Abstract
Study Objectives:
To investigate whether low levels of physical activity were associated with an increased occurrence of obstructive sleep apnea (OSA), OSA-related symptoms, and cardiometabolic risk.
Methods:
A case-control study design was used. OSA cases were patients referred to a sleep clinic for suspected OSA (n = 2,340). Controls comprised participants from the Busselton community (n = 1,931). Exercise and occupational activity were derived from questionnaire data. Associations were modelled using logistic and linear regression and adjusted for confounders.
Results:
In comparison with moderate exercise, the high, low, and nil exercise groups had an odds ratio (OR) for moderate-severe OSA of 0.6 (95% CI 0.5–0.8), 1.6 (95% CI 1.2–2.0), and 2.7 (95% CI 1.9–3.7), respectively. Relative to men in heavy activity occupations, men in medium, light and sedentary occupations had an OR for moderate-severe OSA of 1.7 (95% CI 1.1–2.5), 2.1 (95% CI 1.4–3.2), and 1.8 (95% CI 1.2–2.8), respectively. Relative to women in medium activity occupations, women in light and sedentary occupations had an OR for moderate-severe OSA of 4.2 (95% CI 2.6–7.2) and 3.5 (2.0–6.0). OSA patients who adequately exercised had lower: levels of doctor-diagnosed depression (p = 0.047); symptoms of fatigue (p < 0.0001); systolic (p = 0.015) and diastolic blood pressure (p = 0.015); and C-reactive protein (CRP) (p = 0.003).
Conclusions:
Low levels of physical activity were associated with moderate-severe OSA. Exercise in individuals with OSA is associated with lower levels of depression, fatigue, blood pressure and CRP.
Citation:
Simpson L, McArdle N, Eastwood PR, Ward KL, Cooper MN, Wilson AC, Hillman DR, Palmer LJ, Mukherjee S. Physical inactivity is associated with moderate-severe obstructive sleep apnea. J Clin Sleep Med 2015;11(10):1091–1099.
Keywords: weight, sleep disordered breathing, cardio metabolic, occupation, exercise
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction during sleep.1 OSA is prevalent to a clinically significant degree in at least 2% of women and 4% of men.2 OSA leads to impaired quality of life, and is associated with an increased risk of hypertension, myocardial infarction, stroke, heart failure, metabolic syndrome, depression, and motor vehicle accidents.3–5 Studies examining the effect of exercise on OSA demonstrate that the incidence of OSA is higher among those who rarely undertake vigorous activity.6 Sedentary activities have been recognized to contribute to the risk of type 2 diabetes and cardiovascular disease,7 but their impact on OSA is yet to be examined.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Physical inactivity is increasingly recognised as a health risk factor but it is largely unknown whether such inactivity is associated with OSA and, if so, the health consequences for patients with this condition.
Study Impact: Vigorous exercise may protect against the development of moderate-severe OSA while low or no exercise could contribute to its occurrence. Health benefits of exercise for individuals with OSA appear to include lower levels of depression, fatigue, blood pressure, and the inflammatory biomarker CRP.
Exercise may be a modifiable factor that reduces the risk of OSA. Adequate exercise is defined by US and Australian guidelines as moderate intensity physical activity for at least 150 minutes each week.8,9 Longitudinal data suggests reduced incidence of OSA in those who exercise regularly.6 Two cross-sectional studies have reported lower odds of moderate-severe OSA in those with high exercise levels.10,11 To date, most exercise studies have not captured range of exercise intensities, preferring to dichotomize samples by comparing the effect of vigorous exercise with all other activity levels taken as baseline.6,11 Only one community sample of OSA subjects has defined nil exercise as a separate category from low levels of exercise where, using nil exercise as baseline, there were progressive reduction in odds of moderate-severe OSA across low, moderate, and vigorous exercise categories.10 Further, the investigation of the effect of exercise on OSA related symptoms such as excessive daytime sleepiness, fatigue, and depression is warranted.
Time spent in exercise, regardless of vigor, usually represents a small proportion of the total hours in a week.12 Low levels of activity at other times may independently increase cardio metabolic risk.7,13–15 Sitting, or states of low energy expenditure (< 1.5 METs),16 have been postulated to adversely affect lipoprotein metabolism and other metabolic processes.14,17 Observational studies have shown increased odds of abnormal glucose metabolism and metabolic syndrome in adults who watch more TV—a finding which was independent of whether the adults achieved recommended guidelines for exercise.7 Longitudinal measurement of time spent watching TV and time in automobiles suggests that these inactive behaviors increase cardiovascular disease-related mortality by up to 80%.7
Inactive occupations are increasingly prevalent. In 1960, 50% per cent of US workers undertook at least moderate physical intensity activities.12 By 2006 this had decreased to 20% of workers.12 A Swedish study examined the relationship between occupation and risk of hospitalization due to OSA,18 hypothesizing that socioeconomic status would affect hospitalization rates. Although physical activity was not directly measured in that study, men in occupations with high activity (e.g., farmers, gardeners, wood workers) had lower standardized incidence ratios for hospitalization for OSA.18 Conversely, some low activity occupations (e.g., administrator/manager, sales agents, shop assistants, drivers) demonstrated higher standardized incidence ratios of hospitalization for OSA.18 Thus occupation may characterize sedentary behavior and also be a determinant of OSA.
The current study tested the hypothesis that low levels of occupational activity and low levels of exercise activity would be associated with: (1) the presence of moderate-severe OSA; (2) increased symptoms of excessive daytime sleepiness, fatigue, and depression in OSA; and (3) increased biomarkers of cardiometabolic risk in OSA.
METHODS
Study Participants
OSA cases comprised participants in the Western Australian Sleep Health Study (WASHS) recruited between January 2006 and June 2009.19 Obstructive sleep apnea severity was defined by the AHI1 derived from laboratory-based overnight polysomnography ([PSG] Profusion 2, Compumedics, VIC, Australia). Apnea-hypopnea index was defined as the number of apnea plus hypopnea events per hour of sleep. Patients were categorized as mild OSA: 5.00–14.99 events/h; moderate OSA: 15.00–29.99 events/h; or severe OSA: ≥ 30.00 events/h. Patients with AHI < 5 events/h and those on therapy were excluded. Patients with prior diagnosis of any comorbidity likely to cause low activity levels (e.g., other sleep disorders, lung disease causing moderate-severe ventilatory impairment, heart disease with impaired exercise tolerance and neurode-generative disorders) were also excluded.
The control sample was retrospectively generated from the 2005–2007 crosssectional component of the Busselton Health Study (BHS): a sex- and age-stratified random sample (n = 2,935) of the 2005 Busselton electoral roll.20 The Busselton population is a subset of the catchment region of the WASHS. Data from the Australian Bureau of Statistics suggest that Busselton was representative of Western Australia in terms of age and sex distribution. There were minor underlying differences in the distribution of occupations, with Busselton having slightly more trade and labor-based occupations, while Perth, the main population center of Western Australia, had more professional and administrative occupations (see Table S1, supplemental material). Laboratory PSGs were not performed on controls. However, a subset with low risk of moderate-severe OSA was identified by the criteria: self-reported snoring < 12 times per month or the absence of hypertension (measured using manual sphygmomanometer). These criteria had 92% post-test probability of AHI < 15 events/h in a general population sample21 but did not alter the occupation or exercise profile of the control sample (see Table S2, supplemental material).
Study protocols and informed consent documents were approved by the Sir Charles Gairdner Hospital and University of Western Australia Human Research Ethics Committees. Participation was voluntary. Data were de-identified prior to analysis.
Measurements and Parameters
In cases the symptom profile were derived from questionnaire responses. Excessive daytime sleepiness was defined as an Epworth Sleepiness Score (ESS) > 10.22 Fatigue after waking was measured using the following frequency scale (never, rarely: < 1 day/week, sometimes: 1–2 days/week, frequently: 3–4 days/week, always: 5–7 days/week). Self-report of doctor diagnosed depression (yes/no) was recorded. Smokers were categorized as never, former or current smokers, and pack-years calculated. Systolic and diastolic blood pressure (mm Hg), height (m), weight (kg), and neck circumference (cm) were measured directly according to standard procedures by a respiratory physician. Levels of C-reactive protein ([CRP] mg/L) were measured in just under half of all OSA cases; 524 twelve-hour fasting blood samples were available to assess cholesterol, high-density lipoprotein (HDL), low-density lipo-protein (LDL), and triglycerides (mmol/L).
Alcohol exposure was measured differently in cases and controls. Cases recorded frequency scales for the number of days per week they consumed alcohol (0–7) and the typical number of standard (10 g alcohol) drinks per night consumed (1–2, 3–5, 6–9, 10+). The mean number of alcoholic drinks per night was used to calculate total standard drinks per week. Controls were asked to recall standard drinks per week for different beverage types (light beer, mid beer, white wine, etc.) and these were summed.
Exercise activity was measured using the Active Australia Survey.23 Self-report of total weekly time spent in walking, moderate, and vigorous physical activity was recorded. Metabolic equivalents (METS)16 is a unit of resting metabolic rate equivalent to 3.5 mL of oxygen uptake per kilogram of body weight per minute (kcal/kg−1/min−1). MET minutes of exercise were calculated as intensity adjusted total time in minutes spent in activity. Specifically, MET minutes per week = (3 × total minutes walking) + (4 × total minutes of moderate exercise) + (7.5 × total minutes of vigorous exercise). This formula was used to create 4 exercise categories (nil, low, moderate, and high) that were derived from published thresholds.16
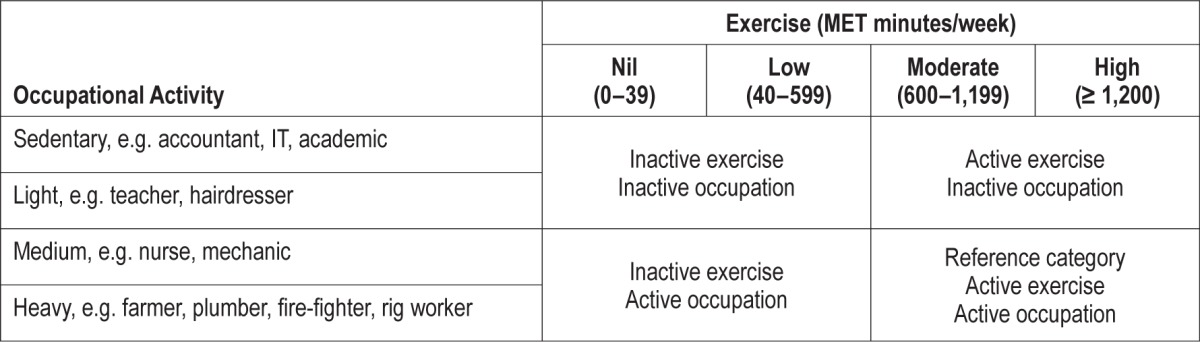
Occupational activity categories were assigned using the US Department of Labor Dictionary of Occupational Titles, where the usual activities of each occupation title (such as standing, walking, sitting, lifting, carrying, pushing, pulling, and manipulation of controls) have been assessed by an occupational analyst. Job-title derived occupational activity has shown good agreement with accelerometry-validated physical activity questionnaires.24 Questionnaire response to job title was matched to the activity category for that same job title in the dictionary, creating 4 occupational categories (sedentary, light, medium, and heavy). Those who listed their occupation as home duties, retired, unemployed, or students were not included in analyses. Table 1 shows the 4 exercise categories and occupational categories, as well as the “combination” categories used.
Table 1.
Summary of methods to characterize activity measures.
Statistical Analysis
Categorical variables were described using cross tabulations. Normally distributed continuous variables were described using mean and standard deviation and variables with skewed distribution using the median and interquartile range (IQR). Univariate differences between cases and controls were tested using either χ2, Student t test, or a Mann-Whitney U test, depending upon the distribution of the independent variable being compared.
The study involved 4 separate analytic investigations, all employing generalized linear modelling, but different sample groupings. Firstly, when comparing OSA cases to controls, cases with an AHI < 15 events/h were excluded to remove potential overlap with undiagnosed mild OSA in the control group. Consequently, the ORs from logistic regression of case and control data refer to odds of having moderate-severe OSA (AHI ≥ 15 events/h). Secondly, the relationship between activity levels and severity of OSA were examined using natural log transformed AHI as the outcome. This analysis used only cases of OSA and the full spectrum of severity was considered, such that all cases with an AHI ≥ 5 events/h were included. Thirdly, to investigate inactivity in relation to OSA symptom profile all cases with AHI ≥ 5 events/h were dichotomized into either active or inactive and compared using logistic regression. Fourthly, to examine the effect of exercise activity on cardiometabolic biomarkers in all cases with OSA (AHI ≥ 5 events/h), each biomarker was used as an outcome variable in a linear regression and the estimated marginal means reported after adjustment for age, sex, ethnicity, years of education, alcohol consumption, smoking (pack-years), and BMI.
All regression analyses were multivariate and employed a forwards variable entry method. Interaction terms between alcohol and tobacco intake were considered as were gender interactions with exercise and occupational activity categories. Interactions were retained where analysis of deviance suggested improvement in the model. Where there was evidence of gender interaction with activity or occupation, sex-stratified analyses were reported. Data were analyzed using R version 3.0.1 (2013, The R Foundation for Statistical Computing), and PASW Statistics GradPack17.0 (2009).
RESULTS
Characteristics of Cases and Controls
Table 2 summarizes characteristics of the cases and control samples. The full case sample comprised 2,340 participants with OSA (63% male). Men had more severe OSA with a median AHI of 31.7 events/h of sleep (IQR 17.7–55.0) compared with a median AHI of 21.6 events/h of sleep (IQR 12.3–37.4) in women (p < 0.001). Moderate or severe OSA was present in 76% of the case sample (n = 1,769; of which 68% were men). Sleep efficiency, ESS, proportion reporting depression, and fatigue symptoms, exercise, and occupational activity did not differ statistically between all cases and those with moderate-severe OSA (AHI ≥ 15 events/h).
Table 2.
Summary of OSA cases and controls.
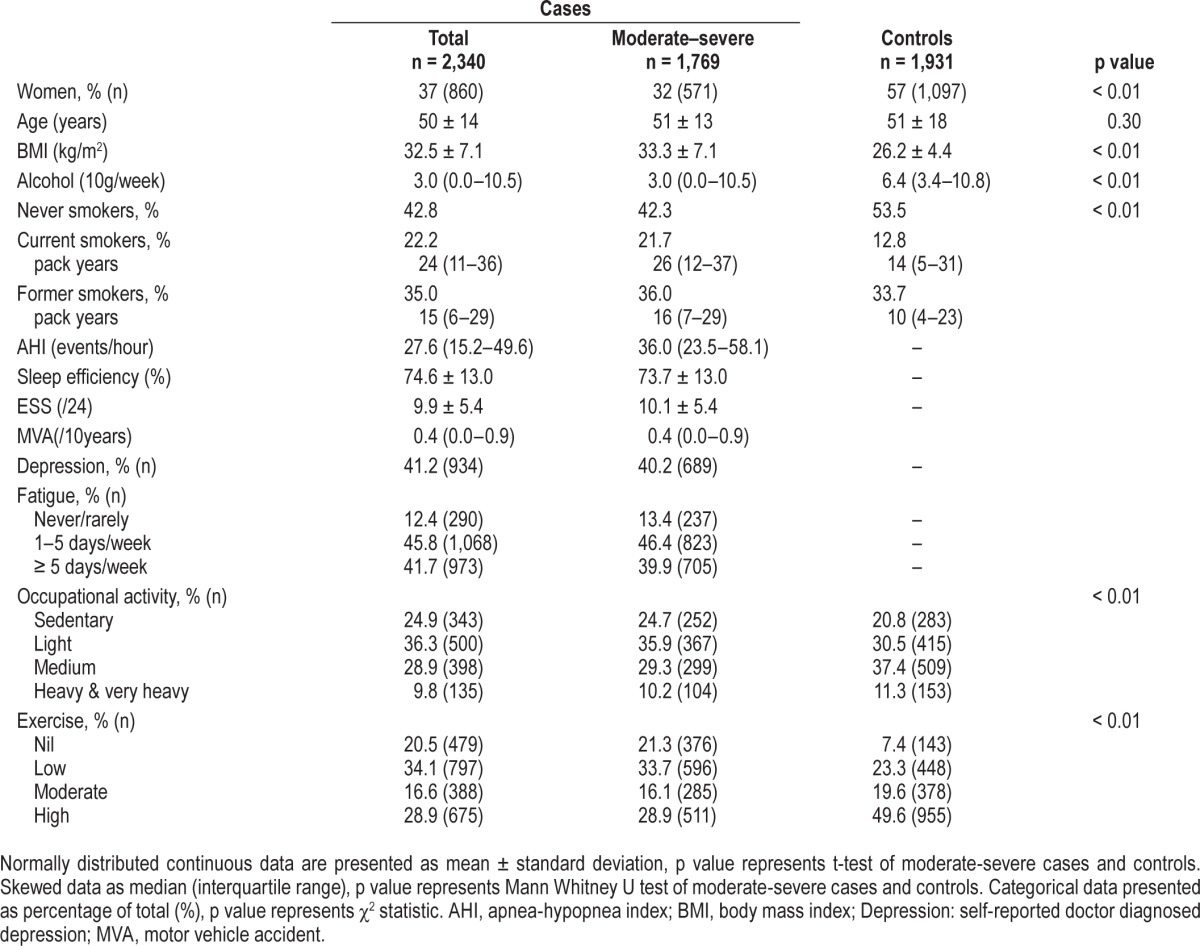
Case and control comparisons were made using cases with AHI ≥ 15 events/h. Body mass index was a major point of difference between cases and controls (Table 2), with mean BMI in moderate-severe cases in the obese range (31.6 ± 6.0 kg/m2 in men and 33.9 ± 8.6 kg/m2 in women), whereas in controls mean BMI was in the overweight range (27.5 ± 3.9 kg/m2 in men and 26.6 ± 5.3 kg/m2 in women). The moderate-severe cases had higher rates of morbid obesity than the controls. Among cases, 9.1% of men and 19.8% of women were morbidly obese (BMI ≥ 40 kg/m2), compared to less than 1% of controls. Thus moderate-severe cases were more obese than general population controls and BMI was a significant covariate in all multivariate models. The case sample had greater tobacco consumption, while the control sample had greater alcohol in-take (Table 2). Although age was similar, dispersion around the mean was greater in controls (Table 2). As a result, in all multivariate case-control analyses the effect of age, sex, BMI, smoking, and alcohol were investigated using forwards step regression and retained where an association with case-control status were indicated by a p value of < 0.05.
Activity and Odds of Moderate-Severe Obstructive Sleep Apnea
Exercise was lower among the moderate-severe OSA cases, in comparison with controls (Table 2). Adequate exercise (≥ 150 min/week of moderate exercise) was reported in 48% of male and 42% of female moderate-severe OSA cases. By comparison, in the controls 69% of males and 66% of females reported this level of activity. Nil exercise was undertaken by 22% of men with moderate-severe OSA and by 9% of men in the control group (p < 0.001). In women, this was 21% of moderate-severe OSA cases versus 7% of controls (p < 0.001).
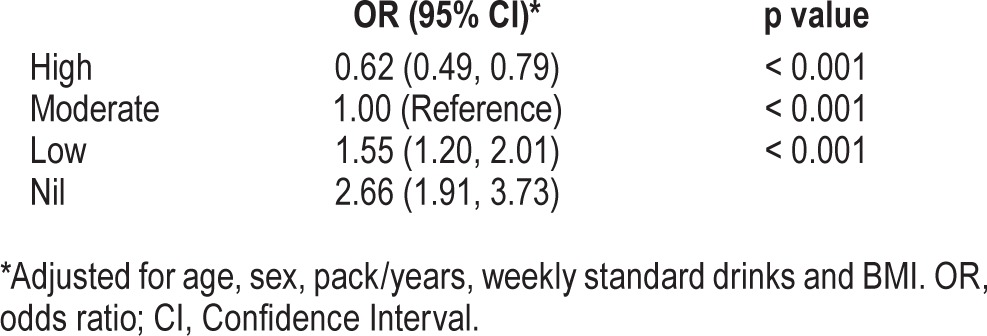
Table 3 shows the odds of moderate-severe OSA among different exercise categories. Moderate exercise is the reference group. There was a clear relationship between exercise and odds of OSA, which persisted after adjustment for confounders (Table 3). Those reporting high levels exercise had reduced odds of moderate-severe OSA (OR = 0.6, 95% CI 0.5–0.8), while low and nil exercise were associated with 1.6 and 2.7 odds of moderate-severe OSA.
Table 3.
Association of exercise with odds of moderate-severe obstructive sleep apnea.
Occupational activity differed between moderate-severe cases and controls (Table 1). Sedentary work was undertaken in a similar proportion of case and control men (23% versus 19%) but a greater proportion of cases were engaged in work of light activity (33% versus 27% of controls). Thus, men with moderate-severe OSA were more likely to be employed in light activity occupations than general population controls (p < 0.001). In women, sedentary occupations were more common in moderate-severe OSA cases than controls (31% versus 21%; p < 0.001).
Logistic regression modelling of the association between case-control status and occupational activity suggested that there was a significant gender interaction between occupational activity and gender (likelihood-ratio χ2 = 13.3, DF = 3, p = 0.004) and therefore further analyses were sex-stratified. In men all occupational activity categories carried higher odds of moderate-severe OSA in comparison with the heavy activity category. Medium activity occupations were associated with a 1.67-fold increase in the odds of developing moderate-severe OSA, while light and sedentary occupations were associated with a 2.1- and 1.8-fold increase (Table 4). In women the medium activity occupation group were used as the reference category due to low numbers (n = 35) in the heavy category. No statistical difference was detected between the medium and heavy category in women, probably in consequence of low power. However, light and sedentary activity occupations were associated with a 4.2- and 3.5-fold increase in the odds of moderate-severe OSA (Table 4). These findings were signifi-cant after adjusting for age and BMI.
Table 4.
Association of occupational activity with odds of moderate-severe obstructive sleep apnea.
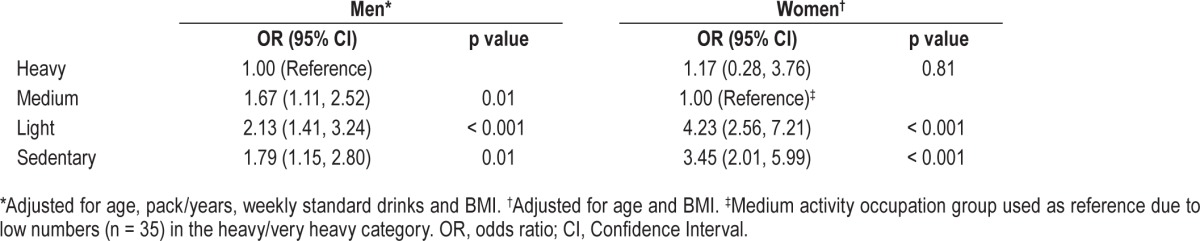
Figure 1 shows the sex stratified ORs for moderate-severe OSA for the combined categories of occupational activity and exercise. The reference group are those who were active in both occupation and exercise. Men who achieved the recommended national guidelines for physical activity but had an inactive occupation had significantly higher odds of having OSA (OR = 1.68, 95% CI: 1.2–2.4), but the magnitude of risk was lower than the other 2 groups who did not engage in exercise. Men who had active occupations but did not exercise had an OR of 3.18, which was comparable to the least active men (inactive in both occupation and exercise) who had an OR of 3.68. These estimates were adjusted for the effect of age, smoking, alcohol intake, and BMI.
Figure 1. The adjusted odds (and 95% confidence intervals) for moderate-severe OSA in men and women based on activity in occupation and exercise.
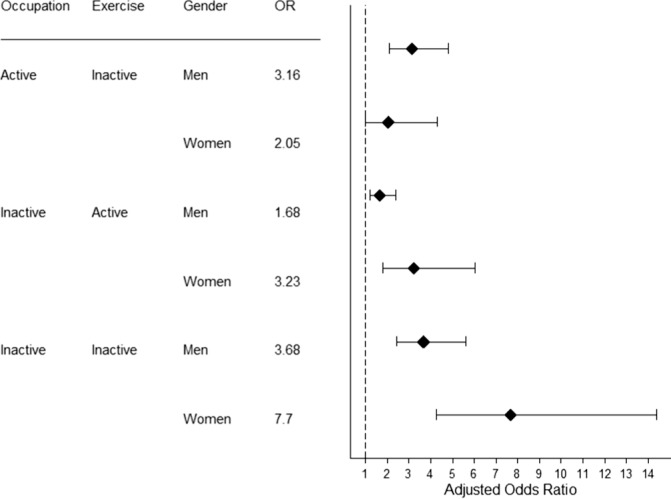
The reference category is active in both occupation and exercise. Estimates are shown after adjustment for age, sex, BMI, alcohol consumption and smoking.
Similar to the analyses of men, women who were inactive in both occupation and exercise had the highest odds of moderate-severe OSA (OR = 7.70, 95% CI: 4.3–14.4). However, in women, occupational activity appeared to modulate risk. Women in an active occupation who did not exercise during recreation did not have significantly higher odds of moderate-severe OSA than those in an active occupation who did exercise (Figure 1). In contrast, women in an inactive occupation who undertook moderate or vigorous physical exercise had an OR of 3.2 (95% CI: 1.8–6.0).
Activity and Severity of Obstructive Sleep Apnea
To examine whether activity was related to log-transformed AHI as a continuous measure of OSA severity, all OSA cases with an AHI > 5 events/h were included in the model. The demographics of this sample are outlined in Table 2 under the column headed “Total” for cases. There was no evidence of a relationship between exercise and log transformed AHI in regression analyses. Similarly, there were no relationships between occupational activity and log-transformed AHI. Further, no statistically significant associations were found when examining the relationships between the combined categories of occupation and exercise activity and severity of OSA.
Activity and Symptom Profile in Obstructive Sleep Apnea Cases
Symptoms reported by OSA cases differed depending upon levels of activity. Fatigue was a common complaint of OSA cases (Table 2), particularly among those who did not engage in any exercise. Multivariate logistic regression suggested that doing no exercise was associated with increased odds (OR = 1.4; 95% CI 1.1, 1.7) of reporting fatigue every day after adjusting for age, AHI, BMI, and sex. There was no association between occupation activity and fatigue symptoms.
Doctor diagnosed depression was common in OSA cases (Table 2). Depression was reported in a greater proportion of cases who did low or nil exercise in comparison with those who did any sort of exercise (57% versus 53%: χ2(DF = 1) = 2.9, p = 0.047). Similarly, a higher proportion of depression was reported in OSA patients with a sedentary or light activity occupations in comparison with those where occupational activity was medium or greater (43% versus 31%: χ2(DF = 1) = 16.2, p < 0.0001). Multivariate logistic regression comparing the most active cases (exercisers with an active occupation) with the least active (non-exercisers with an inactive occupation) revealed an association between low activity levels and depression after adjusting for age, AHI, BMI, and sex (OR = 1.9; 95% CI 1.3, 2.8). Self-reported perception of sleepiness did not differ among the different exercise or occupation categories.
Activity and Cardiometabolic Risk Profile in Obstructive Sleep Apnea Cases
Table 5 presents the estimated marginal means for cardio-metabolic risk factors for occupational activity and exercise (dichotomized into those reporting at least moderate exercise and those reporting less than moderate exercise). The models were adjusted for age, sex, ethnicity, years of education, alcohol consumption, smoking, log-transformed AHI, and BMI. BMI was not used as a covariate when calculating the estimated marginal means of weight, neck circumference, and BMI. The estimated marginal means for occupational activity were adjusted for exercise and, likewise, the estimated marginal means for exercise were adjusted for occupational activity.
Table 5.
The independent association of cardiometabolic risk factors with exercise and occupational activity.
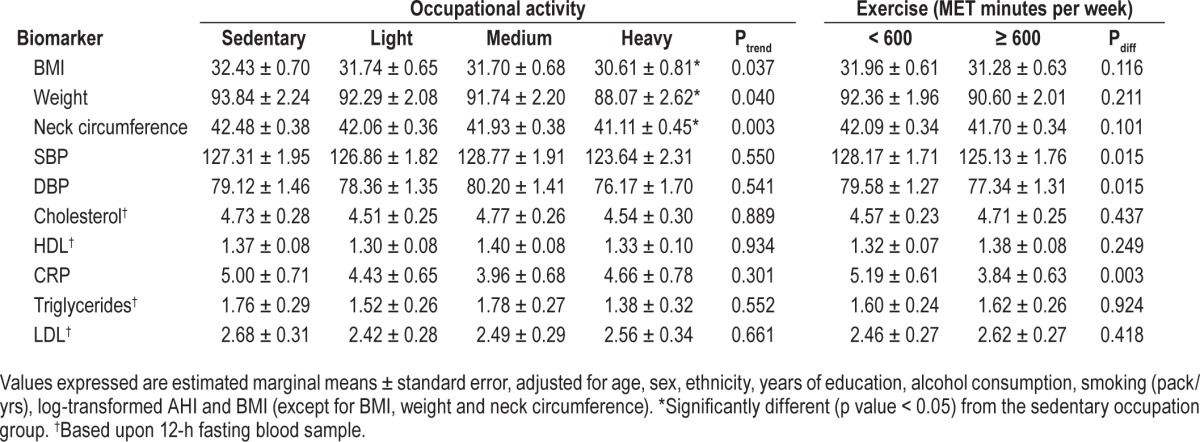
BMI was not observed to differ between those engaging in at least moderate exercise and those not. However, a trend test for mean BMI across the categorical levels of occupational activity (p = 0.037) would suggest that increased physical activity at work was associated with decreased BMI. Estimated mean BMI was significantly lower (p = 0.026) in persons with heavy activity occupations in comparison with those with a sedentary occupation. This relationship was observed in the other measures of adiposity: weight and neck circumference, such that those in physically active occupations were estimated to be almost 6 kg lighter and have 1.4 cm smaller necks (Table 5). There were no comparative differences in the estimated marginal means across the occupational activity categories for systolic and diastolic blood pressure, cholesterol, HDL, triglycerides, or LDL. Exercise was observed to modulate systolic and diastolic blood pressure and levels of CRP (Table 5). Systolic blood pressure was just over 3 mm Hg lower and diastolic blood pressure was 3 mm Hg lower in those who undertook at least moderate levels of weekly exercise. Levels of CRP were significantly lower in those who undertook at least moderate levels of weekly exercise (β −1.35, SE 0.44, p = 0.003).
DISCUSSION
The current study has shown that high levels of occupational activity and exercise were associated with reduced odds of moderate-severe OSA in a large, wellcharacterized, cross-sectional case-control design. Sex-specific relationships between occupation, exercise and moderate-severe OSA were observed. In men, exercise reduced the odds of having moderate-severe OSA, whereas in women, occupational activity reduced the odds of having moderate-severe OSA. These associations were independent of BMI, suggesting that the relationships between activity and OSA risk are not simply explained by the effect of activity on weight. The study also suggests that a lack of exercise activity in those with OSA is associated with fatigue, depression, higher blood pressure, and higher levels of the inflammatory biomarker CRP.
These findings are consistent with those of the Sleep Heart Health Study, which found that three hours of moderately vigorous or vigorous physical activity was associated with an adjusted OR of 0.80 (95% CI, 0.66–0.96) for moderate-severe OSA.11 However, unlike the Sleep Heart Health Study,23 the present study examined occupational activity and exercise, employed a single validated exercise activity questionnaire, and had a larger OSA group. As a result, the present study was able to define nil and low exercise categories. Both of these categories were associated with increased odds of moderate-severe OSA in comparison to the group who undertook recommended levels of exercise activity (moderate). Longitudinal data from the Wisconsin Sleep Cohort Study suggests that decreasing exercise results in worsening of AHI.6 Using nil exercise as the baseline, the present study showed a progressive reduction in odds of moderate-severe OSA with increasing exercise. The OR estimates were not all statistically significant after adjustment for BMI; however, trend tests across these categories were significant. Additionally, a statistically significant trend, which was independent of body habitus, was observed between decreasing mean AHI and increasing hours per week of exercise. Consistent with the findings of this study, previous large community-based samples have reported an association between exercise and reduced risk of OSA. In some of these previous findings, such an association did not persist after accounting for body habitus,6,11 while in others an association remained.10 By comparing a large group of moderate and severe OSA cases to a community sample with low risk of moderate-severe OSA the present study revealed a strong association between OSA and exercise that was independent of body habitus.
This is the first study to report that inactivity during occupation is associated with an increased risk of moderate-severe OSA, independent of the effect of BMI and self-reported exercise activity. Although inactivity in both occupation and exercise were associated with the greatest odds of moderate-severe OSA in both men and women, there were sex differences. In men, a lack of exercise was associated with increased risk for OSA regardless of whether the occupation was active or inactive. In women, exercise only marginally modulated risk when occupation was inactive, and lack of exercise did not increase risk when occupation was active. The mechanisms behind sex differences on activity and disease risk are not clear, although other studies have also noted protective effects of vigorous exercise, particularly in men.11 Intervention studies using prescribed exercise suggest that exercise reduces OSA severity,25,26 and the absence of the protective effect of exercise may explain the mechanisms by which reduced activity causes sleep apnea. Exercise activity may positively modulate OSA by increase of upper airway tonus via the recruitment of upper airway muscles for breathing work or head stability.27,28 Studies examining the effect of physical activity on OSA have not measured muscle tonus,6,10,11 but instead have examined the mediating effect of obesity. Similarly, methods that have been developed to increase upper airway tonus for the treatment of OSA have not been proven effective. Nasal resistance, which can contribute to upper airway collapse during sleep, is decreased during exercise29; however, this effect is not persistent: endurance athletes and sedentary adults have similar nasal resistance at rest.30 In the current study, having an inactive occupation but actively doing exercise was associated with increased odds of moderate-severe OSA, suggesting that inactive work environments had an adverse effect that was not counterbalanced by exercise. Physical inactivity has been associated with adverse cardiometabolic risk,7,14,17,31 as has OSA. Fat distribution is implicated in upper airway stability: fat in the peripharyngeal area of the neck can directly compress the upper airway,32,33 and recumbent abdominal obesity is likely to be associated with increased cranial displacement of the diaphragm, decreasing longitudinal tracheal traction, and increasing propensity for upper airway collapse.34 Exercise, which produces a favourable distribution of fat, may protect against OSA; conversely, inactivity may promote OSA via unfavourable distribution of fat. Distribution of body fat can vary among individuals, despite no difference in BMI, and this has been associated with severity of OSA.35
Inactivity may exert distinct biological consequences which lead to OSA. For example, a sedentary state can lead to greater fluid accumulation in the legs.25 Redolfiet al. observed that fluid displacement from the legs into the neck increased neck circumference and the magnitude of displacement was proportional to the time spent sitting the previous day.36 This may contribute to upper airway collapsibility independent of body weight. OSA patients have an increased susceptibility to pharyngeal obstruction due to rostral fluid redistribution.37 The general health consequences of inactivity have only recently been recognized,17,31 and the mechanisms by which inactivity exert an influence on OSA require further research.
The available data do not rule out a complex relationship whereby OSA contributes to a reduction in activity, perhaps due to OSA symptoms such as sleepiness and fatigue. The cross-sectional nature of the current study limits inference regarding the direction of causality. Indeed, it may be bidirectional. CPAP therapy increased exercise tolerance in obese individuals with OSA,38 suggesting that OSA may exert a physiological influence that blunts capacity to engage in vigorous exercise activity. Conversely, longitudinal data suggest that exercise independently decreases the incidence of moderate-severe OSA.6
Despite finding that activity was associated with risk of having moderate-severe OSA, within all OSA cases (AHI ≥ 5 events/h) there was no evidence of an association between exercise or occupational activity after adjusting for BMI. This may be due to reliance upon AHI to characterize the severity of OSA. AHI is a non-linear, imperfect metric where apneas and hypopneas are given equal weight. However, in OSA cases inactivity were related to symptoms of fatigue and depression as well as higher blood pressure and higher levels of CRP. Other studies have observed lower levels of CRP in active subjects,39 and physical activity is a recognized modifier of depressive symptoms and blood pressure. Increased occupational activity related to lower measures of adiposity such as BMI, weight, and neck circumference. Taken together, these findings would suggest that sleep physicians should continue to encourage patients to exercise and reduce sedentary time, especially where there is comorbid symptomology of depression, fatigue, inflammation, hypertension, or obesity.
Limitations
There are several limitations to the current study. Estimates of exercise were restricted to recall of structured physical activity leaving activity from activities like housework and gardening unquantified. Similarly, occupational activity was derived from job title recorded in a questionnaire and therefore limited to current occupation, with no detail captured on weekly hours, work, or years spent in the occupation. Questionnaire-based methods, though economical and practical in large populations, may be prone to recall bias and lack quantifiable objectivity.40 Despite these limitations, the method used to quantify occupational activity has been previously validated.24 Moreover, the method is unlikely to be subject to systematic recall bias associated with the presence of OSA. Those who listed their occupation as “home duties,” along with those who were retired, unemployed or students were excluded. This methodology increased the accuracy of the activity scale but limited the generalizability of these findings to those in fiscal occupation. Men are more likely to be employed full time and engage in structured physical activity, and women, by contrast, to be engaged in greater proportions of part-time work and incidental physical activity related to the home and family.
The two study populations used for our analyses were not entirely contemporaneous.40 The 2006 census data indicate the Busselton community is representative of Western Australia with respect to sex and age distribution but more likely to have been born in Australia and married. With respect to occupation, Busselton residents were marginally more likely to be employed part-time, and there were a slightly higher proportion of technicians, trades workers and labourers; whereas in Western Australia generally there were a greater proportion of professionals, clerical, and administrative workers (Table S1). Differences in the distributions of types of occupations between the Busselton community and the general population of Western Australia were minor yet may have influenced the ORs generated from the comparison of OSA cases with BHS controls. Inferences about the impact of occupational activity come with this caveat.
In addition, it would have been desirable to have PSG on the control sample; however, such data were not available. Instead, the study was limited to the questionnaire-based method to screen out moderate-severe OSA. Our group has previously demonstrated that this method produces a post-test probability of AHI < 15 events/h of 92%,21 and the selection method was independent of exposure to occupational and exercise activity (Table S2); therefore, the use of questionnaire data to screen out undiagnosed cases did not alter the exposure profile of the control population. The preponderance of fatigue symptoms and obesity among cases are probably significant factors that led to their referral to the sleep center. Since obesity and fatigue may be both cause and result in poor exercise, and since this study is cross-sectional in design, this study cannot determine causality between exercise status and the outcome of OSA.
The availability of 12-hour fasting blood samples was available on only a subset of OSA cases and the results relating to the cardiometabolic biomarkers of cholesterol, LDL, HDL, and triglycerides therefore had reduced power to observe modest differences.
CONCLUSION
The findings of this study suggest a protective role for vigorous exercise and a contributory role for low or no exercise in the development of moderate-severe OSA. Occupational activity levels appeared to have a particular protective effect in women, whereas in men non occupation-related exercise seemed more protective, perhaps reflecting sex differences in the relative vigor applied to these pursuits. The finding of an association between low activity and OSA-related symptoms of fatigue and depression, suggests that increased physical activity might confer symptomatic relief where OSA exists. The study also suggests that in OSA cases exercise is associated with decreased blood pressure and lower levels of the inflammatory biomarker CRP and that occupational activity is associated with lower adiposity. These findings underline the important role of activity in on the overall health of OSA patients.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. McArdle has received research support from ResMed. Dr. Hillman has received research support from and is on the speakers' bureau for ResMed. The other authors have indicated no financial conflicts of interest. The West Australian Sleep Health Study was supported by Sir Charles Gairdner Hospital Research Foundation and the Hollywood Private Hospital Research Foundation. Dr. Simpson was supported by an Australian Postgraduate Award. Prof. Eastwood was supported by an Australian National Health and Medical Research Council Senior Research Fellowship (No. 1042341).
ACKNOWLEDGMENTS
The authors kindly acknowledge the participants of the Busselton Health Studies and the West Australian Sleep Health Study, the Sir Charles Gairdner Hospital Research Foundation, and the Hollywood Private Hospital Research Foundation.
ABBREVIATIONS
- CI
confidence interval
- GDM
gestational diabetes mellitus
- OR
odds ratio
- RLS
restless legs syndrome
- SE
standard error
SUPPLEMENTAL MATERIAL
Australian Bureau of Statistics 2006 census Quickstats data for sex, age, country of birth, and marital status for residents of Busselton and Western Australia.
Characteristics of the reference population and subgroups obtained using the screening questions: blood pressure and snoring frequency.
REFERENCES
- 1.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 4.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 6.Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125:485–90. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exercise and Sport Sciences Reviews. 2010;38:105–13. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Western Australian Department of Health. Fact sheet for the public: find thirty every day. 1999. May, [Last accessed February 1, 2011]. http://www.findthirtyeveryday.com.au/
- 9.Center for Disease Control and Prevention. How much physical activity do adults need? 2012. [Last accessed September 1, 2015]. http://www.cdc.gov/physicalactivity/everyone/guidelines/adults.html.
- 10.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 11.Quan SF, O'Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–57. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 12.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DM, Raynor HA, Ciccolo JT. A review of TV viewing and its association with health outcomes in adults. Am J Lifestyle Med. 2008;2:250–9. [Google Scholar]
- 14.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–67. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 15.Boyle T, Fritschi L, Heyworth J, Bull F. Long-term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol. 2011;173:1183–91. doi: 10.1093/aje/kwq513. [DOI] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551:673–82. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9:129–36. doi: 10.1016/j.sleep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee S, Hillman D, Lee J, et al. Cohort profile: the Western Australian Sleep Health Study. Sleep Breath. 2011;16:205–15. doi: 10.1007/s11325-011-0491-3. [DOI] [PubMed] [Google Scholar]
- 20.Busselton Population Medical Research Institute. The Busselton Health Study: surveying the people of Busselton for the health of the world since 1966 Busselton Population Medical Research Institute. 2011. [Last accessed September 1, 2015]. http://www.busseltonhealthstudy.com/
- 21.Simpson L, Hillman DR, Cooper MN, et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2012;17:967–73. doi: 10.1007/s11325-012-0785-0. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 23.Australian Institute of Health and Welfare (AIHW) The Active Australia Survey: a guide and manual for implementation, analysis and reporting. AIHW. 2003. [Last accessed September 1, 2015]. http://www.aihw.gov.au/publication-detail/?id=6442467449.
- 24.Boyle T, Leong S. Comparing ratings of occupational physical activity. Epidemiology. 2012;23:934–6. doi: 10.1097/EDE.0b013e31826d08e4. [DOI] [PubMed] [Google Scholar]
- 25.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–40. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 27.Vincent HK, Shanely RA, Stewart DJ, et al. Adaptation of upper airway muscles to chronic endurance exercise. Am J Respir Crit Care Med. 2002;166:287–93. doi: 10.1164/rccm.2104120. [DOI] [PubMed] [Google Scholar]
- 28.Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol. 2000;89:590–8. doi: 10.1152/jappl.2000.89.2.590. [DOI] [PubMed] [Google Scholar]
- 29.Dallimore N, Eccles R. Changes in human nasal resistance associated with exercise, hyperventilation and rebreathing. Acta Otolaryngol. 1977:84. doi: 10.3109/00016487709123985. [DOI] [PubMed] [Google Scholar]
- 30.Bussieres M, Perusse L, Leclerc JE. Effect of regular physical exercise on resting nasal resistance. J Otolaryngol. 2000;29:265–9. [PubMed] [Google Scholar]
- 31.Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32:161–6. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kairaitis K, Parikh R, Stavrinou R, et al. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–6. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 33.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47:101–5. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–8. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 35.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33:467–74. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 37.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Pendharkar SR, Tsai WH, Eves ND, Ford GT, Davidson WJ. CPAP increases exercise tolerance in obese subjects with obstructive sleep apnea. Respir Med. 2011;105:1565–71. doi: 10.1016/j.rmed.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Leon-Latre M, Moreno-Franco B, Andres-Esteban EM, et al. Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Rev Esp Cardiol. 2014;67:449–55. doi: 10.1016/j.rec.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S, Lash TL. Modern epidemology. Sydney, NSW: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Australian Bureau of Statistics 2006 census Quickstats data for sex, age, country of birth, and marital status for residents of Busselton and Western Australia.
Characteristics of the reference population and subgroups obtained using the screening questions: blood pressure and snoring frequency.


