Abstract
Objective:
Restless legs syndrome (RLS) is a burdensome sensorimotor disorder that has been linked to diabetes and obesity. However, the relationship of RLS to gestational diabetes mellitus (GDM), a common pregnancy complication strongly associated with obesity and a harbinger of diabetes, remains unknown. In this study, we examined the association of RLS to history of GDM in a sample of older female primary care patients.
Methods:
Participants were community-dwelling women aged ≥ 40 years drawn from an anonymous survey study of West Virginia adult primary care patients. Data gathered included detailed information on demographics, lifestyle factors, reproductive history, sleep patterns, and medical history; the survey also included an RLS diagnostic questionnaire. Women who were pregnant or had missing data on key variables were excluded from the analyses.
Results:
Of the 498 participants included in the final analytic sample, 24.5% met diagnostic criteria for RLS (17.9% with symptoms at least once/week). After adjustment for demographics, lifestyle characteristics, body mass index, diabetes and other comorbid conditions, parity, and other factors, those reporting history of GDM were almost three times as likely to meet criteria for RLS (odds ratio [OR] = 2.7, 95% confidence interval [CI] = 1.3, 5.3). This association increased in magnitude with increasing symptom frequency (adjusted OR for RLS symptoms ≥ 3×/week = 4.8, CI 2.1, 11.2, p for trend = 0.004).
Conclusions:
History of GDM was strongly and positively related to RLS in this study of older female primary care patients, offering further support for a possible role of metabolic dysregulation in RLS development.
Citation:
Innes KE, Kandati S, Flack KL, Agarwal P, Selfe TK. The association of restless legs syndrome to history of gestational diabetes in an Appalachian primary care population. J Clin Sleep Med 2015;11(10):1121–1130.
Keywords: gestational diabetes, metabolic syndrome, pregnancy complications, restless legs syndrome, sleep, Willis-Ekbom disease
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a burdensome sleep disorder affecting an estimated 10–15% of the general adult population, and up to 20% of primary care patients in the United States and Western Europe.1–3 RLS is characterized by an irresistible urge to move the legs that is typically accompanied by distressing, often painful sensations in the legs, begins or worsens during periods of inactivity, is worse during the evening and nighttime hours, and is partially or totally relieved by movement.4 Recently a fifth criterion has been added, stating that symptoms cannot be caused solely by another condition (e.g., leg cramps, positional discomfort).5 RLS can have profound negative effects on quality of life and daily functioning, comparable to those reported in other serious disorders, and is associated with significant economic, social, and health care burdens.6–10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome (RLS) is a burdensome sensorimotor disorder with prevalence rates that are higher in women and further elevated during pregnancy. Although the etiology of RLS remains unclear, RLS has been associated with diabetes, obesity, and related chronic conditions, suggesting a possible role of metabolic dysregulation. However, the relationship of RLS to history of gestational diabetes (GDM), a common pregnancy complication strongly linked to obesity and a harbinger of diabetes, remains unknown.
Study Impact: In this study of older female primary care patients, prior history of GDM was strongly and positively related to RLS, offering further support for a possible role of metabolic dysregulation in RLS development. Better understanding regarding the factors underlying the etiology and progression of RLS will aid in identifying cost-effective treatments for this challenging and still incurable condition.
Although the etiology of RLS remains unclear, recent studies suggest that metabolic dysregulation may play an important role.2 RLS has been significantly associated with diabetes, obesity, and other conditions characterized by insulin resistance and/or glucose intolerance and linked to the metabolic syndrome,2 although the direction of these relationships remains unclear. RLS is also more common in women and during pregnancy, and is positively associated with parity (a factor in turn linked to increased risk for obesity),11,12 insulin resistance, and glucose intolerance,13 suggesting that pregnancy-related metabolic changes may increase risk for RLS in women. One established gestational marker of metabolic dysfunction is gestational diabetes mellitus (GDM), a common complication of pregnancy that affects 2–14% of pregnancies in the United States, and that continues to increase in prevalence globally.14,15 GDM is a powerful predictor of the subsequent development of type 2 diabetes and the metabolic syndrome in women, and thus may be considered an early marker for these chronic insulin resistance conditions.16,17 For example, in a recent meta-analysis of 20 prospective studies, women with a history of GDM were at 7.5-fold increased risk overall for the development of type 2 diabetes.18 GDM has likewise been shown to be a strong predictor of the metabolic syndrome,17,19–21 with risk estimates ranging from threefold19 to more than fivefold.20 However, although several studies have examined risk factors for RLS during pregnancy,22–26 as well as pregnancy-related risk factors for RLS in nonpregnant women,27–29 no studies to our knowledge have yet examined the relationship of RLS to history of GDM. In this study, we examined the association of RLS to history of GDM in a sample of older female primary care patients.
METHODS
Study Population and Survey Administration
Participants for this study were nonpregnant women 40 years of age and older, drawn from an anonymous survey study of ambulatory, community-dwelling, English-speaking adults visiting one of four Morgantown area primary care clinics affiliated with the West Virginia University (WVU) Health Sciences Center. We restricted our sample to women at least 40 years of age to reduce likelihood of including women with undetected pregnancy or early-onset RLS,30–32 which is thought by some to differ in etiology from late-onset RLS.30,32
Survey methods are described elsewhere in detail.3 Briefly, surveys were offered to each adult patient in the clinic waiting area. Those who wished to participate but were unable to read were assisted by research staff. The survey was presented as a study regarding sleep habits in adults, including both those who slept well and those who did not. Over a period of 12 weeks, surveys were distributed during 3- to 4-hour time blocks. Survey administration times were systematically rotated in each clinic, to ensure similar coverage across clinics and reduce the probability of selection bias based on timing of survey administration. Overall survey response rates were excellent, with 919 of 1,198 women (76.7%) returning completed surveys to the drop box provided.3 The survey, consisting of 41 self-report items, required 5–10 minutes to complete. Detailed information was gathered regarding: demographic characteristics; lifestyle; current height and weight; history of physician diagnosed chronic mental and physical health conditions, current medications for these conditions; sleep duration and quality during the past 7 days; and reproductive history, including history of gestational diabetes. This anonymous survey study was approved by the West Virginia University Institutional Review Board.
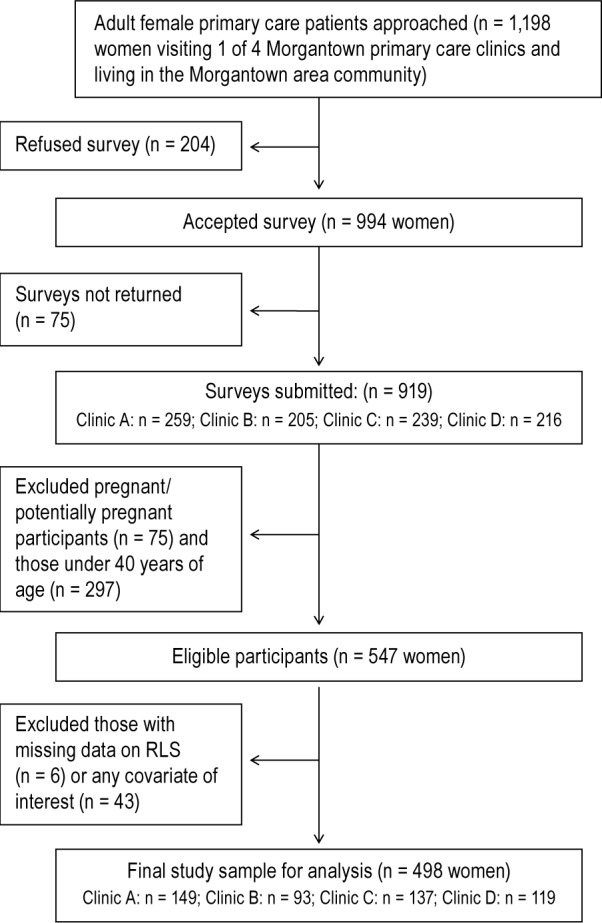
As illustrated in Figure 1, excluding women who were pregnant (n = 65), unsure about their pregnancy status (n = 10), or aged less than 40 years (n = 297) left a sample of 547 eligible adult female primary care patients. Exclusion of those with missing data on RLS symptoms (n = 6, 1.1%) or on pregnancy history, demographics, lifestyle characteristics, and other covariates of interest (n = 43, 9.0%), yielded a final analytic sample of 498 female primary care patients. Participants with missing data were more likely to be older (62.9 ± 2.0 versus 56.9 ± 0.5 y, p = 0.001); they were less likely to: be college graduates (8% vs. 31%, p = 0.0001), be physically active at least 1 h/w (29.0% versus 48.7%, p = 0.03), and report an annual income over $25,000 (31% versus 74%, p < 0.001) than those without missing data. Relative to those included in the analysis, participants with incomplete data did not differ in other demographic or lifestyle characteristics, or in health profiles, sleep patterns, reproductive history, prevalence of RLS, or other factors (p > 0.1).
Figure 1. Study flow diagram.
Measurement of Outcomes, Indices of Burden, and Major Covariates
Ascertainment of RLS
RLS, the primary outcome of interest, was assessed using a previously validated seven item questionnaire3 adapted from the self-completed Cambridge-Hopkins diagnostic questionnaire (CH-RLSq)33 and designed to capture the four International Restless Legs Syndrome Study Group's (IRLSSG) diagnostic criteria in effect at the time of the study,4 determine presence of accompanying unpleasant sensations, and exclude common RLS mimics (e.g., positional discomfort, leg cramps). The seven questions were as follows: (1) “Do you ever have an urge to move your legs that is difficult to resist?”; If YES: (2) Will simply changing leg position by itself once without continuing to move usually relieve these feelings?”; (3) “Is this urge to move usually accompanied by unpleasant sensations in the legs?”; (4) “Are the unpleasant sensations due to leg cramping?”; (5) “Does the urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting?”; (6) “Is the urge to move or the unpleasant sensations partially or totally relieved by movement, such as walking or stretching?”; and (7) “Does the urge to move or the unpleasant sensations only occur or get worse in the evening or night?” An eighth item questioned RLS symptom frequency: “How often do you experience these symptoms?” (Circle one: less than once/month, 1–3 days/month, 1–2 days/week, and 3 or more days/week). To be classified as RLS required an affirmative response to all four RLS diagnostic questions (#1, 5–7, above) and a negative response to questions 2 and 4. Because unpleasant sensations often accompany RLS symptoms, but are not an essential diagnostic criterion for RLS,4 we analyzed the prevalence and correlates of RLS with unpleasant sensations separately. Another section of the survey also asked participants if they had received a physician diagnosis of RLS and/ or were taking medications for RLS.
Demographic and Lifestyle Characteristics
Information regarding demographic characteristics was collected via self-report survey questions, and included age, sex, race/ethnicity, education (< 12 years, high school graduate, some college, ≥ 4 years post high school [college graduate]), employment status (employed, unemployed/out of work, homemaker, retired, student, disabled), marital status (single, married or cohabiting, widowed, divorced), and annual household income (< $25,000, $25,000–50,000, $50–75,000, > $75,000). Lifestyle factors assessed included smoking history and current smoking status (five items), participation in physical activity (two items), and caffeine consumption (two items).
Reproductive History, Including GDM and Other Health-Related Factors
Reproductive history was assessed via self-report survey questions. History of GDM was scored as positive if the participant answered “yes” to the following two questions (1) “Have you ever been pregnant?” and (2) “If yes, were you ever told by a doctor that you had gestational diabetes?” Information was also gathered on menopausal status (one item), previous and current use of hormone replacement therapy (two items), current pregnancy status (one item), gestational hypertension/ preeclampsia (one item), and parity (completed pregnancies, two items).
Obesity was defined as body mass index (BMI) ≥ 30, with BMI calculated as (weight in kg/height in m2). History of a specific chronic health disorder (including diabetes, anemia, hypertension, high cholesterol, heart disease, stroke, kidney disease, osteoarthritis, rheumatoid arthritis, and other serious conditions) was ascertained via self-report and defined as reported physician diagnosis of, and/or currently taking prescription medications for this disorder; depression and sleep apnea were ascertained in a similar fashion. Metabolic syndrome was defined as the presence of at least three of the four following conditions: diabetes, hypertension, obesity, and high cholesterol. Sleep patterns were assessed using six questions adapted from items designed to evaluate sleep quality and duration and included in the 2005–2006 National Health and Nutrition Examination Survey (NHANES) cycle.3,34 Five survey items ascertained the number of days (0–7) during the past week that the participant experienced specific sleep problems (difficulty falling asleep, difficulty staying asleep, early awakening, insufficient rest, and daytime fatigue/somnolence); the composite five-item scale, as described in detail elsewhere, has been shown to be both reliable and useful.3 An additional, open-ended question was used to ascertain sleep duration (in hours and minutes) during the past week.
Analysis
All data were analyzed using IBM SPSS version 21. Potential differences between participants with and without missing data were evaluated using the Student t-test or Mann-Whitney U test for continuous or ordinal variables, and χ2 test for categorical variables. We used multiple logistic regression to determine the independent association of RLS to history of GDM, and to assess the influence of potential confounders and effect modifiers; linear trends were assessed using polynomial contrasts. Unless stated otherwise, multivariable models were adjusted for demographic factors and lifestyle characteristics, as well as BMI, reproductive history (menopause, parity, use of hormone replacement therapy [HRT]) and comorbid conditions, including anemia, kidney disease, heart conditions, hypertension, high cholesterol, arthritis, stroke, and diabetes. Additional analyses also adjusted for cancer, sleep apnea, and depression. All p values presented are two-sided. We also assessed the joint effects of GDM and specific common chronic conditions associated with both RLS and GDM, including obesity, diabetes, and the metabolic syndrome.
In addition, we also conducted several sensitivity analyses to assess the robustness of the observed associations. We restricted RLS cases to those also reporting unpleasant sensations and examined the association of GDM to RLS severity, as indicated by frequency of reported symptoms (no RLS (referent category), RLS with symptoms less than once a week, RLS with symptoms one to two times a week, and RLS with symptoms at least three times a week). We also conducted analyses excluding participants with a history of kidney disease, anemia, stroke, neurological conditions, and diabetes and performed additional analyses excluding patients on medications that might influence RLS symptoms, including those for depression and high cholesterol.
RESULTS
Study population characteristics by RLS status are presented in Table 1. Participants were predominantly non-Hispanic white (92%), and married or cohabiting (67%). Only 31% had completed at least 4 years of college, and approximately 50% reported an annual household income of less than $50,000. Almost 75% of the women were perimenopausal or postmenopausal, 23% reported taking HRT for more than 6 months at some point in their lives, and 9% were nulliparous. Seventy-five percent of participants reported at least one co-morbid health condition, with over 52% reporting at least two comorbid conditions, and approximately one third reporting three or more comorbid disorders (Table 1). As indicated in Table 2, reported sleep impairment was high in this population, with 48% of women surveyed indicating sleep problems daily, and almost one-third reporting an average of less than 5 hours of sleep per night. Prevalence of several chronic conditions was also elevated in this sample, including obesity (43.8%), diabetes (20.8%), hypertension (48.7%), high cholesterol (40.7%), metabolic syndrome (23.6%), arthritis (26.5%), sleep apnea (9.1%), and depression (29.3%).
Table 1.
Demographic, lifestyle, reproductive, and health-related characteristics in 498 nonpregnant women ≥ 40 y of age attending 1 of 4 primary care clinics, stratified by RLS.
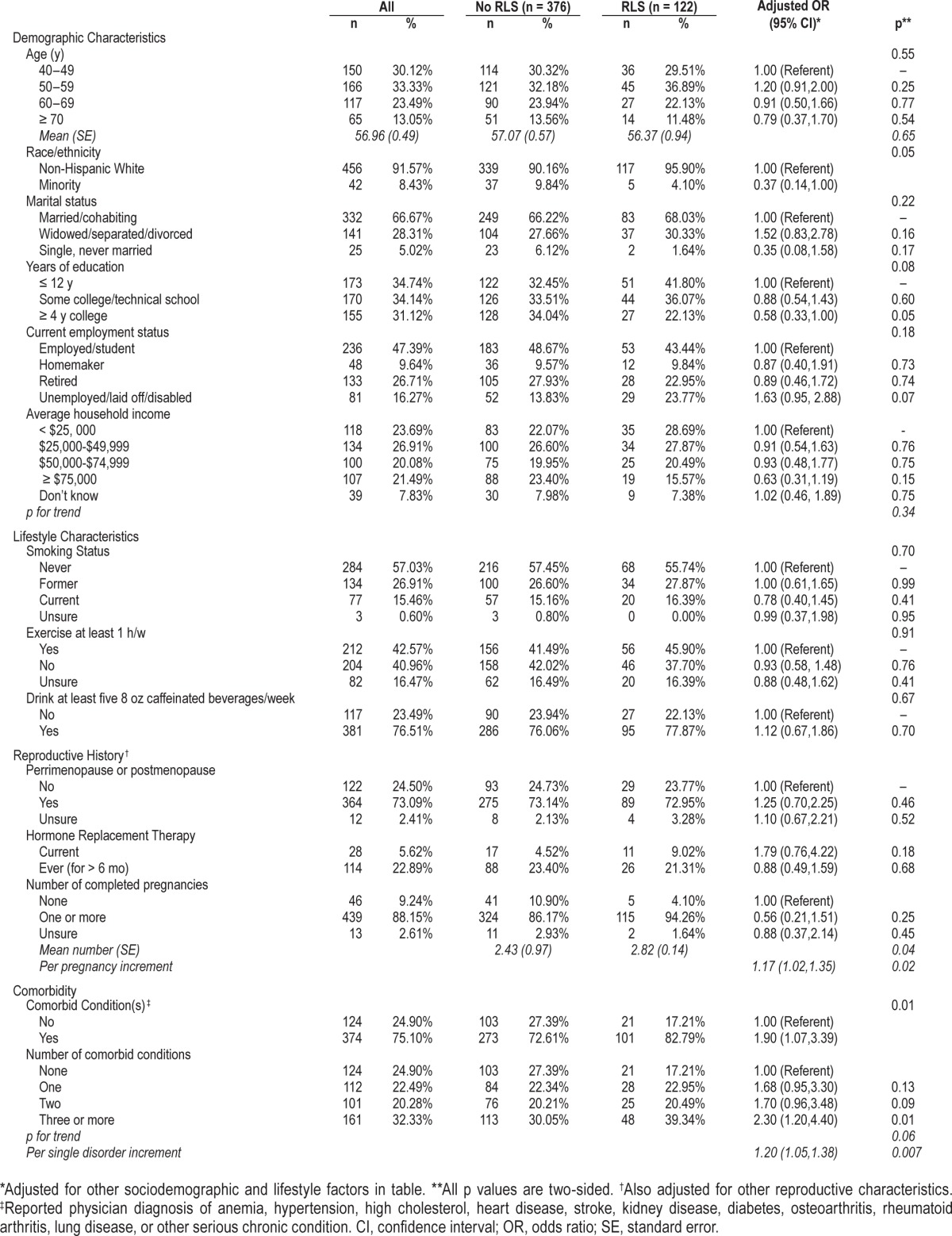
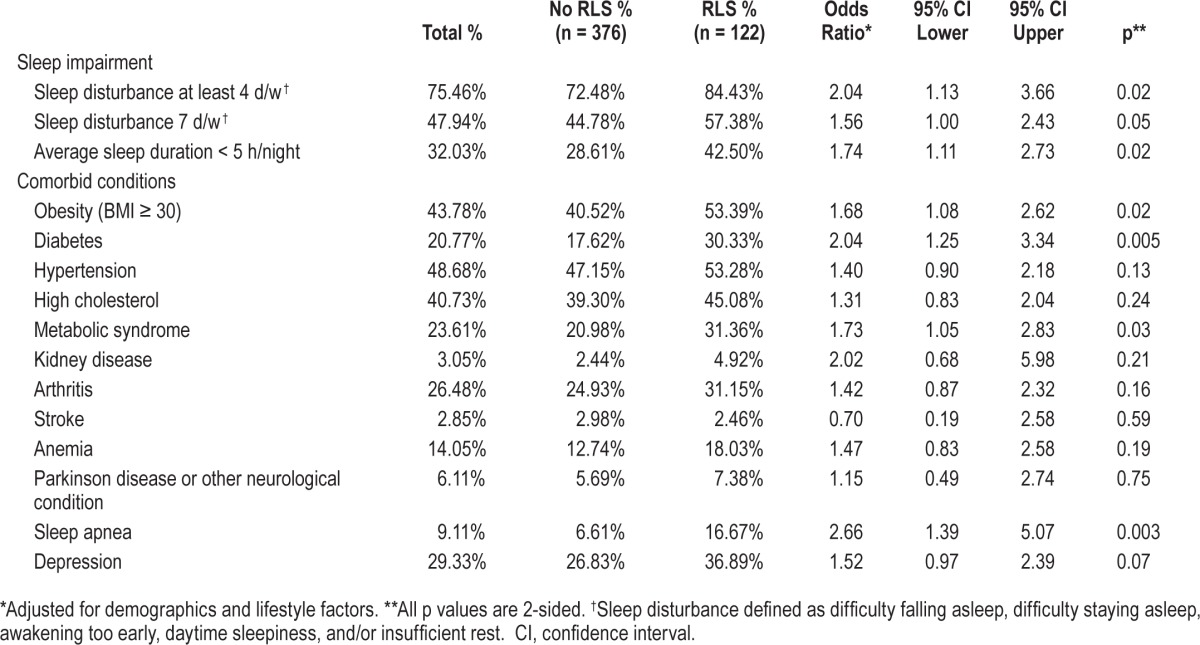
Table 2.
Prevalence of sleep impairment and specific health conditions in 498 nonpregnant women ≥ 40 y of age attending 1 of 4 primary care clinics, stratified by restless legs syndrome status.
Of the 498 women included in this analysis, 45 (9%) reported a history of GDM. One hundred twenty-two participants (24.5%) met the four IRLSSG diagnostic criteria and did not report symptoms due to leg cramps or positional discomfort; of the 122 respondents with RLS, 73% (17.9% of total participants) reported experiencing symptoms at least once a week, and 24.6% (11.9% total participants) indicated symptoms three or more times a week. Of the 107 respondents with RLS who also reported unpleasant sensations, 64% indicated symptoms at least once a week, and 49.5% reported symptoms three or more times a week. Only 41 of those women meeting criteria for RLS (33.6%) had received a physician diagnosis, of whom 18 (14.8%) were on RLS medications.
Participants with RLS were more likely to be unemployed or disabled and less likely to be college-educated and from minority populations after adjustment for other demographic and lifestyle factors (Table 1). After adjustment for other factors, RLS also remained significantly and positively related to both parity and comorbidity, with likelihood of RLS rising with increasing number of completed pregnancies, and rising number of comorbid conditions. As indicated in Table 2, those with obesity, diabetes, or the metabolic syndrome were also more likely to meet criteria for RLS (adjusted ORs, respectively = 1.7, 95% confidence interval [CI] 1.1, 2.6; 2.0, CI 1.3, 3.3; and 1.7, CI 1.1, 2.8), as were women who indicated a history of sleep apnea or depression (adjusted ORs, respectively = 2.7, CI 1.4, 5.1 and 1.5, CI 1.0, 2.4). Relative to those without RLS, participants with RLS were significantly more likely to report sleeping less than 5 hours per night (adjusted OR = 1.7, CI 1.1, 2.7), and to indicate sleep problems at least 4 days per week (adjusted OR = 2.0, CI 1.1, 3.7), as well as daily sleep problems (adjusted OR = 1.6, CI 1.0, 2.4).
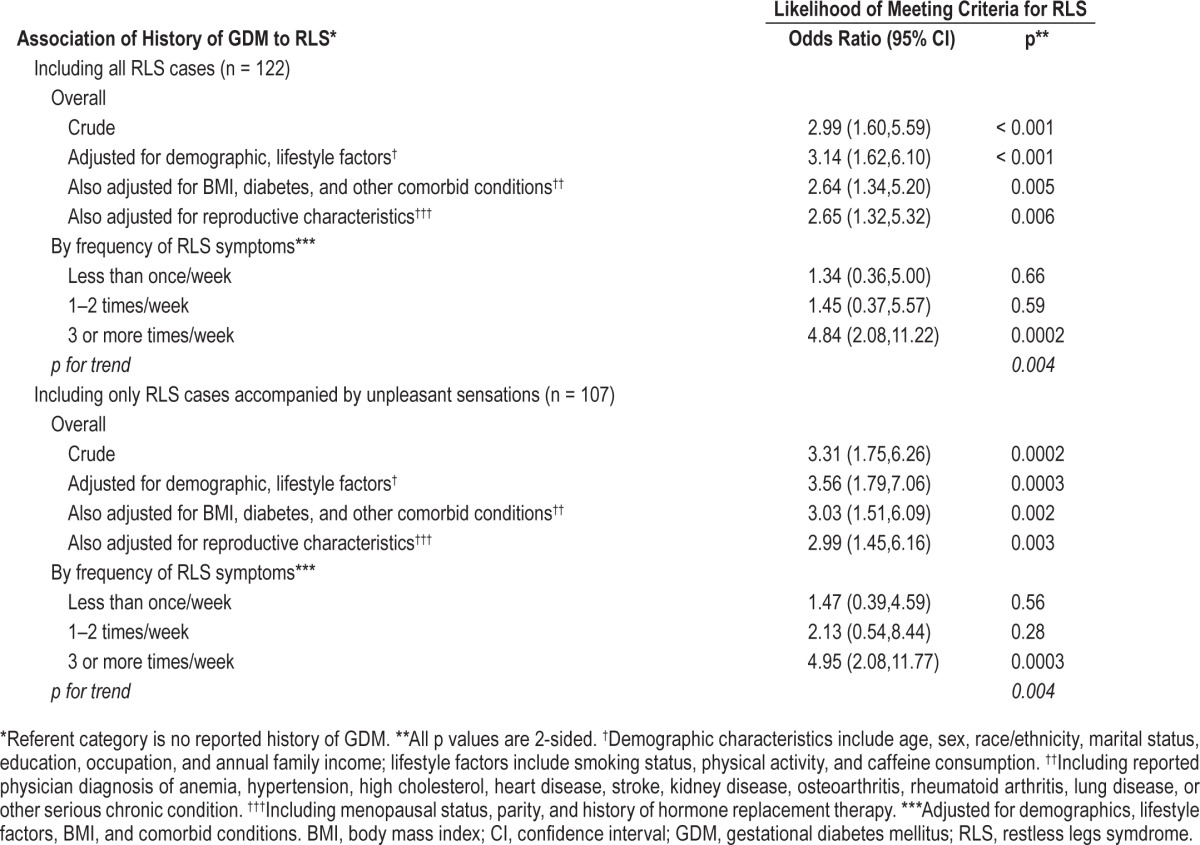
As illustrated in Table 3, reported history of GDM was associated with significantly increased likelihood of RLS in this population. Women who indicated a prior diagnosis of GDM were more than three times as likely to meet criteria for RLS (OR adjusted for demographic and lifestyle characteristics = 3.1, CI 1.6, 6.1). Further adjustment for BMI, reproductive history, and comorbid conditions including diabetes and anemia only slightly attenuated this association (OR = 2.7, CI 1.3, 5.3). The association of GDM to RLS increased in magnitude with rising RLS severity; GDM was associated with an approximately fivefold increase in risk for RLS with symptoms at least three times a week (OR = 4.8, CI 2.1, 11.8, p for trend = 0.004). As indicated in Table 3, restricting the analysis to RLS cases accompanied by unpleasant sensations (n = 107) slightly strengthened the association of RLS to GDM (adjusted OR = 3.0, CI 1.5, 6.2; adjusted OR for symptoms three or more times a week = 5.0, CI 2.1, 11.8)).
Table 3.
Association of history of gestational diabetes mellitus to restless legs syndrome and restless legs syndrome severity in 498 nonpregnant women ≥ 40 y of age attending 1 of 4 WVU primary care clinics.
Sensitivity analyses: Additional adjustment for depression and sleep apnea did not alter the relation with RLS (OR = 2.7, CI 1.4, 5.3), nor did inclusion of diagnosed cancer in the full model (OR = 2.8, CI 1.4, 5.5). Likewise, additional adjustment for sleep impairment or gestational hypertension/preeclampsia did not appreciably change the relation of GDM to RLS (ORs, respectively = 2.9, CI 1.5, 5.8 and 2.6, CI 1.2, 4.9). Excluding women with a history of kidney disease, anemia, stroke, neurological conditions, sleep apnea, or diabetes did not materially alter these findings, nor did including as RLS cases the seven women who indicated receiving a diagnosis of RLS but did not meet RLS criteria. In addition, excluding those on medication for high cholesterol or depression did not alter risk estimates (adjusted OR = 2.8, CI 1.4, 5.4), nor did specifically adjusting for antihypertensive and other medications in the model (adjusted OR = 2.7, CI 1.4, 5.2).
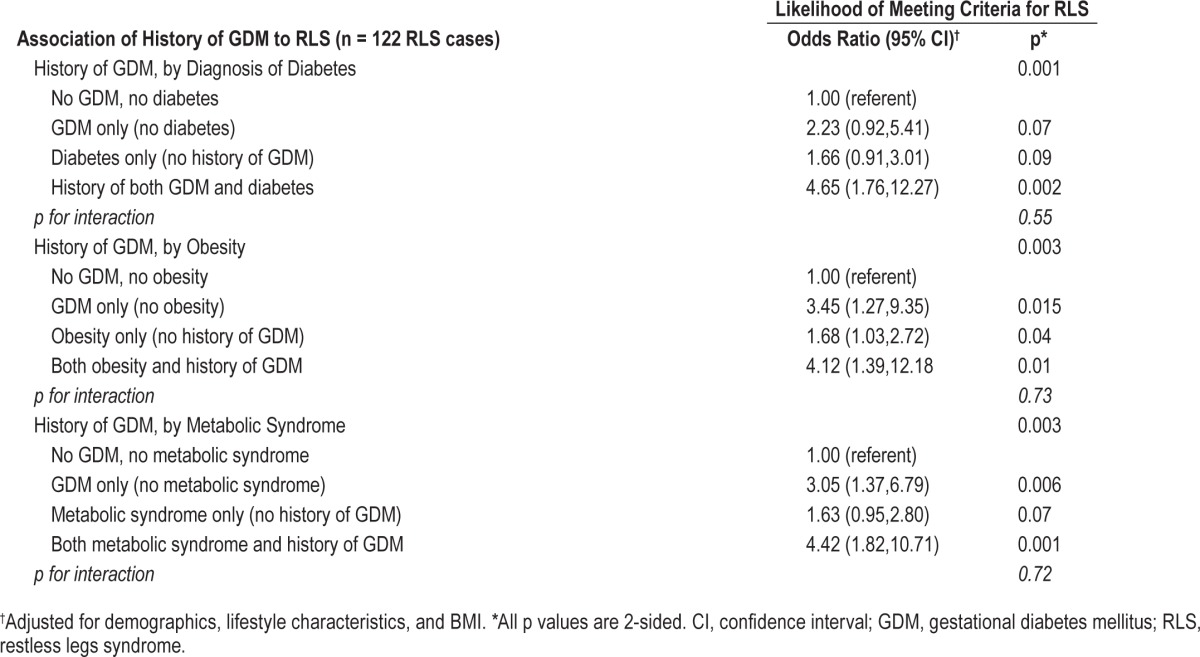
We found no evidence of significant interaction by obesity status, or by presence of diabetes, metabolic syndrome, hyper-tension, or high cholesterol (p > 0.1). As illustrated in Table 4, GDM remained strongly related to RLS even in the absence of obesity, diabetes, or the metabolic syndrome. However, the combination of prior GDM with any one of these conditions appeared to further increase the likelihood of RLS, suggesting these factors may have an additive effect on RLS risk.
Table 4.
Association of restless legs syndrome to history of gestational diabetes mellitus alone and in combination with diabetes, obesity, and the metabolic syndrome in 498 nonpregnant women ≥ 40 y of age attending 1 of 4 WVU primary care clinics.
DISCUSSION
Literature regarding RLS and pregnancy complications remains sparse. A recent cross-sectional study of 79 Peruvian women during the peripartum period documented a higher prevalence of preeclampsia in mothers with RLS,35 although findings were based on small numbers and estimates were not adjusted for GDM, chronic hypertension, diabetes, or certain other conditions linked to increased risk for the development of RLS and/or preeclampsia.36–38 Moreover, the relation of chronic RLS to history of any pregnancy complication, including GDM, has not been explored in depth.
To our knowledge, this is the first study to examine the association of RLS to history of GDM. In brief, findings of this study in older female primary care patients suggest that prior history of GDM is strongly and positively related to RLS. In this population, women who reported a history of GDM were approximately three times as likely to meet criteria for RLS, and five times as likely to experience RLS symptoms at least three times per week. This relationship was not explained by demographic or lifestyle factors, parity and other reproductive characteristics, current BMI, or by reported history of diabetes, metabolic syndrome, apnea, or other comorbid conditions.
Consistent with our findings, most, although not all, prior studies assessing the association of RLS to diabetes have suggested a positive relationship between these conditions, with risk estimates ranging from 1.6 to 4.7.2 In addition, two of the three studies evaluating the association of RLS to measures of glucose tolerance in adults without diabetes reported strongly increased likelihood of impaired glucose tolerance and elevated glycemia levels in those with RLS relative to those without RLS,39–41 suggesting that RLS can precede the development of diabetes.2
The prevalence of RLS was high in this sample of older women, consistent with findings of our previously published study3 and likely at least in part reflecting the elevated rates of obesity, diabetes, and other chronic conditions characterizing this Appalachian population.2,3 Approximately 18% of participants met criteria for RLS and reported symptoms at least once a week, with 11.9% indicating symptoms at least three times a week. Unfortunately, direct comparisons of RLS prevalence across studies are challenging due to variation in RLS diagnostic criteria, definition of clinically significant RLS, and characteristics of target populations.1,2 Nonetheless, the prevalence of RLS observed in this study was higher than that reported in most,42–44 although not all41,45,46 prior studies of Western primary care and older adult populations that used similar criteria and/or clinical confirmation to define RLS.1
Reported incidence of GDM was also elevated in this population, paralleling the high rates of obesity, inactivity, diabetes, and related chronic conditions in West Virginia adults.2,47–49 As has been repeatedly documented in previous studies,2,3,50,51 RLS was positively associated with both depression and sleep impairment in this sample of older women. Those with sleep apnea were also more likely to meet criteria for RLS, as has been reported in other populations.41,52,53
Consistent with prior investigations, multiparity was significantly and positively associated with RLS in this study. Parity has been associated with RLS24,28,29,54–56 and with RLS symptom severity57 in both pregnant24,54,55 and nonpregnant women.28,29,56 Although multiparity has also been related to increased risk for GDM58 and for obesity,11,12 also a strong risk factor for GDM, parity did not explain the association of RLS to GDM in this study. Similarly, although obesity, the metabolic syndrome, and diabetes are strongly linked to GDM,18,58 and were significantly associated with RLS in this population, adjustment for these factors only slightly attenuated the observed association of RLS to GDM.
Although underlying causal relationships cannot be determined in this cross-sectional study, the robust, independent positive association observed between RLS and history of GDM, coupled with previously published findings regarding glucose intolerance and RLS, provide further support for a role of metabolic dysregulation in the etiology of RLS. Glucose intolerance may in part reflect underlying activation of the hypothalamic pituitary adrenal (HPA) axis and contribute to the autonomic dysfunction documented in women with prior GDM.59,60 HPA axis and autonomic dysfunction have, in turn, been strongly linked to the pathogenesis of diabetes and related disorders, and more recently implicated in the development of RLS.2 Unfortunately, we lacked information on the timing of GDM and RLS onset, and thus cannot preclude the possibility that in some women, RLS may have preceded the development of GDM. However, these participants are likely few, given that we excluded women younger than 40 years and that RLS generally develops later in life, with prevalence typically peaking in the sixth decade, as has been previously documented in this and other populations.1,3,61
Strengths and Limitations
Strengths of this clinic-based survey study include the high study participation rates and the relatively large number of participants in our sample. Established international criteria to define RLS4 and validated questions based on an established diagnostic questionnaire33 were used; we also collected information on symptom frequency, and asked specific questions regarding the presence of potential mimics to reduce risk of misclassification.62 Symptom frequency data allowed evaluation of potential dose-response associations. In addition, participants provided comprehensive self-report information on numerous potential correlates and confounders, including demographic and lifestyle characteristics, mood and sleep patterns, medical and reproductive history, and other health-related factors. The survey was presented as a study to examine typical sleep patterns and correlates in Morgantown area residents, with no mention of sleep deficits, pregnancy complications, or RLS; thus, participation bias associated with either RLS or pregnancy history, while possible, is unlikely.
Limitations of this pilot survey are also several. Our sample was restricted to older female primary care patients from a predominantly Appalachian community. Therefore, findings may not be generalizable to other populations. We did not collect data on alcohol consumption, which has been positively associated with RLS53,63 but inversely associated with GDM in some studies. We also lacked data on blood levels of hemoglobin, ferritin, and other analytes potentially related to RLS, although we did have information on diagnosed anemia. However, iron-deficiency anemia has been negatively associated, and elevated serum levels of hemoglobin, ferritin, and related blood markers positively linked to GDM64,65; thus, any undetected iron deficiency would be expected to attenuate the observed relationships. In addition, although we collected information on medication use for a number of specific chronic health conditions, including depression, anxiety, hypertension, dyslipidemia, and others, we did not gather data on specific medication types or on medication use for some uncommon but serious mental health conditions (e.g., schizophrenia or psychosis), which could potentially exacerbate (e.g., tricyclic antidepressants, selective serotonin reuptake inhibitors, statins) or attenuate RLS symptoms (e.g., certain beta-blockers and alpha-2 agonists).2,62 However, excluding those on medication for high cholesterol or depression did not appreciably alter risk estimates, nor did specifically adjusting for antihypertensive, antidepressant, and other medications, suggesting that medication use is unlikely to explain the associations observed in this study. Finally, we did not have information on family history of RLS, an RLS risk factor that could potentially modify the association between RLS and prior history of GDM. We also lacked data on history of transient RLS in pregnancy, a factor recently linked to subsequent risk for chronic RLS.27 If GDM predisposes to RLS during pregnancy, pregnancy-associated RLS may in part mediate the observed increased risk for chronic RLS associated with history of GDM.
Exclusion of participants with missing data on covariates may have introduced selection bias. Unmeasured confounding might also help explain our findings, although our ability to control for a large number of both known and potential factors linked to GDM and RLS renders this possibility less probable. Seven respondents reporting a physician diagnosis of RLS did not meet symptom criteria. Some of these participants may have experienced RLS, for example, only during pregnancy or in association with prior anemia, and others may have been misdiagnosed. One of the seven also reported taking RLS medications, which may have led to symptom relief. Although based on validated questions adapted from an established questionnaire35 and incorporating questions designed to exclude common mimics, ascertainment of RLS was reliant on self-report; some measure of diagnostic error is thus possible. However, requiring an affirmative response to all four essential criteria, along with absence of discomfort due to leg cramps or positional discomfort, renders misdiagnosis less likely.62,66 Ascertainment of GDM and other medical diagnoses was based on self-report, potentially introducing bias. Although our target population comprised WVU primary care patients, who are more likely to seek and receive medical care and thus may be better informed about their health and medical conditions than a general West Virginia population, misclassification remains possible. A validation study of a broader Appalachian population in the Ohio valley using medical records showed adequate concordance (74%) and excellent specificity (more than 95%) for self-reported diabetes, and very high concordance (> 99%) for self-reported cancers (unpublished data). Nonetheless, self-report of chronic illness has been shown to be vulnerable to misclassification,67–69 potentially influencing our findings.
As in most other epidemiological studies of RLS, our findings, while suggestive, are based on cross-sectional data, precluding determination of causality. Clearly, prospective studies are needed to confirm these findings, to investigate potential causal associations, and to explore possible underlying mechanisms.
CONCLUSIONS
Prior history of GDM was strongly and positively related to RLS in this study of older female primary care patients. This association remained robust after adjustment for demographic and lifestyle factors, parity and other reproductive characteristics, current BMI, and diabetes, anemia, and other comorbid conditions. Larger prospective studies are needed to further investigate the relationship of this common pregnancy complication to the development of RLS, and to determine possible causal pathways.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the National Center for Complementary and Integrative Health [Grant Number 1-K01-AT004108 to KEI] and West Virginia University (Faculty Incentive Award). The contents are solely the responsibility of the authors and do not represent the official views of West Virginia University or the National Institutes of Health. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was performed at West Virginia University.
ABBREVIATIONS
- BMI
body mass index
- CH-RLSq
Cambridge-Hopkins diagnostic questionnaire
- CI
confidence interval
- GDM
gestational diabetes mellitus
- HRT
hormone replacement therapy
- IRLSSG
International Restless Legs Syndrome Study Group
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- RLS
restless legs syndrome
REFERENCES
- 1.Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623–34. doi: 10.1016/j.sleep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev. 2012;16:309–39. doi: 10.1016/j.smrv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Innes KE, Flack KL, Selfe TK, Kandati S, Agarwal P. Restless legs syndrome in an appalachian primary care population: prevalence, demographic and lifestyle correlates, and burden. J Clin Sleep Med. 2013;9:1065–75. doi: 10.5664/jcsm.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15:860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Reinhold T, Müller-Riemenschneider F, Willich SN, Brüggenjürgen B. Economic and human costs of restless legs syndrome. Pharmacoeconomics. 2009;27:267–79. doi: 10.2165/00019053-200927040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10:295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11:807–15. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Reese JP, Stiasny-Kolster K, Oertel WH, Dodel RC. Health-related quality of life and economic burden in patients with restless legs syndrome. Expert Rev Pharmacoeconomics Outcomes Res. 2007;7:503–21. doi: 10.1586/14737167.7.5.503. [DOI] [PubMed] [Google Scholar]
- 10.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 11.Luoto R, Mannisto S, Raitanen J. Ten-year change in the association between obesity and parity: results from the National FINRISK Population Study. Gend Med. 2011;8:399–406. doi: 10.1016/j.genm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Davis EM, Zyzanski SJ, Olson CM, Stange KC, Horwitz RI. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am J Public Health. 2009;99:294–9. doi: 10.2105/AJPH.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med. 1989;321:1214–9. doi: 10.1056/NEJM198911023211802. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. 2011;28:652–7. doi: 10.1111/j.1464-5491.2010.03205.x. [DOI] [PubMed] [Google Scholar]
- 16.England LJ, Dietz PM, Njoroge T, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(4) doi: 10.1016/j.ajog.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21:149–57. doi: 10.1080/14767050801929430. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 19.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–10. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 20.Noussitou P, Monbaron D, Vial Y, Gaillard RC, Ruiz J. Gestational diabetes mellitus and the risk of metabolic syndrome: a population-based study in Lausanne, Switzerland. Diabetes Metab. 2005;31:361–9. doi: 10.1016/s1262-3636(07)70205-7. [DOI] [PubMed] [Google Scholar]
- 21.Verma A, Boney CM, Tucker R, Vohr BR. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2002;87:3227–35. doi: 10.1210/jcem.87.7.8684. [DOI] [PubMed] [Google Scholar]
- 22.Sarberg M, Josefsson A, Wirehn AB, Svanborg E. Restless legs syndrome during and after pregnancy and its relation to snoring. Acta Obstet Gynecol Scand. 2012;91:850–5. doi: 10.1111/j.1600-0412.2012.01404.x. [DOI] [PubMed] [Google Scholar]
- 23.Hubner A, Krafft A, Gadient S, Werth E, Zimmermann R, Bassetti CL. Characteristics and determinants of restless legs syndrome in pregnancy: a prospective study. Neurology. 2013;80:738–42. doi: 10.1212/WNL.0b013e318283baf3. [DOI] [PubMed] [Google Scholar]
- 24.Neau JP, Marion P, Mathis S, et al. Restless legs syndrome and pregnancy: follow-up of pregnant women before and after delivery. Eur Neurol. 2010;64:361–6. doi: 10.1159/000322124. [DOI] [PubMed] [Google Scholar]
- 25.Alves DAG, de Carvalho LBC, de Morais JF, do Prado GF. Restless legs syndrome during pregnancy in Brazilian women. Sleep Med. 2010;11:1049–54. doi: 10.1016/j.sleep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Sikandar R, Khealani BA, Wasay M. Predictors of restless legs syndrome in pregnancy: a hospital based cross sectional survey from Pakistan. Sleep Med. 2009;10:676–8. doi: 10.1016/j.sleep.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Cesnik E, Casetta I, Turri M, et al. Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology. 2010;75:2117–20. doi: 10.1212/WNL.0b013e318200d779. [DOI] [PubMed] [Google Scholar]
- 28.Manconi M, Ulfberg J, Berger K, et al. When gender matters: restless legs syndrome. Report of the “RLS and woman” workshop endorsed by the European RLS Study Group. Sleep Med Rev. 2012;16:297–307. doi: 10.1016/j.smrv.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Pantaleo NP, Hening WA, Allen RP, Earley CJ. Pregnancy accounts for most of the gender difference in prevalence of familial RLS. Sleep Med. 2010;11:310–3. doi: 10.1016/j.sleep.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittom S, Dauvilliers Y, Pennestri MH, et al. Age-at-onset in restless legs syndrome: a clinical and polysomnographic study. Sleep Med. 2007;9:54–9. doi: 10.1016/j.sleep.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Allen RP. Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med. 2007;120(1 Suppl 1) doi: 10.1016/j.amjmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Winkelman JW. Considering the causes of RLS. Eur J Neurol. 2006;3:8–14. doi: 10.1111/j.1468-1331.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 33.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10:1097–1100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4:129–39. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez JO, Cabrera SAS, Hidalgo H, et al. Is preeclampsia associated with restless legs syndrome? Sleep Med. 2013;14:894–6. doi: 10.1016/j.sleep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Nerenberg KA, Johnson JA, Leung B, et al. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J Obstet Gynaecol Can. 2013;35:986–94. doi: 10.1016/S1701-2163(15)30786-6. [DOI] [PubMed] [Google Scholar]
- 37.Schneider S, Freerksen N, Rohrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum Dev. 2012;88:179–84. doi: 10.1016/j.earlhumdev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Keckeis M, Lattova Z, Maurovich-Horvat E, et al. Impaired glucose tolerance in sleep disorders. PLoS One. 2010;5:e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco D, Plastino M, Fava A, et al. Role of the Oral Glucose Tolerance Test (OGTT) in the idiopathic restless legs syndrome. J Neurol Sci. 2009;287:60–3. doi: 10.1016/j.jns.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Juuti AK, Läär E, Rajala U, et al. Prevalence and associated factors of restless legs in a 57-year-old urban population in northern Finland. Acta Neurol Scand. 2010;122:63–9. doi: 10.1111/j.1600-0404.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 42.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–7. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study.[see comment] Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Möller C, Wetter TC, Köster J, Stiasny-Kolster K. Differential diagnosis of unpleasant sensations in the legs: prevalence of restless legs syndrome in a primary care population. Sleep Med. 2010;11:161–6. doi: 10.1016/j.sleep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 45.O'Keeffe ST, Egan D, Myers A, Redmond S. The frequency and impact of restless legs syndrome in primary care. Ir Med J. 2007;100:539–42. [PubMed] [Google Scholar]
- 46.Celle S, Roche F, Kerleroux J, et al. Prevalence and clinical correlates of restless legs syndrome in an elderly French population: the synapse study. J Gerontol A Biol Sci Med Sci. 2010;65:167–73. doi: 10.1093/gerona/glp161. [DOI] [PubMed] [Google Scholar]
- 47.Thoenen E. Charleston: West Virginia Bureau for Public Health February; 2006. The burden of arthritis in West Virginia. [Google Scholar]
- 48.Centers for Disease Control and Prevention and Health Promotion, Division of Diabetes Translation. 2012. http://www.cdc.gov/diabetes/data/
- 49.National Center for Chronic Disease Prevention and Health Promotion. Chronic disease. State Profiles. http://www.cdc.gov/chronicdisease/states/west_virginia.htm.
- 50.Hornyak M. Depressive disorders in restless legs syndrome: epidemiology, pathophysiology and management. CNS Drugs. 2010;24:89–98. doi: 10.2165/11317500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Shaya FT, Khoury ACE. Developing benchmarks for adherence studies. Expt Rev Pharmocoecon Outcomes Res. 2005;5:229–32. doi: 10.1586/14737167.5.3.229. [DOI] [PubMed] [Google Scholar]
- 52.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 53.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosomat Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 54.Manconi M, Govoni V, De Vito A, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Med. 2004;5:305–8. doi: 10.1016/j.sleep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Ismaillogullari S, Ozturk A, Mazicioglu MM, Serin S, Gultekin M, Aksu M. Restless legs syndrome and pregnancy in Kayseri, Turkey: a hospital based survey. Sleep Biol Rhythms. 2010;8:137–43. [Google Scholar]
- 56.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 57.Ghorayeb I, Bioulac B, Scribans C, Tison F. Perceived severity of restless legs syndrome across the female life cycle. Sleep Med. 2008;9:799–802. doi: 10.1016/j.sleep.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynecol Obstet. 2001;75:221–8. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 59.Gasic S, Winzer C, Bayerle-Eder M, Roden A, Pacini G, Kautzky-Willer A. Impaired cardiac autonomic function in women with prior gestational diabetes mellitus. Eur J Clin Invest. 2007;37:42–7. doi: 10.1111/j.1365-2362.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 60.Weissman A, Lowenstein L, Peleg A, Thaler I, Zimmer EZ. Power spectral analysis of heart rate variability during the 100-g oral glucose tolerance test in pregnant women. Diabetes Care. 2006;29:571–4. doi: 10.2337/diacare.29.03.06.dc05-2009. [DOI] [PubMed] [Google Scholar]
- 61.Szentkiralyi A, Fendrich K, Hoffmann W, Happe S, Berger K. Incidence of restless legs syndrome in two population-based cohort studies in Germany. Sleep Med. 2011;12:815–20. doi: 10.1016/j.sleep.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Borreguero D, Stillman P, Benes H, et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011;11:28. doi: 10.1186/1471-2377-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults.[see comment] Arch Intern Med. 2000;160:2137–41. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 64.Lao TT, Ho LF. Impact of iron deficiency anemia on prevalence of gestational diabetes mellitus. Diabetes Care. 2004;27:650–6. doi: 10.2337/diacare.27.3.650. [DOI] [PubMed] [Google Scholar]
- 65.Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabetes Complications. 2009;23:194–8. doi: 10.1016/j.jdiacomp.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Benes H, Walters AS, Allen RP, Hening WA, Kohnen R. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(Suppl 18):S401–8. doi: 10.1002/mds.21604. [DOI] [PubMed] [Google Scholar]
- 67.Goebeler S, Jylha M, Hervonen A. Self-reported medical history and self-rated health at age 90. Agreement with medical records. Aging Clin Exp Res. 2007;19:213–9. doi: 10.1007/BF03324692. [DOI] [PubMed] [Google Scholar]
- 68.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56:148–54. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 69.Molenaar EA, Van Ameijden EJ, Grobbee DE, Numans ME. Comparison of routine care self-reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health. 2007;17:199–205. doi: 10.1093/eurpub/ckl113. [DOI] [PubMed] [Google Scholar]


