Abstract
The pigments found in plants, animals and humic substances are well described and classified. In humans considerable progress has been made with the main pigment melanin in defining its biochemistry, the different types and function. However, analytical techniques to show these differences in vivo are still not readily available. NMR and IR spectroscopy are relatively insensitive and reveal only major structural differences. Techniques utilising MS are useful in determining elemental content but require further studies to optimise conditions for accurate mass analysis. How the components may be structurally organised seems to be the most problematic with scanning TEM and the improved FTIR of use in this respect. As regards understanding the nature of the pigment related to HGA seen in patients with Alkaptonuria (AKU), it is still thought of as a melaninlike pigment simply because of its colour and likewise thought to be a polymer of undetermined size. It is important that detailed analysis be carried out to define more accurately this pigment. However, observations suggest it to be the same as the HGA-derived pigment, pyomelanin, produced by bacteria and containing both quinone and phenolic groups. The interesting developments in alkaptonuria will be to understand how such a polymer can cause such profound collagen and connective tissue damage and how best to reverse this process.
Introduction
Pigmentation in humans is a well-controlled phenomenon where both structure and function of the pigment are interconnected. When control is lost, with a lack of pigmentation, albinism results and when in excess, various conditions have been described, including associated disorders of pituitary function, various inflammatory conditions as reviewed (Nordlund et al. 2006), hyperkeratosis hyperpigmentation (Figueras et al. 1993) and the rare genetic disease Alkaptonuria (AKU) (Phornphutkul et al. 2002).
AKU has been associated with many terms including black bone disease to ochronosis (yellow colouration) which suggest a process with diverse effects unlike normal pigmentation. However, the AKU pigment is still relatively poorly studied, with knowledge more than 40 years old needing updating as pigmentation is thought to be the basis for morbidity of the condition. In this review we aim to explain the nature and function of pigment material in plants, bacteria and humans and from this how we should proceed to effective analysis and classification of the pigment seen in AKU. Thus, if we can understand this pigmentation process better, it may allow further insights into the pathophysiology of AKU and yield novel therapeutic targets.
Background
In the study of pigmentation or coloured biological compounds in living matter, it is important that their nature is clearly defined in order to optimise chemical analysis. The term biological pigment is used for all coloured natural substances whose chemical structure determines the actual colour, so if yellow (570–590 nm) by reflecting this wavelength and absorbing the remaining wavelengths of white light (375–780 nm). Obviously if a pigment is black as in the melaninlike types, all the wavelengths are absorbed and none is reflected. Distinction is usually made between a pigment, which is insoluble (resulting in a suspension), and a dye, which can be both a liquid and soluble in the respective material. The structural chemistry of dyes is well established with both conformation and specific groups producing different colours (Kassinger 2003; Venkataraman 2012). However, for pigmented tissue the final colour may be a composite of more than one component and relate to both composition and structure with specific chemical groups and if metals are involved, e.g. Fe or Cu ions. The structure and interaction with the underlying tissue may also be relevant in particular if located superficially or in deeper parts of the tissue. For example, pigment located deep within the tissue may appear as a darker shade than the superficial layers showing selective reflection and a different colouration. The actual structure of the pigments in tissue may also be more complex than any defined chemical form as a result of polymerisation and interaction with other proteins, e.g. collagen and keratin, affecting the final visual appearance.
In order to understand the structural and compositional basis of colour in human pigmentation, awareness of how other natural pigments are coloured is important. This will then help determine how analysis will be best achieved. The plant pigments comprise a variety of different kinds of molecule, including porphyrins (chlorophyll), carotenoids, anthocyanins and betalains (Delgado-Vargas et al. 2000). The carotenoids have a distinctive structure based on the 5 carbon 2-methyl-1,3-butadiene isoprene unit producing the unsaturated hydrocarbons formula C40Hx. These are synthesised only by plants, shown as the orange pigment carotene found in carrots; lutein, a yellow pigment found in fruits and vegetables; and lycopene, the red pigment responsible for the colour of tomatoes. Anthocyanins (literally “flower blue”) are water-soluble flavonoid pigments that appear red to blue according to pH and are produced from the condensation of 4-hydroxy cinnamoyl CoA with phenylalanine. They occur in all tissues of higher plants, providing colour in leaves, plant stem, roots, flowers and fruits, though not always in sufficient quantities to be noticeable. Betalains are aromatic indole derivatives synthesised from tyrosine and complexed with a sugar to give red or yellow pigments. The betalains are water-soluble compounds responsible for the deep red colour of beets and Bougainvillea bract colour and are used commercially as food-colouring agents.
These compounds have defined structures to give their overall colour and are nonprotein in nature. In contrast coloured photopigment rhodopsin is a protein opsin with a reversibly covalently bound cofactor retinal. The rhodopsin absorbs green-blue light and, therefore, appears reddish-purple, so-called visual purple (Stuart and Brige 1996).
The coloured pigments/substances in plants are thus both well characterised and chemically well defined. However, pigmentation in humans primarily associated with the production of melanin has still many unanswered questions, in particular knowledge about the chemical structure and what are the most reliable analytical procedures to describe differences (D’Ischia et al. 2013). A variety of definitions and models are found in the literature, which seems to reflect an arbitrary use of terminology as well as several assumptions and speculations that have never been proven on experimental grounds. This lack of standardised procedures (D’Ischia et al. 2013) makes identification of the pigment associated with the genetic disease alkaptonuria even more challenging.
The following is an update of melanins, their structure and how better understanding of these will help in the interpretation of the nature of the pigment seen in alkaptonuria.
Melanins
The family of melanins, related to the metabolism of tyrosine (Fig. 1), were first described as a black animal pigment and subsequently used to indicate any black or dark brown organic pigment material. A classification was proposed (Nicolaus 1969) indicating three main groups: eumelanin, pheomelanin and allomelanins. A more recent classification (Fig. 1a) of melanins comprises four main classes: (a) eumelanins, black-to-brown insoluble pigments derived from the oxidative polymerisation of l-dopa via 5,6-dihydroxyindole (DHI) intermediates; (b) pheomelanins, yellow-to-reddish brown; (c) neuromelanins, dark pigments produced within substantia nigra neurons; and (d) allomelanins (pyomelanin). These could be further classified in at least five main types according to the source: animal, plant, fungal, bacterial and synthetic melanin (Solano 2014).
Fig. 1.
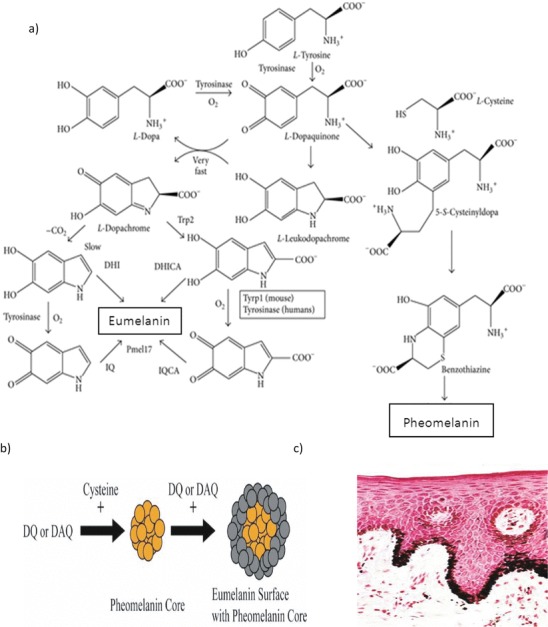
(a) Raper–Mason pathway for eumelanin and pheomelanin formation. The initial phase until l-dopaquinone is common to pheomelanin and eumelanin through the oxidation of l-tyrosine catalysed by the two activities of tyrosinase, tyrosine hydroxylase and dopa oxidase. l-Dopa quinone is the pivotal intermediate. Addition of l-cysteine gives place to benzothiazine units and then to pheomelanins (right side of the pathway). In the absence of l-cysteine, eumelanogenesis takes place since l-dopaquinone spontaneously cycled to l-leukodopachrome. This indoline reacts with l-dopaquinone in a very fast spontaneous reaction to yield l-dopachrome. l-Dopachrome is converted to DHI and DHICA mixtures, according to Trp2 activity and decarboxylation rate. Further oxidation of these dihydroxyindoles by tyrosinase or Trp1 gives place to indolequinones, and subsequent cross-link reactions between hydroxy and quinone forms lead to the polymer (Solano 2014). (b) Representation of eumelanin stacking on top of a core of pheomelanin. DQ, dopaquinone, and DAQ, dopamine quinone, from Ito and Wakamatsu (2008) and Sulzer and Zecca (1999). (c) Fontana–Masson stain shows melanin granules by reduction of silver nitrate to metallic silver at pH 4.0, shown as accumulation of black material in the cytoplasm of skin keratinocytes
Melanin is mainly synthesised in melanocytes, a heterogeneous group of cells originating from embryonic neural crest cells (Cichorek et al. 2013). The melanocytes predominate in the skin epidermis but are also found in the hair and iris where they give colour to these structures and in the inner ear, nervous system and heart (Wakamatsu et al. 2008; Takeda et al. 2007). Other cells as well as melanocytes may produce melanin, e.g. cells of pigmented epithelium of retina, epithelia of iris and ciliary body of the eye, some neurones and adipocytes (Takeda et al. 2007 and Sakamoto et al. 2005). Melanin is made within small membrane-bound packages, the melanosomes. As they become full of melanin, they move into the melanocytes, from where they are transferred to the keratinocytes, the predominant cell in the epidermis or outer layer of the skin. Under normal conditions, melanosomes cover the upper part of the keratinocytes as a barrier to UV-associated genetic damage (Jablonski 2012).
Microphthalmia-associated transcription factor (MITF) acts as a master regulator of melanocyte development, function and survival by modulating various differentiations and cell-cycle progression genes (Levy et al. 2006). The premelanosome protein or melanocyte-specific glycoprotein Pmel17 is synthesised as an integral membrane protein of melanosomal compartments, which drives the formation of striations from within multivesicular bodies (MVBs) and is thus directly involved in the biogenesis of premelanosomes (Berson et al. 2001). The Pmel17 protein and MVBs create the unique architecture of the premelanosome where Pmel17 is enriched in the lumen of premelanosomes and where it associates with characteristic striations of unknown composition upon which melanin is deposited.
Thus, melanin is produced and stored in specialised lysosome-related organelles called melanosomes. The members of the tyrosinase-related family (tyrosinase and tyrosinase-related proteins TRP-1 (DHICAoxidase) and TRP-2, DOPAchrome tautomerase (Del Marmol and Beermann 1996) are also involved in the process of melanogenesis leading to the production of either eumelanin (brown-black) or pheomelanin (yellow-red). This difference in colour development in cell-specific pigment is regulated by MITF through transactivation of the promoters of the tyrosinase gene family (Murisier and Beermann 2006). The overall production of melanin is orchestrated by the pituitary through secretion of melanocyte-stimulating hormone and other cleavage products of a large precursor peptide proopiomelanocortin (Pritchard et al. 2002). The fragment α-MSH is the most important melanocortin for pigmentation and is a fragment of the ACTH part of the precursor molecule. In diseases with oversecretion of ACTH because of pituitary tumours or failure of cortisol production as in Addison’s disease, marked increases in pigmentation (bronzing) are often a clinical presentation because of the concomitant production of α-MSH (Pritchard et al. 2002; Barber et al. 2010).
People have different skin colours, mainly because their melanocytes produce different amounts and kinds of melanin. Natural skin colour can also darken after exposure to intense sunlight as an adaptation to provide partial protection against the UV light-mediated damage to the skin cell DNA (Jablonski and Chaplin 2010).
Melanin Types
Eumelanin has two types: black and brown (Fig. 1). The associated polymers have long been thought to comprise numerous cross-links comprising 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylated acid (DHICA). A small amount of black eumelanin in the absence of other pigments causes grey hair, and brown eumelanin in the absence of other pigments causes yellow (blonde) hair. People with darker skin tones have more of eumelanin as a photo-protectant against harmful UV rays.
Pheomelanin (Fig. 1) is a reddish colour pigment, with very weak absorptive properties of UV radiation. It acts as a photosensitiser by enhancing the skin sensitivity toward sunlight and increases during the aging process. Females tend to have more of this pigment in their skin than men. It is found in particularly large quantities in red hair, lips, nipples, glands of the penis and vagina (Valverde et al. 1995).
The eumelanin-to-pheomelanin ratio determines the skin tone more eumelanin the skin looks darker dependent on the spread, size and number of melanosomes.
Neuromelanin is so called because of black/brown pigmented granules, different from melanosomes, found in the brain, particularly dopaminergic neuronal cells of the substantia nigra, causing their characteristic dark appearance. The pigment is composed of aggregates of 30-nm diameter spheres with a pheomelanin core, a eumelanin surface and a pheomelanin-to-eumelanin ratio of 3:1 of indeterminate size. The complex has been shown to bind heavy metals (Zecca et al. 2008). A recent study using proteomics of the melanin complex systems within neurones suggested the presence of endoplasmic reticulum-derived chaperones, especially the transmembrane protein calnexin, located in lysosome-related melanosomes suggested to be a melanogenic chaperone (Tribl et al. 2005).
Other cells if the necessary enzymes are present will also produce melanins, e.g. epithelia of the retina and iris, some neurones and adipocytes (Ito and Wakamatsu 2008; Murisier and Beermann 2006).
Biosynthetic Pathways of Eumelanin and Pheomelanin
The genetic mechanism behind colouration of human skin, eyes and hair shades is mainly regulated by the activity of tyrosinase which catalyses the first step of the biosynthetic pathway for both eumelanins and pheomelanins (Fig. 1a). The initial phase consists of the oxidation of l-tyrosine catalysed by the two activities of tyrosinase, tyrosine hydroxylase and dopa oxidase with l-Dopaquinone, the key intermediate. Addition of l-cysteine gives place to benzothiazine units and then to pheomelanins. In the absence of l-cysteine, the formation of eumelanin occurs as l-dopaquinone spontaneously cycles to l-leukodopachrome. This indoline reacts with l-dopaquinone in a very fast spontaneous reaction to yield l-dopachrome.The l-dopachrome is then converted to DHI and DHICA mixtures, according to Trp2 activity and the rate of decarboxylation. Further oxidation of these dihydroxyindoles by tyrosinase or Trp1 gives place to indolequinones; subsequent cross-link reactions between hydroxy and quinone forms lead to the polymer (Solano 2014).
Thus, tyrosine is hydroxylated to l-dopa via tyrosinase and the intermediate dopaquinone can then combine with cysteine by two pathways to benzothiazines and pheomelanins:
Dopaquinone + cysteine → 5-S-cysteinyldopa → benzothiazine intermediate → pheomelanin
Dopaquinone + cysteine → 2-S-cysteinyldopa → benzothiazine intermediate → pheomelanin
The dopaquinone can also be converted to leucodopachrome (cyclo-dopa, 2-carboxy-2,3-dihydro-5,6-dihydroxyindole) and follow two more pathways to the eumelanins:
Dopaquinone → leucodopachrome → the cyclised product dopachrome(5,6-Dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid) → 5,6-dihydroxyindole-2-carboxylic acid → quinone → eumelanin
Dopaquinone → leucodopachrome → dopachrome → 5,6-dihydroxyindole → quinone → eumelanin
Studies of the early stages of melanogenesis (involving dopaquinone and cysteine) indicate that mixed melanogenesis proceeds in three distinct stages: the initial production of cysteinyldopas, followed by their oxidation to produce pheomelanin and finally by the production of eumelanin. A model was proposed in which a preformed pheomelanic core is covered by a eumelanic surface (Ito and Wakamatsu 2008) creating the final colour of the melanin. This was supported by kinetic studies (Bush et al. 2006) of pigments containing a mixture of pheomelanin and eumelanin, of which neuromelanin is an example, where pheomelanin formation occurs first with eumelanin formation predominantly occurring only after cysteine levels are depleted. Such a kinetic model would predict a structure with pheomelanin at the core and eumelanin at the surface (Fig. 1b).
The final reactions in forming the pigments may be nonenzymatic mediated by conditions of pH and precursor concentrations (Land et al. 2003). In this final phase of polymerisation, the Pmel17 protein located in the eumelanosome seems to have a role in the regulation and deposition of the polymer on that organelle.
The proposed structural basis for these polymers and a relationship to black appearance are shown (Fig. 2). However, the unsaturated backbone is not planar but if they form layers will result in similar light properties (Tran et al. 2006). These melanin pigments with or without nitrogen systems have defined pathways which can be found in the KEGG (Kyoto Encyclopedia of Genes and Genomes) database.
Fig. 2.
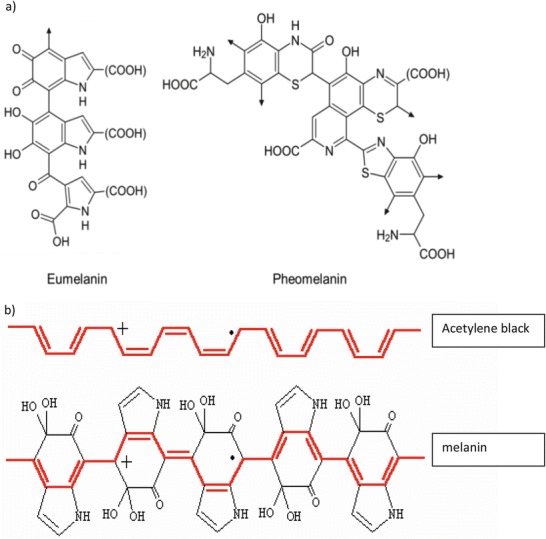
(a) Part of the structural formula of eumelanin or pheomelanin. “(COOH)” can be COOH or H or (more rarely) other groups. The arrow denotes where the polymer continues. (b) Structure of repeating acetylene groups produces a black colour (top) similar to the structure of repeating units in melanin (bottom)
The primary function of melanin is the protection of tissues from sunburn by ultraviolet radiation, and there is a direct correlation between the geographic distribution of UV radiation (UVR) and the distribution of indigenous skin pigmentation around the world. Reducing this pigmentation can be achieved by inhibiting tyrosinase activity (Videria et al. 2013).
Pigmentation in AKU: The Nature of the Pigment
The nonnitrogenous melanin (Fig. 3), based on the oxidation of homogentisic acid and subsequent chemical modification/polymerisation, causes the ochronosis seen in the genetic disease Alkaptonuria (Ranganath et al. 2013). This is explained by a marked accumulation of HGA as a result of a defective homogentisate 1,2-dioxygenase (HGD) enzyme that is unable to metabolise HGA. The production of the precursor benzoquinone (BQA) (Zannoni et al. 1969) shown in Fig. 3 is the pivotal first step in pigment formation similar to the formation of dopaquinone in melanin synthesis (Turick et al. 2010). The oxidation product of HGA in aqueous solution was shown to be stable in acidic, neutral and weak alkaline media using cyclic voltammetry and that the subsequent chemical dimerisation is more probable for the oxidised form of HGA (Eslami et al. 2014).
Fig. 3.

Proposed pathway of the pyomelaninlike AKU pigment based on the formation of benzoquinone enzymatically or by auto-oxidation and then subsequent dimerisation as well as alternative metabolic routes to, HGA mercaptouric acid and HGA glucuronide and subsequent protein binding. NB the relative proportions of these processes are not known (Williams et al. 2012; Zannoni et al. 1969). NB the enzyme “polyphenol oxidase” cited in the figure is reported in protein database as the enzyme EC:1.10.3.1, catalysing the oxidation of ortho- but NOT para-diphenols. Polyphenol oxidases are enzymes generally found in plants but not in humans, as this enzyme does not exist in the sequenced human genome. An enzyme able to oxidise p-diphenols is the “laccase” (EC:1.10.3.2), acting both on o-quinols and p-quinols and often acting also on aminophenols and phenylenediamine
The question in human metabolism relates to how this pigment forms as there is relatively low activity of tyrosinase to oxidise HGA. However, studies (Martin and Batkoff 1987) showed that homogentisic acid oxidation can occur nonenzymatically between pH 6.8 and 9.5 in the presence of oxygen, proportional to the concentration of HGA and optimal at 37°C. Formation of the oxidised product, benzoquinoneacetic acid (BQA), was inferred by decreased absorption at 290 (HGA) and increase at 250 and 315 nm, respectively. This was inhibited by the reducing agents – NADH, reduced glutathione and ascorbic acid – and accelerated by SOD and manganese pyrophosphate. These studies also indicated that the viscosity of buffered solutions of hyaluronic acid treated with homogentisic acid up to 100 μM was decreased by 40–50%, with maximal loss after 40–60 min incubation. Hyaluronic acid solutions incubated without homogentisic acid showed no change in viscosity. This may be highly relevant to the damaging process as hyaluronic acid is the chief viscous element of synovial fluid and the backbone of cartilage collagen (Martin and Batkoff 1987). Wolff et al. (1989) were able to measure both HGA and the benzoquinone acetate (BQA) by HPLC and EC detection and indicated that ascorbic acid administration reduced the output of BQA but not HGA, suggesting the importance of vitamin C treatment as BQA was thought to be the damaging agent rather than HGA. Subsequent clinical studies on the effectiveness of vitamin C in reducing pigmentation have been inconclusive, and Forslind et al. (1984) concluded that “ascorbic acid is not effective in the treatment of symptomatic ochronosis.” However, studies from an in vitro model using human serum treated with 0.33 mM HGA showed that the reducing agents, ascorbic acid (ASC) and other antioxidants including N-acetyl cysteine, were able to prevent HGA oxidation to BQA and formation of both protein adducts with the benzoquinone and the ochronotic pigment, as well as reactive oxygen-mediated cell damage (Braconi et al. 2010, 2011).
Unfortunately, ASC may also auto-co-oxidise with HGA, i.e. act together as prooxidants to produce additional oxidative damage. To counteract this, a second reducing agent in combination, N-acetylcysteine (NAC) that specifically protects cell glutathione ,was added to reduce the negative effects of excess HGA (0.33 mM) and restore viability, proliferation and chondrocyte anabolism (Tinti et al. 2010). On the basis of these in vitro cell models, in particular the human articular primary chondrocytes, Tinti et al. (2010) suggested a possible way forward for the treatment of ochronotic arthropathy. Also further studies have shown that the combination of antioxidants vitamin C and N-acetylcysteine improves red cell viability during storage (Pallotta et al. 2014) and protects from indoxyl sulphate-induced oxidative stress and antiproliferative effects attributed to mitochondrial dysfunction and impaired biogenesis (Lee et al. 2015).
However, data from an acute human inflammatory model of muscle injury (Childs et al. 2001) indicated that administration of vitamin C and NAC immediately post injury may increase tissue damage and oxidative stress. In addition, because ascorbic acid serves as a cofactor for 4-hydroxyphenylpyruvate dioxygenase, the vitamin may increase production of HGA in alkaptonuria (Phornphutkul et al. 2003). Furthermore, the large doses of ascorbic acid actually used in alkaptonuria might contribute to the formation of renal oxalate stones in patients with this disorder, who are already at increased risk for kidney stones.
Thus, the antioxidant approach to treatment still seems controversial, but being able to block the formation of HGA complexes is clearly advisable as formation of plasma-soluble melanin-type complexes similar to lipofuscin were also suggested to be cytotoxic (Hegedus 2000).
Unfortunately detailed chemical analysis outlining the structure of the AKU pigment is still lacking, but similar products have been well described in bacteria and other species. In bacteria an HGA oxidase is present and the HGA-derived pigment pyomelanin can be shown in at least three clinically isolated strains of Pseudomonas aeruginosa (Ogunnariwo and Hamilton-Miller 1975). Similarly the pigment formed by Vibrio cholerae was related to oxidation of homogentisic acid (Ruzafa et al. 1995). A recent study (Carreira et al. 2001) showed that the organism Yarrowia lipolytica was responsible for the brown colouration of various cheeses, ewes, Camembert and gorgonzola, by the production of homogentisic acid and oxidation to form a brown pigment. The single hydroxyl acids, p-hydroxyphenylethanol (p-HEA) and p-hydroxyphenylacetic acid (p-HPAA), did not oxidise in this way, indicating that it was related to the presence of the dihydroxy form. The pigment formation was also dependent on the release of HGA from within the cell. The HGA pigment in bacteria seems to have specific functions involved in the protection from sunlight and scavenging of trace or toxic elements (Przemyslaw et al. 2006). The organism Burkholderia cenocepacia C5424 produces a pigment using an HGA intermediate and was shown to help protect the organism from oxidative damage by host cells (Keith et al. 2007). The loss of pigment production resulted in the generation of a B. cenocepacia strain that was more sensitive to oxidative stress in vitro.
In AKU the process of pigmentation seems to be relatively random being dependent on localised concentrations of HGA able to produce pigment products which then bind to tissue proteins. The exposure to the pigment increases with age and hence the severity of the condition. Linear regression analysis indicated that the radiographic score for the severity of disease began increasing after the age of 30 years, with a more rapid increase in men than in women (Phornphutkul et al. 2002). The ochronotic chondrocyte pigmentation of a mouse model was also shown to follow an age-related pattern with the first signs of pigmentation restricted to the pericellular matrix surrounding individual chondrocytes, followed by eventual progression to the intracellular compartment (Preston et al. 2014). The cartilaginous tissues and bone seem to be particularly susceptible resulting in widespread arthritis and joint destruction (Keller et al. 2005) as well as deterioration of cardiac valves (Lok et al. 2013). In fact a lack of HGD activity in human cardiac tissue was suggested as the cause of localised production of ochronotic pigment in AKU heart (Lok et al. 2013). It could be that other tissues affected by the disease with an enzyme defect have similar increased HGA and pigment production contributing to the induction of ochronotic arthropathy (Laschi et al. 2012).
The composition of ochronotic pigment was investigated by energy-dispersive X-ray spectroscopy (EDS, EDX or XEDS or energy-dispersive X-ray analysis, EDXA) microanalyses on the dark surface of a heart valve from a patient with AKU (Millucci et al. 2014). The data revealed that pigmented areas are composed of C, O, N, S, Na and variable amount of Ca. The white solid deposits in the pigmented valves were shown to contain P and Ca, indicating the presence of hydroxyapatite and a process of endochondral ossification. The SEM images showed an active process with collagen appearing as delicate bundles of fibres with a random orientation and bone-like concretions (Millucci et al. 2014). The unexpected finding of sulphur in cardiac valve tissue pigment was thought to be related to the ability of BQA (oxidised HGA) to form adducts with protein thiols. The rapid formation of such protein complexes has been shown to be dependent on the oxidation of HGA, i.e. through production of BQA (Hegedus 2000). It is thought that products generated by HGA/BQA-induced lipid oxidation are involved in the inflammatory process present in the AKU heart valves as HGA induces SAA (secondary A-amyloid [AA]) amyloidosis and proinflammatory cytokines (Millucci et al. 2014). SAA amyloidosis is a serious complication of chronic inflammatory conditions such as rheumatoid arthritis, and its amyloid deposition process involves a cleaved product of the acute-phase protein serum amyloid A (SAA) (Momohara et al. 2008). Indeed it was suggested that alkaptonuria is a novel-type II AA amyloidosis, if so will open new important perspectives for its therapy, particularly as methotrexate treatment significantly reduced in vitro HGA-induced amyloid A aggregates ( Millucci et al. 2012).
Even though this suggests evidence of an inflammatory component, the underlying chemical mechanism for the damaging effect on cartilage is still not proven, and it may be that damage has already occurred and pigmentation is a secondary consequence. The development of nitisinone [2(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), a potent inhibitor of p-hydroxyphenylpyruvate dioxygenase (which catalyses the formation of homogentisic acid from p-hydroxyphenylpyruvic acid) (Suzuki et al. 1999)], is now used to reduce circulating HGA in AKU patients (Introne et al. 2011). The subsequent effects of such treatment on pigment production and damage will need to be carefully monitored in particular if the osteoarthritis is controlled. Such a programme will need to establish the optimal dose to properly assess how reducing HGA affects pigmentation in the long term for adults (Ranganath et al. 2014). Unfortunately the drug is not yet licensed to treat children with AKU, and as a result, it will be impossible to assess how treatment might affect the development of ochronosis in the earlier years of life.
As regards the chemical structures in the AKU pigment, these are still not clearly defined but maybe represented by forms similar to melanin, i.e. with polymers of varying repeat units (Fig. 3). However, several simple compounds that have similar structures to HGA (MW 168) can result in colouration similar to ochronosis type pigmentation. The rust-coloured perspiration of the hippopotamus contains pigments, conjugated three-ring structures, the red hipposudoric acid (MW 328.3) and the orange norhipposudoric acid (MW 284.3) (Hashimoto et al. 2007). The two compounds are formed from the oxidative dimerisation of homogentisic acid (Kai et al. 2006). They absorb light in the UV–visible range (200–600 nm) and so are thought to protect the hippo’s dermis from the sun. Additionally, low concentrations of hipposudoric acid inhibit the growth of bacteria. Both compounds are highly reactive and tend to polymerise when removed from the hippo and or a water source (Galasso and Pichierri 2009). An unknown agent in hippo mucus keeps the compounds from polymerising for several hours, even after the hippo sweat dries. Further examples of low-molecular-weight-coloured substances are the active ingredients of the henna plant (Lawsonia inermis) 2-hydroxy 1,4 naphthoquinone (MW 174.15) which forms yellow ochre-like prisms when pure but behaves as a red-orange dye. It absorbs UV light and can be used as a sunscreen. The plant extract has been used for over 5,000 years to dye fabrics as well as skin and hair. Another such compound is juglone or 5,hydroxyl 1,4 naphthalenedione (MW 174.2), a brown pigment found in walnuts and naturally in leaves, roots, husks and bark of plants (Juglandaceae family). It is used as a dye, food and cosmetic colourant. In other words the ochronotic pigment may not necessarily be a molecule of polymer proportion.
Analysis/Detection of the Melanin Types
The chemical composition of melanin differs at every location, and the exact information regarding the individual pathways and structures need to be evaluated accordingly. Usually the location is a key to identification, although it can be difficult to decide whether a brown/dark pigment is lipofuscin, hemosiderin or melanin. Melanin stains are Fontana–Masson (stains melanin black) and Schmorl’s method based on the reduction of ferricyanide to ferrocyanide, which in the presence of ferric ions forms prussian blue (i.e. stains melanin blue green) (Bancroft and Stevens 1955). The argentaffin cells, chromaffin cells and some lipofuscins will also stain blue and the nuclei red. Prior bleaching of tissue slides with potassium permanganate or hydrogen peroxide is used to remove melanin and thus confirms the type of complex. It is worth noting that the pseudomelanin of melanosis coli caused by accumulation of lipofuscin in macrophages of the colon is periodic acid Schiff’s stain (PAS) positive, i.e. stains for mucopolysaccharides and true melanin does not. The Fontana–Masson stain is useful for melanin and the “argentaffin granules” of the digestive tract. At pH4, melanin granules reduce silver nitrate to metallic silver, a histochemical reaction that shows accumulations of black material wherever melanin is located. This is seen in the cytoplasm of skin keratinocytes (Fig. 1c).
However, the stains for melanin are simply oxidising agents that are reduced by polyphenol-type reducing substances with defined colour changes and are only indicative not confirmative of melanin. Chemically, melanin is insoluble and amorphous, and it cannot be studied as a crystal form or as a solution. The understanding of the chemical composition of melanin remains limited, due to a paucity of direct measurements. However, to improve understanding of relevant structures, partial degradation processes have been devised for each component, e.g. for eumelanin fragments 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylicacid (DHCI).
Avian feathers have an unparalleled diversity of melanin-based colour mirroring their complex chemistry (Liu et al. 2014). Elucidation of the chemical composition of avian melanin samples of black, brown, grey and iridescent feathers was carried out using laser desorption synchrotron post-ionisation (synchrotron-LDPI) mass spectrometry. The post-ionisation was achieved by tunable vacuum ultraviolet (VUV) radiation to ensure that the internal energy gained during laser desorption caused minimal fragmentation of molecules. The fragment molecules detected were able to distinguish eumelanin-to-pheomelanin ratios based upon m/z 311, 399, 443 and 487 of various redox forms of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) representing eumelanin and m/z 284 and 383 for pheomelanin. Structures indicative of pheomelanin fragments were defined as functionalised benzothiazole, benzothiazine and pyrrole-2,3,5-tricarboxylic acid TPCA moieties (Liu et al. 2014).
Solid-state NMR spectroscopy has also been used to study the structural properties and motional behaviour of natural eumelanin and pheomelanin extracted from black and red human hair (Thureau et al. 2012). Several 1D and 2D NMR spectroscopic techniques were combined to highlight the differences between the two forms of the pigment. It was clear that not only structural features inherent in the pure pigment, but also the role of the matrix structure in defining the overall melanin supramolecular arrangement should be taken into account to explain their functions. The characterisation of eumelanin revealed subunits organised into small planar oligomeric sheets (4–8 monomers), which stack to create nanoaggregates (Fig. 1b and Littrell et al. 2003).
A recent study, using low-voltage high-resolution transmission electron microscopy (TEM), showed the existence of eumelanin protomolecules that stack to form onion-like nanostructures with intersheet spacing between 3.7 and 4.0 Å (Watt et al. 2009). The recent developments in the optimisation of Fourier transform infrared (FTIR) spectroscopy indicate the increasing potential of this technique to highlight compositional differences, in particular a unique chemical profile in the outer segments of pigmented retina compared to other retinal layers, and this profile was altered in albino animals (Levine et al. 1999). Further developments using mid-infrared beamline IRENI (InfraRed ENvironmental Imaging) enabled study of changes in saturated and unsaturated fatty acid ester content in the neuronal layers of the retina. The analysis of amyloid plaques from the model Alzheimer mice showed elevated lipids surrounding and within the dense core of amyloid plaques, suggesting processes of inflammation and aggregation in Alzheimer’s disease (Kastyak-Ibrahim et al. 2011). Further improvements in software analysis (Baker et al. 2014) will hopefully enable this technique to be more applicable to unstained tissue sections, for example, from patients with Alkaptonuria.
Although pheomelanin is less widespread in nature, interest in its characterisation has grown due to its correlation susceptibility to skin cancer as a result of underexpression of “melanocortin 1 receptor” (alpha melanocyte-stimulating hormone receptor) or MC1R gene. This was shown to favour the production of pheomelanin rather than eumelanin and an increased sensitivity to UV radiation (Millington 2006).
Analysis of Pigment Derived from HGA
The major issue in the analysis of the pigment seen in alkaptonuria is ready access to sufficient amounts to carry out detailed structural compositional studies. The possibility of using model systems therefore to produce the pigment would be of considerable value in optimising analytical procedures. In an AKU mouse model, the presence of extensive chondrocytic pigmentation present throughout the femoral and tibial calcified cartilage was observed using Schmorl’s stain (Preston et al. 2014). This study showed how such a model can represent a source of tissue pigmentation and as a result now forms part of a detailed research programme (FP7-HEALTH 2012). Other sources of pigment are possible, for example, the fungus Cryptococcus neoformans is able to produce eumelanin from catecholamine precursors, such as l-dopa, epinephrine and norepinephrine and importantly the ochronotic type pigment (allomelanin/pyomelanin) from HGA (Frases et al. 2007). Unlike other fungi, melanisation in C. neoformans occurs only when the organism is exposed to precursor compounds; thus, it is ideal as a ready supply of the different melanin types. The forms allomelanin and melanin were produced by H99 C. neoformans cells grown with 1 mM of HGA at 22°C for 5 days and/or l-dopa, respectively. The separate pigments were oxidised with permanganate and analysed by high-performance liquid chromatography. The chromatograms showed the presence of peaks matching those of pyrrole-2,3-dicarboxylic acid, 1,3-thiazole-2,4,5-tricarboxylic acid (TTCA) and 1,3-thiazole-4,5-dicarboxylic acid (TDCA) in HGA melanin particles. The presence of pyrrole-2,3,5-tricarboxylic acid (PTCA) was consistent with the presence of permanganate-oxidised degradation products from l-dopa melanin. These separate oxidative products therefore imply different molecular structures for HGA and l-dopa-derived melanins. The diameters and the thicknesses of the shells of the particles recovered from HGA-pigmented cells were also less and were more negatively charged than l-dopa-derived particles. Furthermore, HGA-derived particles were fluorescent when illuminated with several wavelengths, while no fluorescence was produced by similarly irradiated l-dopa particles.
HGA melanin type can also be prepared from pure solutions of HGA (Turick et al. 2009) with molecular weight ranging from 10 to 14 kDa (Turick et al. 2002) smaller than other melanin pigments. The synthetic pyomelanin has a relative greater purity and provides for a consistent structure as microbial pigments often contain metabolic residues such as proteins, amino acids and carbohydrates (David et al. 1996). The synthetic form is therefore a more suitable material for detailed analysis. Several possible polymeric structures, based on Fourier transform infrared (FTIR) spectroscopy data, indicated the presence of quinones and phenols. The FTIR analyses of pyomelanin produced from the auto-oxidation of HGA (HGA melanin) (David et al. 1996) showed absorbance peaks consistent with the OH stretch of polymeric structures, aliphatic CH bonds, aromatic C=C bonds conjugated with C=O and/or COO- groups as well as phenolic OH groups. The presence of quinones in the pyomelanin structure was then confirmed by cyclic voltammetry indicating a two-step oxidation followed by a two-step reduction (Turick et al. 2010). The electron-transfer properties of melanin polymers, including pyomelanin, also constitute a mechanism of electron transfer to solid electron acceptors like metal oxides and electrodes (Ellis and Griffiths 1974; Turick et al. 2003, 2010). This explains how staining shows the presence of reducing substances in tissues through the coupling of hydroxybenzene depigmenting-compound oxidation caused by reduction of ferricyanide (Mentner and Willis 1997).
The application of UV–visible spectral analysis of solutions of HGA and urine from patients with alkaptonuria has been well described (Chen et al. 2014; Tokuhara et al. 2014). It was suggested that such scans could offer a quick and easy procedure to indicate the presence of the ochronotic pigment (Tokuhara et al. 2014). However, we were interested in the use of this technique to test whether we could show evidence of the presence of precursors as well as the presence of pigment. Samples taken from freshly prepared HGA solutions and urine from a patient with alkaptonuria, before and after alkalinisation, show how rapidly HGA is oxidised to the benzoquinone BZQ (Fig. 4). The latter then undergoes further change to possible pigment types with a UV–visible scan similar to synthetic pyomelanin and that produced by bacteria (Fig. 4) with no clear absorption spectra in the visible range. The loss of BZQ was slowed by maintaining pH at 6.0. Unfortunately we were not able confirm the two characteristic possible pigment peaks at 406 and 430 nm observed in urine from alkaptonuria patients incubated with NaOH (Tokuhara et al. 2014). The scan data also suggest the compounds were not similar to melanin derived from dopamine (Chen et al. 2014) where peaks in the visible region of 320, 450, 600 and 700 nm were observed. However, these latter peaks decreased markedly as the complexity of the melanin increased, i.e. via formation of increasing layers of the pigment producing the typical broadband absorption profile. This spectrum is similar to the broad spectrum of eumelanin ((Tran et al. 2006) and explains why visibly it appears black/dark brown (Fig. 2). In fact it is argued that eumelanin consists of many chemically distinct species, and the broadband absorption spectrum is a result of averaging the spectra of these species. The sharp peaks due to 5,6-dihydroxyindole (DHI) and its oligomers would therefore be eliminated after this process. Whether such scans could be used for monitoring the intermediate quinone seems unlikely as conditions favour the reaction of BZQ to form complexes with amino acids, e.g. cysteine (Bender et al. 2007).
Fig. 4.
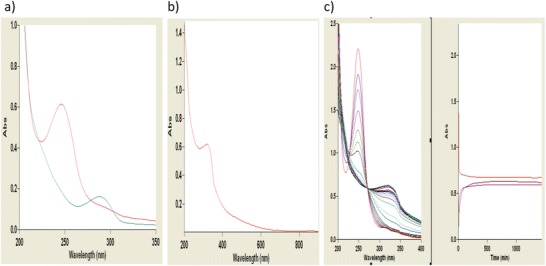
Spectral scans of solutions of HGA, pigment and a urine from a patient with AKU effect of pH and time. All procedures followed were in accordance with the ethical standards of the local hospital ethics committee. Informed consent was obtained from patient(s) included in the study. (a) Pre- and post-alkalinisation, i.e. to pH 10 (1 pt10 M/L NaOH and 50 pts urine), of untreated AKU urine (approximately 20 mmol/L HGA): blue line pre and red line post. (b) HGA solution10 mmol/L scanned 24 h after alkalinisation with ammonia (20 mM/L final concentration). (c) A kinetic study of the alkalinisation of an HGA solution over 24 h absorbances at 250 nm (BQA=red), 290 nm (HGA=brown) and 318 nm (purple). Showing the rapid disappearance of BZQ peak 250 nm and slower loss of peak at 316. Scanning urine or synthetic pyomelanin at pH’s 10.0, 7.0 and or 2.5 shows a similar profile with no definite peaks as in Fig. 4a (blue line) and b. However, for solution at pH 10.0 solutions, the peak at 250 nm was much less and was relatively stable at pH 6.0. Solutions/samples were diluted usually 1 pt to 100 pts of diluent deionised water or buffer solution and scanned with Cary UV–vis scanning spectrometer (Agilent, UK) giving a full scan (200–800 nm) every 1 min
The use of MALDI ESIMS as another approach was also investigated to assess the purity of any preparation and as a measurement of molecular weight. Analysis was carried out on pyomelanin synthesised from HGA metabolising bacteria and produced from solutions of HGA stored at pH 10 for 1 week (Turick et al. 2009).The m/z spectra (Fig. 5), after direct injection without any matrix-modifying agent, show a range of masses from 800 to 2,300 with the majority of around 1,100. These appeared as apparently single-charged entities suggesting a mixture of different molecular weights. Similar mass ranges were found with humic substances (Peña-Méndez et al. 2005). The use of a matrix modifier showed marked differences in the spectra with alpha cyanocinnamic acid giving masses around 900 and dihydroxy benzoic acid modifier a spectra of low mass components. These differences suggest inherent problems of complex molecular breakup within the spectrometer as a result of instrument conditions (Novotny et al. 2014). This suggests that further systematic studies are required to fully utilise the use of ESIMS on the AKU pigment.
Fig. 5.

(a) The pyomelanin was prepared from bacteria fed with increased tyrosine. Also synthetic pyomelanin was prepared from a solution of 20 mmol/L HGA at pH 10 and left for 7 days at 20°C. The samples were added to 10–15 kDa dialyser pack and dialysed against pure water for 24 h with two changes of the dialysate. The samples were then dried before analysis using a Micromass M@LDI™ linear MALDI-TOF -MS, with conditions MCP voltage 1,850, laser power 85, pulse voltage 1,500 and nitrogen UV laser 337 nM; (a) the m/z spectrum observed after straight injection into the MS with no modifier present, (b) MALDI MS with matrix modifier alpha cyanocinnamic acid and (c) MALDI MS with matrix modifier dihydroxy benzoic acid. For scans (b) and (c), top is the control matrix alone, middle synthetic and bottom bacterial pyomelanin. The pyomelanin and scans were provided by Dr CE Turick Savannah River National Laboratory, South Carolina USA
Investigation into the composition of the humic acids may give further clues as to how to proceed in the analysis of AKU pigment. These acids are formed by condensation with polyphenol quinones and carbohydrates/sugars to produce a brown, melanin-tinted mixture of polymers, found in lignin, peat and soils (Novotny et al. 2014). Humic acid has the average chemical formula C187H186O89N9S1 and is insoluble in strong acid (pH 1). A 1:1 hydrogen-to-carbon ratio indicates a significant presence of benzene rings in the structure, whereas a low oxygen-to-carbon ratio indicates fewer acidic functional groups than occur in fulvic acid, the other acidic organic polymer extracted from humus. Separation procedures of these compounds are available, but still more studies are required to elucidate the proposed intermolecular interactions that link humic components into supramolecular associations (Sutton and Sposito 2005) similar to the pigmentation process in AKU.
In conclusion pigments found in plants, animals and humic substances are well described and classified. In humans considerable progress has been made with the main pigment melanin in defining its biochemistry and the different types and functions. However, analytical techniques to show these differences in vivo are still not readily available. NMR and IR spectroscopy are relatively insensitive and reveal only major structural differences. Techniques utilising MS are useful in determining elemental content but require further studies to optimise conditions for accurate mass analysis. How the components may be structurally organised seems to be the most problematic, and scanning TEM as well as the improved FTIR seems to be of use in this respect. As regards understanding the nature of the pigment related to HGA seen in patients with AKU, it is still thought of as a melaninlike pigment simply because of its colour and likewise thought to be a polymer of undetermined size. It is important that detailed analysis be carried out to define more accurately this pigment. However, observations suggest it to be the same as the HGA-derived pigment pyomelanin, produced by bacteria containing both quinone and phenolic groups. The interesting developments in alkaptonuria will be to understand how such a polymer can cause such profound collagen and connective tissue damage and how best to reverse this process.
Compliance with Ethics Guidelines
All procedures reported in this review were in accordance with the ethical standards of the local hospital ethics committee and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from patient(s) wherever included in this review.
Conflict of Interest
NB Roberts, SACurtis, A Milan and LR Ranganath have no conflict of interest.
NB Roberts was the main author who reviewed the literature and completed the review.
SA Curtis was responsible for the UV–visible spectra.
AM Milan, a senior colleague in the AKU group, reviewed and made corrections to the manuscript.
LR Ranganath is the director of the AKU group and was responsible for the commissioning of this review and made corrections to the manuscript.
Footnotes
Competing interests: None declared
Contributor Information
N. B. Roberts, Email: n.b.roberts@liverpool.ac.uk
Collaborators: Johannes Zschocke
References
- Baker MJ, Trevisan J, Bassan P, et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nature Prot. 2014;9:1771–1791. doi: 10.1038/nprot.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and practice of histological techniques. 2. New York: Churchill Livingstone; 1955. [Google Scholar]
- Barber TM, Adams E, Ansorge O, Byrne JV, Karavitaki N, Wass JAH (2010) Review Nelson’s syndrome. Eur J Endo 163:495–507 [DOI] [PubMed]
- Bender RP, Ham AJL, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: adduction of cysteine residues 392 and 405. Biochem. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson JF, Harper DC, Tenza D, Raposo GA, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Molec Biol Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi D, Laschi M, Amato L, Bernardini G, Millucci L, Marcolongo R, Cavallo G, Spefico A, Santucci A. Evaluation of anti-oxidant treatments in an in vitro model of alkaptonuric ochronosis. Rheum. 2010;49:1975–1983. doi: 10.1093/rheumatology/keq175. [DOI] [PubMed] [Google Scholar]
- Braconi D, Bianchini C, Bernardini G, Laschi M, Millucc L, Spreafico A, Santucci A. Redox-proteomics of the effects of homogentisic acid in an in vitro human serum model of alkaptonuric ochronosis. J Inherit Metab Dis. 2011;34:1163–1176. doi: 10.1007/s10545-011-9377-6. [DOI] [PubMed] [Google Scholar]
- Bush WD, Gargullo J, Zucca FA, et al. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. PNAS. 2006;103:14785–14789. doi: 10.1073/pnas.0604010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira A, Ferreira LM, Loureiro V. Brown pigments produced by Yarrowia lipolytica result from extracellular accumulation of homogentisic acid. Appl Environ Microbiol. 2001;67:3463–3468. doi: 10.1128/AEM.67.8.3463-3468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-T, et al. Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat Commun. 2014;5:3859–3866. doi: 10.1038/ncomms4859. [DOI] [PubMed] [Google Scholar]
- Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl- cysteine increases oxidative stress in humans after an acute muscle induced by eccentric exercise. Free Rad Biol Med. 2001;31:745–753. doi: 10.1016/S0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postep Derm Alergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ischia M, Wakamatsu K, Napolitano A, et al. Melanins and melanogenesis: methods, standards, protocols. Pig Cell Melan Res. 2013;26:616–633. doi: 10.1111/pcmr.12121. [DOI] [PubMed] [Google Scholar]
- David C, Daro A, Szalai E, Atarhouch T, Mergeay M. Formation of polymeric pigments in the presence of bacteria and comparison with chemical oxidative coupling-II. Catabolism of tyrosine and hydroxyphenylacetic acid by Alcaligenes eutrophus CH34 and mutants. Eur Polym J. 1996;32:669–697. doi: 10.1016/0014-3057(95)00207-3. [DOI] [Google Scholar]
- Del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Delgado-Vargas F, Jiménez AR, Paredes-López O. Natural pigments: carotenoids, anthocyanins, and betalains — characteristics, biosynthesis, processing, and stability. Crit Revs Food Sci Nutr. 2000;40(3):173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Ellis DH, Griffiths DA. The location and analysis of melanins in cell walls of some soil fungi. Canad J Micro. 1974;20:1379–1386. doi: 10.1139/m74-212. [DOI] [Google Scholar]
- Eslami M, Zare HR, Namazia M. The effect of solvents on the electrochemical behaviour of homogentisic acid. J Electrchem. 2014;720:76–83. [Google Scholar]
- Figueras LE, Rodriguez-Catellanos MA, Gonzalez-Mendoza A, Cantu JM. Hyperkeratosis-hyperpigmentation syndrome: a confirmative case. Clin Genet. 1993;43(2):73–75. doi: 10.1111/j.1399-0004.1993.tb04430.x. [DOI] [PubMed] [Google Scholar]
- Forslind B, Roomans GM, Carlsson LF, Malmquist KG, Akselsson KR. Elemental analysis on freeze-dried sections of human skin: studies by electron microscopy and particle induced X-ray emission analysis. Scann Elec Micro. 1984;11:755–759. [PubMed] [Google Scholar]
- FP7-HEALTH (2012) DEVELOPAKURE: clinical development of nitisinone for alkaptonuria; Project reference: 304985 Cordis.europ.eu/project/rcn/106157
- Frases S, Salazar A, Dadachova E, Casadeval A. Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl Environ Micro. 2007;73:615–621. doi: 10.1128/AEM.01947-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso V, Pichierri F. Probing the molecular and electronic structure of norhipposudoric and hipposudoric acids from the red sweat of hippopotamus amphibius: a DFT investigation. J Phys Chem A. 2009;113(11):2534–2543. doi: 10.1021/jp809138s. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Saikawa Y, Nakata M. Studies on the red sweat of the Hippopotamus amphibius. Pure Appl Chem. 2007;79(4):507–517. doi: 10.1351/pac200779040507. [DOI] [Google Scholar]
- Hegedus ZL. The probable involvement of soluble and deposited melanins their intermediates and reactive oxygen side products in human disease and ageing. Toxic. 2000;145:85–101. doi: 10.1016/S0300-483X(00)00157-8. [DOI] [PubMed] [Google Scholar]
- Introne WJ, Perry MB, Troendle J et al (2011) A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 103(4):307–314 [DOI] [PMC free article] [PubMed]
- Ito S, Wakamatsu K. Chemistry of mixed melanogenesis–pivotal roles of dopaquinone. Photochem Photobiol. 2008;84(3):582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- Jablonski N. Living color. Berkeley/Los Angeles/London: University of California Press; 2012. [Google Scholar]
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. PNAS. 2010;107:8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M, Masanori M, Yoko S, et al. Properties of the enzyme responsible to the synthesis of hipposudoric acid and norhipposudoric acids, the pigments in the red sweat of the hippopotamus. Nippon Kagakkai Koen Yokoshu. 2006;86(2):1314–1318. [Google Scholar]
- Kassinger RG (2003) Dyes from sea snails to synthetics 21st century books. www.Millbrookpress.com
- Kastyak-Ibrahim MZ, Nasse MJ, Rak M, et al. Biochemical label-free tissue imaging with subcellular-resolution synchrotron FTIR with focal plane array detector. Neuro Image. 2011;60(1):376–383. doi: 10.1016/j.neuroimage.2011.11.069. [DOI] [PubMed] [Google Scholar]
- Keith KE, Killip L, He P, Moran GR, Valvano MA. Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J Bacteriol. 2007;189:9057–9065. doi: 10.1128/JB.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JM, Macaulay W, Nercessian OA, Jaffe IA. New developments in ochronosis: review of the literature. Rheum Int. 2005;25:81–85. doi: 10.1007/s00296-004-0498-1. [DOI] [PubMed] [Google Scholar]
- Land EJ, Ramsden CA, Riley PA. Tyrosinase autoactivation and the chemistry of ortho-quinone amines. Acc Chem Res. 2003;36(5):300–308. doi: 10.1021/ar020062p. [DOI] [PubMed] [Google Scholar]
- Laschi M, Tinti L, Braconi D, et al. Homogentisate 1,2 dioxygenase is expressed in human osteoarticular cells: implications in alkaptonuria. J Cell Physiol. 2012;227(9):3254–3257. doi: 10.1002/jcp.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Li LC, Chen JB, Chang HW (2015) Indoxyl sulfate-induced oxidative stress, mitochondrial dysfunction, and impaired biogenesis are partly protected by vitamin C and N-acetylcysteine. Sci World J 2015:620826 [DOI] [PMC free article] [PubMed]
- Levine N, Dorr RT, Ertl GA, Brooks C, Alberts DS. Effects of a potent synthetic melanotropin, Nle4-D-Phe7alpha-MSH (Melanotan) on tanning: dose ranging study. J Dermatol Treat. 1999;10:127–132. doi: 10.3109/09546639909056014. [DOI] [Google Scholar]
- Levy C, Khaled M, Fisher DE. Review MITF: master regulator of melanocyte development and melanoma oncogene. Trends in Molec Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Littrell KC, Gallas JM, Zajac GW, Thiyagarajan P. Structural studies of bleached melanin by synchrotron small-angle X-ray scattering. Photochem Photobiol. 2003;77:115–120. doi: 10.1562/0031-8655(2003)077<0115:SSOBMB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Liu SY, Shawkey MD, Parkinson D, TroyTP AM. Elucidation of the chemical composition of avian melanin. RSC Adv. 2014;4:40396–40399. doi: 10.1039/C4RA06606E. [DOI] [Google Scholar]
- Lok ZS, Goldstein J, Smith JA. Alkaptonuria-associated aortic stenosis. J Card Surg. 2013;28:417–420. doi: 10.1111/jocs.12129. [DOI] [PubMed] [Google Scholar]
- Martin JP, Batkoff B. Homogentisic acid autooxidation and oxygen radical generation: implications for the etiology of Alkaptonuria arthritis. Free Rad Biol Med. 1987;3:241–250. doi: 10.1016/S0891-5849(87)80031-X. [DOI] [PubMed] [Google Scholar]
- Mentner LM, Willis I. Electron transfer and photoprotective properties of melanins in solution. Pigm Cell Res. 1997;10:214–217. doi: 10.1111/j.1600-0749.1997.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Millington GWM. Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors. Clin Expt Dermat. 2006;31:407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- Millucci L, Spreafico A, Tiniti A, et al. Alkaptonuria is a novel human secondary amyloidogenic disease. BBA Molec Basis Dis. 2012;1822:1682–1691. doi: 10.1016/j.bbadis.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millucci L, Ghezzi L, Paccagnini E et al (2014) Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediat Inflamm 12. Article ID 258471 [DOI] [PMC free article] [PubMed]
- Momohara S, Okamoto H, Yamanaka H. Chondrocyte of rheumatoid arthritis serve as a source of intra-articular acute-phase serum amyloid A protein. Clin Chim Acta. 2008;398:155–156. doi: 10.1016/j.cca.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histo Histopath. 2006;21(5):567–578. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- Nicolaus RA (1969) In: Lederer E (ed) Melanins chemistry of natural products. Hermann, Paris
- Nordlund JJ, Boissy R, Hearing VJ, King RA (2006) In: Oetting W, Ortonne J-P (eds) The pigmentary system, 2nd edn. Blackwell, Oxford, 1229 pp
- Novotny NR, Capley EN, Stenson AC. Fact or artifact: the representativeness of ESI-MS for complex natural organic mixtures. J Mass Spect. 2014;49:316–326. doi: 10.1002/jms.3345. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J, Hamilton-Miller MT. Brown and red pigmented Pseudomonas aeruginosa differentiation between melanin and pyrorurin. J Med Microbiol. 1975;8:199–203. doi: 10.1099/00222615-8-1-199. [DOI] [PubMed] [Google Scholar]
- Pallotta V, Gevi F, D'Alessandro A, Zolla L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 2014;12:367–387. doi: 10.2450/2014.0266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Méndez EM, Josef Havel J, Patočka J. REVIEW Humic substances compounds of still unknown structure:applications in agriculture, industry, environment, and biomedicine. J Appl Biomed. 2005;3:13–24. [Google Scholar]
- Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347:2111–2121. doi: 10.1056/NEJMoa021736. [DOI] [PubMed] [Google Scholar]
- Phornphutkul C, IntroneWJ, Way P, William A, Gahl WA (2003) Alkaptonuria. N Engl J Med 348:1408
- Preston AJ, Keenan CM, Sutherland H et al (2014) Chronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann Rheum Dis 73:284–289 [DOI] [PubMed]
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endo. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- Przemyslaw M, Plonka L, Grabacka M. Review Melanin synthesis in microorganisms – biotechnological and medical aspects. Acta Biochim Pol. 2006;53:429–443. [PubMed] [Google Scholar]
- Ranganath LR, Jarvis JC, Gallagher JA. Recent advances in management of alkaptonuria (invited review, best practice article) J Clin Pathol. 2013;66(5):367–373. doi: 10.1136/jclinpath-2012-200877. [DOI] [PubMed] [Google Scholar]
- Ranganath LR, Milan AM, Hughes AT. Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-206033. [DOI] [PubMed] [Google Scholar]
- Ruzafa C, Sanchez-Amat A, Solano F. Characterization of the melanogenic system in Vibrio cholerae, ATCC 14035. Pigment Cell Res. 1995;8(3):147–152. doi: 10.1111/j.1600-0749.1995.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyv NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22(12):3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- Solano F (2014) Melanins: skin pigments and much more – types, structural models, biological functions, and formation routes. New J Sci 28. doi:10.1155/2014/498276. Article ID 498276
- Stuart JA, Brige RR (1996) Characterization of the primary photochemical events in bacteriorhodopsin and rhodopsin. In: Lee AG (ed) Rhodopsin and G-protein linked receptors, part A, vol 2. JAI Press, Greenwich, pp 33–140
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res. 1999;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- Sutton R, Sposito G. Molecular structure in soil humic substances: the new view. Environ Sci Technol. 2005;39(23):9009–9015. doi: 10.1021/es050778q. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Oda K, Yoshikawa Y, Maeda Y, Suzuki T. A novel therapeutic trial of homogentisic aciduria in a murine model of alkaptonuria. J Hum Genet. 1999;44(2):79–84. doi: 10.1007/s100380050114. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takahashi NH, Shibahara S. Neuroendocrine functions of melanocytes: beyond the skin-deep melanin maker. Tohuku J Exp Med. 2007;211(3):201–221. doi: 10.1620/tjem.211.201. [DOI] [PubMed] [Google Scholar]
- Thureau P, Ziarelli F, Thvand A, et al. Probing the motional behavior of eumelanin and pheomelanin with solid-state NMR spectroscopy: new insights into the pigment properties. Chemta Indicat Eur J. 2012;18:10689–10700. doi: 10.1002/chem.201200277. [DOI] [PubMed] [Google Scholar]
- Tinti L, Spreafico A, Braconi D, Millucci L, Bernardini G, Chellini F, Cavallo G, Selvi E, Galeazzi M, Marcolongo R, Gallagher JA, Santucci A. Evaluation of antioxidant drugs for the treatment of ochronotic alkaptonuria in an in vitro human cell model. J Cell Physiol. 2010;225:84–91. doi: 10.1002/jcp.22199. [DOI] [PubMed] [Google Scholar]
- Tokuhara Y, Shukuya K, Tanaka M, et al. Detection of novel visible-light region absorbance peaks in the urine after alkalization in patients with alkaptonuria. PLoS One. 2014;9(1):e86606. doi: 10.1371/journal.pone.0086606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran ML, Powell BJ, Meredith P. Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance. Biophys J. 2006;90:743–752. doi: 10.1529/biophysj.105.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribl F, Gerlach M, Marcus K, et al. Subcellular proteomics of neuromelanin granules isolated from the human. Brain Molec Cell Proteom. 2005;4:945–957. doi: 10.1074/mcp.M400117-MCP200. [DOI] [PubMed] [Google Scholar]
- Turick CE, Tisa LS, Caccavo F., Jr Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol. 2002;68:2436–2444. doi: 10.1128/AEM.68.5.2436-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turick, CE, Caccavo F Jr, Tisa LS (2003) Electron transfer to Shewanella algae BrY toHFO is mediated by cell-associated melanin. FEMS Microbiol Lett 220:99–104 [DOI] [PubMed]
- Turick CE, Beliaev A, Ekechukwu AA, Poppy T, Maloney A, Lowy DA. The role of 4-hydroxyphenylpyruvate dioxygenase in enhancement of solid-phase electron transfer by Shewanella oneidensis MR-1. FEMS Microbiol Ecol. 2009;68:223–235. doi: 10.1111/j.1574-6941.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- Turick CE, Knox AS, Becne JMl, Ekechukwu AA, Milliken CE (2010) Properties and function of pyomelanin. In: Elnashar MM (ed) Biopolymers. Sciyo Janeza Trdine, Croatia, Chap. 26
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte – stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nature Genet. 1995;11(3):328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Venkataraman K (ed) (2012) The chemistry of synthetic dyes. Elsevier, London
- Videria IFS, Moura DFL, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88(1):76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu K, Hu DN, McCormick SA, Ito S. Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pig Cell Melan Res. 2008;21(1):97–105. doi: 10.1111/j.1755-148X.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Watt AAR, Bothma JP, Meredith P. The supramolecular structure of melanin. Soft Matter. 2009;5:3754–3760. doi: 10.1039/b902507c. [DOI] [Google Scholar]
- Williams DP, Lawrence A, Meng X. Pharmacological and toxicological considerations of homogentisic acid in alkaptonuria. Pharmacology. 2012;3:61–74. [Google Scholar]
- Wolff JA, Barshop B, Nyhan WL et al (1989) Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res 26:140–144 [DOI] [PubMed]
- Zannoni VG, Lomtevas N, Goldfinger SO. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim Biophys Acta. 1969;177:94–105. doi: 10.1016/0304-4165(69)90068-3. [DOI] [PubMed] [Google Scholar]
- Zecca L, Bellei C, Costi P et al (2008) New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. PNAS 105:17567–17572 [DOI] [PMC free article] [PubMed]


