Abstract
Background: Alkaptonuria (AKU) is a serious genetic disease due to a defect in tyrosine metabolism, leading to increased serum levels of homogentisic acid (HGA). Nitisinone decreases HGA in AKU, but the concentration–response relationship has not been previously reported.
Objectives: To determine the relationship between serum concentrations of nitisinone and the effect on both HGA and tyrosine; secondly to determine steady-state pharmacokinetics of nitisinone in AKU patients.
Method: Thirty-two patients with AKU received either 1, 2, 4, or 8 mg nitisinone daily. Urine and serum HGA and serum tyrosine and nitisinone were measured during 24 h at baseline (before first dose) and after 4 weeks of treatment.
Results: Nitisinone pharmacokinetics (area under the curve [AUC] and maximum concentrations [Cmax]) were dose proportional. The median oral clearance determined in all patients, irrespective of dose, was 3.18 mL/h·kg (range 1.6–6.7).
Nitisinone decreased urinary excretion of HGA in a concentration-dependent manner, with a maximum effect seen at average nitisinone concentrations of 3 μmol/L. The association between nitisinone and tyrosine concentrations was less pronounced. Serum levels of HGA at Week 4 were below the limit of quantitation in 65% of samples, which prevented determination of the relationship with nitisinone concentrations.
Conclusion: Nitisinone exhibits dose-proportional pharmacokinetics in the studied dosage interval. Urinary excretion of HGA decreases in a concentration-dependent manner, while the increase in tyrosine is less clearly related to nitisinone concentrations.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2015_412) contains supplementary material, which is available to authorized users.
Introduction
Alkaptonuria (AKU, OMIM reference 203500) is a rare autosomal recessive disorder caused by a deficiency in homogentisate 1,2-dioxygenase (HGD, EC 1.13.11.5), the third enzyme in the tyrosine metabolism pathway (Fig. 1). It converts homogentisic acid (HGA, 2,5-dihydroxyphenylacetic acid) to 4-maleylacetoacetic acid. Inability to metabolize HGA leads to urinary excretion of at least 90% of the compound, or 3–6 g per day, in patients with AKU (Garrod 1902; Lustberg et al. 1969; Introne et al. 2011). Despite this pronounced renal elimination, some HGA is oxidized to a melanin-like polymeric pigment via benzoquinone acetic acid. The pigment is deposited in connective tissue, especially in cartilage, a process called ochronosis (Zannoni et al. 1969). This leads to early onset arthritis of the spine and synovial joints and other debilitating symptoms (O’Brien et al. 1963; Ranganath et al. 2013). There is currently no approved pharmacological treatment for AKU.
Fig. 1.
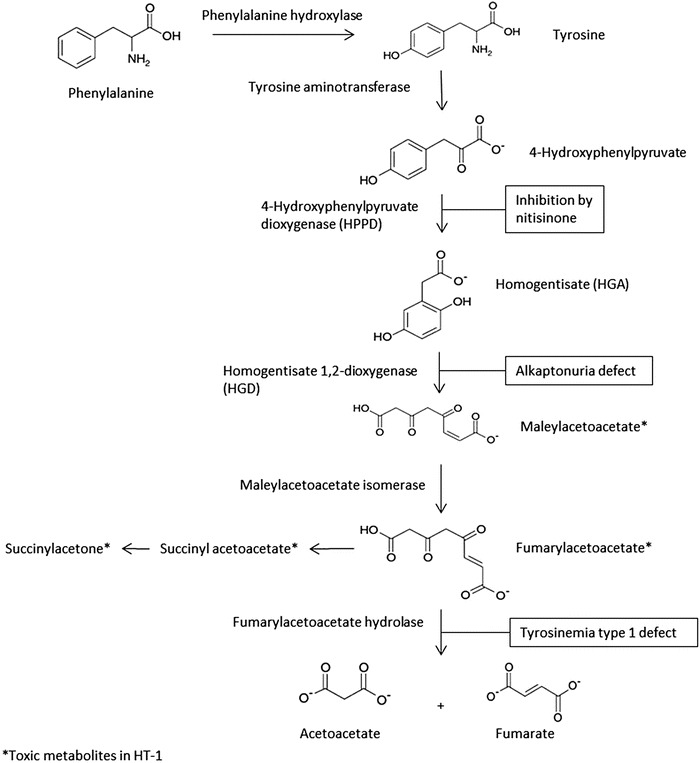
Schematic presentation of tyrosine metabolism, with AKU and HT-1 defects, and nitisinone site of action
Nitisinone (also known as NTBC, an abbreviation of its full chemical name), a potent competitive inhibitor of the enzyme 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) (Fig. 1), has been proposed as a treatment for AKU (Anikster et al. 1998; Lock et al. 2014). This drug is registered in several countries for the treatment of hereditary tyrosinemia type I (HT-1, OMIM reference 276700) which is caused by a defect further downstream in the tyrosine metabolism pathway compared to AKU. In HT-1, nitisinone prevents the formation of the highly toxic metabolites maleylacetoacetate, fumarylacetoacetate, and succinylacetone (Lindstedt et al. 1992), and in combination with a tyrosine-restricted diet, it serves as a successful therapeutic intervention. In AKU, nitisinone can effectively reduce HGA and prevent ochronosis in mice (Suzuki et al. 1999; Preston et al. 2013) and reduce HGA in patients (Introne et al. 2011).
A 4-week dose–response study (“SONIA 1”) in patients with AKU to investigate the effect of nitisinone on 24-hour urinary excretion of HGA (u-HGA24), and serum concentrations of HGA and tyrosine, as well as the safety of this treatment has been performed and recently reported (Ranganath et al. 2014). In short, u-HGA24 decreased in a dose-dependent manner, with the highest dose (8 mg) reducing u-HGA by more than 98%. The fasting predose serum concentrations of HGA (s-HGA) also decreased, and predose serum tyrosine increased with increasing doses. All doses were well tolerated by the patients.
Previous studies with nitisinone have not determined its pharmacokinetics (PK) after repeated dosing nor the relationship between serum exposure and pharmacological effect. Therefore, the SONIA 1 study also aimed at determining nitisinone PK at steady state and to test for PK dose proportionality, as well as describing the relationship between serum concentrations and the effect on HGA and tyrosine in patients with AKU.
Materials and Methods
Patients
Patients with AKU were verified by increased urine HGA excretion and HGD gene mutation identification and were eligible for participation. Detailed inclusion and exclusion criteria have been presented earlier (Ranganath et al. 2014).
Study Design and Treatments
SONIA 1 was an international, multicenter, open-label, parallel-group, randomized study in 40 AKU patients, of which 32 (8 per dose group) received nitisinone in doses of either 1, 2, 4, or 8 mg nitisinone once daily for 4 weeks, administered as an oral suspension containing 4 mg nitisinone per milliliter. Another eight patients served as an untreated control group (data not included).
Measurements
Measurement of serum nitisinone, s-HGA, and tyrosine was performed over 24 h after 4 weeks of treatment (Week 4). The first sample was collected after breakfast, just prior to administration of the last dose of nitisinone. Subsequent postdose samples at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 15, 18, and 24 h were taken. Full s-HGA and tyrosine profiles were also determined at baseline, before administration of the first dose of study medication.
Measurement of u-HGA24 was performed at baseline and at Weeks 2 and 4 (see details in Ranganath et al. 2014). Only baseline and Week 4 data are discussed here, since no 24-hour serum profiles were collected at Week 2.
The urine and serum samples were analyzed according to the liquid chromatography–mass spectrometry methods described by Hughes et al. (2014, 2015).
Calculations and Statistics
Based on nitisinone serum concentrations, the following PK variables were calculated using Phoenix WinNonlin 6.3 (Certara L.P., St. Louis, MO, USA): maximum and minimum concentrations (Cmax, Cmin), the area under the 24-hour concentration vs. time curve (AUC24, calculated by the linear trapezoidal rule), and oral clearance (CL/F, calculated as dose/AUC24). The same software was used to determine Cmax and AUC24 for s-HGA and serum tyrosine.
The average concentrations during 24 h (Cav) were calculated as AUC24/24 for nitisinone, s-HGA, and tyrosine.
The dose proportionality of nitisinone AUC24 and Cmax, respectively, was evaluated using a power model (Gough et al. 1995).
Results
Demographics
Demographics for all 40 patients in SONIA 1 have previously been presented (Ranganath et al. 2014). The 32 patients who were treated with nitisinone, and are included in this report of the exposure–response relationships, had a mean age of 47.5 years (range 19–62 years), mean body weight of 79.9 kg (59–112 kg), and mean height of 168.0 cm (164–180 cm). Twenty-three of these 32 patients (72%) were male.
Nitisinone Pharmacokinetics
The AUC24 and Cmax data indicate that exposure to nitisinone is dose proportional within the studied dose range, as indicated by the 95% confidence intervals for the regression coefficients, 0.90–1.26 for AUC24 and 0.85–1.21 for Cmax (Table 1). The oral clearance was similar across the dose groups and ranged from 1.6 to 6.7 mL/h·kg with an overall median of 3.18 mL/h·kg.
Table 1.
Dose proportionality of nitisinone PK parameters
| Estimate (beta) | 95% CI lower | 95% CI upper | |
|---|---|---|---|
| AUC24 (μmol · h/L) | 1.08 | 0.90 | 1.26 |
| C max (μmol/L) | 1.03 | 0.85 | 1.21 |
AUC 24 area under the 24-hour serum concentration profile, CI confidence interval, C max maximum serum concentration
The median Cmax/Cmin ratio for all patients was 1.80, ranging from 1.3 to 3.8 in individual patients (data not shown). Mean serum concentration profiles for the four doses are shown in Supplementary Figure S1.
Relationship Between Nitisinone Exposure and the Effect on HGA and Tyrosine
At baseline, u-HGA24 ranged from 14 400 to 69 500 μmol across all dose groups (Ranganath et al. 2014). At Week 4, u-HGA24 decreased in relation to nitisinone concentrations up to a Cav of about 3 μmol/L, with no further decrease in u-HGA24 above this level (Fig. 2). In the seven patients with concentrations above 3 μmol/L (all receiving 8 mg nitisinone), median u-HGA24 was 135.7 μmol (range 83–213 μmol). The change in u-HGA24 from baseline was 99.4–99.7% in these patients.
Fig. 2.
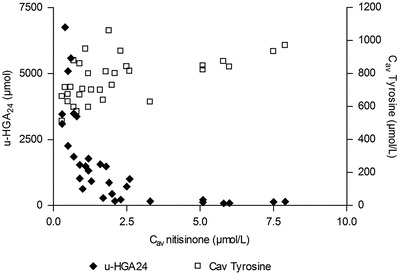
Individual urinary excretion of HGA and daily average serum tyrosine concentrations vs. daily average serum nitisinone concentrations at Week 4 in AKU patients treated with nitisinone (N = 32)
Serum concentrations of HGA at Week 4 were below the lower limit of quantification (LLOQ, 3.1 μmol/L) in 65% of all samples from treated patients, and only four patients (three on 1 mg and one on 2 mg nitisinone) had HGA above the LLOQ in all samples. The number of patients with s-HGA below the LLOQ increased with increasing nitisinone dose, and therefore, no analysis of individual s-HGA data vs. nitisinone concentrations has been performed. However, for all patients on 1 mg, Cav could be reasonably well estimated, and a comparison of the median results at baseline and Week 4 (Table 2) indicates that a dose of 1 mg nitisinone decreased s-HGA by approximately 88%.
Table 2.
Serum concentration data (median and range) for HGA, tyrosine, and nitisinone in patients with AKU treated with nitisinone (N = 8 per dose group)
| Time | 1 mg | 2 mg | 4 mg | 8 mg | |
|---|---|---|---|---|---|
| Nitisinone | |||||
| C av (μmol/L) | Week 4 | 0.47 (0.3–0.8) | 1.04 (0.7–1.6) | 1.94 (1.3–2.6) | 5.49 (2.3–7.9) |
| C min (μmol/L) | Week 4 | 0.30 (<0.2–0.5) | 0.70 (0.5–1.2) | 1.40 (1.0–2.3) | 4.55 (1.5–6.2) |
| C max (μmol/L) | Week 4 | 0.70 (0.4–1.1) | 1.55 (0.9–1.9) | 2.50 (1.9–3.0) | 7.30 (3.3–9.7) |
| CL/F (mL/h·kg) | Week 4 | 2.80 (1.8–6.7) | 3.49 (1.7–4.6) | 3.49 (1.9–5.2) | 2.27 (1.6–5.0) |
| HGA | |||||
| C av (μmol/L) | Baseline | 32.96 (22.5–44.1) | 34.21 (25.1–29.1) | 37.69 (28.9–49.9) | 38.61 (25.2–47.7) |
| Week 4 | 3.93 (0.5–7.3) | 3.32 (<3.1–5.8) | <1.3 (<1.3–1.3) | <0.4 (<0.4–0.4) | |
| C max (μmol/L) | Baseline | 51.30 (40.8–69.9) | 52.85 (39.8–72.9) | 61.70 (48.4–88.3) | 57.05 (38.8–78.2) |
| Week 4 | 6.60 (4.3–10.7) | 5.10 (<3.1–7.5) | 3.40 (<3.1–4.7) | <3.1 (<3.1–3.2) | |
| Tyrosine | |||||
| C av (μmol/L) | Baseline | 56.0 (50–61) | 62.6 (46–79) | 58.0 (47–75) | 59.3 (50–82) |
| Week 4 | 667.3 (511–879) | 702.1 (596–948) | 805.9 (639–1,059) | 860.0 (627–970) | |
| C max (μmol/L) | Baseline | 71.0 (57–113) | 77.5 (59–100) | 80.5 (57–114) | 75.0 (62–120) |
| Week 4 | 721.0 (594–978) | 835.5 (662–1,010) | 889.0 (696–1,155) | 984.0 (715–1,066) | |
C av average serum concentration during the 24-hour dosing interval (C av = AUC24/24), C min minimum serum concentration, C max maximum serum concentration, CL/F oral clearance
Median Cav values for serum tyrosine increased in relation to dose (Table 2), but no clear relationship between nitisinone exposure and individual tyrosine data could be seen (Fig. 2). At the highest nitisinone serum concentrations, above 5 μmol/L, daily average tyrosine concentrations were in the range of 800–1,000 μmol/L. However, all treated patients had daily tyrosine averages above 500 μmol/L, and values above 800 μmol/L were seen at nitisinone concentrations as low as 0.7 μmol/L and a dose of 1 mg.
Similar profiles were seen for serum tyrosine and HGA at baseline. After intake of breakfast (time = 0), mean serum tyrosine concentrations decreased from 58 μmol/L right after breakfast to 50 μmol/L at 4 h, thereafter to increase to a maximum of 65 μmol/L at 12 h. The s-HGA profile followed the tyrosine profile, except that it showed a slight increase between 0 and 2 h. Mean s-HGA concentrations after breakfast (t = 0) were 29 μmol/L, and maximum concentrations at 12 h were 47 mmol/L (Supplementary Figure S2).
Discussion
The nitisinone serum concentrations increased in proportion to dose, as shown by the power model analysis of AUC and Cmax data and supported by the similar clearance in all dose groups The median oral clearance in all 32 patients, 3.2 mL/h·kg, is in reasonable accordance with the data in HT-1 patients; 4.0 mL/h·kg (0.0956 L/kg · day). The fluctuation in nitisinone serum concentrations (Cmax/Cmin ratio) was relatively low, supporting previous reports of a long half-life (Hall et al. 2001).
We found that nitisinone decreased urinary excretion of HGA in a concentration-dependent manner, up to nitisinone serum concentrations of about 3 μmol/L. This concentration was reached with a dose of 8 mg daily in seven of the eight patients in that dose group, and these had a reduction in u-HGA24 from baseline of at least 99.4%. Despite this near 100% decrease in u-HGA, the amount excreted at the highest nitisinone concentrations, or dose, is still about 50 times higher than the highest amount (2.92 μmol/24 h) found in a recent study in 22 normal subjects, using the same analytical method as in our study (Davison et al. 2014). It is therefore possible that even higher doses and concentrations of nitisinone could lead to a complete normalization, which would correspond to a decrease in u-HGA from baseline of 99.98–100.00% in individual patients.
Assuming that the reduction in u-HGA reflects the degree of inhibition of HPPD, the results indicate that the enzyme is almost completely inhibited at a nitisinone concentration of 3 μmol/L. Nitisinone is extensively bound to serum albumin with a free fraction of about 4.5% at normal serum albumin concentrations (unpublished data). Thus, a total concentration of 3 μmol/L corresponds to an unbound concentration of approximately 135 nmol/L. This is in agreement with the maximum inhibition of HPPD by nitisinone seen in vitro (Ellis et al. 1995).
The results indicate a dose–response relationship between s-HGA and nitisinone, as the number of patients with s-HGA below the LLOQ increased with dose. An analysis of the relationship between Cav for s-HGA and nitisinone concentrations could not be performed due to the nonquantifiable s-HGA values. The decrease in s-HGA, from baseline to Week 4, which could be estimated for the 1-mg dose (approximately 88%), is in line with the previously reported decrease in u-HGA24 for that dose (Ranganath et al. 2014).
The s-HGA profile at baseline shows the expected increase with feeding due to a continuous supply of tyrosine. The circulating concentrations of both HGA and tyrosine show a fall after the last meal and true fasting late in the evening and night, reaching a nadir prior to feeding in the morning.
At baseline, serum tyrosine was normal in the AKU patients, despite the defect in HGD. Normal, non-AKU subjects had fasting serum tyrosine concentrations up to 88 μmol/L (Davison et al. 2014). The maximum daily average in AKU patients at baseline was 82 μmol/L.
In patients with AKU, renal clearance of HGA is 4–5 times creatinine clearance suggesting active secretion of HGA (Lustberg et al. 1969). An ongoing study of proximal tyrosine metabolites in samples from SONIA 1 may throw more light on this issue, but such data are as yet unavailable. The importance of a normal renal function has also been illustrated in an AKU patient with renal failure. He had exacerbated ochronosis and s-HGA levels about twice those of other AKU patients. After a renal transplant, the levels decreased to those normally seen in AKU, and u-HGA increased by 2–3 g per day (Introne et al. 2002).
Inhibiting HPPD leads to pronounced tyrosinemia even at low nitisinone doses. High serum concentrations of tyrosine are known to cause eye lesions in some patients due to high concentrations in the aqueous humor (Hanhart 1947; Lock et al. 2014). In the treatment of HT-1 with nitisinone, it is therefore recommended that serum tyrosine be kept below 400–500 μmol/L, by using a diet low in tyrosine and phenylalanine (Mayorandan et al. 2014; SmPC for Orfadin). Even the lowest dose in our study, 1 mg daily, resulted in tyrosine levels above this limit in every patient, indicating that diet restrictions may be required also if treating AKU patients with nitisinone, at least if such patients would develop eye symptoms due to tyrosinemia.
In conclusion, nitisinone exhibits dose-proportional pharmacokinetics in the studied dosage interval. Urinary excretion of HGA decreases in a concentration-dependent manner, while the increase in tyrosine is less clearly related to nitisinone concentrations.
Electronic Supplementary Material
Figure S1. Mean (SD) steady-state serum concentrations of nitisinone in AKU patients (N = 8 per dose group)
Figure S2. Mean (SD) serum concentrations of HGA and tyrosine in AKU patients before treatment with nitisinone (N = 32)
Acknowledgements
We wish to thank Jean Devine and Jeannette Usher in the Clinical Biochemistry and Metabolic Medicine for the handling of the serum and urine samples. We also want to thank Helen Bygott, Emily Luangrath-Nicholson, Richard Fitzgerald, and Asad Ullah at the Clinical Trials Units in Royal Liverpool University Hospital and Oľga Lukačová, Eva Vrtíková, and Vanda Mlynariková in the National Institute of Rheumatic Disease in Piešťany for the diligence shown in carrying out the study.
This study was part of the DevelopAKUre program, which received funding from the European Commission 7th Framework Program (FP7).
Synopsis (Take-Home Message)
In patients with alkaptonuria, nitisinone decreases urinary excretion of HGA in a concentration-dependent manner, while the increase in serum tyrosine is less clearly related to nitisinone concentrations.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (6).
Informed consent was obtained from all patients before being included in the study.
Animal rights: Not applicable for this clinical study.
The study EudraCT number is 2012-005340-24 and is registered at ClinicalTrials.gov with number NCTO1828463 and the title “Dose Response Study of Nitisinone in Alkaptonuria (SONIA1).”
Conflict of Interest
B Olsson and J Szamosi are Sobi employees and shareholders. All other authors declare that they have no conflict of interest.
Contributors
BO, LRR, AKH, TFC, JR contributed to the study design.
LRR, JR undertook medical procedures.
ATH, AMA developed analytical methods and analyzed study samples.
TFC, EEP, JS, BO contributed to the statistical analyses including PK calculations.
BO drafted the first version of the manuscript.
All authors contributed to the interpretation of data and writing and revision of the manuscript.
All authors approved the manuscript for publication.
Footnotes
Competing interests: None declared
Contributor Information
Birgitta Olsson, Email: birgitta.olsson@sobi.com.
Collaborators: Johannes Zschocke
References
- Anikster Y, Nyhan WL, Gahl WA. NTBC and alkaptonuria. Am J Hum Genet. 1998;63:920–921. doi: 10.1086/302027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AS, Milan AM, Hughes AT, et al. Serum concentrations and urinary excretion of homogentisic acid and tyrosine in normal subjects. Clin Chem Lab Med. 2014 doi: 10.1515/cclm-2014-0668. [DOI] [PubMed] [Google Scholar]
- Ellis MK, Whitfield AC, Gowans LA, et al. Inhibition of 4-hydroxyphenylpyruvate dioxygenase by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione and 2-(2-chloro-4-methanesulfonylbenzoyl)-cyclohexane-1,3-dione. Toxicol Appl Pharmacol. 1995;133:12–19. doi: 10.1006/taap.1995.1121. [DOI] [PubMed] [Google Scholar]
- Garrod AE. About alkaptonuria. Med Chir Trans. 1902;85:69–78. [PMC free article] [PubMed] [Google Scholar]
- Gough K, Hutchison M, Keene O, et al. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmaceutics UK joint working party. Drug Inf J. 1995;29:1039–1048. [Google Scholar]
- Hall MG, Wilks MF, Provan WM, et al. Pharmacokinetics and pharmacodynamics of NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione, inhibitors of 4-hydroxyphenyl pyruvate dioxygenase (HPPD) following a single dose to healthy male volunteers. Br J Clin Pharmacol. 2001;52:169–177. doi: 10.1046/j.0306-5251.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanhart E. Neue sonderformen von keratosis palmo-plantaris, u.a. eine regelmässig-dominante mit systematisierten lipomen, ferner 2 einfach-rezessive mit schwachsinn und z.T. mit hornhautveränderungen des auges (ektodermalsyndrom) Dermatologica. 1947;94:286–308. doi: 10.1159/000255917. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Milan AM, Christensen P et al (2014) Urine homogentisic acid and tyrosine: simultaneous analysis by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 963:106–112. doi:10.1016/j.jchromb.2014.06.002 [DOI] [PubMed]
- Hughes AT, Milan AM, Davison AS et al (2015) Serum markers in alkaptonuria: simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann Clin Biochem pii:0004563215571969 [DOI] [PubMed]
- Introne WJ, Phornphutkul C, Bernardini I, et al. Exacerbation of the ochronosis of alkaptonuria due to renal insufficiency and improvement after renal transplantation. Mol Genet Metab. 2002;77:136–142. doi: 10.1016/S1096-7192(02)00121-X. [DOI] [PubMed] [Google Scholar]
- Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103:307–314. doi: 10.1016/j.ymgme.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt S, Holme E, Lock EA, et al. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- Lock E, Ranganath LR, Timmis O. The role of nitisinone in tyrosine pathway disorders. Curr Rheumatol Rep. 2014;16:457. doi: 10.1007/s11926-014-0457-0. [DOI] [PubMed] [Google Scholar]
- Lustberg TJ, Schulman JD, Seegmiller JE (1969) Metabolic fate of homogentisic acid-1–14 C (HGA) in alkaptonuria and effectiveness of ascorbic acid in preventing experimental ochronosis. Arthritis Rheum 12:678
- Mayorandan S, Meyer U, Gokcay G, et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014;9:107. doi: 10.1186/s13023-014-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WM, La Du BN, Bunim JJ. Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: review of world literature (1584–1962) Am J Med. 1963;34:813–838. doi: 10.1016/0002-9343(63)90089-5. [DOI] [Google Scholar]
- Preston AJ, Keenan CM, Sutherland H, et al. Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann Rheum Dis. 2013;73(1):284–289. doi: 10.1136/annrheumdis-2012-202878. [DOI] [PubMed] [Google Scholar]
- Ranganath LR, Jarvis JC, Gallagher JA. Recent advances in management of alkaptonuria (invited review; best practice article) J Clin Pathol. 2013;66:367–373. doi: 10.1136/jclinpath-2012-200877. [DOI] [PubMed] [Google Scholar]
- Ranganath LR, Milan AM, Hughes AT (2014) Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicenter, randomized, open-label, no-treatment controlled, parallel-group, dose–response study to investigate the effect of once daily nitisinone on 24-hour urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis 1–6. doi:10.1136/annrheumdis-2014-206033 [DOI] [PubMed]
- SmPC for Orfadin capsules. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000555/WC500049195.pdf
- Suzuki Y, Oda K, Yoshikawa Y, et al. A novel therapeutic trial of homogentisic aciduria in a murine model of alkaptonuria. J Hum Genet. 1999;44:79–84. doi: 10.1007/s100380050114. [DOI] [PubMed] [Google Scholar]
- Zannoni VG, Lomtevas N, Goldfinger S. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim Biophys Acta. 1969;177:94–105. doi: 10.1016/0304-4165(69)90068-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean (SD) steady-state serum concentrations of nitisinone in AKU patients (N = 8 per dose group)
Figure S2. Mean (SD) serum concentrations of HGA and tyrosine in AKU patients before treatment with nitisinone (N = 32)


