | 0–56 | 24 | 0.11 |
| Domain 1 | Mean, 95%CI | 5a | 0–23 | 0–23 | 24 | 0.11 |
| Domain 2 | Mean, 95%CI | 8 | 0–23 | 0–23 | 24 | 0.015 |
| Domain 3 | Mean, 95%CI | 3a | 0–16 | 0–18 | 24 | 0.97 |
| Myopathy score | Mean, 95%CI | 3 | 0–7 | 0–7 | 24 | 0.57 |
| Encephalopathy score | Median, IQR | 2 | 1–5 | 0–11 | 24 | 0.25 |
| Diabetes mellitus (severity) | Mean, 95%CI | 2 | 0–5 | 0–5 | 24 | 0.31 |
| Cardiomyopathy (severity) | Median, IQR | 0 | 0–2 | 0–5 | 24 | 0.06 |
| Stroke-like episodes (severity) | Median, IQR | 0 | 0–0 | 0–5 | 24 | 0.055 |
| QoL mental | Mean, 95%CI | 44 | 27–61 | 25–61 | 24 | 0.055 |
| QoL physical | Mean, 95%CI | 38 | 18–59 | 17–60 | 24 | 0.081 |
| [GDF15] (pg/mL) | Mean, 95%CI | 1,687a | 483–5,791 | 420–5,981 | 24 | 0.41 |
| [FGF21] (pg/mL) | Median, IQR | 321 | 252–768 | 4–1,491 | 24 | 0.084 |
Patient characteristics for those adult carriers (n = 24) for whom myocardial strain was measured. The difference at baseline between this cohort and all carriers was calculated (chi-square test (2 sided); Fisher’s exact test (2 sided), Mann-Whitney U test; independent sample t-test). The presence of diabetes mellitus was obtained from the NMDAS scale (score on diabetes mellitus item ≥3)
BMI body mass index, 95%CI 95% confidence interval, Domain 1 current function, Domain 2 system-specific involvement, Domain 3 current clinical assessment, Encephalopathy score sum of the encephalopathic symptoms of the NMDAS (psychiatric symptoms, migraine, seizure, stroke-like episodes, encephalopathy, and cognition), GDF15 growth and differentiation factor 15, IQR interquartile range, Myopathy score sum of the myopathic symptoms of the NMDAS (exercise intolerance, respiratory muscle weakness, ptosis, external ophthalmoplegia, and myopathy), n = number of carriers of which data were available at that specific time point, NMDAS Newcastle mitochondrial disease adult scale, UEC urinary epithelial cells
aLognormal distribution
Table 4.
Echocardiographic characteristics of the cohort of all adult m.3243A>G carriers of which myocardial strain was reported
| Cardiac ultrasound findings | n | % |
|---|---|---|
| Normal | 10 | 42 |
| Left ventricular hypertrophy without systolic dysfunction | 8 | 33 |
| Left ventricular systolic dysfunction only | 2 | 8 |
| Ejection fraction <55% | 3 | 13 |
| Left ventricular diastolic dysfunction | 8 | 33 |
| Dilated left ventricle | 2 | 8 |
| Aortic valve insufficiency (mild) | 2 | 8 |
| Mitral valve insufficiency (mild) | 1 | 4 |
| Dilated left atrium | 2 | 8 |
The correlation coefficient between the global longitudinal strain and the concentration of GDF15 was 0.55 (p = 0.006; n = 23; Pearson’s correlation coefficient). There was no correlation between global circumferential or radial strain and the concentration of GDF15 (r = 0.17; p = 0.47; n = 21 and r = 0.20; p = 0.37; n = 22, respectively; Spearman’s correlation coefficients).
Family-Matched Controls
The family-matched controls (n = 30) were similar to their maternal relatives carrying the m.3243A>G mutation with respect to age (p = 0.52; independent sample t-test), gender (p = 1.0), and smoking (p = 0.29; Fisher’s exact test), but not with respect to BMI (p = 0.004; Mann-Whitney U test) and the presence and severity of DM (p = 0.001; Fisher’s exact test) (see Table 1 and 2). The mean GDF15 concentration in the family-matched control group was 490 pg/mL (95%CI, 272–1,616 pg/mL; range, 236–1,687 pg/mL), which was significantly lower than their relatives carrying the m.3243A>G mutation (1,525 pg/mL (95%CI, 411–5,691 pg/mL); range, 333–7,421 pg/mL; p < 0.001; independent samples t-test). Only one maternal family member had an elevated concentration of GDF15 (1,560 pg/mL in an 18-year-old female) compared to age- and gender-matched controls. None of the maternal relatives was pregnant or known to have cancer, renal dysfunction, or cardiac problems.
Discussion
This study explored the value of serum GDF15 in indicating and monitoring mitochondrial disease severity and disease progression in adult m.3243A>G carriers. We found that the concentration of serum GDF15 correlates moderately to disease severity but does not correlate to disease progression in m.3243A>G carriers. Analysis of data obtained in general patient care indicated that GDF15 might be a surrogate biomarker of left ventricular myocardial strain.
So far, a few studies have found promising results regarding the diagnostic properties of GDF15 in patients with mitochondrial disease (Kalko et al. 2014; Yatsuga 2014). None of these studies focused on the value of GDF15 as a surrogate marker for predicting or monitoring disease progression. We found normal (age- and gender-matched (Ho et al. 2012)) concentrations of GDF15 in 47% of our carriers, including both symptomatic and asymptomatic individuals and in 97% of our family-matched controls. The family-matched controls (bearing the same nuclear genetic and environmental background as the carriers, but without the m.3243A>G detectable in UEC, leukocytes, or buccal mucosa cells) had significantly lower GDF15 concentrations compared to their maternal relatives carrying the m.3243A>G mutation.
GDF15 has also been reported as a nonspecific biomarker for many diseases, including cancer, cardiac, pulmonary, renal, and gynecological disease (Izumiya et al. 2014; Kempf and Wollert 2013; Trovik et al. 2014; Yang et al. 2014). Since several of these conditions are highly prevalent in m.3243A>G carriers (de Laat et al. 2012; Hirano et al. 2002), including kidney failure (Breit et al. 2012), diabetes mellitus (Dominguez-Rodriguez et al. 2014), and cardiomyopathy (Montoro-Garcia et al. 2012), the contribution of these conditions to the concentration of GDF15 was studied in more detail. We observed higher concentrations of GDF15 in carriers with renal abnormalities (mainly microalbuminuria) and diabetes mellitus. The myocardial strain measured in echocardiograms collected as part of general patient care with a maximum of 1 year apart from GDF15 sampling indicates that GDF15 may be a promising surrogate marker for myocardial deformity in patients with the m.3243A>G mutation. The correlation between the concentration of GDF15 and longitudinal myocardial strain, but not between the concentration of GDF15 and circumferential or radial strain (Lok et al. 2012; St John Sutton et al. 2014), might be explained by the observation that changes in longitudinal myocardial strain precede changes in ejection fraction and global longitudinal strain is now part of the recommendations in the assessment of cardiac function, e.g., in monitoring chemotherapy (Bates et al. 2013; Hollingsworth et al. 2012; Plana et al. 2014; St John Sutton et al. 2014). In summary, GDF15 is a nonspecific marker that seems to be highly influenced by several other symptoms frequently seen in this patient group.
The previously proposed biomarker FGF21 had no additional value to GDF15 in predicting and monitoring disease severity in m. 3243A>G carriers. It seems neither of the two parameters is useful as a surrogate marker for disease severity and disease progression.
Strengths of our study include the high number of carriers with different levels of the same mutation in their mitochondrial genome, resulting in a heterogeneous multisystem disease that we quantified systematically using a standardized and quantitative follow-up, enabling us to draw tentative conclusions regarding the value of GDF15 as a prognostic biomarker for monitoring disease progression. Several limitations to our study are worth mentioning. The period of follow-up is relatively short for this slowly progressive and often oscillating disease. The NMDAS used in the follow-up of disease severity is not very accurate and lacks sensitivity, making it unfit to establish subtle changes, and in this aspect the study is underpowered. The quality of the serum may have been influenced by the storage and previous use (freeze thawing) of the samples used in our study. Since the echocardiograms for 2D strain analysis were collected as part of clinical care, the level of standardization was suboptimal and samples for GDF15 analysis were not taken at the same moment as the echocardiography. Therefore, the results of this pilot study, including the responsivity of GDF15 as a biomarker for myocardial strain, need to be confirmed in a prospective study. Finally, since only carriers of the m.3243A>G were included in this study, one may not extrapolate these findings to other causes of mitochondrial failure
The mitochondrial disease field is diligently looking for easy-to-measure biomarkers, both for diagnostic and prognostic purposes. In this study, we have demonstrated that the concentration of serum GDF15 is moderately related to disease severity, but not to disease progression. Although the current study does not focus on the diagnostic applicability of GDF15, the lack of correlation between the concentration of GDF15 and disease progression in this 2-year follow-up study makes this biomarker unsuitable as a prognostic biomarker. The moderate correlation between myocardial strain and the concentration of GDF15 is a promising starting point for finding a prognostic biomarker for myocardial deformity but warrants further confirmation, preferably in a longitudinal study including an in-depth evaluation of renal function, glucose tolerance, and the effects of pharmacological interventions.
Acknowledgments
This work was partly supported by the Netherlands Organisation for Scientific Research (the NWO Centres for Systems Biology Research initiative), ZonMW (AGIKO grants Saskia Koene and Dennis Vriens), and Stichting Energy4All. We thank Inge Konijnenberg-Kramer for sample handling. Jan Smeitink is the CEO of Khondrion BV.
Take-Home Message
In adult m.3243A>G carriers, serum GDF15 levels correlate to mitochondrial disease severity and myocardial strain, but not to disease progression
Conflict of Interest
Saskia Koene received research support from the Netherlands Organisation for Scientific Research (NWO).
Paul de Laat received research support from the Stichting Energy4all.
Doorlène H. van Tienoven reports no disclosures.
Gert Weijers reports no disclosures.
Dennis Vriens received research support from the Netherlands Organisation for Scientific Research (NWO) and the Dutch Cancer Society, not related to the current study.
Prof. Fred C.G.J. Sweep reports no disclosures.
Janneke Timmermans reports no disclosures.
Livia Kapusta reports no disclosures.
Dr. Mirian C.H. Janssen reports no disclosures.
Prof. Smeitink is the founder and CEO of Khondrion and is funded by the Netherlands Organisation for Scientific Research and by ongoing Marie Curie and Eurostars grants and grants of Stichting Energy4All, none related to the current study.
This study was not industry sponsored.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Details of the Contributions of Authors
Saskia Koene: manuscript preparation, study coordination, and statistical analyses
Paul de Laat: sample collection and patient inventory
Doorlène H. van Tienoven: ELISA and sample handling
Gert Weijers: strain analysis and manuscript revision
Dennis Vriens: statistical analysis, figures, and manuscript revision
Fred C.G.J. Sweep: ELISA, preparations of the analyses, and manuscript revision
Janneke Timmermans: echocardiography
Livia Kapusta: strain analyses and manuscript revision
Mirian C.H. Janssen: patient collection and manuscript revision
Jan A.M. Smeitink: manuscript preparation, study design, and manuscript revision
Footnotes
Competing interests: None declared
Contributor Information
Saskia Koene, Email: Saskia.Koene@radboudumc.nl.
Collaborators: Johannes Zschocke
References
- Association WM (2013) Declaration of Helsinki – ethical principles for medical research involving human subjects. 64th WMA General Assembly, Fortaleza, Brazil
- Bates MG, Hollingsworth KG, Newman JH, et al. Concentric hypertrophic remodelling and subendocardial dysfunction in mitochondrial DNA point mutation carriers. Eur Heart J Cardiovasc Imaging. 2013;14:650–658. doi: 10.1093/ehjci/jes226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit SN, Carrero JJ, Tsai VW, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol Dial Transplant. 2012;27:70–75. doi: 10.1093/ndt/gfr575. [DOI] [PubMed] [Google Scholar]
- Bulten BF, Mavinkurve-Groothuis AM, de Geus-Oei LF, et al. Early myocardial deformation abnormalities in breast cancer survivors. Breast Cancer Res Treat. 2014;146:127–135. doi: 10.1007/s10549-014-2997-4. [DOI] [PubMed] [Google Scholar]
- de Laat P, Koene S, van den Heuvel LP, Rodenburg RJ, Janssen MC, Smeitink JA. Clinical features and heteroplasmy in blood, urine and saliva in 34 Dutch families carrying the m.3243A>G mutation. J Inherit Metab Dis. 2012;11:1059–1969. doi: 10.1007/s10545-012-9465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. Usefulness of growth differentiation factor-15 levels to predict diabetic cardiomyopathy in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;2:01367–01368. doi: 10.1016/j.amjcard.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Emma F, Bertini E, Salviati L, Montini G. Renal involvement in mitochondrial cytopathies. Pediatr Nephrol. 2012;27:539–550. doi: 10.1007/s00467-011-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Konishi K, Arata N, et al. Renal complications in a patient with A-to-G mutation of mitochondrial DNA at the 3243 position of leucine tRNA. Intern Med. 2002;41:113–118. doi: 10.2169/internalmedicine.41.113. [DOI] [PubMed] [Google Scholar]
- Ho JE, Mahajan A, Chen MH, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;58:1582–1591. doi: 10.1373/clinchem.2012.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, Hwang SJ, Wollert KC, et al. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59:1613–1620. doi: 10.1373/clinchem.2013.205716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth KG, Gorman GS, Trenell MI, et al. Cardiomyopathy is common in patients with the mitochondrial DNA m.3243A>G mutation and correlates with mutation load. Neuromuscul Disord. 2012;22:592–596. doi: 10.1016/j.nmd.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Hanatani S, Kimura Y, et al. Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30:338–344. doi: 10.1016/j.cjca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Kalko SG, Paco S, Jou C, et al. Transcriptomic profiling of TK2 deficient human skeletal muscle suggests a role for the p53 signalling pathway and identifies growth and differentiation factor-15 as a potential novel biomarker for mitochondrial myopathies. BMC Genomics. 2014;15:91. doi: 10.1186/1471-2164-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf T, Wollert KC. Risk stratification in critically ill patients: GDF-15 scores in adult respiratory distress syndrome. Crit Care. 2013;17:173. doi: 10.1186/cc12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene S, de Laat P, van Tienoven DH, et al. Serum FGF21 levels in adult m.3243A>G carriers: clinical implications. Neurology. 2014;6:125–133. doi: 10.1212/WNL.0000000000000578. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim J, Kim HD, Lee JS, Lee YM. Initial experiences with proton MR spectroscopy in treatment monitoring of mitochondrial encephalopathy. Yonsei Med J. 2010;51:672–675. doi: 10.3349/ymj.2010.51.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok SI, Winkens B, Goldschmeding R, et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail. 2012;14:1249–1256. doi: 10.1093/eurjhf/hfs120. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro-Garcia S, Hernandez-Romero D, Jover E, et al. Growth differentiation factor-15, a novel biomarker related with disease severity in patients with hypertrophic cardiomyopathy. Eur J Intern Med. 2012;23:169–174. doi: 10.1016/j.ejim.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Moore AG, Brown DA, Fairlie WD, et al. The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab. 2000;85:4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- Pfeffer G, Horvath R, Klopstock T, et al. New treatments for mitochondrial disease-no time to drop our standards. Nat Rev Neurol. 2013;9:474–481. doi: 10.1038/nrneurol.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, Phoenix C, Elson JL, McFarland R, Chinnery PF, Turnbull DM. Mitochondrial disease in adults: a scale to monitor progression and treatment. Neurology. 2006;66:1932–1934. doi: 10.1212/01.wnl.0000219759.72195.41. [DOI] [PubMed] [Google Scholar]
- St John Sutton M, Ky B, Regner SR, et al. Longitudinal strain in Friedreich Ataxia: a potential marker for early left ventricular dysfunction. Echocardiography. 2014;31:50–57. doi: 10.1111/echo.12287. [DOI] [PubMed] [Google Scholar]
- Suomalainen A. Biomarkers for mitochondrial respiratory chain disorders. J Inherit Metab Dis. 2011;34:277–282. doi: 10.1007/s10545-010-9222-3. [DOI] [PubMed] [Google Scholar]
- Trovik J, Salvesen HB, Cuppens T, Amant F, Staff AC. Growth differentiation factor-15 as biomarker in uterine sarcomas. Int J Gynecol Cancer. 2014;24:252–259. doi: 10.1097/IGC.0000000000000037. [DOI] [PubMed] [Google Scholar]
- Xu X, Li Z, Gao W. Growth differentiation factor 15 in cardiovascular diseases: from bench to bedside. Biomarkers. 2011;16:466–475. doi: 10.3109/1354750X.2011.580006. [DOI] [PubMed] [Google Scholar]
- Yang CZ, Ma J, Zhu DW, et al. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Ann Oncol. 2014;24:24. doi: 10.1093/annonc/mdu120. [DOI] [PubMed] [Google Scholar]
- Yatsuga S, Koga Y (2014) Growth differentiation factor 15 and fibroblast growth factor 21: novel biomarkers for mitochondrial diseases United Mitochondrial Disease Foundation Conference 2014


