Abstract
Purpose
Previous studies have reported that survivors of non-Hodgkin lymphoma (NHL) have an increased risk of developing cutaneous melanoma; however, risks associated with specific treatments and immune-related risk factors have not been quantified.
Patients and Methods
We evaluated second melanoma risk among 44,870 1-year survivors of first primary NHL diagnosed at age 66 to 83 years from 1992 to 2009 and included in the Surveillance, Epidemiology, and End Results-Medicare database. Information on NHL treatments, autoimmune diseases, and infections was derived from Medicare claims.
Results
A total of 202 second melanoma cases occurred among survivors of NHL, including 91 after chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and 111 after other NHL subtypes (cumulative incidence by age 85 years: CLL/SLL, 1.37%; other NHL subtypes, 0.78%). Melanoma risk after CLL/SLL was significantly increased among patients who received infused fludarabine-containing chemotherapy with or without rituximab (n = 18: hazard ratio [HR], 1.92; 95% CI, 1.09 to 3.40; n = 10: HR, 2.92; 95% CI, 1.42 to 6.01, respectively). Significantly elevated risks also were associated with T-cell activating autoimmune diseases diagnosed before CLL/SLL (n = 36: HR, 2.27; 95% CI, 1.34 to 3.84) or after CLL/SLL (n = 49: HR, 2.92; 95% CI, 1.66 to 5.12). In contrast, among patients with other NHL subtypes, melanoma risk was not associated with specific treatments or with T-cell/B-cell immune conditions. Generally, infections were not associated with melanoma risk, except for urinary tract infections (CLL/SLL), localized scleroderma, pneumonia, and gastrohepatic infections (other NHLs).
Conclusion
Our findings suggest immune perturbation may contribute to the development of melanoma after CLL/SLL. Increased vigilance is warranted among survivors of NHL to maximize opportunities for early detection of melanoma.
INTRODUCTION
Treatment advances have substantially improved prognosis after diagnosis of non-Hodgkin lymphoma (NHL), with 5-year relative survival increasing from 47% to 71% during the past four decades.1 However, second malignancy is an important cause of morbidity and mortality among the more than 700,000 survivors of NHL in the United States today.2 Compared with the general population, survivors of NHL have an increased risk of developing melanoma, with particularly elevated risks among survivors of more indolent NHLs, specifically chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL; standardized incidence ratio, 1.92) and, to a lesser extent, follicular lymphoma (FL; standardized incidence ratio, 1.60).3
Factors that may explain the increased risk of developing melanoma after NHL remain unknown. In addition to immune deficits, chemotherapy for NHL has been implicated, but previous studies have lacked detailed treatment data or sufficient numbers of second melanoma cases to investigate associations with specific chemotherapeutic agents.3–21 Increased melanoma risk in immunosuppressed patients22–27 supports a potential role for immune dysfunction in the development of melanoma after NHL, either independently or in conjunction with UV radiation via sun exposure, a major risk factor for melanoma.5,15–21 However, no previous study has had data on these factors to investigate the hypothesized associations. We therefore used the Surveillance, Epidemiology, and End Results-Medicare (SEER-Medicare) linkage to quantify the risk of developing second cutaneous melanoma in relation to NHL treatments and immune-related medical conditions among 44,870 survivors of first primary NHL in the US older adult population.
PATIENTS AND METHODS
Study Population
Eligible patients were identified through the SEER-Medicare linkage,28 including men and women diagnosed with first primary NHL between age 66 and 83 years from 1992 to 2009. First primary NHL cases were identified in SEER (Table 1).29–31 Patients must have had continuous fee-for-service Medicare parts A and B coverage for 12 months or longer before and after NHL diagnosis for ascertainment of treatment and medical conditions. NHL diagnoses and follow-up time at age 85 years and older were excluded because of under-ascertainment of second malignancies at older ages.32 Patients who died less than 1 year after NHL diagnosis (N = 602), developed a second cancer less than 1 year after NHL diagnosis (n = 947), had HIV (n = 291), or underwent solid organ transplantation before NHL diagnosis (n = 124) were excluded. We required 1 year or longer of follow-up after NHL to allow sufficient time for completion of initial treatment before being at risk for a second melanoma, and to minimize inclusion of incidental cancer diagnoses identified during heightened medical surveillance immediately after NHL diagnosis. Second primary invasive cutaneous melanomas that developed 1 year or longer after first primary NHL were identified through SEER and classified according to site and thickness (Table 1).33
Table 1.
Selected Characteristics of 44,870 1-Year Survivors of First Primary NHL, Overall and by NHL Subtype, Diagnosed at Age 66 to 83 Years, 16 SEER Registries, 1992 to 2009
| Variable | Total NHL |
First Primary NHL Subtype* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL |
DLBCL |
FL |
MZL |
Other |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of 1-year survivors | 44,870 | 13,950 | 10,311 | 7,437 | 3,516 | 9,656 | ||||||
| Age at NHL diagnosis, years | ||||||||||||
| 66-69 | 9,673 | 21.6 | 2,901 | 20.8 | 2,169 | 21.0 | 1,828 | 24.6 | 767 | 21.8 | 2,008 | 20.8 |
| 70-74 | 13,120 | 29.2 | 3,982 | 28.5 | 3,038 | 29.5 | 2,243 | 30.2 | 988 | 28.1 | 2,869 | 29.7 |
| 75-79 | 12,754 | 28.4 | 4,030 | 28.9 | 2,934 | 28.5 | 1,985 | 26.7 | 958 | 27.3 | 2,847 | 29.5 |
| 80-83 | 9,323 | 20.8 | 3,037 | 21.8 | 2,170 | 21.1 | 1,381 | 18.6 | 803 | 22.8 | 1,932 | 20.0 |
| Sex | ||||||||||||
| Male | 22,097 | 49.3 | 7,590 | 54.4 | 4,725 | 45.8 | 3,229 | 43.4 | 1,439 | 40.9 | 5,114 | 53.0 |
| Female | 22,773 | 50.8 | 6,360 | 45.6 | 5,586 | 54.2 | 4,208 | 56.6 | 2,077 | 59.1 | 4,542 | 47.0 |
| Race | ||||||||||||
| White | 40,752 | 90.8 | 12,841 | 92.1 | 9,222 | 89.4 | 6,888 | 92.6 | 3,090 | 87.9 | 8,711 | 90.2 |
| Other/unknown | 4,118 | 9.2 | 1,109 | 7.9 | 1,089 | 10.6 | 549 | 7.4 | 426 | 12.1 | 945 | 9.8 |
| Year of NHL diagnosis | ||||||||||||
| 1992-1997† | 8,127 | 18.1 | 2,755 | 19.8 | 1,798 | 17.4 | 1,345 | 18.1 | 233 | 6.6 | 1,996 | 20.7 |
| 1998-2003 | 15,978 | 35.6 | 5,002 | 35.9 | 3,680 | 35.7 | 2,618 | 35.2 | 1,317 | 37.5 | 3,361 | 34.8 |
| 2004-2009 | 20,765 | 46.3 | 6,193 | 44.4 | 4,833 | 46.9 | 3,474 | 46.7 | 1,966 | 55.9 | 4,299 | 44.5 |
| Residence at time of NHL diagnosis‡ | ||||||||||||
| North | 20,348 | 45.4 | 6,691 | 48.0 | 4,473 | 43.4 | 3,206 | 43.1 | 1,511 | 43.0 | 4,467 | 46.3 |
| Central | 12,778 | 28.5 | 3,727 | 26.7 | 3,107 | 30.1 | 2,260 | 30.4 | 1,041 | 29.6 | 2,643 | 27.4 |
| South | 11,744 | 26.2 | 3,532 | 25.3 | 2,731 | 26.5 | 1,971 | 26.5 | 964 | 27.4 | 2,546 | 26.4 |
| Median age at NHL, years | 74.0 | 75.0 | 74.0 | 74.0 | 75.0 | 74.0 | ||||||
| Mean person-years at risk | 5.5 | 5.6 | 5.4 | 5.8 | 5.6 | 5.3 | ||||||
| No. of second melanomas | 202 | 91 | 34 | 34 | 10 | 33 | ||||||
| Median interval from NHL to melanoma, years | 3.0 | 3.3 | 2.9 | 2.8 | 3.0 | 2.6 | ||||||
| Site of melanoma§ | ||||||||||||
| Face/head/neck | 73 | 36.1 | 37 | 40.7 | < 10 | — | 11 | 32.3 | < 10 | — | 14 | 42.4 |
| Trunk | 56 | 27.7 | 23 | 25.3 | 13 | 38.2 | < 10 | — | < 10 | — | < 10 | — |
| Upper/lower extremities, other/unspecified | 73 | 36.1 | 31 | 34.1 | 13 | 38.2 | 14 | 41.2 | < 10 | — | 11 | 33.3 |
| Thickness of melanoma, mm | — | |||||||||||
| < 1.0 | 104 | 51.4 | 41 | 45.1 | 25 | 73.5 | 16 | 47.1 | < 10 | — | 16 | 48.5 |
| > 1.0 | 70 | 34.7 | 39 | 42.9 | < 10 | — | 13 | 38.2 | < 10 | — | < 10 | — |
| Unknown | 28 | 13.9 | 11 | 12.1 | < 10 | — | < 10 | — | < 10 | — | < 10 | — |
NOTE. Counts and percentages are not reported for fewer than 10 melanoma cases to protect patient confidentiality.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; ICD-O-3 International Classification of Diseases for Oncology, 3rd Edition; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma.
First primary NHL subtype defined by ICD-O-3 as DLBCL (9678-9680, 9684 [B cell]), FL (9690-9691, 9695, 9698), CLL/SLL (9670, 9823), MZL (9689, 9699) and other NHL (9590-9596, 9671, 9673, 9675, 9684 [non B cell], 9687, 9700-9702, 9705, 9708-9709, 9714-9719, 9727-9729, 9827 [primary site, C42.0-42.1, C42.4]).
1992-1997 includes 13 SEER registries, whereas 1998-2003 and 2004-2009 include 16 SEER registries.
Residence defined by SEER registry areas, including north (Connecticut, Detroit, Iowa, Seattle, and New Jersey), central (San Francisco, Utah, San Jose, Greater California, and Kentucky), and south (Hawaii, New Mexico, Atlanta, Los Angeles, Rural Georgia, Greater Georgia, and Louisiana).
Melanoma site defined by ICD-O-3 as face/head/neck (C44.0-C44.4), trunk (C44.5), upper extremities (C44.6), lower extremities (C44.7), and other/unspecified (C44.8-C44.9).
NHL Treatments and Medical Conditions
Information on NHL treatments (infused chemotherapy, radiotherapy, and hematopoietic stem-cell transplant) and immune-related medical conditions (nonhematologic autoimmune diseases and selected infections) was derived from Medicare claims (Appendix Tables 1, 2, and 3, online only). Data on oral chemotherapeutic agents were not available from Medicare throughout the study period and, thus, were excluded. Patients who did not receive therapy (eg, observed) or who received oral chemotherapy agents exclusively were included in our referent group.
Table 2.
Risk of Melanoma After First Primary NHL, by Subtype, in Relation to Autoimmune Conditions and Infections
| Medical Conditions | Before NHL Diagnosis |
After NHL Diagnosis* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total NHL |
Melanoma Cases |
Total NHL |
Melanoma Cases |
|||||||||
| No. | % | No. | % | HR† | 95% CI | No. | % | No. | % | HR† | 95% CI | |
| Patients diagnosed with first primary CLL/SLL | ||||||||||||
| B-cell–activating conditions | 2,263 | 16.2 | < 10 | — | 0.68 | 0.31 to 1.49 | 3,637 | 26.1 | 13 | 14.3 | 0.90 | 0.45 to 1.83 |
| T-cell–activating conditions | 5,488 | 139.3 | 36 | 39.6 | 2.27 | 1.34 to 3.84 | 6,749 | 48.4 | 49 | 53.9 | 2.92 | 1.66 to 5.12 |
| Autoimmune conditions, by organ system‡ | ||||||||||||
| Systemic/connective tissue | 1,687 | 12.1 | < 10 | — | 0.85 | 0.37 to 1.98 | 1,912 | 13.7 | < 10 | — | 1.39 | 0.66 to 2.93 |
| Cardiovascular | 1,367 | 9.8 | < 10 | — | 1.62 | 0.76 to 3.45 | 2,370 | 17.0 | 15 | 16.5 | 2.04 | 1.08 to 3.85 |
| Chronic rheumatic heart disease | 1,219 | 8.7 | < 10 | — | 1.88 | 0.88 to 4.00 | 2,187 | 15.7 | 15 | 16.5 | 2.31 | 1.23 to 4.36 |
| Endocrine | 931 | 6.7 | < 10 | — | 2.56 | 1.21 to 5.42 | 1,213 | 8.7 | < 10 | — | 1.43 | 0.57 to 3.61 |
| Graves' disease | 783 | 5.6 | < 10 | — | 2.66 | 1.20 to 5.91 | 872 | 6.3 | < 10 | — | 1.88 | 0.74 to 4.75 |
| Skin | 2,222 | 15.9 | 18 | 19.8 | 1.64 | 0.95 to 2.83 | 2,402 | 17.2 | 21 | 23.1 | 1.87 | 1.02 to 3.43 |
| Localized scleroderma | 1,809 | 13.0 | 12 | 13.2 | 1.21 | 0.65 to 2.27 | 1,924 | 13.8 | 13 | 14.3 | 1.43 | 0.72 to 2.84 |
| Psoraisis | 445 | 3.2 | < 10 | — | 2.68 | 1.21 to 5.91 | 431 | 3.1 | < 10 | — | 2.78 | 0.99 to 7.71 |
| GI | 1,435 | 10.3 | < 10 | — | 0.72 | 0.29 to 1.81 | 2,926 | 21.0 | < 10 | — | 0.81 | 0.37 to 1.78 |
| Nervous system | 110 | 0.8 | < 10 | — | 0 | 0.0 | 180 | 1.3 | < 10 | — | 2.33 | 0.32 to 17.09 |
| Asthma | 1,919 | 13.8 | 13 | 14.3 | 2.14 | 1.13 to 4.02 | 2,484 | 17.8 | 18 | 19.8 | 3.24 | 1.75 to 6.00 |
| Infections, by organ system | ||||||||||||
| Respiratory, upper airway | 5,847 | 41.9 | 35 | 38.5 | 1.11 | 0.70 to 1.77 | 5,850 | 41.9 | 35 | 38.5 | 0.87 | 0.46 to 1.63 |
| Pharyngitis | 2,141 | 15.4 | 10 | 11.0 | 0.87 | 0.45 to 1.71 | 2,143 | 15.4 | 11 | 12.1 | 1.01 | 0.48 to 2.12 |
| Sinusitis | 4,074 | 29.2 | 24 | 26.4 | 1.28 | 0.78 to 2.11 | 4,238 | 30.4 | 29 | 31.9 | 1.36 | 0.75 to 2.45 |
| Respiratory, lower airway | 6,122 | 43.9 | 37 | 40.7 | 1.27 | 0.79 to 2.07 | 8,347 | 59.8 | 41 | 45.1 | 0.89 | 0.49 to 1.63 |
| Acute bronchitis | 4,526 | 32.4 | 27 | 29.7 | 1.38 | 0.84 to 2.25 | 5,081 | 36.4 | 34 | 37.4 | 1.47 | 0.81 to 2.64 |
| Pneumonia | 2,872 | 20.6 | 16 | 17.6 | 1.19 | 0.67 to 2.11 | 6,414 | 46.0 | 21 | 23.1 | 0.85 | 0.47 to 1.53 |
| Skin | 2,125 | 15.2 | 17 | 18.7 | 1.67 | 0.96 to 2.90 | 3,490 | 25.0 | 21 | 23.1 | 1.47 | 0.84 to 2.59 |
| Cellulitis | 1,191 | 8.5 | 11 | 12.1 | 1.95 | 1.02 to 3.74 | 1,948 | 14.0 | 11 | 12.1 | 1.37 | 0.67 to 2.78 |
| Urinary tract | 6,227 | 44.6 | 39 | 42.9 | 1.65 | 0.99 to 2.76 | 7,942 | 56.9 | 46 | 50.6 | 2.12 | 1.19 to 3.77 |
| Cystitis/pyelonephritis, UTI | 5,630 | 40.4 | 34 | 37.4 | 1.81 | 1.08 to 3.01 | 7,599 | 54.5 | 40 | 44.0 | 2.17 | 1.24 to 3.78 |
| Prostatitis§ | 1,554 | 20.5 | 17 | 23.0 | 1.29 | 0.73 to 2.28 | 1,199 | 15.8 | 14 | 18.9 | 1.73 | 0.80 to 3.78 |
| Gastrohepatic | 2,084 | 14.9 | 11 | 12.1 | 1.06 | 0.55 to 2.02 | 2,594 | 18.6 | 12 | 13.2 | 1.27 | 0.64 to 2.52 |
| Gastroenteritis | 2,020 | 14.5 | 11 | 12.1 | 1.07 | 0.56 to 2.05 | 2,445 | 17.5 | 11 | 12.1 | 1.19 | 0.58 to 2.43 |
| Patients diagnosed with first primary NHL other than CLL/SLL | ||||||||||||
| B-cell–activating conditions | 6,013 | 19.5 | 16 | 14.4 | 1.14 | 0.65 to 1.98 | 8,078 | 26.1 | 24 | 21.6 | 1.56 | 0.88 to 2.76 |
| T-cell–activating conditions | 13,142 | 42.5 | 36 | 32.4 | 0.93 | 0.59 to 1.45 | 15,201 | 49.2 | 35 | 31.5 | 1.13 | 0.67 to 1.88 |
| Autoimmune conditions, by organ system | ||||||||||||
| Systemic/connective tissue | 4,798 | 15.5 | < 10 | — | 0.59 | 0.28 to 1.23 | 4,734 | 15.3 | < 10 | — | 0.69 | 0.28 to 1.73 |
| Cardiovascular | 3,212 | 10.4 | < 10 | — | 0.78 | 0.36 to 1.70 | 5,527 | 17.9 | < 10 | — | 0.50 | 0.20 to 1.24 |
| Endocrine | 2,419 | 7.8 | < 10 | — | 0.34 | 0.08 to 1.38 | 2,947 | 9.5 | < 10 | — | 1.19 | 0.51 to 2.78 |
| Skin | 5,648 | 18.3 | 23 | 20.7 | 1.38 | 0.85 to 2.24 | 5,394 | 17.5 | 18 | 16.2 | 1.21 | 0.63 to 2.32 |
| Localized scleroderma | 4,265 | 13.8 | 22 | 19.8 | 1.88 | 1.15 to 3.06 | 4,110 | 13.3 | 17 | 15.3 | 1.62 | 0.85 to 3.12 |
| Gastrointestinal | 3,458 | 11.2 | 10 | 9.0 | 1.15 | 0.59 to 2.24 | 6,043 | 19.5 | 15 | 13.5 | 1.42 | 0.77 to 2.64 |
| Pernicious anemia | 2,635 | 8.5 | < 10 | — | 1.58 | 0.78 to 3.19 | 5,068 | 16.4 | 15 | 13.5 | 1.75 | 0.94 to 3.24 |
| Nervous system | 194 | 0.6 | < 10 | — | 5.44 | 1.29 to 23.00 | 379 | 1.2 | < 10 | — | 2.01 | 0.27 to 14.82 |
| Asthma | 4,338 | 14.0 | < 10 | — | 0.92 | 0.45 to 1.86 | 5,133 | 16.6 | 13 | 11.7 | 1.75 | 0.89 to 3.44 |
| Infections, by organ system | ||||||||||||
| Respiratory, upper airway | 13,016 | 42.1 | 41 | 36.9 | 1.08 | 0.70 to 1.65 | 11,944 | 38.6 | 40 | 36.0 | 1.31 | 0.76 to 2.25 |
| Otitis media | 3,400 | 11.0 | 11 | 9.9 | 1.07 | 0.57 to 2.01 | 2,857 | 9.2 | < 10 | — | 1.11 | 0.48 to 2.58 |
| Pharyngitis | 4,800 | 15.5 | 18 | 16.2 | 1.29 | 0.77 to 2.17 | 4,299 | 13 | 12 | 10.8 | 0.89 | 0.42-1.86 |
| Sinusitis | 9,109 | 29.5 | 30 | 27.0 | 1.12 | 0.72 to 1.74 | 8,120 | 26.3 | 26 | 23.4 | 1.09 | 0.59 to 2.01 |
| Respiratory, lower airway | 13,047 | 42.2 | 41 | 36.9 | 1.34 | 0.86 to 2.10 | 16,711 | 54.1 | 46 | 41.4 | 1.51 | 0.91 to 2.49 |
| Acute bronchitis | 9,819 | 31.8 | 30 | 27.0 | 1.14 | 0.73 to 1.79 | 9,358 | 30.3 | 27 | 24.3 | 1.17 | 0.67 to 2.07 |
| Pneumonia | 5,667 | 18.3 | 27 | 24.3 | 2.23 | 1.39 to 3.58 | 12,383 | 40.1 | 25 | 22.5 | 1.16 | 0.68 to 1.98 |
| Skin | 4,501 | 14.6 | 13 | 11.7 | 1.00 | 0.55 to 1.82 | 7,025 | 22.7 | 15 | 13.5 | 1.01 | 0.56 to 1.81 |
| Cellulitis | 2,465 | 8.0 | < 10 | — | 0.59 | 0.28 to 1.70 | 3,762 | 12.2 | 11 | 9.9 | 1.59 | 0.84 to 3.01 |
| Urinary tract | 14,162 | 45.8 | 53 | 47.8 | 1.57 | 1.02 to 2.44 | 17,462 | 56.5 | 54 | 48.7 | 1.33 | 0.76 to 2.32 |
| Cystitis/pyelonephritis, UTI | 12,837 | 41.5 | 39 | 35.1 | 1.32 | 0.84 to 2.07 | 16,759 | 54.2 | 51 | 46.0 | 1.66 | 1.00 to 2.73 |
| Prostatitis§ | 3,168 | 21.8 | 19 | 25.0 | 1.20 | 0.70 to 2.06 | 2,154 | 14.9 | < 10 | — | 0.65 | 0.23 to 1.85 |
| Gastrohepatic | 4,880 | 15.8 | 14 | 12.6 | 1.18 | 0.66 to 2.11 | 5,926 | 19.2 | 21 | 18.9 | 2.17 | 1.28 to 3.69 |
| Gastroenteritis | 4,689 | 15.2 | 13 | 11.7 | 1.11 | 0.61 to 2.03 | 5,556 | 18.0 | 19 | 17.1 | 1.91 | 1.10 to 3.33 |
NOTE. Counts and percentages are not reported for fewer than 10 melanoma cases to protect patient confidentiality.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; HR, hazard ratio; NHL, non-Hodgkin lymphoma; UTI, urinary tract infection.
New claims occurring after NHL but before second cancer, death, end of study, or loss to follow-up, with no claims before NHL.
Individuals with no history of the condition of interest comprise the referent group for each analysis. Hematologic autoimmune conditions (eg, autoimmune hemolytic anemia, thrombocytopenia) were excluded from consideration because of difficulty distinguishing these diagnoses from manifestations of chemotherapy toxicity. HR (95% CI) adjusted for sex, race, residence, Charlson comorbidity index, socioeconomic status, and follow-up time (time-dependent covariate) and stratified by calendar year. Age was used as the time scale.
B-cell activating conditions include rheumatoid arthritis, Sjogren's syndrome, discoid lupus erythematosus, reactive arthritis, Felty's syndrome, chronic thryoiditis, systemic/discoid lupus erythematosus, pernicious anemia, and myasthenia gravis. T-cell–activating conditions include ankylosing spondylitis, dermatomyositis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, rheumatic fever, chronic rheumatic heart disease, giant cell arteritis, systemic vasculitis, Addison's disease, Graves' disease, primary biliary cirrhosis, alopecia areata, localized scleroderma, dermatitis herpetiformis, psoriasis, celiac disease, Crohn's disease, ulcerative colitis, amyotrophic sclerosis, multiple sclerosis, and asthma.
Among males only.
Table 3.
Risk of Melanoma After First Primary NHL, by Subtype, in Relation to NHL Treatments
| NHL Treatment | Total NHL |
Melanoma Cases |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | HR* | 95% CI | |
| Patients diagnosed with first primary CLL/SLL | ||||||
| Infused chemotherapy | ||||||
| None recorded | 8,899 | 63.8 | 52 | 57.1 | 1.00 | Referent |
| Model A† | ||||||
| Any rituximab | 3,744 | 26.8 | 27 | 29.7 | 1.43 | 0.77 to 2.67 |
| Any fludarabine | 2,958 | 21.2 | 28 | 30.8 | 1.90 | 1.08 to 3.37 |
| Any cyclophosphamide | 2,402 | 17.2 | 19 | 20.9 | 1.11 | 0.59 to 2.08 |
| Model B‡ | ||||||
| Fludarabine without rituximab | 917 | 6.6 | 10 | 11.0 | 2.92 | 1.42 to 6.01 |
| Rituximab without fludarabine | 1,703 | 12.2 | < 10 | — | 1.63 | 0.79 to 3.38 |
| Fludarabine + rituximab | 2,041 | 14.6 | 18 | 19.8 | 1.92 | 1.09 to 3.40 |
| Radiotherapy | ||||||
| No | 12,925 | 92.7 | 85 | 93.6 | 1.00 | Referent |
| Yes | 1,025 | 7.3 | < 10 | — | 0.86 | 0.31 to 2.40 |
| Patients diagnosed with first primary NHL other than CLL/SLL | ||||||
| Infused chemotherapy | ||||||
| None recorded | 10,040 | 32.5 | 34 | 30.6 | 1.00 | Referent |
| Model A† | ||||||
| Any rituximab | 15,726 | 50.9 | 53 | 47.8 | 1.06 | 0.62 to 1.84 |
| Any fludarabine | 2,518 | 8.1 | < 10 | — | 1.22 | 0.57 to 2.61 |
| Any cyclophosphamide | 16,786 | 54.3 | 68 | 61.3 | 1.44 | 0.89 to 2.35 |
| Model B‡ | ||||||
| Cyclophosphamide without rituximab | 4781 | 15.5 | 24 | 21.6 | 1.78 | 0.97 to 3.25 |
| Rituximab without cyclophosphamide | 3,721 | 12.0 | < 10 | — | 0.99 | 0.45 to 2.19 |
| Cyclophosphamide + rituximab | 12,005 | 38.8 | 44 | 39.6 | 1.27 | 0.77 to 2.12 |
| Radiotherapy | ||||||
| No | 21,051 | 68.1 | 76 | 68.5 | 1.00 | Referent |
| Yes | 9,869 | 31.9 | 35 | 31.5 | 1.11 | 0.73 to 1.69 |
NOTE. Treatments received by fewer than 10 melanoma cases are not reported here but are included in Appendix Table 1 (eg, other alkylating agents, epipodophyllotoxins, and hematopoietic stem cell transplantation). Counts and percentages are not reported for fewer than 10 melanoma cases to protect patient confidentiality.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; HR, hazard ratio; NHL, non-Hodgkin lymphoma.
HR (95% CI) adjusted for sex, race, residence, Charlson comorbidity index, socioeconomic status, and follow-up time (time-dependent covariate) and stratified by calendar year. Age was used as the time scale. Time-dependent covariates were used to indicate receipt of any radiotherapy or chemotherapy during follow-up on the basis of timing of initiation of therapy. Model A represents risk for patients who received any of the three main chemotherapy agents with separate indicator variables for each agent. The categories in model B are mutually exclusive. The HRs for chemotherapy in models A and B were additionally adjusted for receipt of other alkylating agents. The HR for radiotherapy was additionally adjusted for chemotherapy using model B.
Percentages do not add up to 100% because groups are not mutually exclusive.
Percentages do not add up to 100% because a small number of patients received other agents.
Occurrences of autoimmune diseases and infections were defined as having one or more Medicare claims at any time during follow-up, considering diagnoses occurring before NHL separately from those occurring after NHL. Diagnoses of these conditions occurring before 1992 (study start) or Medicare enrollment (age 65 years) were not captured. We evaluated all specific autoimmune conditions and infections with 10 or more second melanoma cases. Because many diagnoses were rare, we grouped them by the tissue and organ systems involved, and (for autoimmune conditions) by whether they activate B or T cells (Table 2).34–38 Hematologic autoimmune conditions (eg, autoimmune hemolytic anemia and thrombocytopenia) were not considered because of difficulty distinguishing these diagnoses from manifestations of chemotherapy toxicity.
Because sun exposure is a key melanoma risk factor, we grouped survivors of NHL according to their residence at the time of NHL on the basis of the SEER registry (Table 1). These categorizations, on the basis of UV-B radiation flux, have been shown to be a valid proxy for recent sun exposure in a melanoma risk model.39
Statistical Analysis
Follow-up began 1 year after NHL and continued until the earliest of the following: second cancer diagnosis, age 85 years, death, loss to follow-up, or end of study (December 31, 2009). We used Cox proportional hazards regression to compute hazard ratios (HRs) and 95% CIs to assess the association between specific risk factors and development of second melanoma after first primary NHL. All analyses used age as the time scale, were adjusted for demographic factors (sex, race [white or other], residence [North, Central, or South], socioeconomic status [derived using census tract–level information on household income and educational attainment], number of comorbidities40 [0, 1, or 2+], and follow-up time as a time-dependent covariate [1 to 1.9, 2 to 2.9, 3 to 3.9, 4 to 4.9, 5 to 6.9, and ≥ 7 years]), and were stratified by calendar year of NHL diagnosis. Stratification by year and use of age as the time scale adjusted for differences in the gap between entrance into Medicare and NHL diagnosis. Time-dependent covariates were used to indicate receipt of radiotherapy or infused chemotherapy during follow-up on the basis of the timing of therapy initiation. For autoimmune conditions/infections occurring before NHL, we defined the follow-up period from age at enrollment into Medicare to age of NHL diagnosis. Autoimmune conditions and infections diagnosed after NHL were evaluated as time-dependent covariates on the basis of the timing of diagnoses of these conditions. Because CLL/SLL treatment patterns differ from other NHLs,41 and because CLL/SLL survivors have a higher risk for developing melanoma,3 we calculated risks separately for CLL/SLL and other NHL subtypes combined.
Second melanoma risk associated with NHL treatments was evaluated in multivariable models, including both chemotherapy and radiotherapy. We modeled infused chemotherapy-related risks a priori using two different approaches. First (model A), we examined risks for patients who received any of the three main chemotherapy agents (cyclophosphamide, rituximab, and fludarabine) with separate indicator variables for each agent. Second (model B), we assessed risk according to the most frequently received combinations of these agents (Table 3). For both analytic approaches, we used patients with no Medicare chemotherapy claims (ie, no infused chemotherapy) as our referent group and excluded other treatments (eg, other alkylating agents and epipodophyllotoxins) because few patients (n < 10) received them. In secondary analyses, we considered other agents (Appendix Table 1). A two-sided Wald χ2 P < .05 comparing models with and without the factor of interest identified melanoma risk factors. For all melanoma risk factors we identified in our primary analyses, exploratory analyses investigated differences in risks by residence because of the hypothesized interaction between immune dysfunction and UV radiation.5,15–21 To evaluate if increased melanoma risk was influenced by increased medical surveillance, we also investigated differences by melanoma thickness.42 Finally, we calculated cumulative incidence of melanoma by age at diagnosis, taking into account death, loss to follow-up, and diagnosis of other second cancers as competing risks.43 All analyses were conducted using SAS 9.3 (Cary, NC).
RESULTS
Our study population of 44,870 1-year survivors of first primary NHL included 13,950 individuals with CLL/SLL, 10,311 with diffuse large B-cell lymphoma (DLBCL), 7,437 with FL, 3,516 with marginal zone lymphoma, and 9,656 with other lymphoma subtypes (Table 1). The median age at diagnosis was 74 years, and most survivors (91%) were white. Greater than half of all survivors of CLL/SLL were male (54%), whereas 54% of survivors of DLBCL and 57% of survivors of FL were female. At the time of NHL diagnosis, 45% of survivors resided in northern regions, 29% in central regions, and 26% in southern regions.
During 247,883 total person-years of follow-up (mean follow-up time, 5.5 years), 202 second melanomas were diagnosed. The median interval from NHL to melanoma diagnosis was 3 years (range, 1 to 14.8 years). Melanoma risks were higher among males, non-Hispanic whites, patients residing in southern regions, and for survivors of CLL/SLL than for survivors of other NHL subtypes (Appendix Table 4, online only). Nearly half (n = 91, 45%) of melanomas were diagnosed after CLL/SLL, with 40.7% of these occurring on the face, head, or neck and 42.9% with 1-mm thickness or greater (Table 1). In contrast, among survivors of all other NHL subtypes combined, melanomas with specified site and depth occurred most frequently on the trunk (29.7%), and 27.9% were 1-mm thick or greater.
A total of 5,051 (36.3%) survivors of CLL/SLL received infused chemotherapy during follow-up, of whom 2,712 (53.7%) initiated chemotherapy within 12 months of diagnosis. Rituximab (26.8%), fludarabine (21.2%), and cyclophosphamide (17.2%; Table 3 and Appendix Table 1) were the most common received infused agents. Initial analyses showed second melanoma risk was significantly increased among 2,958 patients who were treated for CLL/SLL with any fludarabine-containing chemotherapy (HR, 1.90; 95% CI, 1.08 to 3.37) compared with patients not recorded as receiving infused chemotherapy or who received oral agents alone (Table 3, model A). Further analyses showed significant increased risk of developing melanoma among patients with CLL/SLL who received fludarabine-containing infused chemotherapy with rituximab (HR, 1.92; 95% CI, 1.09 to 3.40) or without rituximab (HR, 2.92; 95% CI, 1.42 to 6.01; Table 3, model B). In models adjusting for chemotherapy, treatment of CLL/SLL with radiotherapy was not associated with melanoma risk.
Among survivors of non-CLL/SLL, a total of 20,880 (67.5%) patients received infused chemotherapy during follow-up. Of those patients, 18,694 (89.5%) began chemotherapy within the first 12 months of NHL diagnosis. Rituximab (50.9%) and cyclophosphamide (54.3%) were the most common received infused agents, whereas fewer patients received fludarabine (8.1%) or other agents (7.6%; Table 3 and Appendix Table 1). Melanoma risks were not significantly increased after treatment with any rituximab-, fludarabine-, or cyclophosphamide-containing chemotherapy (model A), or after radiotherapy (model B). In more detailed models, nonsignificant increased risks were observed among patients who received cyclophosphamide-containing chemotherapy without rituximab (model B; HR, 1.78; 95% CI, 0.97 to 3.25). These risks were particularly increased among individuals who also received doxorubicin (cyclophosphamide and doxorubicin without rituximab: n = 20; HR, 2.09; 95% CI, 1.11 to 3.92; cyclophosphamide without doxorubicin/rituximab: n = 4; HR, 1.01; 95% CI, 0.34 to 3.00).
T-cell–activating autoimmune diseases diagnosed before and after CLL/SLL were associated with significant increased risk of melanoma when compared with patients without T-cell–activating autoimmune diseases (before CLL/SLL: HR, 2.27; 95% CI, 1.34 to 3.84; after CLL/SLL: HR, 2.92; 95% CI, 1.66 to 5.12), whereas nonsignificant decreases in melanoma risk were observed for B-cell–activating conditions occurring before and after CLL/SLL (Table 2). Among T-cell–activating autoimmune conditions, risk of developing melanoma was particularly increased for individuals diagnosed before CLL/SLL with Graves' disease (HR, 2.66; 95% CI, 1.20 to 5.91) or psoriasis (HR, 2.68; 95% CI, 1.21 to 5.91), and after CLL/SLL diagnosis with chronic rheumatic heart disease (HR, 2.31; 95% CI, 1.23 to 4.36), skin-related autoimmune conditions (HR, 1.87; 95% CI, 1.02 to 3.43), and before and after CLL/SLL with asthma (before CLL/SLL: HR, 2.14; 95% CI, 1.13 to 4.02; after CLL/SLL: HR, 3.24; 95% CI, 1.75 to 6.00). Despite numerous infections diagnosed among patients with CLL/SLL, only cellulitis diagnosed before CLL/SLL (HR, 1.95; 95% CI, 1.02 to 3.74) and cystitis/pyelonephritis urinary tract infections diagnosed before and after CLL/SLL were associated with significantly elevated melanoma risk (before CLL/SLL: HR, 1.81; 95% CI, 1.08 to 3.01; after CLL/SLL: HR, 2.17; 95% CI, 1.24 to 3.78).
In contrast to CLL/SLL, among patients without CLL/SLL, diagnosis of B-cell– or T-cell–activating autoimmune conditions was not significantly related to subsequent melanoma risk (Table 2). In detailed analyses, the occurrence of localized scleroderma (T-cell–activating condition) before non-CLL/SLL diagnosis was associated with an increased melanoma risk (HR, 1.88; 95% CI, 1.15 to 3.06) and nervous system autoimmune conditions (HR, 5.44; 95% CI, 1.29 to 23.00). Among infections, melanoma risk was linked to pneumonia (HR, 2.23; 95% CI, 1.39 to 3.58) and urinary tract infections (HR, 1.57; 95% CI, 1.02 to 2.44) diagnosed before non-CLL/SLL and after non-CLL/SLL with gastrohepatic infections (mainly gastroenteritis; HR, 1.91; 95% CI, 1.10 to 3.33) and cystitis/pyelonephritis urinary tract infections (HR, 1.66; 95% CI, 1.00 to 2.73). Significant decreases in melanoma risk were not observed for any specific autoimmune conditions or infections.
Risk estimates for the associations described above were materially unchanged in multivariable analyses that included all chemotherapeutic agents as well as autoimmune conditions and infections with P < .05 in the same model. Risk estimates also were similar for melanomas less than 1-mm thick and 1-mm thick or greater, regardless of residence, and for DLBCL and FL survivors, although our sample size was limited for subgroup analyses.
Cumulative incidence of melanoma was higher for survivors of CLL/SLL than for survivors of non-CLL/SLL (1.37% v 0.78%, attained at age 85 years; Fig 1). Among survivors of CLL/SLL, the cumulative incidence of melanoma was higher for patients who received fludarabine-containing chemotherapy versus those who did not (1.66% v 1.22%), and for those with a diagnosis of T-cell–activating autoimmune conditions (1.64% v 1.03%).
Fig 1.
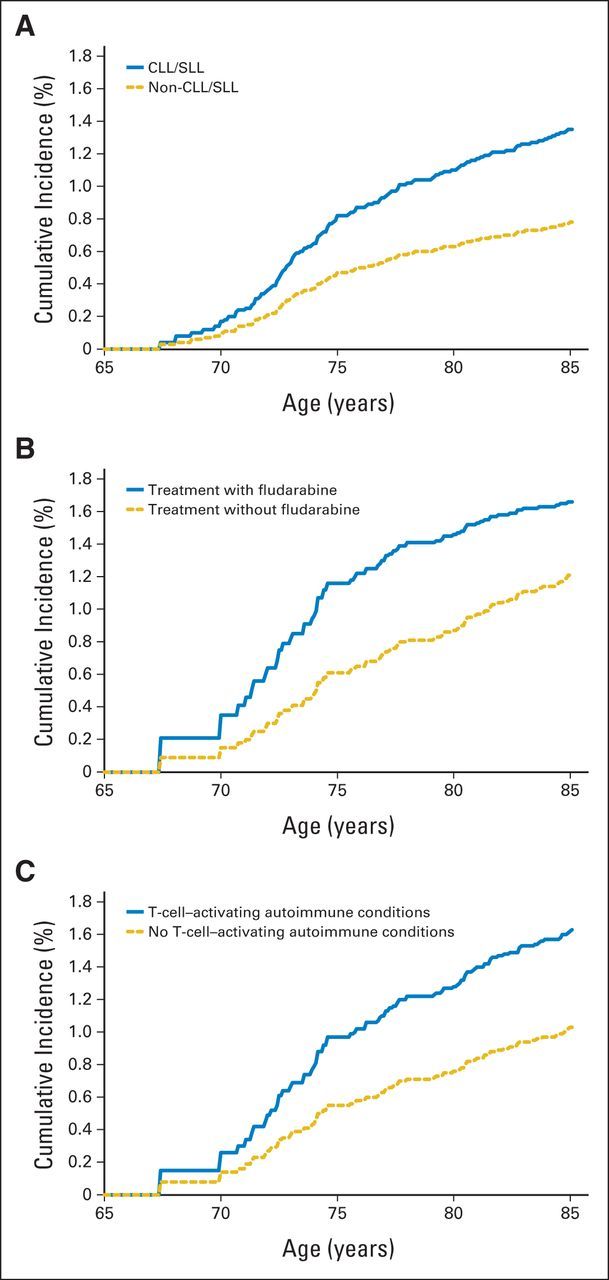
(A) Cumulative incidence of subsequent melanoma after non-Hodgkin lymphoma by subtype, (B) by fludarabine treatment among chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) survivors, and (C) by diagnosis of T-cell–activating autoimmune conditions among CLL/SLL survivors.
DISCUSSION
In a large-scale, population-based study, we show, for the first time, to our knowledge, that patients with CLL/SLL who were treated with fludarabine-containing chemotherapy (with or without rituximab) or who were diagnosed with T-cell–activating autoimmune conditions have an approximately two-fold increased risk of developing cutaneous melanoma. In contrast, survivors of non-CLL/SLL had no evidence of heighted melanoma risk associated with either T-cell or B-cell autoimmune diseases, and specific chemotherapy regimens did not seem to strongly increase the risk of melanoma. In general, most infections did not seem to be related to melanoma risks. Our study results provide direct evidence for the importance of immune perturbation in explaining the excess of melanoma diagnoses observed in survivors of CLL/SLL.
Patients with CLL/SLL experience profound and prolonged immune dysfunction characterized by defective B-cell and T-cell function, which contributes to increased incidence of infections and autoimmune diseases.44–46 In the first quantification of second melanoma risk associated with specific infused chemotherapy agents administered for CLL/SLL, including both initial and subsequent chemotherapy, we demonstrated that fludarabine-containing regimens, with or without rituximab, are linked to a significant excess risk of melanoma after CLL/SLL. Increased secondary malignancy risks, including treatment-related acute myeloid leukemia and solid malignancies, particularly skin cancers, have been reported in fludarabine-treated CLL/SLL cohorts,9,13,47–53 but no previous study has directly compared melanoma risks in patients treated with and without fludarabine. The exact mechanism of action of fludarabine in the induction of cutaneous melanoma is unclear, but may be a result of inherent predisposition to malignancy among patients with CLL/SLL coupled with the immunosuppressive and DNA-damaging effects of fludarabine.47,54,55
Increased melanoma risks also have been observed in other immunosuppressed populations, such as solid organ and bone marrow transplant recipients, particularly those who received T-cell–depleting therapies,23,26 as well as in persons with HIV/AIDS.22,56 In addition, the critical role of T cells in the antitumor response in patients with melanoma is supported by the effectiveness of immunotherapy directed at T-cell checkpoints in treating metastatic melanoma.57–59 In our study, the melanomas occurring after CLL/SLL were more likely to be 1-mm thick or greater compared with those occurring after other NHLs, which is consistent with previous studies reporting these melanomas as more advanced and more aggressive than melanomas that arise in the general population (Robbins et al, submitted for publication.3,25,60–62 Patients with CLL/SLL are commonly diagnosed with hematologic autoimmune conditions (eg, autoimmune hemolytic anemia or thrombocytopenia); however, nonhematologic autoimmune diseases are reportedly rare (Robbins et al, submitted for publication).62 In a new finding, to our knowledge, we show that melanoma risk is approximately two-fold increased for patients with T-cell–activating autoimmune conditions, including Graves' disease, psoriasis, chronic rheumatic heart disease, localized scleroderma/psoriasis, and asthma. Combined with previous literature, our findings support the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL.
Although cutaneous melanoma has been reported in excess after all NHLs combined in numerous studies,3–21 limited data by NHL subtype have suggested a modestly increased risk of melanoma after FL, whereas risk after DLBCL did not exceed unity.3 In contrast to our CLL/SLL results, we found no significant associations between melanoma risk and treatment for non-CLL/SLL NHL subtypes, although a 70% nonsignificant increase in risk was seen among patients treated with cyclophosphamide-based regimens without rituximab. Although cyclophosphamide has been associated with immunosuppression at higher doses and can increase the risk of bacterial infections, the association is because of ensuing neutropenia and not reduced T-cell function.63–65 Our investigation had relatively few melanoma cases after specific NHLs other than CLL/SLL; thus, future studies with larger numbers will be needed to evaluate treatment- and immune-related risk factors for non-CLL/SLL NHL.
We found little evidence that T-cell– or B-cell–activating autoimmune conditions were related to melanoma after non-CLL/SLL NHL, raising the possibility that these conditions may only contribute to the development of melanoma after NHL in the context of profound and prolonged immunosuppression, such as that seen after CLL/SLL. As the single exception, melanoma risk was significantly increased among individuals who developed localized scleroderma before NHL. We also observed increased risk for melanoma among individuals diagnosed with pneumonia, urinary tract infections, and gastrohepatic infections after non-CLL/SLL NHL, a different pattern of infections than that observed for survivors of CLL/SLL in whom melanoma risk was significantly associated with cellulitis and urinary tract infections. Although it is plausible that these infections could be markers of immune perturbation, and T-cell dysfunction, in particular, for pneumonia,66,67 our data overall did not demonstrate strong associations with the occurrence of infections.
The primary strength of this study was analysis of a large, population-based cohort to identify specific risk factors for second melanoma development by NHL subtype, considering detailed treatment and nontreatment risk factors. The cohort, records-based study design also provided long-term follow-up for exposure assessment and second melanoma occurrence, and eliminated selection bias.
Despite these strengths, several key limitations should be considered in the interpretation of our results. Most notable, we lacked data on dose and duration of chemotherapy, and information on oral chemotherapy agents was not available for the duration of follow-up; thus, we could not ascertain receipt of chlorambucil or other oral alkylators.49 Chlorambucil is myelosuppressive but does not typically result in profound T-cell depletion; nevertheless, our fludarabine-related risk estimates are likely to be conservative because patients who received only oral chemotherapy are included in our referent group.
In addition, because of the nature of the Medicare claims database, some exposures may have been misclassified (eg, because of missing information on infections and autoimmune diseases diagnosed in patients before enrollment into Medicare, or for mild infections for which the patient did not seek medical care). Because we did not include persons younger than 65 years at NHL diagnosis, our study findings may not be generalizable to younger populations. We also were restricted to assessing potential risk factors that generate a medical claim, excluding key melanoma risk factors, such as genetic susceptibility or a direct measure of sun exposure. However, our analyses were adjusted for residence (a proxy for sun exposure), although this risk prediction model of melanoma incidence has not been validated in a cancer survivor population.39
We provide, to our knowledge, the first evidence that specific chemotherapeutic agents and immune-related medical conditions are related to subsequent melanoma risk among older survivors of CLL/SLL, but not among patients with other NHL subtypes combined. Our findings identify high-risk survivors of NHL who may benefit most from regular full skin examinations to maximize opportunities for early detection of cutaneous melanoma. Further research is needed to understand the biologic mechanisms behind the immune perturbations that lead to melanoma development and to determine if similar risks extend to younger survivors of NHL.
Supplementary Material
Appendix
Table A1.
Frequency of Receipt and Medical Claim Codes for Specific Chemotherapeutic Agents
| Chemotherapy | HCPCS/CPT Codes | Total NHL |
Melanoma Cases* |
||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Patients diagnosed with first primary CLL/SLL | |||||
| Most common agents | |||||
| Cyclophosphamide | J8530, J9070, J9080, J9090-J9097 | 2,402 | 17.2 | 19 | 20.9 |
| Fludarabine | J9185 | 2,958 | 21.2 | 28 | 30.8 |
| Rituximab | J9310 | 3,744 | 26.8 | 27 | 29.7 |
| Plant alkaloids | |||||
| Vincristine | J9370, J9375, J9380 | 1,590 | 11.4 | 13 | 14.3 |
| Patients diagnosed with first primary NHL other than CLL/SLL | |||||
| Most common agents | |||||
| Cyclophosphamide | J8530, J9070, J9080, J9090-J9097 | 16,786 | 54.3 | 68 | 61.3 |
| Rituximab | J9310 | 15,726 | 50.9 | 53 | 47.8 |
| Topoisomerases II inhibitors | |||||
| Doxorubicin | J9000-J9001 | 12,115 | 39.2 | 56 | 50.5 |
| Plant alkaloids | |||||
| Vincristine | J9370, J9375, J9380 | 16,365 | 52.9 | 63 | 56.8 |
| Colony stimulating factors | |||||
| G-CSF | J1440-J1441 | 7,027 | 22.7 | 26 | 23.4 |
NOTE. Information on oral chemotherapy agents not captured in study include capecitabine, chlorambucil, cyclophosphamide, levamisole, procarbazine, and temozolomide.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CPT, Current Procedural Technology; G-CSF, granulocyte colony-stimulating factor; HCPCS, Health Care Common Procedure Coding System; NHL, non-Hodgkin lymphoma.
Counts and percentages are not reported when fewer than 10 melanoma cases to protect patient confidentiality. Chemotherapy agents with fewer than 10 melanoma cases include aldesleukin, alemtuzumab, idarubicin, ixabepilone, asparaginase, azacitidine, bendamustine, bevacizumab, bleomycin, bortezomib, busulfan, carboplatin, carmustine, cetuximab, cisplatin, cladribine, clofarabine, cytarabine, dacarbazine, dactinomycin, daunorubicin, decitabine, denileukin, diftitox, docetaxel, epirubicin, etoposide, floxuridine, fluorouracil, gefitinib, gemcitabine, gemtuzumab, G-CSF, ibritumomab, ifosfamide, interferons (1B, 2A, 2B, A1, N3), irinotecan, leucovorin, lomustine, mechlorethamine, melphalan, methotrexate, mitomycin, mitoxantrone, nelarabine, oxaliplatin, paclitaxel, panitumumab, pegaspargase, pemetrexed, pentostatin, plicamycin, streptozocin, temsirolimus, teniposide, thiotepa, topotecan, tositumomab, trastuzumab, valrubicin, vinblastine, and vinorelbine.
Table A2.
Frequency of Diagnosis and Medical Claim Codes for Autoimmune Conditions
| Autoimmune Conditions | HCPCS/CPT Codes | Total NHL |
Melanoma Cases |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before NHL |
After NHL* |
Before NHL |
After NHL* |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Patients diagnosed with first primary CLL/SLL | |||||||||
| B-cell–activating conditions† | Conditions below | 2,263 | 16.2 | 3,637 | 26.1 | < 10 | — | 13 | 14.3 |
| T-cell–activating conditions† | Conditions below | 5,488 | 39.3 | 6,749 | 48.4 | 36 | 39.6 | 49 | 53.9 |
| By organ system involved | |||||||||
| Systemic/connective tissue | 1,687 | 12.1 | 1,912 | 13.7 | < 10 | — | < 10 | — | |
| Ankylosing spondylitis | 720 | 321 | 2.3 | 377 | 2.7 | < 10 | — | < 10 | — |
| Dermatomyositis/polymyositis | 710.3, 710.4 | 37 | 0.3 | 39 | 0.3 | < 10 | — | < 10 | — |
| Felty's syndrome | 714.1 | 15 | 0.1 | 15 | 0.1 | < 10 | — | < 10 | — |
| Systemic lupus erythematosus | 710 | 348 | 2.5 | 424 | 3.0 | < 10 | — | < 10 | — |
| Polymyalgia rheumatica | 725 | 256 | 1.8 | 295 | 2.1 | < 10 | — | < 10 | — |
| Reactive arthritis | 99.3 | < 10 | — | < 10 | — | < 10 | — | < 10 | — |
| Rheumatic fever | 390-392 | 46 | 0.3 | 52 | 0.4 | < 10 | — | < 10 | — |
| Rheumatoid arthritis | 714 | 981 | 7.0 | 1,037 | 7.4 | < 10 | — | < 10 | — |
| Sarcoidosis | 135 | 35 | 0.3 | 43 | 0.3 | < 10 | — | < 10 | — |
| Sjogren's syndrome | 710.2 | 123 | 0.9 | 128 | 0.9 | < 10 | — | < 10 | — |
| Systemic sclerosis/scleroderma | 710.1 | 17 | 0.1 | 24 | 0.2 | < 10 | — | < 10 | — |
| Cardiovascular | 1,367 | 9.8 | 2,370 | 17.0 | < 10 | — | 15 | 16.5 | |
| Chronic rheumatic heart disease | 393-398 | 1,219 | 8.7 | 2,187 | 15.7 | < 10 | — | 15 | 16.5 |
| Giant cell arteritis | 446.5 | 127 | 0.9 | 134 | 1.0 | < 10 | — | < 10 | — |
| Systemic vasculitis | 446,447.60 | 174 | 1.3 | 239 | 1.7 | < 10 | — | < 10 | — |
| Endocrine | 931 | 6.7 | 1,213 | 8.7 | < 10 | — | < 10 | — | |
| Addison's disease | 255.4 | 71 | 0.5 | 242 | 1.7 | < 10 | — | < 10 | — |
| Chronic thyroiditis/Hashimoto thyroiditis | 245.2 | 103 | 0.7 | 134 | 1.0 | < 10 | — | < 10 | — |
| Graves' disease | 242 | 783 | 5.6 | 872 | 6.3 | < 10 | — | < 10 | — |
| Primary biliary cirrhosis | 571.6 | 12 | 0.1 | 23 | 0.2 | < 10 | — | < 10 | — |
| Skin | 2,222 | 15.9 | 2,402 | 17.2 | 18 | 19.8 | 21 | 23.1 | |
| Alopecia areata | 704.1 | 25 | 0.2 | 55 | 0.4 | < 10 | — | < 10 | — |
| Dermatitis herpetiformis | 694 | 56 | 0.4 | 114 | 0.8 | < 10 | — | < 10 | — |
| Discoid lupus erythematosus | 695.4 | 34 | 0.2 | 38 | 0.3 | < 10 | — | < 10 | — |
| Localized scleroderma | 701 | 1,809 | 13.0 | 1,924 | 13.8 | 12 | 13.2 | 13 | 14.3 |
| Psoriasis | 696 | 445 | 3.2 | 431 | 3.1 | < 10 | — | < 10 | — |
| Gastrointestinal | 1,435 | 10.3 | 2,926 | 21.0 | < 10 | — | < 10 | — | |
| Celiac disease | 579 | 149 | 1.1 | 203 | 1.5 | < 10 | — | < 10 | — |
| Crohn's disease | 555 | 105 | 0.8 | 142 | 1.0 | < 10 | — | < 10 | — |
| Pernicious anemia | 281 | 1,119 | 8.0 | 2,548 | 18.3 | < 10 | — | < 10 | — |
| Ulcerative colitis | 556 | 187 | 1.3 | 255 | 1.8 | < 10 | — | < 10 | — |
| Nervous system | 110 | 0.8 | 180 | 1.3 | < 10 | — | < 10 | — | |
| Amyotrophic sclerosis | 335.2 | 10 | 0.1 | 48 | 0.3 | < 10 | — | < 10 | — |
| Multiple sclerosis | 340 | 54 | 0.4 | 69 | 0.5 | < 10 | — | < 10 | — |
| Myasthenia gravis | 358 | 50 | 0.4 | 69 | 0.5 | < 10 | — | < 10 | — |
| Respiratory (asthma) | 493 | 1,919 | 13.8 | 2,484 | 17.8 | 13 | 14.3 | 18 | 19.8 |
| Autoimmune disease, NOS | 279.4 | 32 | 0.23 | 64 | 0.5 | < 10 | — | < 10 | — |
| Patients diagnosed with first primary NHL other than CLL/SLL | |||||||||
| B-cell–activating conditions † | Conditions below | 6,013 | 19.5 | 8,078 | 26.1 | 16 | 14.4 | 24 | 21.6 |
| T-cell–activating conditions † | Conditions below | 13,142 | 42.5 | 15,201 | 49.2 | 36 | 32.4 | 35 | 31.5 |
| By organ system involved | |||||||||
| Systemic/connective tissue | 4,798 | 15.5 | 4,734 | 15.3 | < 10 | — | < 10 | — | |
| Ankylosing spondylitis | 720 | 790 | 2.6 | 853 | 2.8 | < 10 | — | < 10 | — |
| Dermatomyositis/polymyositis | 710.3, 710.4 | 126 | 0.4 | 113 | 0.4 | < 10 | — | < 10 | — |
| Felty's syndrome | 714.1 | 32 | 0.1 | 31 | 0.1 | < 10 | — | < 10 | — |
| Systemic lupus erythematosus | 710 | 1,146 | 3.7 | 1,189 | 3.9 | < 10 | — | < 10 | — |
| Polymyalgia rheumatica | 725 | 727 | 2.4 | 671 | 2.2 | < 10 | — | < 10 | — |
| Reactive arthritis | 99.3 | 12 | 0.0 | < 10 | — | < 10 | — | < 10 | — |
| Rheumatic fever | 390-392 | 78 | 0.3 | 99 | 0.3 | < 10 | — | < 10 | — |
| Rheumatoid arthritis | 714 | 2,961 | 9.6 | 2,658 | 8.6 | < 10 | — | < 10 | — |
| Sarcoidosis | 135 | 144 | 0.5 | 180 | 0.6 | < 10 | — | < 10 | — |
| Sjogren's syndrome | 710.2 | 446 | 1.4 | 495 | 1.6 | < 10 | — | < 10 | — |
| Systemic sclerosis/scleroderma | 710.1 | 99 | 0.3 | 106 | 0.3 | < 10 | — | < 10 | — |
| Cardiovascular | 3,212 | 10.4 | 5,527 | 17.9 | < 10 | — | < 10 | — | |
| Chronic rheumatic heart disease | 393-398 | 2,771 | 9.0 | 5,071 | 16.4 | < 10 | — | < 10 | — |
| Giant cell arteritis | 446.5 | 368 | 1.2 | 355 | 1.2 | < 10 | — | < 10 | — |
| Systemic vasculitis | 446,447.60 | 521 | 1.7 | 585 | 1.9 | < 10 | — | < 10 | — |
| Endocrine | 2,419 | 7.8 | 2,947 | 9.5 | < 10 | — | < 10 | — | |
| Addison's disease | 255.4 | 146 | 0.5 | 586 | 1.9 | < 10 | — | < 10 | — |
| Chronic thyroiditis/Hashimoto thyroiditis | 245.2 | 307 | 1.0 | 441 | 1.4 | < 10 | — | < 10 | — |
| Graves' disease | 242 | 2,042 | 6.6 | 2,067 | 6.7 | < 10 | — | < 10 | — |
| Primary biliary cirrhosis | 571.6 | 34 | 0.1 | 56 | 0.2 | < 10 | — | < 10 | — |
| Skin | 5,648 | 18.3 | 5,394 | 17.5 | 23 | 20.7 | 18 | 16.2 | |
| Alopecia areata | 704.1 | 47 | 0.2 | 50 | 0.2 | < 10 | — | < 10 | — |
| Dermatitis herpetiformis | 694 | 173 | 0.6 | 214 | 0.7 | < 10 | — | < 10 | — |
| Discoid lupus erythematosus | 695.4 | 151 | 0.5 | 135 | 0.4 | < 10 | — | < 10 | — |
| Localized scleroderma | 701 | 4,265 | 13.8 | 4,110 | 13.3 | 22 | 19.8 | 17 | 15.3 |
| Psoriasis | 696 | 1,465 | 4.7 | 1,357 | 4.4 | < 10 | — | < 10 | — |
| GI | 3,458 | 11.2 | 6,043 | 19.5 | 10 | 9.0 | 15 | 13.5 | |
| Celiac disease | 579 | 364 | 1.2 | 507 | 1.6 | < 10 | — | < 10 | — |
| Crohn's disease | 555 | 291 | 0.9 | 372 | 1.2 | < 10 | — | < 10 | — |
| Pernicious anemia | 281 | 2,635 | 8.5 | 5,068 | 16.4 | < 10 | — | < 10 | — |
| Ulcerative colitis | 556 | 469 | 1.5 | 612 | 2.0 | < 10 | — | < 10 | — |
| Nervous system | 194 | 0.6 | 379 | 1.2 | < 10 | — | < 10 | — | |
| Amyotrophic sclerosis | 335.2 | 30 | 0.1 | 77 | 0.3 | < 10 | — | < 10 | — |
| Multiple sclerosis | 340 | 69 | 0.2 | 145 | 0.5 | < 10 | — | < 10 | — |
| Myasthenia gravis | 358 | 102 | 0.3 | 163 | 0.5 | < 10 | — | < 10 | — |
| Respiratory (asthma) | 493 | 4,338 | 14.0 | 5,133 | 16.6 | < 10 | — | < 10 | — |
| Autoimmune disease, NOS | 279.4 | 120 | 0.4 | 161 | 0.5 | < 10 | — | < 10 | — |
NOTE. Counts and percentages are not reported for fewer than 10 melanoma cases to protect patient confidentiality.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CPT, Current Procedural Technology; HCPCS, Health care Common Procedure Coding System; NHL, non-Hodgkin lymphoma; NOS, not otherwise specified.
New claims occurring after NHL but before second cancer, death, end of study, or loss to follow-up, with no claims before NHL.
B-cell–activating conditions include rheumatoid arthritis, Sjogren's syndrome, discoid lupus erythematosus, reactive arthritis, Felty's syndrome, chronic thryoiditis, systemic/discoid lupus erythematosus, pernicious anemia, and myasthenia gravis. T-cell–activating conditions include ankylosing spondylitis, dermatomyositis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, rheumatic fever, chronic rheumatic heart disease, giant cell arteritis, systemic vasculitis, Addison's disease, Graves' disease, primary biliary cirrhosis, alopecia areata, localized scleroderma, dermatitis herpetiformis, psoriasis, celiac disease, Crohn's disease, ulcerative colitis, amyotrophic sclerosis, multiple sclerosis, and asthma. Hematologic autoimmune conditions (eg, autoimmune hemolytic anemia, thrombocytopenia) were excluded from consideration because of difficulty distinguishing these diagnoses from manifestations of chemotherapy toxicity.
Table A3.
Frequency of Diagnosis and Medical Claim Codes for Infections
| Infections | HCPCS/CPT Codes | Total NHL |
Melanoma Cases |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before NHL |
After NHL* |
Before NHL |
After NHL* |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Patients diagnosed with first primary CLL/SLL | |||||||||
| Respiratory, upper airway | 5,847 | 41.9 | 5,850 | 41.9 | 35 | 38.5 | 35 | 38.5 | |
| Laryngitis | 464-464.4, 476-476.1 | 593 | 4.3 | 614 | 4.4 | < 10 | — | < 10 | — |
| Otitis media | 017.4, 055.2, 381.0-381.4, 382, 383.0-383.1 | 1,621 | 11.6 | 1,514 | 10.9 | < 10 | — | < 10 | — |
| Pharyngitis | 462,472.1 | 2,141 | 15.4 | 2,143 | 15.4 | 10 | 11.0 | 11 | 12.1 |
| Sinusitis | 461,473 | 4,074 | 29.2 | 4,238 | 30.4 | 24 | 26.4 | 29 | 31.9 |
| Respiratory, lower airway | 6,122 | 43.9 | 8,347 | 59.8 | 37 | 40.7 | 41 | 45.1 | |
| Acute bronchitis | 466 | 4,526 | 32.4 | 5,081 | 36.4 | 27 | 29.7 | 34 | 37.4 |
| Influenza | 487 | 783 | 5.6 | 821 | 5.9 | < 10 | — | < 10 | — |
| Pneumonia | 480-486, 770 | 2,872 | 20.6 | 6,414 | 46.0 | 16 | 17.6 | 21 | 23.1 |
| Tuberculosis | 010-018 | 122 | 0.9 | 150 | 1.1 | < 10 | — | < 10 | — |
| Skin | 2,125 | 15.2 | 3,490 | 25.0 | 17 | 18.7 | 21 | 23.1 | |
| Cellulitis | 682.9 | 1,191 | 8.5 | 1,948 | 14.0 | 11 | 12.1 | 11 | 12.1 |
| Herpes zoster | 53 | 1,079 | 7.7 | 1,958 | 14 | < 10 | — | 13 | 14.3 |
| Urinary tract | 6,227 | 44.6 | 7,942 | 56.9 | 39 | 42.9 | 46 | 50.6 | |
| Cystitis/pyelonephritis, UTI | 599 | 5,630 | 40.4 | 7,599 | 54.5 | 34 | 37.4 | 40 | 44.0 |
| Prostatitis† | 601 | 1,554 | 20.5 | 1,199 | 15.8 | 17 | 23.0 | 14 | 18.9 |
| Gastrohepatic | 2,084 | 14.9 | 2,594 | 18.6 | 11 | 12.1 | 12 | 13.2 | |
| Gastroenteritis | 558.9 | 2,020 | 14.5 | 2,445 | 17.5 | 11 | 12.1 | 11 | 12.1 |
| HBV | 070.2-070.3 | 36 | 0.3 | 96 | 0.7 | < 10 | — | < 10 | — |
| HCV | 070.4, 070.5, 070.7 | 51 | 0.4 | 133 | 1.0 | < 10 | — | < 10 | — |
| Patients diagnosed with first primary NHL other than CLL/SLL | |||||||||
| Respiratory, upper airway | 13,016 | 42.1 | 11,944 | 38.6 | 41 | 36.9 | 40 | 36.0 | |
| Laryngitis | 464-464.4, 476-476.1 | 1,467 | 4.7 | 1,390 | 4.5 | < 10 | — | < 10 | — |
| Otitis media | 017.4, 055.2, 381.0-381.4, 382, 383.0-383.1 | 3,400 | 11.0 | 2,857 | 9.2 | 11 | 9.9 | < 10 | — |
| Pharyngitis | 462, 472.1 | 4,800 | 15.5 | 4,299 | 13 | 18 | 16.2 | 12 | 10.8 |
| Sinusitis | 461, 473 | 9,109 | 29.5 | 8,120 | 26.3 | 30 | 27.0 | 26 | 23.4 |
| Respiratory, lower airway | 13,047 | 42.2 | 16,711 | 54.1 | 41 | 36.9 | 46 | 41.4 | |
| Acute bronchitis | 466 | 9,819 | 31.8 | 9,358 | 30.3 | 30 | 27.0 | 27 | 24.3 |
| Influenza | 487 | 1,707 | 5.5 | 1,531 | 5.0 | < 10 | — | < 10 | — |
| Pneumonia | 480-486, 770 | 5,667 | 18.3 | 12,383 | 40.1 | 27 | 24.3 | 25 | 22.5 |
| Tuberculosis | 010-018 | 315 | 1.0 | 454 | 1.5 | < 10 | — | < 10 | — |
| Skin | 4,501 | 14.6 | 7,025 | 22.7 | 13 | 11.7 | 15 | 13.5 | |
| Cellulitis | 682.9 | 2,465 | 8.0 | 3,762 | 12.2 | < 10 | — | 11 | 9.9 |
| Herpes zoster | 53 | 2,295 | 7.4 | 3,980 | 12.9 | < 10 | — | < 10 | — |
| Urinary tract | 14,162 | 45.8 | 17,462 | 56.5 | 53 | 47.8 | 54 | 48.7 | |
| Cystitis/pyelonephritis, UTI | 599 | 12,837 | 41.5 | 16,759 | 54.2 | 39 | 35.1 | 51 | 46.0 |
| Prostatitis† | 601 | 3,168 | 21.8 | 2,154 | 14.9 | 19 | 25.0 | < 10 | — |
| Gastrohepatic | 4,880 | 15.8 | 5,926 | 19.2 | 14 | 12.6 | 21 | 18.9 | |
| Gastroenteritis | 558.9 | 4,689 | 15.2 | 5,556 | 18.0 | 13 | 11.7 | 19 | 17.1 |
| HBV | 070.2-070.3 | 95 | 0.3 | 198 | 0.6 | < 10 | — | < 10 | — |
| HCV | 070.4, 070.5, 070.7 | 196 | 0.6 | 336 | 1.1 | < 10 | — | < 10 | — |
NOTE. Counts and percentages are not reported for fewer than 10 melanoma cases to protect patient confidentiality.
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CPT, Current Procedural Technology; HCPCS, Health care Common Procedure Coding System; HBV, hepatitis B virus; HCV, hepatitis C virus; ICD, International Classification of Diseases; NHL, non-Hodgkin lymphoma; UTI, urinary tract infection.
New claims occurring after NHL but before second cancer, death, end of study, or loss to follow-up, with no claims before NHL.
Among males only.
Table A4.
Risk of Melanoma After First Primary NHL, by Subtype
| Variable | Total NHL |
CLL/SLL |
Other NHL Subtypes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | HR* | 95% CI | No. | % | HR* | 95% CI | No. | % | HR* | 95% CI | |
| Sex | ||||||||||||
| Male | 22,097 | 49.3 | 1.00 | Referent | 7,590 | 54.4 | 1.00 | Referent | 14,507 | 46.9 | 1.00 | Referent |
| Female | 22,773 | 50.8 | 0.31 | 0.23 to 0.43 | 6,360 | 45.6 | 0.24 | 0.14 to 0.41 | 16,413 | 53.1 | 0.37 | 0.24 to 0.55 |
| Race | ||||||||||||
| White | 40,752 | 90.8 | 1.00 | Referent | 12,841 | 92.1 | 1.00 | Referent | 27,911 | 90.3 | 1.00 | Referent |
| Other/unknown | 4,118 | 9.2 | 0.36 | 0.16 to 0.81 | 1,109 | 7.9 | 0.17 | 0.02 to 1.20 | 3,009 | 9.7 | 0.46 | 0.19 to 1.14 |
| Residence at time of NHL diagnosis† | ||||||||||||
| North | 20,348 | 45.4 | 1.00 | Referent | 6,691 | 48.0 | 1.00 | Referent | 13,657 | 44.2 | 1.00 | Referent |
| Central | 12,778 | 28.5 | 0.98 | 0.68 to 1.39 | 3,727 | 26.7 | 1.19 | 0.70 to 2.00 | 9,051 | 29.3 | 0.88 | 0.54 to 1.42 |
| South | 11,744 | 26.2 | 1.38 | 1.00 to 1.92 | 3,532 | 25.3 | 1.40 | 0.85 to 2.29 | 8,212 | 26.6 | 1.36 | 0.88 to 2.11 |
| Charlson comorbidity index | ||||||||||||
| No comorbidities | 11,134 | 24.8 | 1.00 | Referent | 3,322 | 23.8 | 1.00 | Referent | 7,812 | 25.3 | 1.00 | Referent |
| 1 comorbidity | 11,138 | 24.8 | 0.62 | 0.44 to 0.88 | 3,366 | 24.1 | 0.51 | 0.29 to 0.87 | 7,772 | 25.1 | 0.75 | 0.47 to 1.18 |
| 2+ comorbidities | 22,473 | 50.1 | 0.36 | 0.26 to 0.50 | 7,226 | 51.8 | 0.33 | 0.20 to 0.54 | 15,247 | 49.3 | 0.40 | 0.25 to 0.63 |
| Missing | 125 | 0.3 | 2.34 | 0.57 to 9.62 | 36 | 0.3 | 2.59 | 0.34 to 19.63 | 89 | 0.3 | 2.47 | 0.33 to 18.58 |
| Socioeconomic status | ||||||||||||
| Lowest quintile | 9,832 | 21.9 | 1.00 | Referent | 3,239 | 23.2 | 1.00 | Referent | 6,593 | 21.3 | 1.00 | Referent |
| 2nd lowest quintile | 9,792 | 21.8 | 1.17 | 0.72 to 1.92 | 3,087 | 22.1 | 1.27 | 0.60 to 2.65 | 6,705 | 21.7 | 1.13 | 0.58 to 2.19 |
| Middle quintile | 10,062 | 22.4 | 1.30 | 0.80 to 2.10 | 3,140 | 22.5 | 1.23 | 0.58 to 2.61 | 6,922 | 22.4 | 1.39 | 0.73 to 2.63 |
| 2nd highest quintile | 9,295 | 20.7 | 1.52 | 0.95 to 2.44 | 2,742 | 19.7 | 1.59 | 0.78 to 3.27 | 6,553 | 21.2 | 1.47 | 0.78 to 2.78 |
| Highest quintile | 5,374 | 12.0 | 1.93 | 1.17 to 3.17 | 1,540 | 11.0 | 2.70 | 1.31 to 5.56 | 3,834 | 12.4 | 1.43 | 0.71 to 2.86 |
| Missing | 515 | 1.1 | 3.57 | 1.62 to 7.88 | 202 | 1.4 | 4.29 | 1.50 to 12.25 | 313 | 1.0 | 2.95 | 0.85 to 10.29 |
| NHL subtype | ||||||||||||
| DLBCL | 10,311 | 23.0 | 1.00 | Referent | ||||||||
| CLL/SLL | 13,950 | 31.1 | 1.65 | 1.11 to 2.45 | ||||||||
| FL | 7,437 | 16.6 | 1.25 | 0.78 to 2.01 | ||||||||
| MZL | 3,516 | 7.8 | 0.90 | 0.44 to 1.82 | ||||||||
| Other | 9,656 | 21.5 | 0.93 | 0.58 to 1.51 | ||||||||
Abbreviations: CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HR, hazard ratio; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma.
The HRs (95% CI) were calculated using one model including indicator variables for sex, race, residence, Charlson comorbidity index, socioeconomic status, and NHL subtype, then adjusted for follow-up time and stratified by calendar year with age as the time scale.
Residence defined by Surveillance, Epidemiology, and End Results registry areas, including north (Connecticut, Detroit, Iowa, Seattle, and New Jersey), central (San Francisco, Utah, San Jose, Greater California, and Kentucky), and south (Hawaii, New Mexico, Atlanta, Los Angeles, Rural Georgia, Greater Georgia, and Louisiana).
Footnotes
Processed as a Rapid Communication manuscript.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Clara J.K. Lam, Rochelle E. Curtis
Collection and assembly of data: Clara J.K. Lam, Eric A. Engels
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk Factors for Melanoma Among Survivors of Non-Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Clara J.K. Lam
No relationship to disclose
Rochelle E. Curtis
No relationship to disclose
Graça M. Dores
No relationship to disclose
Eric A. Engels
No relationship to disclose
Neil E. Caporaso
No relationship to disclose
Aaron Polliack
No relationship to disclose
Joan L. Warren
No relationship to disclose
Heather A. Young
No relationship to disclose
Paul H. Levine
No relationship to disclose
Angelo F. Elmi
No relationship to disclose
Joseph F. Fraumeni Jr
No relationship to disclose
Margaret A. Tucker
No relationship to disclose
Lindsay M. Morton
No relationship to disclose
REFERENCES
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975-2011. http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 2.de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: Differences by lymphoma subtype. J Clin Oncol. 2010;28:4935–4944. doi: 10.1200/JCO.2010.29.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis LB, Curtis RE, Hankey BF, et al. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422–1427. doi: 10.1093/jnci/84.18.1422. [DOI] [PubMed] [Google Scholar]
- 5.Goggins WB, Finkelstein DM, Tsao H. Evidence for an association between cutaneous melanoma and non-Hodgkin lymphoma. Cancer. 2001;91:874–880. doi: 10.1002/1097-0142(20010215)91:4<874::aid-cncr1076>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Brennan P, Scelo G, Hemminki K, et al. Second primary cancers among 109,000 cases of non-Hodgkin's lymphoma. Br J Cancer. 2005;93:159–166. doi: 10.1038/sj.bjc.6602654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tward JD, Wendland MMM, Shrieve DC, et al. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Lenner P, Sundquist J, et al. Risk of subsequent solid tumors after non-Hodgkin's lymphoma: Effect of diagnostic age and time since diagnosis. J Clin Oncol. 2008;26:1850–1857. doi: 10.1200/JCO.2007.14.6068. [DOI] [PubMed] [Google Scholar]
- 9.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirani M, Marcheselli R, Marcheselli L, et al. Risk for second malignancies in non-Hodgkin's lymphoma survivors: A meta-analysis. Ann Oncol. 2011;22:1845–1858. doi: 10.1093/annonc/mdq697. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Wang H, Zhou S, et al. Risk of second malignant neoplasms after cyclophosphamide-based chemotherapy with or without radiotherapy for non-Hodgkin lymphoma. Leuk Lymphoma. 2013;54:1396–1404. doi: 10.3109/10428194.2012.743657. [DOI] [PubMed] [Google Scholar]
- 12.Hisada M, Biggar RJ, Greene MH, et al. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Vena DA, Barrett J, et al. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999;17:2454–2460. doi: 10.1200/JCO.1999.17.8.2454. [DOI] [PubMed] [Google Scholar]
- 14.Schollkopf C, Rosendahl D, Rostgaard K, et al. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121:151–156. doi: 10.1002/ijc.22672. [DOI] [PubMed] [Google Scholar]
- 15.Travis LB, Curtis RE, Boice JD, Jr, et al. Second cancers following non-Hodgkin's lymphoma. Cancer. 1991;67:2002–2009. doi: 10.1002/1097-0142(19910401)67:7<2002::aid-cncr2820670729>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Travis LB, Curtis RE, Glimelius B, et al. Second cancers among long-term survivors of non-Hodgkin's lymphoma. J Natl Cancer Inst. 1993;85:1932–1937. doi: 10.1093/jnci/85.23.1932. [DOI] [PubMed] [Google Scholar]
- 17.Ellis M, Lishner M. Second malignancies following treatment in non-Hodgkin's lymphoma. Leuk Lymphoma. 1993;9:337–342. doi: 10.3109/10428199309148531. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Hemminki K. Second primary neoplasms among 53,159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: a search for common mechanisms. Br J Cancer. 2001;85:997–1005. doi: 10.1054/bjoc.2001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna DB, Stockton D, Brewster DH, et al. Evidence for an association between cutaneous malignant melanoma and lymphoid malignancy: A population-based retrospective cohort study in Scotland. Br J Cancer. 2003;88:74–78. doi: 10.1038/sj.bjc.6600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royle JS, Baade P, Joske D, et al. Risk of second cancer after lymphohematopoietic neoplasm. Int J Cancer. 2011;129:910–919. doi: 10.1002/ijc.25706. [DOI] [PubMed] [Google Scholar]
- 21.Royle JA, Baade PD, Joske D, et al. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: A population-based study. Br J Cancer. 2011;105:1076–1081. doi: 10.1038/bjc.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemminki K, Jiang Y, Steineck G. Skin cancer and non-Hodgkin's lymphoma as second malignancies: Markers of impaired immune function? Eur J Cancer. 2003;39:223–229. doi: 10.1016/s0959-8049(02)00595-6. [DOI] [PubMed] [Google Scholar]
- 25.Levi F, Randimbison L, Te VC, et al. Non-Hodgkin's lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74:1847–1850. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene MH, Young TI, Clark WH., Jr Malignant melanoma in renal-transplant recipients. Lancet. 1981;1:1196–1199. doi: 10.1016/s0140-6736(81)92359-x. [DOI] [PubMed] [Google Scholar]
- 28.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. International Classification of Diseases for Oncology (ed 3) http://www.who.int/classifications/icd/adaptations/oncology/en/
- 30.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 32.Curtis RE, Freedman DM, Ron E, et al. Bethesda, MD: National Cancer Institute, NIH publication 05-5302; 2006. New malignancies among cancer survivors: SEER Cancer Registries, 1973-2000. [Google Scholar]
- 33. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2009 Sub (1973-2007 varying), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission, 2010.
- 34.Smedby KE, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballotti S, Chiarelli F, de Martino M. Autoimmunity: Basic mechanisms and implications in endocrine diseases—Part II. Horm Res. 2006;66:142–152. doi: 10.1159/000094252. [DOI] [PubMed] [Google Scholar]
- 36.Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J Immunol. 2013;190:1974–1981. doi: 10.4049/jimmunol.1202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Ing S, Fraser A, et al. Follicular helper T cells: New insights into mechanisms of autoimmune diseases. Ochsner J. 2013;13:131–139. [PMC free article] [PubMed] [Google Scholar]
- 38.Porakishvili N, Mageed R, Jamin C, et al. Recent progress in the understanding of B-cell functions in autoimmunity. Scand J Immunol. 2001;54:30–38. doi: 10.1046/j.1365-3083.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- 39.Fears TR, Guerry IVD, Pfeiffer RM, et al. Identifying individuals at high risk of melanoma: A practical predictor of absolute risk. J Clin Oncol. 2006;24:3590–3596. doi: 10.1200/JCO.2005.04.1277. [DOI] [PubMed] [Google Scholar]
- 40.Klabunde CN, Potosky AL, Jegler JM, et al. Development of a comorbidity index using physican claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 41.CLL Trialists' Collaborative Group. Chemotherapeutic options in chronic lymphocytic leukemia: A meta-analysis of the randomized trials. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 42.Baumert J, Schmidt M, Giehl KA, et al. Time trends in tumour thickness vary in subgroups: Analysis of 6475 patients by age, tumour site and melanoma subtype. Melanoma Res. 2009;19:24–30. doi: 10.1097/CMR.0b013e32831c6fe7. [DOI] [PubMed] [Google Scholar]
- 43.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Riches JC, Gribben JG. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Hematol. 2014;51:228–234. doi: 10.1053/j.seminhematol.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Dasanu CA. Intrinsic and treatment-related immune alterations in chronic lymphocytic leukaemia and their impact for clinical practice. Expert Opin Pharmacother. 2008;9:1481–1494. doi: 10.1517/14656566.9.9.1481. [DOI] [PubMed] [Google Scholar]
- 46.Molica S. Second neoplasms in chronic lymphocytic leukemia: Incidence and pathogenesis with emphasis on the role of different therapies. Leuk Lymphoma. 2005;46:49–54. doi: 10.1080/10428190400007524. [DOI] [PubMed] [Google Scholar]
- 47.Keating MJ, O'Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 48.Laurenti L, Tarnani M, Chiusolo P, et al. Low incidence of secondary neoplasia after autotransplantation for lymphoproliferative disease: The role of pre-transplant therapy. Clin Transplant. 2008;22:191–199. doi: 10.1111/j.1399-0012.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 49.Misgeld E, Germing U, Aul C, et al. Secondary myelodysplastic syndrome after fludarabine therapy of a low-grade non-Hodgkin's lymphoma. Leuk Res. 2001;25:95–98. doi: 10.1016/s0145-2126(00)00092-8. [DOI] [PubMed] [Google Scholar]
- 50.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: Results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 51.Tam CS, Seymour JF, Prince HM, et al. Treatment-related myelodysplasia following fludarabine combination chemotherapy. Haematologica. 2006;91:1546–1550. [PubMed] [Google Scholar]
- 52.Armitage JO, Carbone PP, Connors JM, et al. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol. 2003;21:897–906. doi: 10.1200/JCO.2003.07.113. [DOI] [PubMed] [Google Scholar]
- 53.Bowcock SJ, Rassam SM, Lim Z, et al. High incidence of therapy-related myelodysplasia and acute leukaemia in general haematology clinic patients treated with fludarabine and cyclophosphamide for indolent lymphoproliferative disorders. Br J Haematol. 2006;134:242–243. doi: 10.1111/j.1365-2141.2006.06158.x. [DOI] [PubMed] [Google Scholar]
- 54.Lukenbill J, Kalaycio M. Fludarabine: A review of the clear benefits and potential harms. Leuk Res. 2013;37:986–994. doi: 10.1016/j.leukres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Robak T, Lech-Maranda E, Korycka A, et al. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: Mechanism of action and clinical activity. Curr Med Chem. 2006;13:3165–3189. doi: 10.2174/092986706778742918. [DOI] [PubMed] [Google Scholar]
- 56.Lanoy E, Dores GM, Madeleine MM, et al. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23:385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 61.Frankenthaler A, Sullivan RJ, Wang W, et al. Impact of concomitant immunosuppression on the presentation and prognosis of patients with melanoma. Melanoma Res. 2010;20:496–500. doi: 10.1097/CMR.0b013e32833e9f5b. [DOI] [PubMed] [Google Scholar]
- 62.Farma JM, Zager JS, Barnica-elvir V, et al. A collision of diseases: Chronic lymphocytic leukemia discovered during lymph node biopsy for melanoma. Ann Surg Oncol. 2013;20:1360–1364. doi: 10.1245/s10434-012-2740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCune WJ, Golbus J, Zeldes W, et al. Clinical and immunologic effects of monthly administration of intravenous cyclophosphamide in severe systemic lupus erythematosus. N Engl J Med. 1998;318:1423–1431. doi: 10.1056/NEJM198806023182203. [DOI] [PubMed] [Google Scholar]
- 64.Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: A data-driven review. Arthritis Rheum. 2010;62:9–21. doi: 10.1002/art.25061. [DOI] [PubMed] [Google Scholar]
- 65.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39:1475–1482. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 66.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e10000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediate Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


