Abstract
Purpose:
To assess the efficacy of the continuous positive airway pressure (CPAP) on nocturia in patients with obstructive sleep apnea (OSA).
Methods:
A literature review was performed to identify all published clinical trials of CPAP for the treatment of nocturia. The search included the following databases: MEDLINE, Embase, and the Cochrane Controlled Trials Register. The reference lists of the retrieved studies were also investigated.
Results:
Five publications involving a total of 307 patients were used in the analysis, which compared the number of incidents of nocturia before and after CPAP treatment. We found that patients with OSA and nocturia who were treated with CPAP had a significant decrease in the frequency of nocturia and the volume of urine associated with it. The mean number of nocturia incidents (standardized mean difference [SMD], –2.28; 95% confidence interval [CI], –2.42 to –2.15; P<0.00001) and the associated urine volume (SMD, –183.12; 95% CI, –248.27 to –117.98; P<0.00001) indicated that CPAP was effective. Besides, the Epworth Sleepiness Scale (SMD, –5.88; 95% CI, –6.56 to –5.21; P<0.00001) and the CPAP apnea-hypopnea index (SMD, –31.57; 95% CI, –33.87 to –29.28; P<0.00001) indicated that CPAP significantly improved the quality of sleep.
Conclusions:
This meta-analysis indicates that CPAP maybe an effective treatment for reducing nocturia associated with OSA and improving the quality of life of such patients.
Keywords: Continuous Positive Airway Pressure; Nocturia; Sleep Apnea, Obstructive; Meta-Analysis; Clinical Trial
INTRODUCTION
Nocturia has been defined by the International Continence Society as “the complaint that the individual has to wake up at night one or more times to void” [1]. Nocturia is a common problem for patients who suffer from obstructive sleep apnea (OSA) syndrome [2-4]. Schatzl et al. [5] found that the prevalence of nocturia increases with age, from 3.4% in men younger than 30 years to 32.4% in those aged 60 years or older. Generally, older men with nocturia were often assumed to have benign prostatic hyperplasia and women were assumed to have an overactive bladder or reduced bladder capacity. However, Lundgren [6] believe that nocturia may be associated with nocturnal polyuria.
OSA is characterized by repetitive occlusion of breathing in the upper airway. It is one of the most common respiratory disorders, affecting up to 20% of the general population [7]. Krieger et al. [8] found that the rate of nocturia was significantly greater in patients with OSA than in healthy controls. Umlauf et al. [9] reported that nocturnal urine volume and atrial natriuretic peptide (ANP) excretion are elevated in patients with OSA.
Continuous positive airway pressure (CPAP) is an effective treatment for middle-aged patients with moderate-to-severe OSA syndrome [10]. Some articles reported that CPAP treatment not only improves breathing in OSA patients but also decreases the frequency of nocturia and the associated urine volume [11,12]. Although these studies indicate that CPAP is an effective treatment for nocturia, thus far, no meta-analysis has been performed to confirm this claim.
Therefore, we conducted a meta-analysis to evaluate the efficacy of CPAP in treating nocturia among patients with OSA.
MATERIALS AND METHODS
Search Strategy
Clinical studies describing the effectiveness of CPAP in treating nocturia in patients with OSA were included in this review. They were identified by searching the MEDLINE (1966 to July 2015), Embase (1974 to July 2015), and Cochrane Controlled Trials Register databases. We also searched the reference lists of the retrieved studies. The following keywords were used in the search as text word or subject headings: CPAP, nocturia, OSA, meta-analysis, clinical trial. No ethical approval was sought for this study, as it was a systematic review and meta-analysis of published manuscripts.
Inclusion Criteria and Trial Selection
Clinical trials reporting the incidence of nocturia in patients with OSA who underwent CPAP were included. Two reviewers independently selected the articles for inclusion by assessing the eligibility of full papers against the review inclusion criteria. Disagreements were resolved by discussion, if necessary, with a third reviewer. A flowchart of the study selection process is shown in Fig. 1.
Fig. 1.
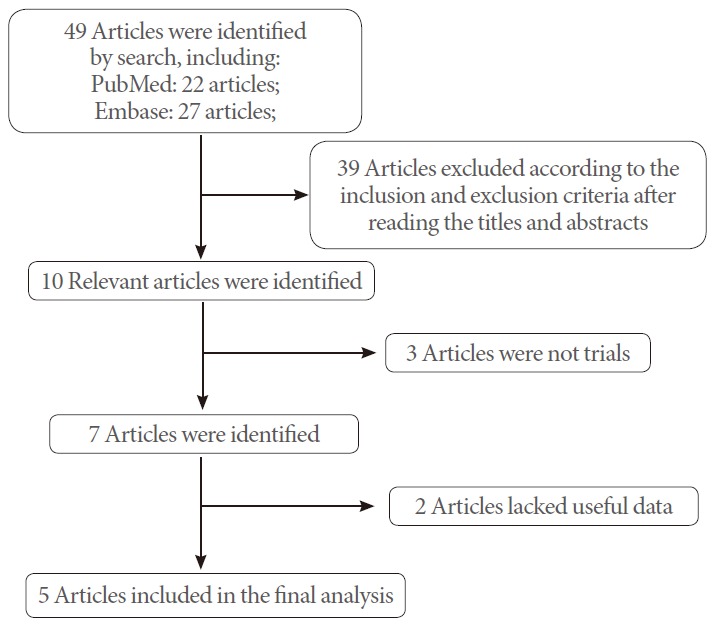
Flowchart of the study selection.
Quality Assessment
The quality of the retrieved clinical trials was assessed using the Jadad scale [13]. All the identified clinical trials were included in the meta-analysis regardless of the quality score. The methodological quality of each study was assessed according to how patients were allocated to the arms of the study, the concealment of allocation procedures, blinding, and data loss due to attrition. The studies were then classified qualitatively according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.1.0 [14]. On the basis of these quality assessment criteria, each study was rated and assigned to one of the following three quality categories: A, if all quality criteria were adequately met, the study was deemed to have a low risk of bias; B, if one or more of the quality criteria was only partially met or was unclear, the study was deemed to have a moderate risk of bias; or C, if one or more of the criteria was not met or not included, the study was deemed to have a high risk of bias.
Data Extraction
The following data were extracted from each eligible study: (1) the name of the clinical trial; (2) the number of patients in each group; (3) the therapy that the patients received; (4) the country in which the study was conducted; and (5) the data collected including the frequency of nocturia and the associated urine volume, the Epworth Sleepiness Scale (ESS), and the apnea-hypopnea index (AHI).
Statistical Analysis and Meta-Analysis
A meta-analysis was performed to assess the outcome of CPAP for patients with OSA. It was carried out using RevMan v.5.1.0 (Cochrane Collaboration, Oxford, UK) [14]. When binary variables were reported, odds ratio (OR) or risk ratio was used for analysis. Continuous parameters were analyzed by computing the mean difference or standardized mean difference (SMD) by using the DerSimonian and Laird random-effects model [15]. The pooled effects were determined using the z test; P<0.05 was considered statistically significant for all analyses. The Cochrane chi-square test and inconsistency (I²) measure were used to evaluate the heterogeneity among studies, thus describing the extent of true inconsistency in results across trials [16]. I²<25% reflects a small level of inconsistency, and I²>50% reflects significant inconsistency.
RESULTS
Characteristics of the Individual Studies
The database search revealed 49 articles largely suitable for inclusion in our meta-analysis. However, based on the selection criteria, 39 articles were excluded after reading the titles and abstracts of the articles. Of the remaining 10 articles, 3 were not trials and 2 lacked useful data. Finally, 5 articles [11,12,17-19] were included in the analysis (Fig. 1). The baseline characteristics of the studies included in our meta-analysis are listed in Table 1.
Table 1.
Study and patient characteristics
| Study | Therapy in experimental group | Country | Sample size (n) |
Duration of treatment (mo) | Inclusion population | |
|---|---|---|---|---|---|---|
| Experimental | Control | |||||
| Guilleminault et al. (2004) [11] | CPAP | USA | 31 | 31 | 1 | Males, 65 years and older, with either (1) snoring and daytime fatigue or sleepiness, with sleep disordered breathing or (2) difficulty initiating sleep or waking up too early, were considered for the study. |
| Margel et al. (2006) [17] | CPAP | Israel | 97 | 97 | 3 | After polysomnography, those found to have OSA and were not to make changes in any existing medical treatment |
| Miyauchi et al. (2015) [12] | CPAP | Japan | 51 | 51 | 1 | Older than 20-year-old subjects undergoing polysomnography owing to suspicious diagnosis of OSAS because of loud snoring, nocturnal choking, or daytime sleepiness. |
| McMillan et al. (2014) [18] | CPAP | UK | 113 | 113 | 12 | Consecutive patients aged 65 years or older with newly diagnosed OSAS. |
| Liu and Liu (2001) [19] | CPAP | China | 15 | 15 | 1 | Patients undergoing polysomnography owing to suspicious diagnosis of OSAS because of loud snoring, nocturnal choking, or daytime sleepiness. |
CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; OSAS, obstructive sleep apnea syndrom.
Quality of the Individual Studies
In all 5 clinical trials, the processes used for randomization were well described. All included a power calculation to determine the optimal sample size (Table 2). The quality score assigned to each included study was ‘B and A’ (Table 2). A funnel plot was used to make a qualitative estimate of the publication bias of the studies, and no evidence of bias was found (Fig. 2).
Table 2.
Quality assessment of individual study
| Study | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow-up | Calculation of sample size | Statistical analysis | Intention-to-treat analysis | Level of quality |
|---|---|---|---|---|---|---|---|---|
| Guilleminault et al. (2004) [11] | A | A | B | 0 | YES | t-test and Kruskal-Wallis test | YES | B |
| Margel et al. (2006) [17] | A | A | B | 0 | YES | Student paired t-test | YES | B |
| Miyauchi et al. (2015) [12] | A | A | B | 0 | YES | Wilcoxon signed rank test | YES | B |
| McMillan et al. (2014) [18] | A | A | A | 0 | YES | t-test and Kruskal-Wallis test | YES | A |
| Liu and Liu (2001) [19] | A | B | B | 0 | YES | Student paired t-test | YES | B |
A, all quality criteria met (adequate): low risk of bias; B, one or more of the quality criteria only partly met (unclear): moderate risk of bias.
Fig. 2.
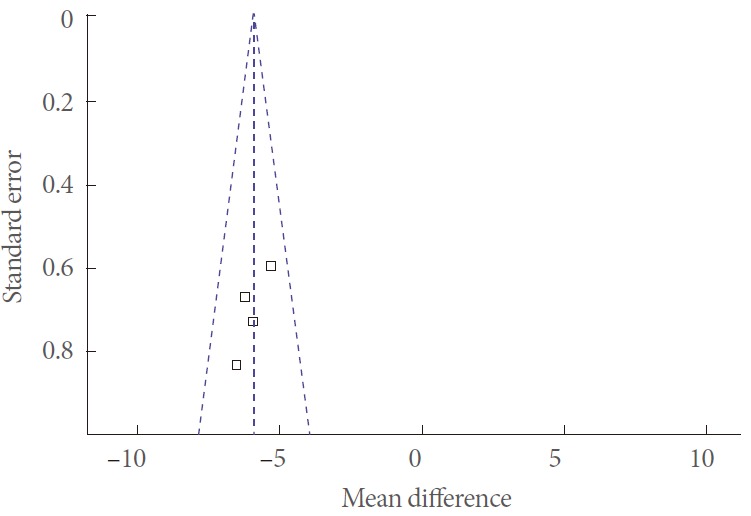
Funnel plot of the studies represented in our meta-analysis.
Efficacy
The mean number of nocturia incidents
Five clinical trials, representing 307 participants, included data on nocturia (Fig. 3). The pooled estimate of SMD between the pre- and posttreatment groups was –2.28, and the 95% confidence interval (CI) was –2.42 to –2.15 (P<0.00001). This result shows that CPAP treatment produced a statistically significant reduction in the mean number of nocturia incidents.
Fig. 3.
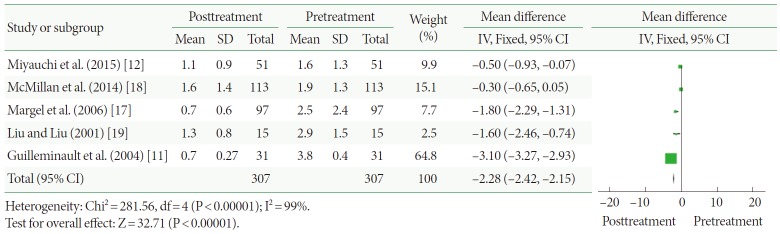
Change in nocturia conditions after continuous positive airway pressure treatment versus before treatment. SD, standard deviation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; Fixed, fixed effect model.
Night-time urine volume (mL)
Two clinical trials, representing 40 participants, included night-time urine volume data (Fig. 4). The pooled estimate of SMD between the pre- and posttreatment groups was –183.12, and the 95% CI was –248.27 to –117.98 (P<0.00001). This result shows that CPAP produces statistically significant reductions in night-time urine volume.
Fig. 4.
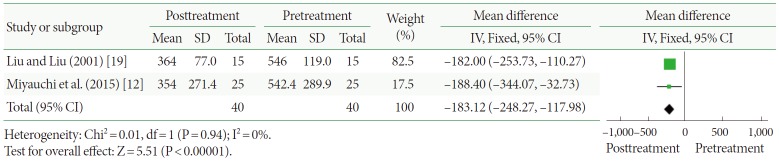
Change in night-time urine volume after continuous positive airway pressure treatment versus before treatment. SD, standard deviation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; Fixed, fixed effect model.
Change of ESS ratings
Four of the clinical trials, representing 131 participants, assessed participants using the ESS (Fig. 5). Based on our analysis, the pooled estimate of SMD between the pre- and posttreatment groups was –5.88, and the 95% CI was –6.56 to –5.21 (P<0.00001). This result shows that CPAP produced statistically significant reductions in study participants’ ESS ratings.
Fig. 5.
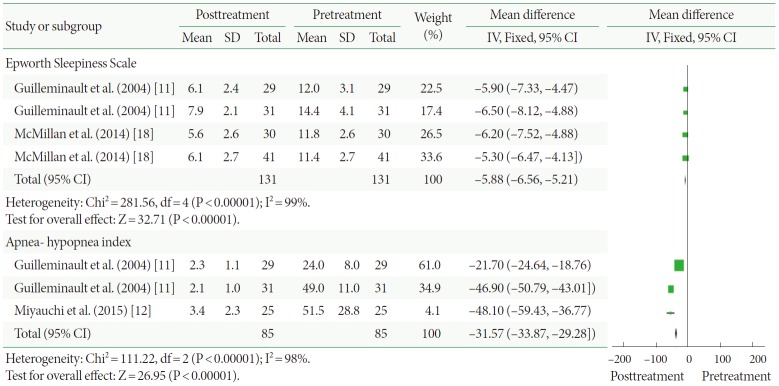
Changes to the Epworth Sleepiness Scale and apnea-hypopnea index after continuous positive airway pressure treatment versus before treatment. SD, standard deviation; CI, confidence interval; df, degrees of freedom; IV, inverse variance; Fixed, fixed effect model.
Change of AHI
Three of the clinical trials, representing 85 participants, included the AHI (Fig. 5). The pooled estimate of SMD between the pre- and posttreatment groups was –31.57, and the 95% CI was –33.87 to –29.28 (P<0.00001). This result shows that CPAP produced statistically significant reductions in the AHI of study participants.
Miyauchi et al. [12] reported that urinary concentrations of Na, Cl, and K were significantly decreased after CPAP treatment (P=0.004, P=0.007, and P=0.025). Moreover, they also found that total International Prostate Symptom Score (IPSS) was significantly decreased after CPAP treatment (P =0.03). Besides, Liu and Liu [19] reported that the osmotic concentration of nocturia urine was significantly increased after CPAP treatment (P<0.05) and the concentration of ANP was significantly decreased after CPAP treatment (P<0.01).
DISCUSSION
Nocturia is a common problem among older adults and appears to negatively impact sleep quality [20,21]. Even though nocturia is often attributed to problems of the bladder, it is common and often serious in patients with OSA [22,23]. OSA is a potential reason of nocturia. In patients with OSA, the frequency with which nocturia occurs more than once in one night is reported to increase by 52%–77%, depending on the severity of the breathing problems associated with the sleep disorder [24,25]. CPAP treatment maintains the airway patency by supplying air with positive pressure, and is hence an effective treatment for OSA. Some studies reported that CPAP treatment improves not only the breathing difficulties associated with the sleep disorder but also the urinary symptoms, including nocturia [26-28].
Our study reveals that nocturia (P<0.00001) and night-time urine volume (P<0.00001) were significantly decreased after CPAP treatment. The mean number of nocturia incidents was decreased by 2.28. Mean night-time urine volume (mL) was decreased by 183.12 mL. The results show that treatment with CPAP provides both statistically significant and, more importantly, clinically relevant improvements in nocturia and reduction in night-time urine volume. Miyauchi et al. [12] reported that reduction in nocturia may be primarily due to reduction in night-time urine volume. Moreover, they also found that total IPSS and quality of life scores were significantly improved after CPAP treatment [12]. An earlier study reported that patients with OSA exhibit greater urinary flows and a reduction in the percentage of filtered sodium that is reabsorbed [29]. In addition, Umlauf et al. [9] found that both night-time urine volume and ANP excretion are elevated in patients with a high RDI (respiratory disturbance index, which was calculated by dividing the total number of events of apnea plus hypopnea by the total sleep time). Therefore, we thought one possible reason for the beneficial effect of CPAP treatment on nocturia may have to do with the reduced release of ANP. CPAP therapy can effectively relieve obstruction of the airway, avoiding excessive expansion of the cardiac atrium, which is induced by intrathoracic negative pressure. At the same time, CPAP can effectively reduce night-time hypoxemia, thereby avoiding pulmonary hypoxic contraction. All of these factors can significantly reduce the secretion of ANP at night. Hence, patients with OSA after CPAP treatment had a significant reduction in nocturia. In other words, CPAP treatment eliminates negative intrathoracic pressure and thereby reduces secretion of ANP. This leads to the reduction of nocturnal urine volume and urinary electrolyte excretion at night and, consequently, relieves the nocturia.
Additionally, our study reveals that significant improvements in the ESS ratings (P<0.00001) and AHI (P<0.00001) were observed after CPAP treatment. As a consequence, sleep quality was improved by a smoother respiratory pattern and a lower probability of arousal from a full bladder.
The positive pressures applied in CPAP were 4–20 cm H2O, which is presumably a measure of pressure using a column of water, which reduces sleepiness and is marginally cost effective. Hence, we believe that CPAP 4–20 cm H2O is an effective treatment for nocturia in patients with OSA.
There are some important limitations to our analysis. Data on the treatment of CPAP were derived from a relatively small-sized sample because cohort sizes of a few of the studies were not large. The long-term safety, efficacy, and persistence of CPAP cannot be extrapolated from this article. In addition, data from unpublished studies were not included in the analysis. Such factors may have resulted in a bias. Besides, CPAP is usually not a sufficient treatment for complex sleep-related breathing disorders, including mixed sleep apnea syndrome with central and obstructive apneas, chronic obstructive pulmonary disease etc. However, the included studies did not distinguish between the mixed sleep apnea syndromes. We think that a carefully layered analysis should be applied, but owing to the limited data available, further studies are required. We suggest that more high-quality trials with larger samples are needed to learn more about the efficacy of CPAP for the treatment of nocturia.
In conclusion, this meta-analysis indicates that CPAP appears to be an effective treatment for reducing nocturia and improving the quality of life of patients with OSA.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90 Suppl 3:11–5. doi: 10.1046/j.1464-410x.90.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Moriyama Y, Miwa K, Tanaka H, Fujihiro S, Nishino Y, Deguchi T. Nocturia in men less than 50 years of age may be associated with obstructive sleep apnea syndrome. Urology. 2008;71:1096–8. doi: 10.1016/j.urology.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Pressman MR, Figueroa WG, Kendrick-Mohamed J, Greenspon LW, Peterson DD. Nocturia. A rarely recognized symptom of sleep apnea and other occult sleep disorders. Arch Intern Med. 1996;156:545–50. doi: 10.1001/archinte.156.5.545. [DOI] [PubMed] [Google Scholar]
- 4.Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99:S750–6. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- 5.Schatzl G, Temml C, Schmidbauer J, Dolezal B, Haidinger G, Madersbacher S. Cross-sectional study of nocturia in both sexes: analysis of a voluntary health screening project. Urology. 2000;56:71–5. doi: 10.1016/s0090-4295(00)00603-8. [DOI] [PubMed] [Google Scholar]
- 6.Lundgren R. Nocturia: a new perspective on an old symptom. Scand J Urol Nephrol. 2004;38:112–6. doi: 10.1080/00365590310020033. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Krieger J, Petiau C, Sforza E, Delanoe C, Hecht MT, Chamouard V. Nocturnal pollakiuria is a symptom of obstructive sleep apnea. Urol Int. 1993;50:93–7. doi: 10.1159/000282460. [DOI] [PubMed] [Google Scholar]
- 9.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–44. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 10.McDaid C, Griffin S, Weatherly H, Duree K, van der Burgt M, van Hout S, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13 doi: 10.3310/hta13040. iii-iv, xi-xiv, 1-119, 143-274. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Lin CM, Goncalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res. 2004;56:511–5. doi: 10.1016/S0022-3999(04)00021-2. [DOI] [PubMed] [Google Scholar]
- 12.Miyauchi Y, Okazoe H, Okujyo M, Inada F, Kakehi T, Kikuchi H, et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology. 2015;85:333–6. doi: 10.1016/j.urology.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Rennie D. The randomized controlled trial gets a middle-aged checkup. JAMA. 1998;279:319–20. doi: 10.1001/jama.279.4.319. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S. London: Cochrane Collaboration; c2015. Cochrane handbook for systematic reviews of interventions. version 5.1.0 [Internet] [updated 2011 Mar] [cited 2015 Apr]. Available from: http://www.cochrane-handbook.org/ [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006;67:974–7. doi: 10.1016/j.urology.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 18.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–12. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Liu L. Effect of treatment with continuous positive airway pressure on nocturnal polyuria in patients with obstructive sleep apnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2001;24:158–60. [PubMed] [Google Scholar]
- 20.Kim SO, Choi HS, Kim YJ, Kim HS, Hwang IS, Hwang EC, et al. Impact of nocturia on health-related quality of life and medical outcomes study sleep score in men. Int Neurourol J. 2011;15:82–6. doi: 10.5213/inj.2011.15.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda ED, Gomes CM, Torricelli FC, de Bessa J Junior, de Castro JE, Ferreira BR, et al. Nocturia is the lower urinary tract symptom with greatest impact on quality of life of men from a community setting. Int Neurourol J. 2014;18:86–90. doi: 10.5213/inj.2014.18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warley AR, Stradling JR. Abnormal diurnal variation in salt and water excretion in patients with obstructive sleep apnoea. Clin Sci (Lond) 1988;74:183–5. doi: 10.1042/cs0740183. [DOI] [PubMed] [Google Scholar]
- 23.Ulfberg J, Thuman R. A non-urologic cause of nocturia and enuresis: obstructive sleep apnea syndrome (OSAS) Scand J Urol Nephrol. 1996;30:135–7. doi: 10.3109/00365599609180904. [DOI] [PubMed] [Google Scholar]
- 24.Kaynak H, Kaynak D, Oztura I. Does frequency of nocturnal urination reflect the severity of sleep-disordered breathing? J Sleep Res. 2004;13:173–6. doi: 10.1111/j.1365-2869.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 25.Oztura I, Kaynak D, Kaynak HC. Nocturia in sleep-disordered breathing. Sleep Med. 2006;7:362–7. doi: 10.1016/j.sleep.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Kiely JL, Murphy M, McNicholas WT. Subjective efficacy of nasal CPAP therapy in obstructive sleep apnoea syndrome: a prospective controlled study. Eur Respir J. 1999;13:1086–90. doi: 10.1034/j.1399-3003.1999.13e24.x. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J Obstet Gynecol. 2006;194:1399–403. doi: 10.1016/j.ajog.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 28.Cruz IA, Drummond M, Winck JC. Obstructive sleep apnea symptoms beyond sleepiness and snoring: effects of nasal APAP therapy. Sleep Breath. 2012;16:361–6. doi: 10.1007/s11325-011-0502-4. [DOI] [PubMed] [Google Scholar]
- 29.Krieger J, Imbs JL, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med. 1988;148:1337–40. [PubMed] [Google Scholar]


