Abstract
Study Objectives:
To perform a meta-analysis of the effect of wakefulness-promoting agents (modafinil and armodafinil) in patients with residual sleepiness after CPAP therapy for obstructive sleep apnea.
Methods:
We conducted a systematic search of MEDLINE (1966 to September 2014), EMBASE (1980 to September 2014) and Cochrane Database for randomized placebo controlled trials on modafinil or armodafinil in patients who met established criteria for diagnosis of obstructive sleep apnea, adequate continuous positive airway pressure use, and who complained of residual sleepiness. Risk of bias was assessed. Primary outcomes were the Epworth Sleepiness Scale and mean sleep latencies on the maintenance of wakefulness test. Secondary outcomes were the Clinical Global Impression of Change, change in daily continuous positive airway pressure use, and the frequency of headaches.
Results:
Out of 118 abstracts screened and 12 full text articles reviewed, we included 6 studies (total of 1,479 participants) in our final meta-analysis: Three evaluated modafinil, and three armodafinil. Risk of bias was unclear in one or more key domains for four studies. When compared with placebo, wakefulness promoting agents decreased Epworth Sleepiness Scale by 2.51 points (95% CI, 2.00–3.02), increased sleep latency in maintenance of wakefulness test by 2.73 minutes (95% CI, 2.12–3.34), increased the reporting of minimal improvement on the Clinical Global Impression of Change by 26% (RR 1.59; 95% CI, 1.36–1.86), and increased the risk of headaches by 8% (RR 1.98; 95% CI, 1.48–2.63). Also, there was a trend for decreased continuous positive airway pressure after treatment with these agents.
Conclusion:
Wakefulness promoting agents improve objective and subjective measures of sleepiness, wakefulness, perception of disease severity in patients with residual sleepiness after CPAP therapy for OSA, and are generally well tolerated.
Citation:
Sukhal S, Khalid M, Tulaimat A. Effect of wakefulness-promoting agents on sleepiness in patients with sleep apnea treated with CPAP: a meta-analysis. J Clin Sleep Med 2015;11(10):1179–1186.
Keywords: modafinil, armodafinil, residual sleepiness, sleep apnea, CPAP
Obstructive sleep apnea (OSA) is a syndrome of recurrent, partial, or complete upper airway collapse during sleep. Excessive daytime sleepiness, fatigue, and altered attention are common symptoms of OSA. It is recognized as an independent risk factor for cardiovascular disease, reduced quality of life, and motor vehicle accidents.1
While several randomized controlled trials have shown that continuous positive airway pressure (CPAP) reduces sleepiness, residual sleepiness still occurs in up to 13% of patients adequately treated with CPAP.2 According to a large multi-center French registry, the prevalence of residual sleepiness in OSA with CPAP use > 3 h/night was 13%, and > 6 h/night was 9%. At the time of diagnosis, patients with residual sleepiness had worse subjective appreciation of their health, felt more fatigued, and complained more frequently of CPAP side effects.3
The mechanism of residual sleepiness despite adequate treatment of OSA remains unknown. One proposed mechanism is that these patients are more susceptible to intermittent hypoxia than patients that are not sleepy and suffer injury to specific neuronal systems that promote wakefulness.4
Currently, modafinil and armodafinil (the longer lasting R-enantiomer of racemic modafinil) are the only wakefulness promoting agents (WPA) approved as adjuvant pharmacotherapy for residual sleepiness in OSA. Their mechanism of action is uncertain, but it is postulated that they modulate glutamate, gamma-aminobutyric acid, histamine, hypocretin, and monoamines in a complex neuronal network which arises in the midbrain reticular formation and innervates the hypothalamus, thalamus, and basal forebrain.5 They are better tolerated and are less addictive than other CNS stimulants. They have been successfully used to reduce sleepiness in patients with narcolepsy and idiopathic hypersomnia.6
This success evoked interest for testing them in treating residual sleepiness in patients with OSA. The effects of WPAs on residual sleepiness in patients with OSA has been studied in a variety of settings and led to the approval by the United States Food and Drug Administration in 2007.7 On the other hand, the European Medicines Agency has restricted the use of modafinil to narcolepsy because the evidence for use in patients with OSA was deemed weak and because of concerns about serious side effects.8
In this review, we aimed to conduct a meta-analysis to evaluate the effect of wakefulness promoting agents (armodafinil and modafinil) in patients with OSA.
METHODS
Data Sources
Using relevant keywords, MeSH terms, and text, we performed a systematic search of MEDLINE (1966 to September 2014) via Ovid, EMBASE (1980 to September 2014) via Scopus, and the Cochrane Central Register of Controlled Trials (see Appendix). We also examined bibliography of included articles to identify additional references.
Study Selection
We considered only double-blind, randomized placebo-controlled trials (RCTs) that compared modafinil or armodafinil to placebo, in adults who met established criteria for the diagnosis of OSA, adequate CPAP use, and who complained of residual sleepiness. Open label extension studies, secondary analyses on existing placebo controlled RCTs, patient populations with comorbid psychiatric conditions (other than depression) were excluded. Studies done with less than 30 patients, animal studies, review articles, case reports, and abstracts in languages other than English were also excluded from analysis.
Primary outcomes of interest were the Epworth Sleepiness Scale (ESS) and the mean sleep latency times on the maintenance of wakefulness test (MWT). Secondary outcomes included the Clinical Global Impression of Change (CGI-C), impact of WPAs on CPAP usage, and the frequency of common adverse effects.
Data Extraction and Synthesis
Two authors independently reviewed titles and abstracts of the identified resources. They obtained the full text of all studies of possible relevance for independent assessment. All the authors decided which trials fit the inclusion criteria. The authors resolved any disagreement by consensus. Two authors performed data extraction independently with specific data extraction forms, and the third confirmed the accuracy. Outcome variables and 95% confidence intervals (CI) were derived from each study and summary statistics were applied as appropriate. In cases where these were not reported in the abstract or full text, we extracted them from the figures using Plot Digitizer 2.6.3 software.
Statistical Analysis
A weighted treatment effect was calculated across trials. For continuous variables, the results were expressed as weighted mean differences with 95% CI using inverse variance method. For dichotomous variables, the results were expressed as risk difference and relative risk (RR) with 95% CIs using inverse variance method. Shared placebo group encountered in one study was split equally between two intervention arms. Statistical heterogeneity was identified and measured by the Q and I2 statistic. We used a p value (< 0.01) based on the Q statistic or I2 magnitude ≥ 50% to define significant heterogeneity. Fixed effects model was used for all analysis. Methods based on random effects model were chosen when significant heterogeneity were present. All statistical analysis was done using RevMan 5.3 software.
Assessment of Risk of Bias
Two authors independently assessed risk of bias in the included studies with specific focus on random sequence generation and treatment allocation concealment. Risk of bias was rated “low” if method of random sequence generation was described, “high” if non-random methods were used for selection, and “unclear” if it was not described. Similarly, we rated risk of performance bias as “low,” “high,” or “unclear,” depending on whether appropriate methods to conceal treatment allocation were described. We also assessed the adequacy of blinding of participants, personnel, and outcome assessors. Disagreements were resolved by consensus.
RESULTS
Study Selection
Out of 118 abstracts screened and 12 full text articles reviewed, we included 6 studies9–14 with a total of 1,479 participants, in our final meta-analysis (Figure 1). We excluded open label extension studies, and studies in which secondary analysis was done on an existing data set to avoid duplication of patients. All RCTs excluded patients with psychiatric comorbidities except Krystal et al.13 They specifically studied patients with treated comorbid depression. All studies were performed in Europe and North America except the study by Inoue et al.14 which was performed in Japan.
Figure 1. PRISMA flow diagram for study selection.
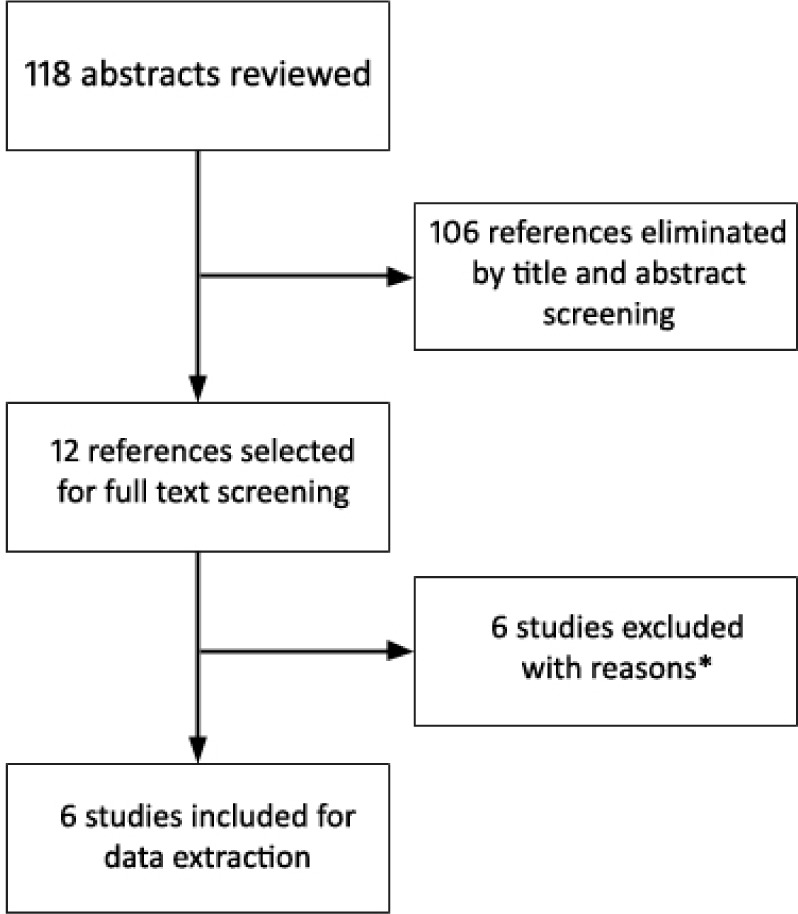
*Four open label extension studies, one with no outcome of interest, one pooled analysis.
Study Characteristics
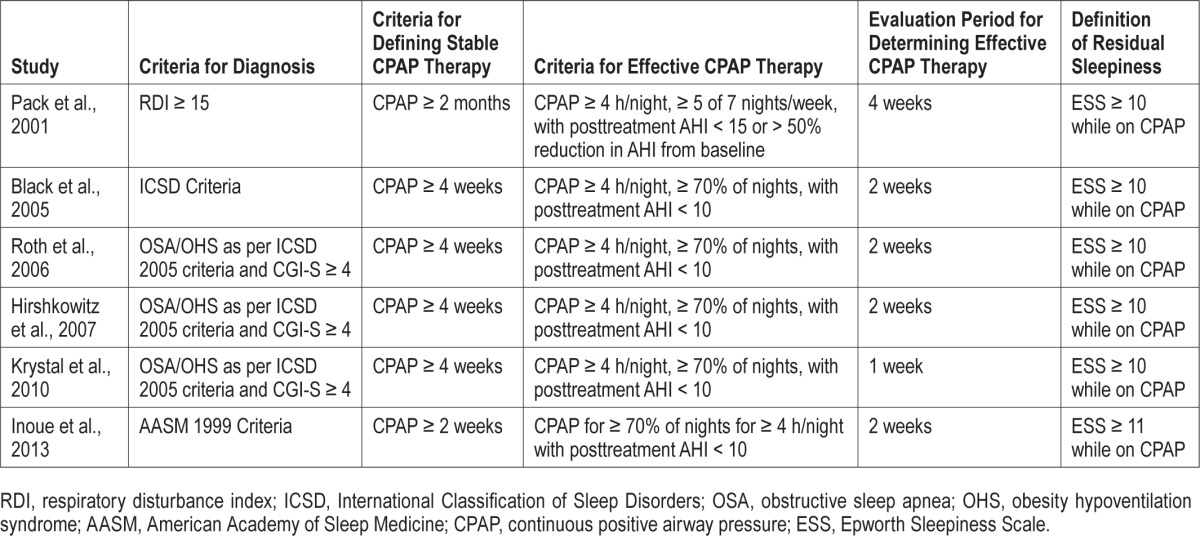
All study participants were adults (age > 18) who had OSA with residual sleepiness and were on stable and effective CPAP therapy. The characteristics of included studies (Table 1), criteria for defining OSA, residual sleepiness, and effective CPAP therapy (Table 2) were similar between all trials. Dosage regimens for modafinil and for armodafinil differed between the trials with the presumed objective of minimizing side effects during the titration to a maximum effective dose.
Table 1.
Characteristics of included studies.
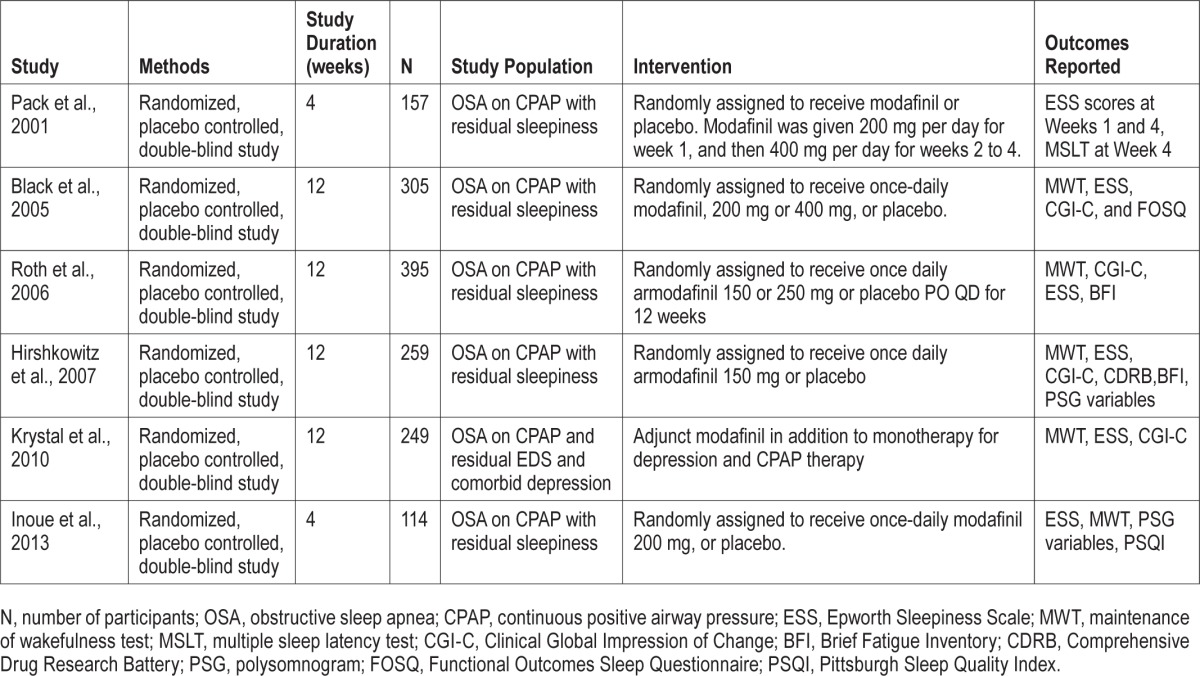
Table 2.
Definitions and criteria for defining OSA, stable and effective CPAP therapy, and residual sleepiness used in the RCTs studied.
Study Quality
Risk of bias was rated unclear in one or more key domains in four of these studies (Figure 2). Pack et al.9 did not mention the method of random allocation. For Black et al.,10 it was unclear if participants and personnel could guess the allocation based on dosage regimen of the study drug. Hirshkowitz et al.12 did not mention if outcome assessment was blinded. For Krystal et al.,13 initial outcome visit was not blinded, and it is not mentioned if placebo dose was also titrated to mimic armodafinil dose titration in the study group.
Figure 2. Risk of bias summary.
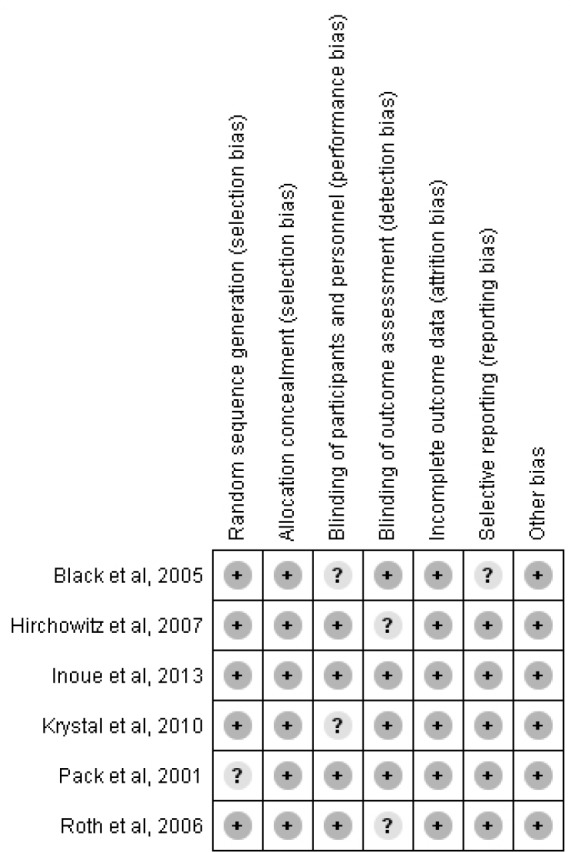
+ = low risk. ? = unclear risk.
Outcomes
Primary Outcomes
EPWORTH SLEEPINESS SCALE (ESS)
This outcome was studied and reported by all 6 RCTs. The mean difference was 2.51 points (95% CI, 2.00–3.02), in favor of WPAs (Figure 3). There was no significant heterogeneity (I2 = 26%, p value of Q statistic 0.23). Data for this outcome was derived from graphs for Pack et al.9 and Hirshkowitz et al.12
Figure 3. Forest plot for Epworth Sleepiness Scale.
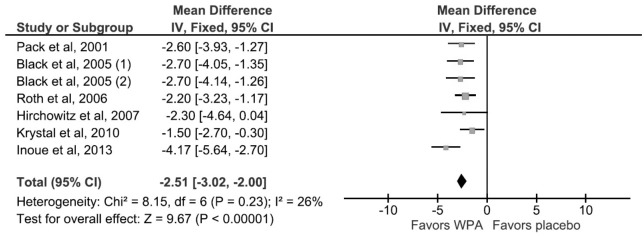
For Pack et al, 2001 and Hirshowitz 2007, standard error of mean (SEM) was derived from graphical data presented by the authors. Black et al, 2005 used a shared placebo group between two different dosage arms of modafinil, 200 mg daily and 400 mg daily, indicated by (1) and (2) in the figure. The shared placebo group was split equally between the two arms for the meta-analysis of continuous variables. WPA, wakefulness promoting agent.
MEAN SLEEP LATENCY TIME IN MAINTENANCE OF WAKEFULNESS TEST (MWT)
This outcome was studied and reported by 5 RCTs. The mean difference was 2.87 minutes (95% CI, 1.86–3.88) in favor of WPAs (Figure 4). There was no significant heterogeneity (I2 = 0%, p value of Q statistic 0.61).
Figure 4. Forest plot for mean sleep latency on the maintenance of wakefulness test.
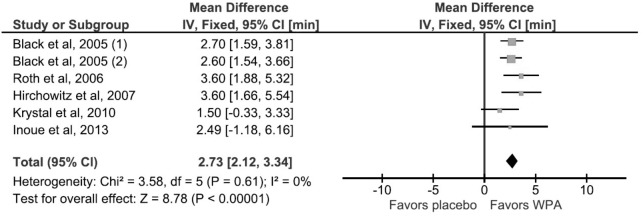
For Black et al, 2005, standard deviation (SD) was derived from p value and sample size in the experimental and control groups. The numbers (1) and (2) indicates subgroup analysis of the 200 mg and 400 mg modafinil dosage arms respectively. WPA, wakefulness promoting agent.
Secondary Outcomes
CLINICAL GLOBAL IMPRESSION OF CHANGE (CGI-C)
This outcome was studied and reported by 5 RCTs. Use of WPAs increased the reporting of minimal improvement on the CGI-C by 26% (RR 1.56; 95% CI, 1.59–1.86) (Figure 5). There was significant heterogeneity across studies (I2 = 53%, p value of Q statistic 0.06) and random effects model was used.
Figure 5. Forest Plot for the Comprehensive Global Inventory of Change.
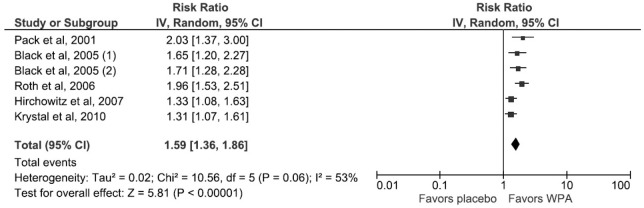
For Black et al, 2005 (1) and (2) indicates subgroup analysis of the 200 mg and 400 mg modafinil dosage arms respectively. WPA, wakefulness promoting agent.
MEAN CHANGE IN DURATION OF CPAP USE
This outcome was reported by 3 RCTs (Table 3). Use of CPAP decreased more in the WPA arms than in the control arms by a weighted mean difference of 0.12 h (95% CI, 0.00–0.24, p = 0.05) (Figure 6). There was no significant heterogeneity (I2 = 14%, p value of Q statistic 0.32).
Table 3.
Mean duration of nasal CPAP use at baseline across studies.
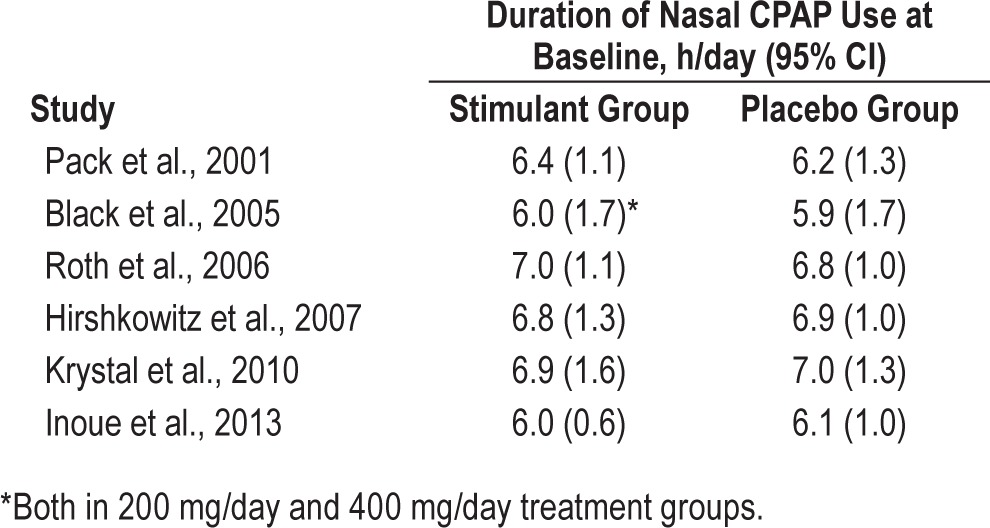
Figure 6. Forest Plot for incidence of headache with WPA treatment.
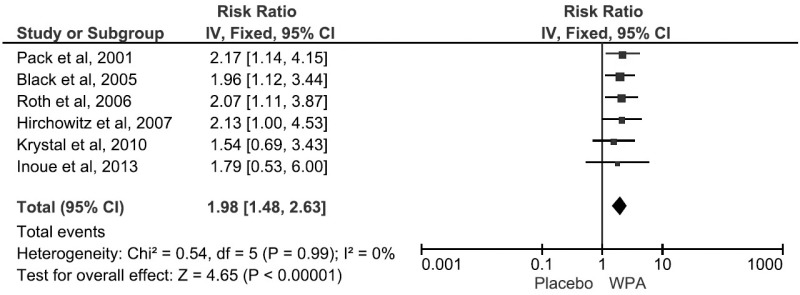
WPA, wakefulness promoting agent.
Adverse Effects
The most common side effects reported were headaches, nausea, and dizziness (Table 4). Use of WPAs increased the risk of headaches by 8% (RR 1.98; 95% CI, 1.48–2.63) (Figure 7). On subgroup analysis, armodafinil was associated with a lower rate of headache than modafinil (p < 0.05).
Table 4.
Frequency of the most common side effects encountered in the intervention arm.
Figure 7. Forest Plot for mean change in duration of nCPAP use at baseline and during WPA treatment.
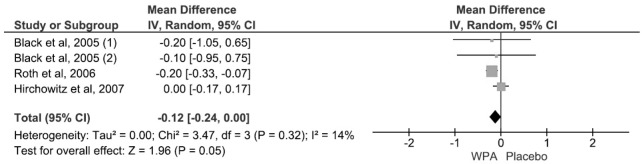
For Pack et al. 2001, and Krystal et al. 2010, complete data on change in nCPAP use during WPA treatment was not available. For Black et al., 2005 (1) and (2) indicates subgroup analysis of the 200 mg and 400 mg modafinil dosage arms respectively. WPA, wakefulness promoting agent.
DISCUSSION
Our meta-analysis suggests that for patients with residual sleepiness despite adequate CPAP, treatment with modafinil or armodafinil reduced sleepiness and improved global impression of illness severity and response to therapy. The use of WPAs was associated with more headaches and a trend toward reduction in the use of CPAP.
The statement by the American Thoracic Society on sleep apnea and driving risk recommends against prescribing stimulants because there is limited evidence that they reduce accidents and because of the concern that they might improve subjective measures of wakefulness more than objective ones, leading drivers to be overconfident despite their impairment.15 The American Academy of Sleep Medicine recommends using WPAs to treat residual sleepiness despite effective CPAP treatment when no other causes for sleepiness are identified,16 while the American College of Physicians17 and the British Thoracic Society18 do not endorse their use.
The European Medicines Agency restricts the use of modafinil to narcolepsy because it considers the evidence weak for indications other than narcolepsy and because of concerns for serious skin reactions (erythema multiforme, Steven-Johnsons syndrome, toxic epidermal necrolysis), suicidal ideation, and hallucinations.8 From the date of initial marketing on December 1998 until January 2007, the United States Food and Drug Administration received reports of six cases of such serious skin reactions prompting a product labeling update.19 Data on occurrence of such severe skin reactions was not reported in the trials included in this meta-analysis. Headaches, nausea, and dizziness were the most common side effects reported.
The pooled estimate for number needed to harm (NNH) for headache was 12, based on a pooled risk difference 0.08 (95% CI: 0.05 to 0.12). The number needed to treat (NNT) for the percentage of patients reporting at least a minimal improvement in CGI-C was 4, based on a pooled risk difference 0.26 (95% CI: 0.19 to 0.33). Therefore, the NNT-to-NNH ratio favors the use of WPAs, with the caveat that the pooled NNT derived from meta-analysis can be misleading given the variation in baseline risk and event rates across trials.20
There were certain limitations of this meta-analysis. First, outcome measures such as polysomnographic parameters, functional outcome questionnaires, and cognitive outcomes, were included in some of the RCTs, but were not analyzed by us because they were not uniformly studied or reported. In addition, some RCTs did not report confidence intervals or standard errors of the mean in the article and we had to derive them from graphical data, which may be a source of error.21
Second, the RCTs were limited to 4 to 12 weeks of follow up, therefore we cannot comment on long term effects of using these agents. Third, we have combined analysis for armodafinil and modafinil and cannot determine if one is preferable to the other, although it appears that armodafinil had a lower incidence of headache. Additionally, dosage regimens across various RCTs were different and it was difficult to determine an optimal dosing strategy based on the available data.
Fourth, there was one study which included patients with treated depression, which is an important confounding factor. Fifth, we were unable to determine the proportion of patients discontinuing the drugs from serious adverse events that were of concern to the European Medicines Agency.
We must mention that all trials included in this analysis received funding in part or whole from drug manufacturers which may be a source of bias. Industry-sponsored drug and device studies are more favorable to the sponsored product when compared to non-industry-sponsored studies and this bias cannot be measured by standard risk of bias assessment tools.22
Since there is a dose-response relationship between CPAP and cognitive function,23 physicians should pay close attention to CPAP use. In the trials included in this analysis, the average daily CPAP use was 6.5 hours. Therefore when a patient uses CPAP for 4–6 hours, the physician should first determine if residual sleepiness is due to short sleep time or because CPAP use is shorter than sleep time and then address each situation accordingly. On the other hand, patients that remain sleepy despite using CPAP for ≥ 6.5 hours are unlikely to increase their use of CPAP and are also unlikely to notice any improvement in their sleepiness. This group might benefit most from using WPAs.
In our practice, we prescribe modafinil to carefully selected patients with whom we discuss the potential risks and benefits of such treatment. Generally these are patients in whom sleepiness interferes significantly with study or work. Our approach begins by ruling out residual OSA, periodic limb movement disorder, depression, and drug-induced sleepiness. We then attempt to maximize CPAP use. We assess the relationship between total sleep time and CPAP use aiming to increase total sleep time and to eliminate any sleep without CPAP. If a patient continues to be sleepy after maximizing sleep time and CPAP use, we prescribe a trial of modafinil. We measure sleepiness before and after treatment with ESS and MWT and continue to treat if the response is satisfactory.
The modest decrease in CPAP use after treatment with WPAs, does not appear to be clinically relevant in the setting of these trials. Yet this trend might be more significant in clinical practice with wider use of these agents for longer periods combined with suboptimal monitoring of CPAP use. In addition, the effect of reduced CPAP on wakefulness might be masked by these agents. Therefore, physicians must monitor objectively and closely adherence with CPAP because it remains the treatment of OSA associated with less cardiovascular deaths— an effect that has not been demonstrated for WPAs
This meta-analysis shows that, with a favorable NNT/NNH ratio, modafinil and armodafinil reduced sleepiness in patients that remained sleepy after adequate use of CPAP. Nevertheless, considering the source of funding of these trials, the concerns about serious adverse effects, and the trend for lesser CPAP use on treatment, we call on independent investigators to conduct longer and larger trials that address these issue as well as the appropriate methods for selecting patients and for monitoring them long-term.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. All authors participated in the creation of this manuscript. This manuscript does not discuss off label use of a drug.
ABBREVIATIONS
- CGI-C
Clinical Global Impression of Change
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- MSLT
multiple sleep latency test
- MWT
maintenance of wakefulness test
- NNH
number needed to harm
- NNT
number needed to treat
- OSA
obstructive sleep apnea
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
randomized controlled trial
- SD
standard deviation
- SEM
standard error of mean
- USFDA
United States Food and Drug Administration
- WPA
wakefulness promoting agent
APPENDIX
Search Strategies
Database: Ovid MEDLINE without Revisions (1996 to September Week 1 2014)
Search Strategy:
modafinil.mp. (1116)
armodafinil.mp. (78)
(obstructive adj sleep adj apnea).mp. (10004)
exp wakefulness/ (8290)
exp Sleep Apnea, Obstructive/ (11165)
OSA.mp. (5240)
Continuous Positive Airway Pressure/ or Sleep Apnea, Obstructive/ (12875)
1 or 2 (1128)
3 or 5 or 6 or 7 (16454)
8 and 9 (70)
from 10 keep 6,20-22,27-28,40,48-49,52,54,66 (12)
Database: EMBASE
(“OSA” OR “Obstructive Sleep Apnea” OR “Obstructive Sleep Apnoea”) AND (“Modafinil” OR “Armodafinil”) AND CPAP
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasa M, Tamisier R, Launois SH, et al. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22:389–97. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 3.Pépin J-L, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33:1062–7. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 4.Vernet C, Redolfi S, Attali V, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38:98–105. doi: 10.1183/09031936.00040410. [DOI] [PubMed] [Google Scholar]
- 5.Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–94. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 6.Szabadi E. Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol. 2006;61:761–6. doi: 10.1111/j.1365-2125.2006.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health & Human Services. US Food and Drug Administration. Drug and Biologic Approval and IND Activity Reports. Efficacy Supplement Approvals in 2004. [Accessed July 6, 2014]. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/drugandbiologicapprovalreports/ucm081887.htm.
- 8.European Medicines Agency. European Medicines Agency recommends restricting the use of modafinil. 2010. Jul 22, [Accessed July 6, 2014]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/07/news_detail_001061.jsp&mid=WC0b01ac058004d5c1.
- 9.Pack AI, Black JE, Schwartz JR, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:1675–81. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 10.Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–71. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 11.Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28:689–706. doi: 10.1016/j.clinthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Hirshkowitz M, Black JE, Wesnes K, Niebler G, Arora S, Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101:616–27. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Krystal AD, Harsh JR, Yang R, Yang R, Rippon GA, Lankford DA. A double-blind, placebo-controlled study of armodafinil for excessive sleepiness in patients with treated obstructive sleep apnea and comorbid depression. J Clin Psychiatry. 2010;71:32–40. doi: 10.4088/JCP.09m05536gry. [DOI] [PubMed] [Google Scholar]
- 14.Inoue Y, Takasaki Y, Yamashiro Y. Efficacy and safety of adjunctive modafinil treatment on residual excessive daytime sleepiness among nasal continuous positive airway pressure-treated Japanese patients with obstructive sleep apnea syndrome: a double-blind placebo-controlled study. J Clin Sleep Med. 2013;9:751–7. doi: 10.5664/jcsm.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay GG, Feldman N. Effects of armodafinil on simulated driving and self-report measures in obstructive sleep apnea patients prior to treatment with continuous positive airway pressure. J Clin Sleep Med. 2013;9:445–54. doi: 10.5664/jcsm.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 17.Qaseem A, Holty J-EC, Owens DK, Dallas P, Starkey M, Shekelle P. Management of obstructive sleep apnea in adults: a Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2013;159:471–83. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health & Human Services. National Guideline Clearinghouse. Management of obstructive sleep apnoea/hypopnoea syndrome in adults. A national clinical guideline. [Accessed December 21, 2014]. http://www.guideline.gov/content.aspx?id=3878.
- 19.US Department of Health & Human Services. US Food and Drug Administration. FDA Drug Safety Newsletter. Postmarketing Reviews - Volume 1, Number 1, Fall 2007. [Accessed July 20, 2014]. http://www.fda.gov/drugs/drugsafety/drugsafetynewsletter/ucm115974.htm#ModafinilmarketedasProvigil:SeriousSkinReactions.
- 20.Marx A, Bucher HC. Numbers needed to treat derived from meta-analysis: a word of caution. Evid Based Med. 2003;8:36–37. [PubMed] [Google Scholar]
- 21.Can data extraction from figures perform a meta-analysis? [Accessed August 26, 2013]. http://2012.colloquium.cochrane.org/sites/2012.colloquium.cochrane.org/files/uploads/posters/025.pdf.
- 22.Bero L. Industry sponsorship and research outcome: a Cochrane review. JAMA Intern Med. 2013;173:580–1. doi: 10.1001/jamainternmed.2013.4190. [DOI] [PubMed] [Google Scholar]
- 23.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]


