Abstract
Study Objectives:
Sleep disturbance is a hallmark feature of cannabis withdrawal. In this study we explored the effects of lithium treatment supplemented with nitrazepam on objective and subjective measures of sleep quality during inpatient cannabis withdrawal.
Methods:
Treatment-seeking cannabis-dependent adults (n = 38) were admitted for 8 days to an inpatient withdrawal unit and randomized to either oral lithium (500 mg) or placebo, twice daily in a double-blind RCT. Restricted nitrazepam (10 mg) was available on demand (in response to poor sleep) on any 3 of the 7 nights. Dependent outcome measures for analysis included repeated daily objective actigraphy and subjective sleep measures throughout the 8 day detox, subjective cannabis withdrawal ratings, and detoxification completion rates.
Results:
Based on actigraphy, lithium resulted in less fragmented sleep compared to placebo (p = 0.04), but no other objective measures were improved by lithium. Of the subjective measures, only nightmares were suppressed by lithium (p = 0.04). Lithium did not have a significant impact on the use of nitrazepam. Sleep bout length (p < 0.0001), sleep efficiency (p < 0.0001), and sleep fragmentation (p = 0.05) were improved on nights in which nitrazepam was used. In contrast, only night sweats improved with nitrazepam from the subjective measures (p = 0.04). A Cox regression with daily repeated measures of sleep efficiency averaged across all people in the study a predictor suggests that a one-unit increase in sleep efficiency (the ratio of total sleep time to the total time in bed expressed as a percentage) resulted in a 14.6% increase in retention in treatment (p = 0.008, Exp(B) = 0.854, 95% CI = 0.759–0.960). None of the other sleep measures, nor use of lithium or nitrazepam were significantly associated with retention in treatment.
Conclusions:
Lithium seems to have only limited efficacy on sleep disturbance in cannabis withdrawal. However the nitrazepam improved several actigraphy measures of sleep disturbance, warranting further investigation. Discord between objective and subjective sleep indices suggest caution in evaluating treatment interventions with self-report sleep data only.
Citation:
Allsop DJ, Bartlett DJ, Johnston J, Helliwell D, Winstock A, McGregor IS, Lintzeris N. The effects of lithium carbonate supplemented with nitrazepam on sleep disturbance during cannabis abstinence. J Clin Sleep Med 2015;11(10):1153–1162.
Keywords: cannabis withdrawal, sleep disturbance, actigraphy, pharmacotherapy, lithium, nitrazepam, cannabis withdrawal scale
Sleep difficulties and cannabis use appears to be a bi-directional relationship. Individuals often start using cannabis when they experience sleep difficulties during stressful times, often for coping reasons1; and this is a risk factor for cannabis lapse (within the first 2 days out of 7) following a self-guided quit attempt.2,3 The most prevalent sleep disturbances with cannabis withdrawal include difficulty getting to sleep, staying asleep, nightmares and strange dreams, and night sweats.4 During the withdrawal process, sleep difficulties predict relapse to cannabis use.5–8 There is some evidence that pharmacotherapy during cannabis abstinence attenuates withdrawal related sleep disturbances,9 including improvements in polysomnographic measures of sleep efficiency (but not latency) with the nonbenzodiazepine GABA(A) receptor agonist zolpidem.10 While there is a plethora of studies exploring self-reported sleep disturbances associated with cannabis withdrawal, there are very few that quantify sleep patterns using objective means, with the exception of the paper by Vandrey et al.10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disturbance is the most consistent, prevalent, and severe symptom of cannabis withdrawal. However, there are currently no evidence-based medicines to assist with cannabis dependence or cannabis withdrawal.
Study Impact: This formal RCT demonstrates that, contrary to positive open label clinical findings, lithium carbonate does not improve cannabis withdrawal related sleep disturbances. However, the hypnotic benzodiazepine rescue medication nitrazepam significantly improved multiple objective measures of sleep disturbance during cannabis withdrawal.
Poor sleep is often the last symptom to remit, and recent trials have started to specifically target sleep disturbances during withdrawal. Haney and colleagues conducted a small laboratory study examining the muscle relaxant baclofen and the antidepressant mirtazapine.11 Mirtazapine improved sleep but was not effective in relation to withdrawal symptoms or relapse rates. Vandrey and colleagues recently ran the first pharmacotherapy trial specifically targeting sleep disruption during cannabis withdrawal in an inpatient setting.10 The researchers randomized 20 adults, using a within-subject crossover design of extended release of zolpidem, a non-benzodiazepine GABA(A) receptor agonist during short periods of cannabis abstinence. Zolpidem was found to attenuate symptoms of sleep disruption during cannabis withdrawal, but there was a trend for sleep to become progressively worse over the short three-day abstinence period.
Sleep disturbances following cannabis cessation have indicated reductions across total sleep time, sleep efficiency, latency, and amount of REM sleep, with these disturbances lasting at least 2 weeks.12,13 It should be noted that premorbid sleep patterns were not assessed in either of these studies; thus it was not clear whether these sleep disturbances were directly related to cannabis cessation. Similar observations were made by Vandrey, who also noted decreased sleep efficiency and increased sleep latency among 20 subjects in a controlled laboratory study.10 An increase, rather than reduction in REM sleep was observed in the Vandrey study. It is uncertain whether the differences between these studies were due to participant variations or research-design differences. Specifically, observations were only taken for 3 days post-withdrawal, whereas Bolla et al. (2008; 2010),12,13 have noted that sleep disturbances were observed two weeks post withdrawal.
Self-reported sleep disturbance is one of the hallmark features of cannabis withdrawal and is considered a potential therapeutic target for cannabis cessation.4,5,9,10 Recent studies have demonstrated high endorsement frequencies on ratings of sleep disturbances across research and clinical treatment seeking samples (67% to 73% of adults and 33% to 43% of adolescents) lasting months after cessation of cannabis use.6,10,15–17 Clinically, this is an important issue warranting further attention as it is likely that sleep disturbance during withdrawal impacts on the success of the quit attempt or is associated with relapse. Thus the development of interventions to ameliorate sleep disturbances may improve outcomes in treatments for cannabis dependence. Possibly due to its effects on stabilising mood and reducing depression (two key symptoms of cannabis withdrawal), lithium carbonate has proven effective at reducing precipitated cannabis withdrawal symptoms in rats15 as well as in humans in an open label inpatient study.16 Owing to the effects of lithium on cannabis withdrawal in rats being blocked by a selective oxytocin receptor antagonist a working mechanistic hypothesis is that lithium may exert its effects on cannabis withdrawal by stimulating oxytocin release.15 Intranasal oxytocin administration in humans significantly improves sleep architecture, including reducing sleep latency, increasing sleep efficiency, and increasing the percentage of REM episodes.17 In addition, lithium has previously been shown to significantly improve sleep disturbances during depressive episodes. Combined these observations make lithium a worthy target for exploring possible amelioration of sleep disturbances in cannabis abstinence in humans.18,19
This paper is a secondary analysis of data from a pharmacotherapy RCT testing the effects of lithium vs. placebo on cannabis withdrawal symptoms in an inpatient setting, which demonstrated no positive benefits of lithium on cannabis withdrawal symptoms.20 As the study protocol allowed for a strictly limited amount of “rescue” medications, and the detox unit that we performed the study in used the hypnotic benzodiazepine nitrazepam as a matter of routine course for sleep disturbances, we opportunistically explore the impact of nitrazepam use on subjective and objective sleep outcomes. Nitrazepam has previously been shown to have dramatic effects on reducing nightmares,21 which are one of the most prevalent and persistent cannabis withdrawal symptoms. The aims of the present paper were: (1) to evaluate the effects of lithium vs. placebo on subjective and objective measures of sleep patterns while controlling for baseline sleep quality (AIS scores, and baseline plasma THC and oxytocin levels), (2) to evaluate if sleep disturbance during cannabis abstinence is predictive of more severe cannabis withdrawal symptoms, (3) to explore whether the benzodiazepine nitrazepam results in better subjective and objective sleep patterns during cannabis withdrawal, and (4) to explore whether sleep quality during the first 7 nights of cannabis abstinence is associated with retention in treatment.
METHODS
Cannabis smokers who were dependent according to the DSM-IV criteria were recruited to an RCT testing the effects of lithium vs. placebo on cannabis withdrawal symptoms in an inpatient setting for 7 nights.20
Participants
Inclusion criteria were: 18 years of age or older; DSM-IVR criteria for cannabis dependence22; and self-reported withdrawal symptoms as a barrier to achieving abstinence from cannabis in previous quit attempts. Exclusion criteria were: current alcohol dependence; more than twice weekly use of drugs other than cannabis, caffeine or tobacco, and/or injecting drug use more than once per week in the prior 30 days, current prescriptions (methadone, buprenorphine, mood stabilizing, antidepressant and/or antipsychotic medications); history of schizophrenia, bipolar affective disorder, recent psychosis, significant suicide risk or admission to mental health unit within the 3 months prior to recruitment; significant renal, thyroid, or hepatic disease; abnormal electrocardiogram in subjects aged over 45 years; lactose or lithium intolerance; and for females, current breastfeeding, pregnant, or planning to become pregnant in the month following study entry.
Procedures
Following a medical examination to assess eligibility, subjects signed written informed consent on day 1 upon arrival at the inpatient unit, which triggered randomization procedures to either the lithium or placebo condition. Placebo medication was administered to both groups on day 1 of their inpatient stay, with lithium dosing commencing for that group on the morning of day 2, lasting until the evening of day 7. Follow-up interviews were undertaken 30 days after discharge to assess for relapse to cannabis use by means of self-report and urine analysis. Study procedures were approved by Royal Prince Alfred Hospital Human Research Ethics Committee (09/RPAH/274) and were in accordance with the 1964 Declaration of Helsinki. The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR) (12610000182099).
Medications
Participants were randomized to receive lithium carbonate 500 mg or placebo (lactose) twice a day (10:00 and 18:00) on Days 2 to 7 of admission. Nitrazepam 10 mg oral as a hypnotic was available on up to 3 nights of the 7-night admission for those participants who had sleep disturbance preventing them from falling asleep within 3 h of unit bedtime, and for whom standard behavioral sleep hygiene measures had proved unsuccessful. There was no strict lights out regime enforced on the unit, although all patients were expected to be in their bedrooms by 23:30. All subjects were given ad hoc advice from nursing staff on sleep hygiene and the potential beneficial effects of minimizing caffeine consumption and maintaining a regular sleep pattern.
Measures
Objective Sleep Patterns using Actigraphy
An actiwatch is a watch-like device containing an accelerometer which records movement over time, and is used extensively in sleep research and circadian rhythm disorders.23 Since the publication of the last American Academy of Sleep Medicine (AASM) practice parameters on the use of actigraphy in 2003, there has been a huge increase in the use of actigraphy. We used the actiwatch to measure sleep efficiency, fragmentation, duration (total sleep time), sleep onset latency, and length of wake and sleep bouts for each of the 7 nights of cannabis abstinence on the ward. Actiwatches were fitted at randomization on day 1, and were worn continuously, day and night, for the duration of the patient's time in the unit. This means that all objective actigraphy outcomes (sleep efficiency, sleep fragmentation, sleep duration, etc.) were measured on a daily basis during the inpatient stay.
Cannabis Withdrawal and Subjective Sleep Patterns
The severity of cannabis withdrawal symptoms (overall withdrawal and separate sleep symptoms– woke up early, nightmares/strange dreams, woke up with night sweats, and trouble getting to sleep) was quantified daily on a 10-point self-report scale (0 = Not at all, 10 = Extremely) for each prior 24-h period using the Cannabis Withdrawal Scale (CWS).4 Subjective sleep assessment at baseline for the week prior to admission was assessed using the Athens Insomnia Scale (AIS).24 The AIS is a commonly used, validated global insomnia symptom questionnaire designed to assess the severity of insomnia based on the ICD-10 diagnostic criteria. It is a self-reported questionnaire consisting of 8 items; the first 5 items assess difficulty with sleep induction, awakening during the night, early morning awakening, total sleep time, and overall quality of sleep, while the last 3 items pertain to the sense of well-being, overall functioning and sleepiness during the day. The usual time frame for responding is the last month. Each item of AIS can be rated 0–3, with 0 corresponding to no problem at all and 3 to very serious problem. The AIS can identify insomniacs with 86% positive predictive validity if a score of 6 or above is given. Mood was assessed using the Depression, Anxiety and Stress Scale (DASS) – 21,25 also at baseline.
Drug Use and Plasma Assays
Self-reported cannabis use was quantified at baseline as the average grams used per day over the previous week using a modified timeline follow-back interview.26 Self-report data was supported by plasma assays of THC levels. THC at baseline was extracted from 0.5 mL plasma samples using Styre Screen SSTHC063 solid phase extraction columns (60 mg/3 mL) from United Chemical Technologies (Pennsylvania, USA) as per manufacturer instructions. Extracts were dried under nitrogen and reconstituted in 50 μL initial mobile phase (40% methanol and 60% 10 mM ammonium acetate) for analysis. Chromatographic separation was performed on a Pinnacle DB Biphenyl column (100 mm × 2.1 mm, 1.9 μm) from Restek Inc (Pennsylvania, USA) via gradient elution at 0.3 mL/min using a Shimadzu Nexera ultra high performance liquid chromatograph (Shimadzu Corp, Kyoto, Japan). Analytes and internal standard ion transitions were acquired via multiple reaction monitoring using a Shimadzu 8030 triple quadrupole mass spectrometer operated in positive atmospheric pressure chemical ionization mode. QC samples covered concentration ranges 0.5–20 ng/mL for THC, 5–200 ng/mL for THC COOH. The limits of quantification were 0.5 and 5 ng/mL, respectively.
Baseline plasma oxytocin levels were assayed using a commercially available oxytocin ELISA kit (ENZO Life Sciences, Ann Arbor, MI). All blood samples were collected in chilled EDTA-treated tubes, centrifuged at 4°C and plasma stored at −80°C. Unextracted samples were diluted 1:4 times in assay buffer.27,28 The oxytocin assay had a sensitivity of 15.7 pg/mL, and intra- and inter-assay coefficient of variation below 10% and 15.5%, respectively.
Retention in Treatment
Retention in treatment was defined as number of nights of inpatient treatment completed (range: 0–7).
Statistical Analysis
To test the effect of treatment allocation (lithium vs. placebo) on sleep patterns, mixed models for repeated measures (MMRMs) were used with an autoregressive covariance structure (AR1).29–31 Separate models were constructed for each sleep quality outcome measure as a dependent variable (i.e. daily scores from the objective actigraphy measures and CWS sleep measures were used as repeated measures outcomes in the MMRM). Independent variables tested were time in treatment (day) and treatment group (lithium vs. placebo) and their interaction. Baseline Athens Insomnia Scale (AIS) scores and baseline plasma THC and oxytocin levels were controlled for in each model but were removed if not a significant predictor. Statistics are reported for the interaction terms of treatment day and treatment group allocation only, unless only a main effect was significant. In the case of significant interactions post hoc analyses explore the interaction adjusting for multiple comparisons using the Bonferroni method.
To assess the effects of sleep patterns on the severity of cannabis withdrawal symptoms, mixed models were again employed with separate models constructed for each DSM5 cannabis withdrawal symptom as dependent variable (obtained from daily CWS data), testing the effects of the independent variables of time in treatment (days), the actiwatch sleep variables: efficiency, fragmentation, duration (total sleep time), onset latency, length of wake and sleep bouts and their interaction with time. All models controlled for experimental drug treatment (lithium vs. placebo), the use of nitrazepam, and baseline sleep quality measured by the AIS.
To test whether the use of nitrazepam resulted in better sleep patterns MMRMs were used with each objective measure of sleep quality as a dependent variable. Day of nitrazepam use and a binary nights using vs. not using nitrazepam were used as the independent measures.
To test the effects of sleep quality during cannabis abstinence on retention in treatment, a cox regression was performed with number of days in treatment as the dependent time variable being predicted, censored at day 7 (last day in the inpatient unit), with predictor variables consisting of daily repeated measures of actiwatch data, including sleep efficiency, fragmentation, duration (total sleep time), sleep onset latency, length of wake and sleep bouts, wake after sleep onset (WASO), and baseline AIS scores as covariates. Daily acti-watch predictor data were entered into the model separately for individual patients in the study, but the algorithm underneath a Cox regression takes averages across people to deliver an overall model response, as with most other inferential statistics. All models controlled for treatment group (lithium vs. placebo) and the use of nitrazepam.
RESULTS
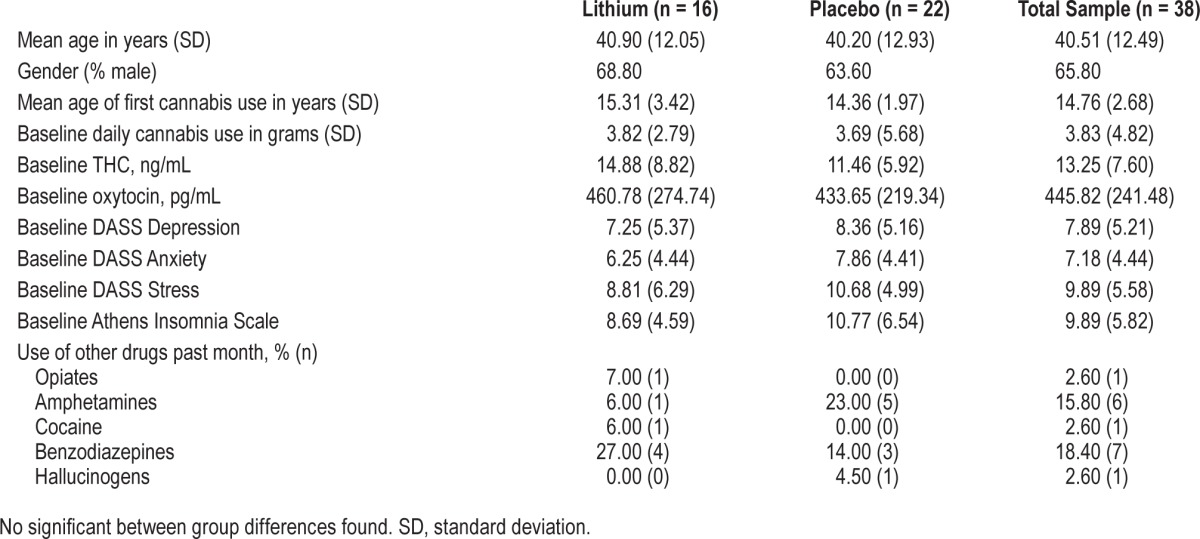
A total of 38 subjects were randomized (placebo n = 17, lithium n = 21) on day 1, and by day 8, only 17 subjects (45%) remained in the hospital (placebo n = 9, lithium n = 8). The benzodiazepine medication nitrazepam was used to aid sleep on at least one occasion during the inpatient stay by 68% (n = 26) of subjects (placebo 82%, n = 14; lithium 57%, n = 12). Demographics and cannabis and other drug use characteristics are listed in Table 1. Baseline AIS scores upon entry into the study were in the high insomnia range for this patient population (9.89 [standard deviation; SD 5.82]), and there was no difference between lithium and placebo treatment groups (lithium = 10.77 [6.54]; Placebo = 8.69 [4.59]; F1,36 = 1.19, p = 0.3). Ten of the 38 subjects reported normal sleep patterns on entry to the study, with the remainder scoring in the insomnia range (scores of 6 or above). Baseline Depression, Anxiety and Stress (DASS-21) scale scores were typically in the moderate range and were not different between the groups (see Table 1). Baseline use of cannabis and other drugs was not significantly different between the groups (Table 1).
Table 1.
Demographics, cannabis use, and psychosocial functioning at baseline.
The Effects of Lithium on Sleep Quality
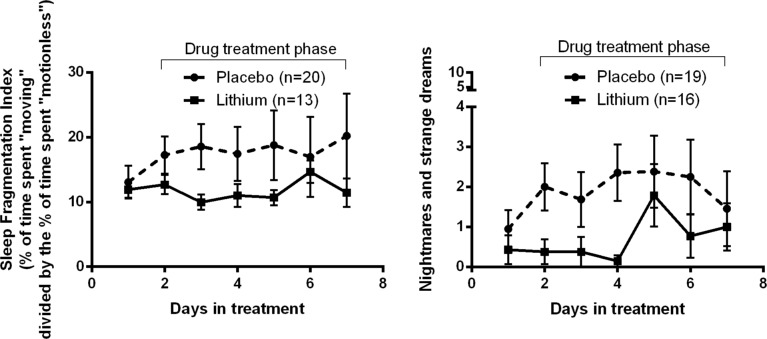
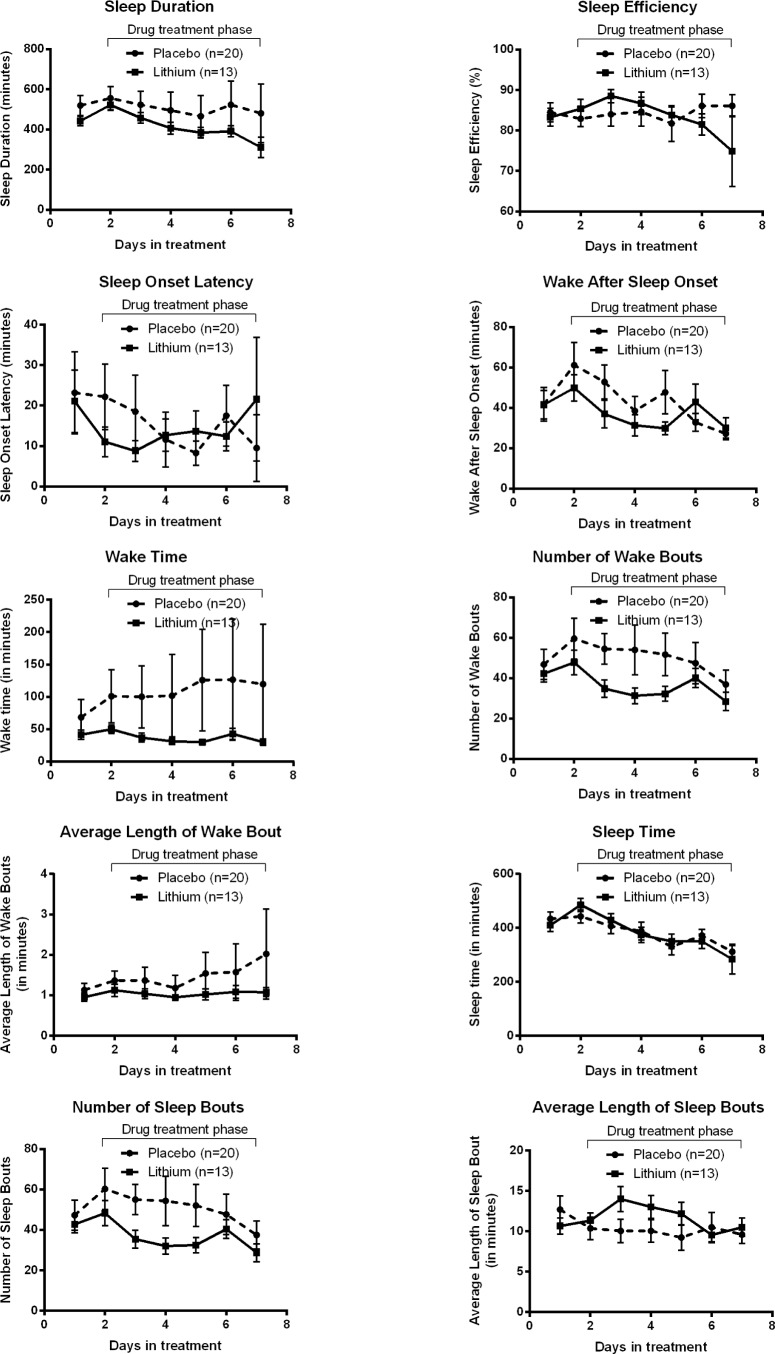
First looking at actigraphy measures of sleep quality, subjects receiving lithium had a significantly less fragmented sleep pattern (F6, 139.54 = 2.23, p = 0.04) than subjects receiv -ing the placebo (Figure 1). None of the other actigraphy sleep measures were significantly different between lithium and placebo allocations, including wake after sleep onset (WASO; F6, 121.18 = 1.31, p = 0.26), total wake time (F6, 143.38 = 1.64, p = 0.14), sleep duration (F6, 138.83 = 0.39, p = 0.89), sleep onset latency (F6, 108.39 = 0.46, p = 0.84), sleep efficiency (F6, 108.61 = 1.89, p = 0.09), average wake bout length (lithium = 1.03 (SD 0.44); placebo = 1.39 (SD 1.53); F6, 137.01 = 0.77, p = 0.59), and average sleep bout length (lithium = 11.69 [SD 4.43]; placebo = 10.57 [SD 5.96]; F6, 129.05 = 1.83, p = 0.09).
Figure 1. Sleep fragmentation (objective) and nightmares and strange dreams (subjective) were significantly suppressed by lithium treatment during inpatient cannabis withdrawal.
Of the subjective sleep measures taken with the daily CWS, only nightmares and strange dreams were suppressed by lithium, with a significant main effect of drug treatment (F1, 46.78 = 4.32, p = 0.04; Figure 2), but not the interaction of treatment with time (F6,141.24 = 0.94, p = 0.47). None of the other subjective sleep ratings were significantly impacted by lithium treatment, including overall insomnia (F6, 139.25 = 0.62, p = 0.71), waking up early (F6, 137.65 = 0.34, p = 0.91), night sweats (F6, 137.12 = 0.64, p = 0.7), or trouble getting to sleep (F6, 134.72 = 1.39, p = 0.22).
Figure 2. All other objective sleep measures from the actiwatch plotted against time in treatment, split by drug treatment group. None of these measures were significantly influenced by drug treatment.
Baseline self-reported sleep quality (AIS scores) and baseline plasma THC and oxytocin levels were tested in all models described above but did not significantly predict any measure of sleep quality during cannabis abstinence so were not retained in final models for reasons of parsimony.
Is Nitrazepam Use Associated with Better Sleep Quality?
Fewer subjects on lithium opted to use the sleep rescue medication nitrazepam during their inpatient stay (n = 12/21; 57%) than those on placebo (n = 14/17; 82%); however, the trend did not reach statistical significance (χ2 = 2.76, p = 0.09). Of subjects using nitrazepam, the placebo group used nitrazepam on a mean of 2.46 (SD 0.88) nights compared to lithium subjects, who took nitrazepam on a mean of 2.83 (SD 0.94) nights (F1,23 = 1.05, p = 0.32).
According to the objective actigraphy data, those nights when nitrazepam was consumed resulted in significantly better sleep quality (Table 2), including improvements in total sleep duration, sleep efficiency, and sleep fragmentation. Importantly the use of nitrazepam resulted in a whole hour more time asleep compared to those nights when nitrazepam was not taken (Table 2). While the remaining actiwatch measures were not statistically significantly different between nitrazepam nights and no nitrazepam nights, they all were in the direction of improved sleep outcomes while taking nitrazepam (Table 2).
Table 2.
Differences in sleep quality measures on nights when nitrazepam was consumed vs. no nitrazepam nights.
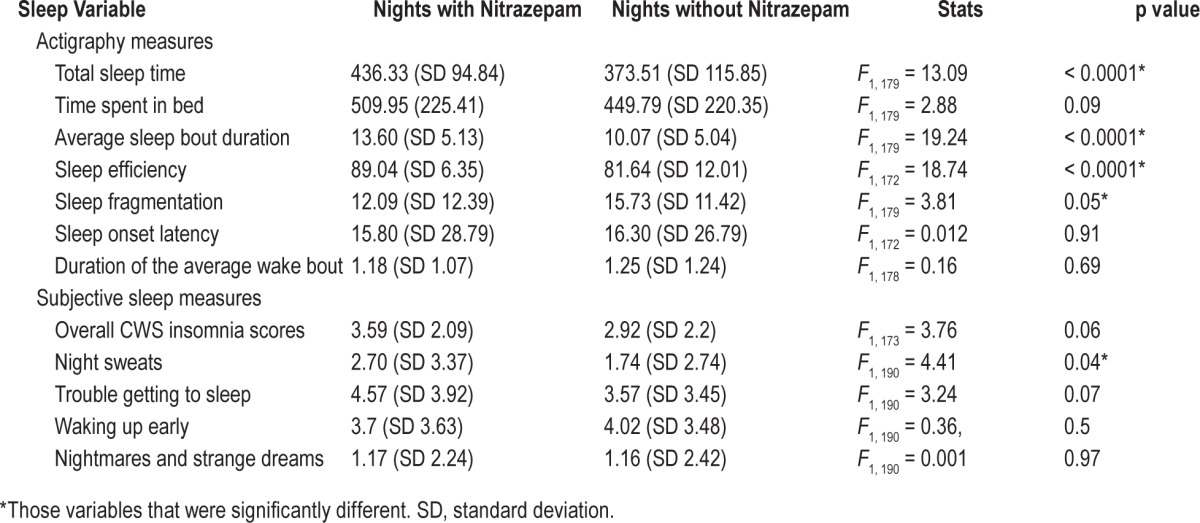
Only night sweats from the subjective measures of sleep quality obtained from the daily CWS were reported as being significantly different for the nights that nitrazepam was used (Table 2). There was a trend for lower overall insomnia scores (F1, 173 = 3.76, p = 0.06) and trouble getting to sleep (F1, 190 = 3.24, p = 0.07) when nitrazepam was used, although these subjective variables were not significant.
Sleep Quality and the Severity of Cannabis Withdrawal
None of the objective measures of sleep quality studied with the actiwatch were significant predictors of overall cannabis withdrawal score (p = 0.18–0.91). Similarly when cannabis withdrawal is broken down into its constituent DSM5 components, none of the objective sleep measures predicted subjective withdrawal features, including cravings for cannabis, restlessness, depression, physical symptoms (e.g., headache, stomachache), anxiety, insomnia, or irritability.
Sleep Quality and Retention in Treatment
A Cox regression revealed that the average daily sleep efficiency (total sleep time in min/total time in bed in min × 100/1, expressed as a percentage) measured repeatedly for all patients during their inpatient stay, was a significant predictor of retention in treatment in this study. Specifically, the cox regression model outputs, which yield average parameter estimates across all patient data, suggests that a one % increase in sleep efficiency resulted in a 14.6% increase in retention in treatment (Wald(1) = 6.9, p = 0.008, Exp(B) = 0.854, 95% CI = 0.759–0.960). None of the other sleep measures recorded were significantly associated with retention in treatment (all p values > 0.05), nor were the use of lithium (p = 0.8), nitrazepam (p = 0.52), or baseline sleep quality measured using the AIS (p = 0.51).
DISCUSSION
This study compares objective and subjective sleep quality in cannabis-dependent treatment seekers during a 7 day inpatient cannabis detox. Sleep disturbances are one of the most severe and enduring symptoms of cannabis withdrawal,4,5,32,33 and there are no evidence-based treatment options to target this pervasive withdrawal symptom. This patient population entered our study reporting significant self-rated insomnia symptoms despite using cannabis right up until study entry (i.e., average AIS score 9.89 [SD 5.82] when a cutoff of 6 is considered predictive of insomnia).
Effects of Lithium on Sleep Quality
In this study we found that the original target of our investigation, lithium carbonate, improved sleep fragmentation and nightmares during cannabis abstinence (Figure 1)—but did not affect a range of other measures of sleep quality (Figure 2). Actigraphic sleep fragmentation can be thought of as a measure of restlessness during the sleep period expressed as a percentage the sleep period spent moving (an defined time period with more than 2 activity counts is considered moving), and the percentage of the number of immobile phases (i.e., an defined time period with no movement). Fragmentation is an indicator of sleep disruption or disturbance34 and closely approximates the polysomnography measure of microarousals, which are associated with poor health outcomes.35 While the findings that most sleep quality measures were not affected by lithium treatment is in line with our previous analysis of poor efficacy of lithium carbonate for the overall cannabis withdrawal indication,20 the effects on fragmentation and nightmares does suggest a possible limited role of lithium in rectifying sleep disturbances during cannabis abstinence treatment. Sleep quality parameters during cannabis abstinence were not affected by the overall quality of self-reported sleep at study entry (baseline AIS scores), nor by baseline measures of plasma THC or oxytocin, similar to their effects on cannabis withdrawal in general.20 These factors may not have influenced sleep during cannabis abstinence owing to limited variability in this population.
Looking in more detail at sleep fragmentation, it is of note that the level of sleep fragmentation in this population was not particular high when compared to clinical sleep populations (e.g., insomnia patients).36 As such the significant effects of lithium on sleep fragmentation in this study may suggest that lithium may have clinical benefits in cannabis withdrawal for people with more severe fragmentation at baseline than that observed in this population. So it would be of great interest for future studies to quantify sleep fragmentation during a baseline period prior to commencing treatment for cannabis withdrawal. A 2-week actigraphy data collection is standard for clinical data collection. It may be that lithium becomes more of an effective treatment option for those individuals entering the study with preexisting insomnia levels of sleep fragmentation (i.e., > 25). Sleep fragmentation may be a particularly pertinent objective indicator of the severity of cannabis withdrawal, as it is frequently associated with periodic leg movement, which is increased in heavy cannabis users37 and during acute cannabis withdrawal.12 The effects of lithium on suppressing withdrawal related nightmares and strange dreams is of interest as increased REM sleep has been documented during cannabis withdrawal, explaining the occurrence of increased dream state.12
Is Nitrazepam Use Associated with Better Sleep Quality?
The use of the hypnotic benzodiazepine nitrazepam was only intended as a “rescue” medication in this study, with a limited 10 mgs available on only three of the seven inpatient nights to avoid possible abuse and/or effects on withdrawal.38 Our secondary analysis demonstrated substantial improvements to sleep efficiency, length of sleep bouts and sleep fragmentation on nights when nitrazepam was used (Figure 3). While other sleep quality indicators were not necessarily significant in the models, most were affected in a positive direction (i.e., improved sleep) by the use of nitrazepam and sample size and power problems may explain lack of more significant results here. While this study was not designed to directly examine the efficacy of nitrazepam upon sleep in cannabis withdrawal, our findings point towards potential benefits of using benzodiazepines for this indication, consistent with previous work of Vandrey and colleagues with the benzodiazepine-like medication zolpidem. These findings suggested further research is warranted to examine the role of benzodiazepines for assisting sleep during cannabis withdrawal. Of interest, the positive effects of nitrazepam on objective measures of sleep quality using the actigraphy were not reflected in the subjective interpretations of the cannabis users own sleep quality. This finding has implications for any future research that attempts to study the effects of interventions on sleep quality during cannabis abstinence, as the majority of existing studies into the phenomena use subjective measures. It may be that while sleep was better on nitrazepam, the requirement to have tried to get to sleep for 3 hours prior to nitrazepam administration means that the sleep was still relatively poor (an average of 7 h sleep on nitrazepam (Table 2), which is at the low end of recommended sleep duration for adults).
Figure 3. Nights when nitrazepam “rescue” medication was used had significantly better objective indicators of sleep quality for length of sleep bouts, sleep efficiency and sleep fragmentation.
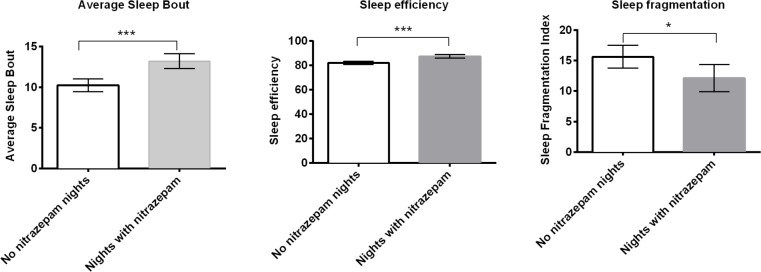
*p ≤ 0.05, ***p ≤ 0.0001.
Sleep Quality and the Severity of Perceived Cannabis Withdrawal
Despite the fact that the use of objective actigraphy data is more precise and accurate than subjective measures of sleep quality39 during cannabis abstinence, actiwatch measures of sleep quality did not predict the severity of subjective cannabis withdrawal symptoms. This is somewhat surprising given that the objective data presented show evidence of fragmented and disturbed sleep during cannabis abstinence, in support of previous studies.10 Such observations are typically associated with negative health outcomes, including mental health disturbances such as increased depression and anxiety,40 two prominent cannabis withdrawal symptoms. It is possible that there may have been insufficient variation between patients objective sleep measures to predict subsequent cannabis withdrawal symptoms, or possibly that withdrawal was not as pronounced in this carefully controlled inpatient setting owing to lack of potential triggers of withdrawal related phenomena typically found in the outpatient environment.4
Sleep Quality and Retention in Treatment
Sleep efficiency predicted retention in treatment, with a 14.6% increase in retention for every percentage unit increase in sleep efficiency. While sleep efficiency was not significantly affected by lithium treatment in this study, it was significantly improved at clinical levels on those nights when nitrazepam was taken (along with a range of other sleep measures). Although nitrazepam use in and of itself did not directly predict retention in treatment, these findings suggest that it could potentially be a mediator of sleep efficiency and its effects on retention in cannabis treatment, warranting further investigations in larger samples and with a more systematic approach to the targeted use of nitrazepam.
Limitations
One of the major limitations of this study is the low sample size which restricts the analyses that can be performed. Clearly some positive effects of nitrazepam on sleep variables during cannabis withdrawal have been highlighted, such as objective sleep efficiency, fragmentation and average sleep bout length. However the analysis also showed effects of lithium on a very narrow set of sleep parameters (fragmentation and nightmares). The limited sample size produces highly unstable and unreliable models when three way interactions are explored, yet there is an obvious need to explore higher-level interactions to fully understand the effect of these drugs in combination. Another notable limitation that pertains specifically to our findings around nitrazepam are that the study protocol to some extent confounds interpretation, as subjects were instructed that they could only have access to the three nights of available doses of nitrazepam on nights when they were experiencing significant problems sleeping. Nevertheless the fact that objective sleep parameters were better on those nights suggests that nitrazepam use may be a particularly potent treatment for cannabis withdrawal induced sleep disturbances. However, nitrazepam would need to be implemented in a well-controlled environment and for a short sustained period only to avoid dependence and potential abuse.
Giving individuals in this study a better understanding of what could be expected in relation to their sleep during the withdrawal process is potentially beneficial. In this study only basic sleep hygiene information was given ad hoc and predominantly relating to caffeine/alcohol consumption and some sleep timing. In a review exploring the relationship between sleep disorders and substance abuse only 16 papers were identified involving the treatment of drug or alcohol dependence.41 Samples were predominantly small with only one with > 30 patients and only one randomized controlled trial. There was no evidence that insomnia treatment prevented subsequent relapse. The need for large randomized double blind studies was emphasized along with consideration of genetic differences in insomnia vulnerability and common traits of psychological dependence relating to other forms of insomnia. Kaplan et al. set out a cognitive behavioral therapy regime in the recovery period based on how common insomnia is during this process and if persistent after abstinence predicts relapse.38 Such interventions need to be a core management component in improving quality of life short and long term. One more potential limitation of this study may be related to the use of only a very limited range of assessments to obtain subjective measures of sleep during the treatment phase (i.e., this study only used 4 questions on sleep from the Cannabis Withdrawal Scale on a daily basis during treatment). It may be that the use of daily sleep diaries or repeat AIS measures throughout treatment could have provided more useful subjective information for correlating to actiwatch measures than the sleep items on the Cannabis Withdrawal Scale.42
CONCLUSIONS
In summary, this work suggests that lithium treatment may not be cost effective for treating cannabis withdrawal as only a small subset of sleep related dysfunction were improved and there was no observed influence on retention in treatment. In contrast, nitrazepam resulted in significant suppression of withdrawal related sleep dysfunction according to objective data collected using the actiwatch. This finding adds support to previous work suggesting also that hypnotic sleep medications may be useful adjuncts to cannabis withdrawal treatment.10 The disconnect between objective sleep indicators and subjectively reported sleep quality in this study suggests that future research in the area of sleep during cannabis treatments may need to go beyond self-reported sleep quality to accurately assess the impact of interventions. While polysomnography is the gold standard for objectively quantifying sleep disturbances, it is a logistically difficult approach demanding much of the patient and is only a one night “snapshot” of sleep. Actigraphy data is a more cost effective alternative,43 especially for longitudinal studies and those in the outpatient environment.
DISCLOSURE STATEMENT
This was not an industry supported study. Lithium was trialled in this study as an off-label experimental drug intervention. This study was funded by the National Health and Medical Research Council (NHMRC Project Grant 556301) and sponsored by The University of Sydney, Camperdown, NSW, Australia. The NHMRC played no role in the design of the study, collection and analysis of the data or the decision to publish. Dr. Lintzeris has recieved research support from and participated in speaking engagements for Reckitt Benckster. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the staff and participating clients from Riverlands Drug and Alcohol Centre, Lismore, NSW, Australia.
REFERENCES
- 1.Bonn-Miller MO, Babson KA, Vujanovic AA, Feldner MT. Sleep problems and PTSD Symptoms interact to predict marijuana use coping motives: a preliminary investigation. J Dual Diagn. 2010;6:111–22. [Google Scholar]
- 2.Babson KA, Boden MT, Harris AH, Stickle TR, Bonn-Miller MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. J Subst Abuse Treat. 2013;44:438–43. doi: 10.1016/j.jsat.2012.08.224. [DOI] [PubMed] [Google Scholar]
- 3.Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2010;74:928–33. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Allsop DJ, Copeland J, Norberg MM, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–8. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copersino ML, Boyd SJ, Tashkin DP, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- 8.Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA. Delta9-tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend. 2010;106:65–8. doi: 10.1016/j.drugalcdep.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71:281–91. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 10.Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38–44. doi: 10.1016/j.drugalcdep.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haney M, Hart CL, Vosburg SK, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2010;211:233–44. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolla KI, Lesage SR, Gamaldo CE, et al. Sleep disturbance in heavy marijuana users. Sleep. 2008;31:901–8. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla KI, Lesage SR, Gamaldo CE, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010;11:882–9. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liappas J, Paparrigopoulos T, Tzavellas E, Christodoulou G. Alcohol detoxification and social anxiety symptoms: a preliminary study of the impact of mirtazapine administration. J Affect Disord. 2003;76:279–84. doi: 10.1016/s0165-0327(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 15.Cui S, Bowen RC, Gu G, Hannesson DK, Yu PH, Zhang X. Prevention of cannabinoid withdrawal syndrom by lithium: involvement of oxytocinergic neuronal activation. J Neurosci. 2001;21:9867–76. doi: 10.1523/JNEUROSCI.21-24-09867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winstock A, Lea T, Copeland J. Lithium carbonate in the management of cannabis withdrawal in humans: an open-label study. J Psychopharmacol (Oxf) 2009;23:84–93. doi: 10.1177/0269881108089584. [DOI] [PubMed] [Google Scholar]
- 17.Braga R, Panaitescu A, Badescu S, Zagrean A, Zagrean L. Intranasal administration of oxytocin alters sleep architechture. Biol Rhythm Res. 2014;45:69–75. [Google Scholar]
- 18.McCall WV, Blocker JN, D'Agostino R, Jr., et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11:822–7. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billiard M. Lithium carbonate: effects on sleep patterns of normal and depressed subjects and its use in sleep-wake pathology. Pharmacopsychiatry. 1987;20:195–6. doi: 10.1055/s-2007-1017102. [DOI] [PubMed] [Google Scholar]
- 20.Johnston J, Lintzeris N, Allsop DJ, et al. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient setting. Psychopharmacology (Berl) 2014;231:4623–36. doi: 10.1007/s00213-014-3611-5. [DOI] [PubMed] [Google Scholar]
- 21.Ellingsen P. Double-blind trial of triazolam 0.5 mg vs. nitrazepam 5 mg in outpatients. Acta Psychiatr Scand. 1983;67:154–8. doi: 10.1111/j.1600-0447.1983.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 24.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–60. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 25.Lovibond SH, Lovibond PF. Manual for the depression anxiety and stress scales. 2nd ed. Sydney: Psychology Foundation; 1995. [Google Scholar]
- 26.Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the Timeline Followback approach: a psychometric evaluation. Drug Alcohol Depend. 2012;121:247–52. doi: 10.1016/j.drugalcdep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Carson DS, Bosanquet DP, Carter CS, Pournajafi-Nazarloo H, Blaszczynski A, McGregor IS. Preliminary evidence for lowered basal cortisol in a naturalistic sample of methamphetamine polydrug users. Exp Clin Psychopharmacol. 2012;20:497–503. doi: 10.1037/a0029976. [DOI] [PubMed] [Google Scholar]
- 28.Szeto A, McCabe PM, Nation DA, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell ML, Fairclough DL. Practical and statistical issues in missing data for longitudinal patient reported outcomes. Stat Methods Med Res. 2014;23:440–59. doi: 10.1177/0962280213476378. [DOI] [PubMed] [Google Scholar]
- 30.Mallinckrodt CH, Clark SW, Carroll RJ, Molenbergh G. Assessing response profiles from incomplete longitudinal clinical trial data under regulatory considerations. J Biopharm Stat. 2003;13:179–90. doi: 10.1081/BIP-120019265. [DOI] [PubMed] [Google Scholar]
- 31.Rubin D. Inference and missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 32.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–8. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 33.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 34.Loewen A, Siemens A, Hanly P. Sleep disruption in patients with sleep apnea and end-stage renal disease. J Clin Sleep Med. 2009;5:324–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas RJ. Sleep fragmentation and arousals from sleep-time scales, associations, and implications. Clin Neurophysiol. 2006;117:707–11. doi: 10.1016/j.clinph.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–65. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan KA, McQuaid J, Primich C, Rosenlicht N. An evidence-based review of insomnia treatment in early recovery. J Addict Med. 2014;8:389–94. doi: 10.1097/ADM.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 39.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Stores G. Clinical diagnosis and misdiagnosis of sleep disorders. J Neurol Neurosurg Psychiatry. 2007;78:1293–7. doi: 10.1136/jnnp.2006.111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth T. Does effective management of sleep disorders reduce substance dependence? Drugs. 2009;69(Suppl 2):65–75. doi: 10.2165/11531120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school-aged children using actigraphy: concordance with polysomnography. J Sleep Res. 2011;20:223–32. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]