Abstract
Purpose
Women with proliferative breast lesions are candidates for primary prevention, but few risk models incorporate benign findings to assess breast cancer risk. We incorporated benign breast disease (BBD) diagnoses into the Breast Cancer Surveillance Consortium (BCSC) risk model, the only breast cancer risk assessment tool that uses breast density.
Methods
We developed and validated a competing-risk model using 2000 to 2010 SEER data for breast cancer incidence and 2010 vital statistics to adjust for the competing risk of death. We used Cox proportional hazards regression to estimate the relative hazards for age, race/ethnicity, family history of breast cancer, history of breast biopsy, BBD diagnoses, and breast density in the BCSC.
Results
We included 1,135,977 women age 35 to 74 years undergoing mammography with no history of breast cancer; 17% of the women had a prior breast biopsy. During a mean follow-up of 6.9 years, 17,908 women were diagnosed with invasive breast cancer. The BCSC BBD model slightly overpredicted risk (expected-to-observed ratio, 1.04; 95% CI, 1.03 to 1.06) and had modest discriminatory accuracy (area under the receiver operator characteristic curve, 0.665). Among women with proliferative findings, adding BBD to the model increased the proportion of women with an estimated 5-year risk of 3% or higher from 9.3% to 27.8% (P < .001).
Conclusion
The BCSC BBD model accurately estimates women's risk for breast cancer using breast density and BBD diagnoses. Greater numbers of high-risk women eligible for primary prevention after BBD diagnosis are identified using the BCSC BBD model.
INTRODUCTION
In 2015, more than 231,000 women in the United States will be diagnosed with breast cancer, and approximately 40,000 will die as a result of breast cancer.1 Both the US Preventive Services Task Force and the American Society of Clinical Oncology recommend routine risk assessment for women to engage in informed decision making about therapies to reduce their risk for breast cancer.2,3 However, risk assessment is not routinely performed, and few eligible women are taking the medications approved for the prevention of breast cancer.4
Approximately 1.6 million women in the United States have a breast biopsy every year.5 Women with proliferative breast lesions may be candidates for primary prevention, but few risk models incorporate benign findings for accurate assessment of breast cancer risk.6,7 Breast density and benign breast disease (BBD) diagnoses are both strong, independent risk factors for incident breast cancer.8 Women with dense breasts and proliferative findings on a breast biopsy are at the highest risk of breast cancer. The Breast Cancer Surveillance Consortium (BCSC) risk prediction model is the only risk assessment tool that uses the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) density categories9 to estimate a woman's risk (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm).10 However, the BCSC model does not account for important breast biopsy results, such as atypical ductal hyperplasia or lobular carcinoma in situ (LCIS).
We previously demonstrated that the BCSC model had greater discriminatory accuracy (area under the receiver operating characteristic curve) than the Breast Cancer Risk Assessment Tool (BCRAT).10 In this article, we build on our prior work using data from more than 1 million ethnically diverse women in the BCSC to update and validate the BCSC risk prediction model with the addition of all forms of BBD diagnoses.
METHODS
Study Population
The National Cancer Institute–funded BCSC11 is a community-based, geographically diverse cohort study that broadly represents the population of women presenting for screening mammography in the United States.12 Our sample consisted of 1,135,977 women age 35 to 74 years old who had at least one mammogram with BI-RADS density reported between 1994 and 2010. We excluded all women who had a diagnosis of breast cancer before their first eligible mammography examination and those with cancers diagnosed in the first 3 months of follow-up. Women were also excluded if they had breast implants or mastectomy.
Each registry obtains annual approval from their institutional review board for consenting processes or a waiver of consent, enrollment of participants, and ongoing data linkage for research purposes. All registries have received a Federal Certificate of Confidentiality that protects the identities of research participants.
Measurement of Risk Factors
Patient information was obtained primarily from self-report at the time of the mammogram, including age, family history of breast cancer in a first-degree relative, race/ethnicity, and history of prior breast biopsies. Race and ethnicity were coded using the expanded definition currently used by SEER and US Vital Statistics (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Native American/Alaskan Native, Hispanic, other).
BBD
Community pathologists at each site classified breast biopsy results using their local practice. We grouped each diagnosis from the pathology reports into one of the following four categories using the taxonomy proposed by Dupont and Page13–15: nonproliferative, proliferative without atypia, proliferative with atypia, and LCIS. Nonproliferative diagnoses included fibroadenomas, cysts, calcifications, fibrocystic changes, nonsclerosing adenosis, lipomas, and fat necrosis. Proliferative diagnoses without atypia included usual ductal hyperplasia, complex fibroadenomas, sclerosing adenosis, and papillomas or papillomatosis. Proliferative diagnoses with atypia included atypical ductal hyperplasia and atypical lobular hyperplasia. If there was more than one diagnosis on a single biopsy or multiple biopsies were performed, we chose the biopsy with the highest grade (LCIS > atypical hyperplasia > proliferative without atypia > nonproliferative) to represent the biopsy for that time period. We classified the biopsy as diagnosis unknown if a woman reported a prior biopsy but pathology results were not available.
Mammographic Breast Density
Community radiologists at each site classified breast density on screening mammograms as part of routine clinical practice using the following four American College of Radiology BI-RADS density categories9: a = almost entirely fat; b = scattered fibroglandular densities; c = heterogeneously dense; and d = extremely dense.
Ascertainment of Breast Cancers
Breast cancer outcomes (17,908 invasive cancers) diagnosed at least 3 months after the index mammogram were obtained at each site through linkage with the regional population-based SEER program, state tumor registries, and pathology databases.
Vital Status
Vital status was obtained through linkage to SEER registries, state tumor registries, and state death records.
Model Development
We estimated the hazard ratios for each risk factor using a partly conditional Cox proportional hazards model for incident invasive breast cancer to incorporate biopsies occurring after study entry.16 We used a robust sandwich estimator for repeated measures survival data to account for multiple observations per woman.17 Women entered the model 3 months after the index mammogram and possibly entered again 3 months after a new, more severe biopsy result. Each observation for a women was censored at the time of death, at diagnosis of ductal carcinoma in situ (DCIS), at mastectomy, at the end of complete cancer capture for her BCSC registry, or at 10 years of follow-up. All models were adjusted for age (continuous), age squared, and race/ethnicity. We included interaction terms in the model if they were statistically significant (P < .05). There were significant interactions between age and the following risk factors: breast density, breast biopsy, family history, and race/ethnicity. We assessed the proportional hazards assumption by calculating interval-specific hazard ratios (ie, 0 to 3 months, 3 to 6 months, 6 months to 1 year, 1 year to 2 years, and so on) for each predictor variable and comparing for clinically meaningful changes over time. The proportional hazards assumption appeared reasonable for all predictors.
We developed absolute risk model estimates for 5- and 10-year risk for invasive breast cancer. We based our estimates of breast cancer incidence on the SEER 18 age- and race/ethnicity-specific risk for invasive breast cancer (2000 to 2010).18 Age-specific incidence for each race/ethnicity group was estimated by fitting a third-order polynomial model to the SEER data. We calculated the baseline risk for the model by adjusting SEER incidence for the population attributable risk for each risk factor subgroup. We estimated the age- and race/ethnicity-specific distribution of family history, breast biopsy, BBD diagnoses, and breast density needed for these calculations using data from a larger set of 4,610,085 mammograms from the BCSC. We used the methods described in Gail et al6 for translating the hazard ratios and risk factor distributions into absolute risks. The age- and ethnicity-specific competing risk of death for women was calculated using 2010 US Vital Statistics data.19 Age-specific mortality for each ethnic group was estimated by fitting an exponential model to the observed total mortality minus deaths from breast cancer. The age- and race/ethnicity-specific competing risk of DCIS was estimated by fitting a third-order polynomial model to SEER breast DCIS rates. We applied the adjustments for whites to women of other/mixed race.
Model calibration was assessed by calculating the ratio of the expected (E) to observed (O) number of breast cancers by age groups, race/ethnicity, and individual risk factor distributions. We calculated the number of cancers observed in each subgroup by multiplying the number in the subgroup by 1 minus the estimated survival rate using the Kaplan-Meier method. We calculated the 95% CIs using the formula based on the Greenwood variance.20,21 Calibration assesses how closely the number of women predicted to develop breast cancer by the model matches the actual number of breast cancers diagnosed in that group. An E/O ratio of 1.0 would indicate perfect calibration.
The discriminatory accuracy of the model was summarized using the area under the time-dependent receiver operating characteristic curve (AUC).22 We performed five-fold cross-validation to confirm the internal validity of the model.23,24 The AUC measures the ability of the model to separate women who will develop breast cancer from those who will not. An AUC of 0.5 is equivalent to chance, and an AUC of 1.0 indicates perfect discrimination between women who develop breast cancer and those who do not.
We used risk reclassification tables25,26 to compare the performance of the model with BBD plus breast density versus the model with breast density alone. Women were cross-classified based on their estimated risks from the two models, using risk categories 0% to 1.66%, 1.67% to 3%, and ≥ 3%. These cut points were chosen because they represent the 5-year risk above which the US Food and Drug Administration recommends consideration of chemoprevention (1.66%) and the risk above which the US Preventive Services Task Force found that the benefits of chemoprevention outweigh the risks (≥ 3%). To avoid bias as a result of miscalibration of the models, we used the Breslow estimator of the 5-year survivor function from the two Cox models for the risk reclassification tables. We used the Kaplan-Meier estimator to estimate the number of breast cancer events and nonevents within each cross-classified risk category.20,21,26
All analyses were performed using R version 2.15.3 (www.r-project.org) and SAS version 9.2 (SAS Institute, Cary, NC). The funding source had no role in the design, conduct, and analysis of this study and did not participate in the decision to submit this article for publication.
RESULTS
At the time of their first mammogram in the BCSC, 49% of women in this study were younger than age 50 years (Table 1). The majority of women were white (76%), but there were more than 60,000 women representing each of the black, Asian, and Hispanic groups. During a mean follow-up of 6.9 years, 17,908 women developed invasive breast cancer. As expected, older age, non-Hispanic white race/ethnicity, a family history of breast cancer, a history of breast biopsies, and dense breasts were all associated with the development of breast cancer (Table 1).
Table 1.
Baseline Characteristics of the Cohort
| Risk Factor | No. of Women (%) |
||
|---|---|---|---|
| No Breast Cancer | Breast Cancer | Total | |
| Age group, years | |||
| 35-39 | 98,666 (8.82) | 770 (4.30) | 99,436 (8.75) |
| 40-44 | 248,569 (22.23) | 2,471 (13.80) | 251,040 (22.10) |
| 45-49 | 200,874 (17.97) | 2,784 (15.55) | 203,658 (17.93) |
| 50-54 | 183,095 (16.38) | 3,014 (16.83) | 186,109 (16.38) |
| 55-59 | 134,279 (12.01) | 2,781 (15.53) | 137,060 (12.07) |
| 60-64 | 102,217 (9.14) | 2,315 (12.93) | 104,532 (9.20) |
| 65-69 | 84,990 (7.60) | 2,030 (11.34) | 87,020 (7.66) |
| 70-74 | 65,379 (5.85) | 1,743 (9.73) | 67,122 (5.91) |
| Race/ethnicity | |||
| White, non-Hispanic | 847,835 (75.83) | 14,587 (81.46) | 862,422 (75.92) |
| Black, non-Hispanic | 77,924 (6.97) | 1,139 (6.36) | 79,063 (6.96) |
| Asian, Pacific Islander | 59,875 (5.36) | 561 (3.13) | 60,436 (5.32) |
| American Indian | 11,457 (1.02) | 106 (0.59) | 11,563 (1.02) |
| Hispanic | 105,758 (9.46) | 1,326 (7.40) | 107,084 (9.43) |
| Other, mixed, unknown | 15,220 (1.36) | 189 (1.06) | 15,409 (1.36) |
| No. of first-degree relatives with breast cancer | |||
| 0 | 983,275 (87.94) | 14,546 (81.23) | 997,821 (87.84) |
| ≥ 1 | 134,794 (12.06) | 3,362 (18.77) | 138,156 (12.16) |
| BI-RADS breast density* | |||
| a: Almost entirely fat | 94,712 (8.47) | 867 (4.84) | 95,579 (8.41) |
| b: Scattered fibroglandular densities | 469,348 (41.98) | 6,829 (38.13) | 476,177 (41.92) |
| c: Heterogeneously dense | 442,771 (39.60) | 8,085 (45.15) | 450,856 (39.69) |
| d: Extremely dense | 111,238 (9.95) | 2,127 (11.88) | 113,365 (9.98) |
| Benign breast disease | |||
| None (no prior biopsy) | 930,924 (83.26) | 13,342 (74.50) | 944,266 (83.12) |
| Prior biopsy, unknown diagnosis | 160,812 (14.38) | 3,908 (21.82) | 164,720 (14.50) |
| Nonproliferative | 18,730 (1.68) | 407 (2.27) | 19,137 (1.68) |
| Proliferative without atypia | 6,204 (0.55) | 177 (0.99) | 6,381 (0.56) |
| Proliferative with atypia | 1,045 (0.09) | 43 (0.24) | 1,088 (0.10) |
| LCIS | 354 (0.03) | 31 (0.17) | 385 (0.03) |
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; LCIS, lobular carcinoma in situ.
Using the BI-RADS density categories: a = almost entirely fat; b = scattered fibroglandular densities; c = heterogeneously dense; and d = extremely dense.
Women commonly reported prior breast biopsies (17%). The incidence of invasive breast cancer among women with a breast biopsy varied significantly by pathologic diagnosis (Fig 1). Women with atypical hyperplasia or LCIS had a two- to four-fold higher incidence of breast cancer compared with those with nonproliferative diagnoses.
Fig 1.
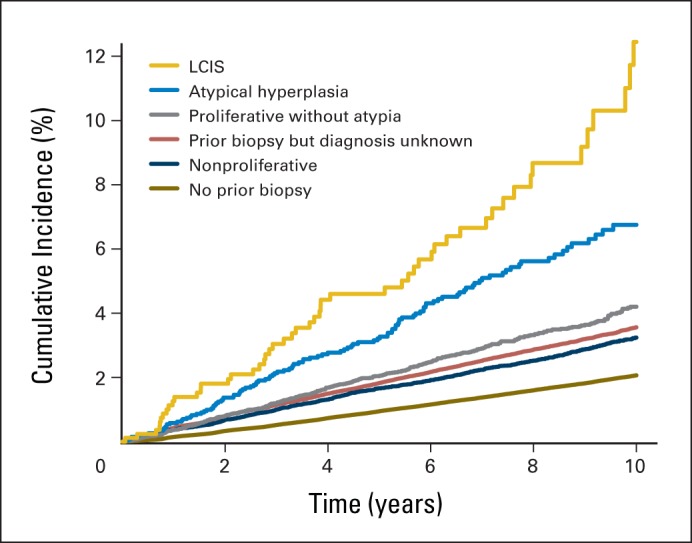
Breast cancer incidence by benign breast disease. LCIS, lobular carcinoma in situ.
There were important interactions of age with the majority of the other risk factors in the model. Because age is a continuous risk factor, we highlighted the relative hazards at representative ages to illustrate the change in risk with age (Table 2). The strength of the association declined with age for race/ethnicity, family history, and BI-RADS density. For example, the relative hazard for the highest density declined from 1.97 at age 40 years to 1.53 at age 70 years (P for interaction < .001). The interaction between BBD and age was curvilinear (significant interactions with both age and age-squared). For example, the relative hazard for LCIS declined from 7.64 at age 40 years to 3.29 at age 60 and then increased to 5.84 at age 70 (P for interaction = .036 with age and .018 with age-squared).
Table 2.
Cox Proportional Hazards Model Results Showing the Interactions of Age With Other Risk Factors on Breast Cancer
| Factor | HR (95% CI) |
P for Interaction With Age | P for Interaction With Age Squared | |||
|---|---|---|---|---|---|---|
| Age 40 Years | Age 50 Years | Age 60 Years | Age 70 Years | |||
| Race/ethnicity | < .001 | |||||
| White, non-Hispanic | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||
| Black, non-Hispanic | 1.20 (1.08 to 1.34) | 1.03 (0.96 to 1.10) | 0.89 (0.82 to 0.95) | 0.76 (0.68 to 0.86) | ||
| Asian | 0.99 (0.85 to 1.16) | 0.88 (0.80 to 0.97) | 0.78 (0.71 to 0.87) | 0.70 (0.59 to 0.83) | ||
| American Indian | 0.76 (0.53 to 1.10) | 0.73 (0.58 to 0.91) | 0.69 (0.56 to 0.86) | 0.66 (0.47 to 0.94) | ||
| Hispanic | 1.02 (0.92 to 1.13) | 0.92 (0.86 to 0.98) | 0.82 (0.77 to 0.88) | 0.74 (0.66 to 0.83) | ||
| Other, mixed | 1.10 (0.86 to 1.40) | 0.95 (0.82 to 1.10) | 0.82 (0.69 to 0.98) | 0.71 (0.53 to 0.95) | ||
| Family history | .007 | .019 | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 1.89 (1.73 to 2.06) | 1.60 (1.52 to 1.68) | 1.47 (1.40 to 1.55) | 1.47 (1.37 to 1.58) | ||
| BI-RADS density | < .001 | |||||
| a: Almost entirely fat | 0.48 (0.41 to 0.58) | 0.54 (0.48 to 0.60) | 0.60 (0.56 to 0.65) | 0.67 (0.61 to 0.74) | ||
| b: Scattered fibroglandular densities | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||
| c: Heterogeneously dense | 1.62 (1.52 to 1.72) | 1.51 (1.45 to 1.57) | 1.40 (1.36 to 1.45) | 1.31 (1.24 to 1.38) | ||
| d: Extremely dense | 1.97 (1.82 to 2.15) | 1.81 (1.72 to 1.91) | 1.66 (1.56 to 1.78) | 1.53 (1.37 to 1.70) | ||
| Benign breast disease | .036 | .018 | ||||
| No prior biopsy | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||
| Prior biopsy, unknown diagnosis | 1.50 (1.37 to 1.64) | 1.44 (1.38 to 1.50) | 1.46 (1.40 to 1.52) | 1.57 (1.49 to 1.66) | ||
| Nonproliferative | 1.31 (1.10 to 1.56) | 1.43 (1.30 to 1.56) | 1.56 (1.41 to 1.72) | 1.70 (1.50 to 1.93) | ||
| Proliferative without atypia | 1.70 (1.29 to 2.25) | 1.66 (1.47 to 1.89) | 1.76 (1.53 to 2.02) | 2.02 (1.71 to 2.38) | ||
| Proliferative with atypia | 3.19 (1.95 to 5.20) | 2.97 (2.35 to 3.74) | 2.77 (2.20 to 3.49) | 2.59 (1.88 to 3.58) | ||
| LCIS | 7.64 (3.50 to 16.67) | 3.60 (2.53 to 5.12) | 3.29 (2.30 to 4.71) | 5.84 (4.01 to 8.53) | ||
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; LCIS, lobular carcinoma in situ.
Calibration of the BCSC Breast Density Model was reasonably accurate across risk factor subgroups for both 5- and 10-year risk (Table 3). The model slightly underestimated breast cancer rates in the youngest women (E/O ratio, 0.86 for women age 35 to 39 years). It also underestimated cancer rates among Asian women (E/O ratio, 0.94) and Hispanic women (E/O ratio, 0.94). Overall, the model slightly overestimated the risk for women in the BCSC cohort (E/O ratio, 1.04).
Table 3.
Calibration of the BCSC BBD Model in Risk Factor Subgroups
| Risk Group | 5 Years |
10 Years |
||||
|---|---|---|---|---|---|---|
| Expected Rate | Observed Rate | E/O Ratio (95% CI) | Expected Rate | Observed Rate | E/O Ratio (95% CI) | |
| Full cohort | 1.20 | 1.15 | 1.04 (1.02 to 1.06) | 2.57 | 2.46 | 1.05 (1.03 to 1.06) |
| Age group, years | ||||||
| 35-39 | 0.42 | 0.49 | 0.86 (0.78 to 0.95) | 1.12 | 1.24 | 0.90 (0.84 to 0.97) |
| 40-44 | 0.65 | 0.66 | 0.98 (0.93 to 1.03) | 1.58 | 1.61 | 0.98 (0.94 to 1.02) |
| 45-49 | 0.99 | 0.98 | 1.02 (0.97 to 1.06) | 2.26 | 2.08 | 1.09 (1.05 to 1.13) |
| 50-54 | 1.28 | 1.12 | 1.14 (1.09 to 1.19) | 2.78 | 2.45 | 1.13 (1.09 to 1.18) |
| 55-59 | 1.55 | 1.53 | 1.01 (0.97 to 1.06) | 3.25 | 3.20 | 1.02 (0.98 to 1.05) |
| 60-64 | 1.79 | 1.69 | 1.05 (1.00 to 1.11) | 3.61 | 3.44 | 1.05 (1.01 to 1.09) |
| 65-69 | 1.97 | 1.82 | 1.08 (1.03 to 1.14) | 3.83 | 3.53 | 1.08 (1.04 to 1.13) |
| 70-74 | 2.10 | 1.98 | 1.06 (1.00 to 1.12) | 3.89 | 3.79 | 1.03 (0.98 to 1.07) |
| Race/ethnicity | ||||||
| White, non-Hispanic | 1.28 | 1.21 | 1.06 (1.04 to 1.08) | 2.73 | 2.53 | 1.08 (1.06 to 1.10) |
| Black, non-Hispanic | 1.11 | 1.04 | 1.06 (0.99 to 1.14) | 2.35 | 2.37 | 0.99 (0.93 to 1.06) |
| Asian | 0.93 | 0.99 | 0.94 (0.85 to 1.04) | 1.96 | 2.21 | 0.88 (0.81 to 0.97) |
| Hispanic | 0.86 | 0.92 | 0.94 (0.88 to 1.00) | 1.85 | 2.05 | 0.90 (0.85 to 0.96) |
| American Indian | 0.94 | 0.75 | 1.26 (1.01 to 1.58) | 1.98 | 1.49 | 1.32 (1.07 to 1.64) |
| Other, mixed, unknown | 1.17 | 0.99 | 1.18 (0.99 to 1.40) | 2.54 | 2.18 | 1.16 (1.01 to 1.34) |
| BI-RADS breast density | ||||||
| a: Almost entirely fat | 0.71 | 0.64 | 1.11 (1.02 to 1.21) | 1.45 | 1.44 | 1.01 (0.94 to 1.08) |
| b: Scattered fibroglandular densities | 1.09 | 1.03 | 1.05 (1.02 to 1.08) | 2.28 | 2.17 | 1.05 (1.03 to 1.08) |
| c: Heterogeneously dense | 1.38 | 1.32 | 1.04 (1.01 to 1.07) | 2.96 | 2.85 | 1.04 (1.02 to 1.07) |
| d: Extremely dense | 1.42 | 1.42 | 1.00 (0.95 to 1.05) | 3.15 | 2.97 | 1.06 (1.01 to 1.11) |
| Family history of breast cancer | ||||||
| 0 | 1.11 | 1.06 | 1.04 (1.02 to 1.06) | 2.36 | 2.27 | 1.04 (1.03 to 1.06) |
| 1+ | 1.89 | 1.82 | 1.04 (1.00 to 1.08) | 4.02 | 3.75 | 1.07 (1.04 to 1.11) |
| Benign breast disease | ||||||
| None (no prior biopsy) | 1.00 | 0.96 | 1.04 (1.02 to 1.07) | 2.16 | 2.12 | 1.02 (1.00 to 1.04) |
| Prior biopsy, unknown diagnosis | 1.90 | 1.85 | 1.03 (0.99 to 1.06) | 3.97 | 3.67 | 1.08 (1.05 to 1.11) |
| Nonproliferative | 1.71 | 1.69 | 1.01 (0.93 to 1.10) | 3.62 | 3.35 | 1.08 (1.00 to 1.17) |
| Proliferative without atypia | 2.19 | 2.08 | 1.05 (0.93 to 1.19) | 4.61 | 4.36 | 1.06 (0.95 to 1.18) |
| Proliferative with atypia | 3.79 | 3.30 | 1.15 (0.91 to 1.45) | 7.87 | 7.11 | 1.11 (0.91 to 1.34) |
| LCIS | 5.90 | 4.80 | 1.23 (0.87 to 1.74) | 11.94 | 13.46 | 0.89 (0.65 to 1.21) |
NOTE. The observed rate uses the Kaplan-Meier estimator to calculate the number of cancers observed in each subgroup (total cohort, N = 1,135,977). The expected rate is the average of the BCSC BBD model predicted risk for each woman in the subcohort. No additional adjustments were performed.
Abbreviations: BBD, benign breast disease; BCSC, Breast Cancer Surveillance Consortium; BI-RADS, Breast Imaging Reporting and Data System; E/O, expected rate divided by the observed rate; LCIS, lobular carcinoma in situ.
The distribution of 5- and 10-year risk with the BCSC BBD model is shown in Figure 2. Almost half of women (47%) had a 5-year risk less than 1%. Only 3% had a 5-year risk greater than 3%. The 10-year risk is similarly skewed to the right.
Fig 2.
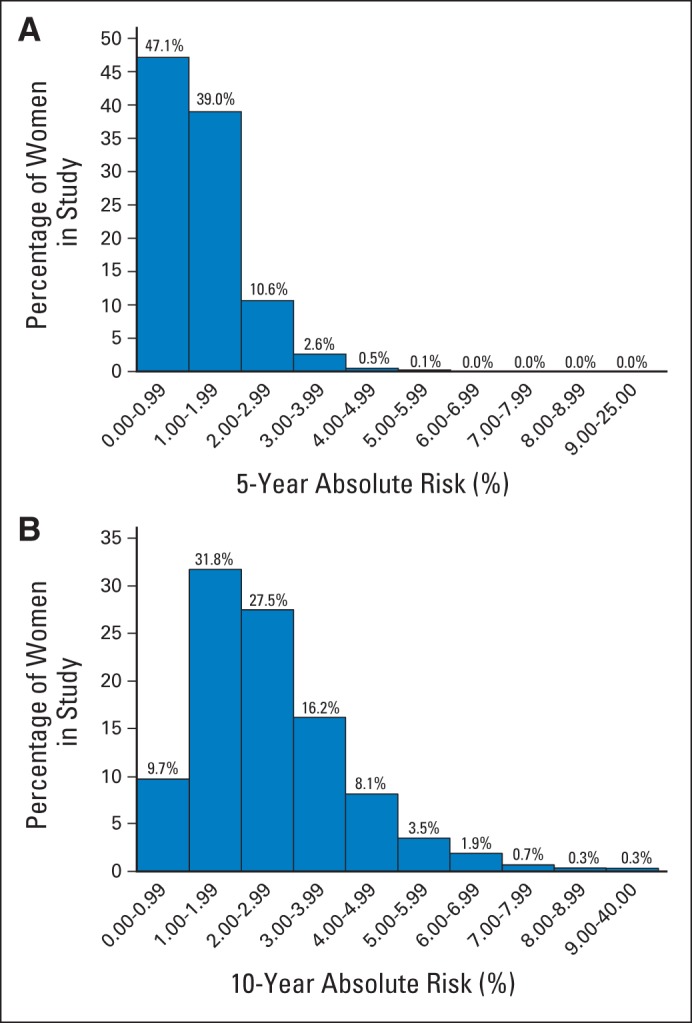
Distribution of (A) 5- and (B) 10-year risk for invasive breast cancer among 1,135,977 women age 35 to 74 years using the Breast Cancer Surveillance Consortium benign breast disease model.
The AUC for the BCSC BBD model discrimination was 0.665 (Appendix Table A1, online only). This was minimally greater than that of the model without BBD (AUC, 0.664). However, among women with a known biopsy result, the AUC increased from 0.650 to 0.660, and the proportion of women estimated to have a 5-year risk for invasive breast cancer ≥ 3% increased from 7% to 14%. Finally, among women with proliferative disease on biopsy, there was a statistically significant increase in the proportion of women estimated to have a 5-year risk for invasive breast cancer ≥ 3% (9.3% to 27.8%; P < .001; Table 4).
Table 4.
Risk Reclassification Table Comparing the BCSC Model With the BCSC BBD Model in Women With Proliferative Disease on Biopsy
| 5-Year Risk in BCSC Model* | 5-Year Risk in BCSC BBD Model* |
Total Correctly Reclassified† |
||||
|---|---|---|---|---|---|---|
| 0% to < 1.67% | 1.67% to < 3% | ≥ 3% | Total (row) | No. | % (95% CI) | |
| 0% to < 1.67% | 53 | 0.65 (0.47 to 0.82) | ||||
| No. of women | 5,463 | 2,348 | 385 | 8,196 | ||
| No. of events | 72 | 37 | 16 | 125 | ||
| No. of nonevents | 5,391 | 2,311 | 369 | 8,071 | ||
| 1.67% to < 3% | 115 | 1.51 (1.23 to 1.78) | ||||
| No. of women | 0 | 4,768 | 2,847 | 7,615 | ||
| No. of events | 0 | 98 | 115 | 213 | ||
| No. of nonevents | 0 | 4,670 | 2,732 | 7,402 | ||
| ≥ 3% | 0 | 0 | ||||
| No. of women | 0 | 0 | 1,615 | 1,615 | ||
| No. of events | 0 | 0 | 81 | 81 | ||
| No. of nonevents | 0 | 0 | 1,534 | 1,534 | ||
| Total | 168 | 0.96 (0.82 to 1.11) | ||||
| No. of women | 5,463 | 7,116 | 4,847 | 17,426 | ||
| No. of events | 72 | 135 | 212 | 420 | ||
| No. of nonevents | 5,391 | 6,981 | 4,635 | 17,006 | ||
Abbreviations: BBD, benign breast disease; BCSC, Breast Cancer Surveillance Consortium.
Based on the 5-year survivor function estimates from the respective Cox models to ensure accurate calibration.
Cases reassigned to higher risk categories, and noncases reassigned to lower risk categories.
We used reclassification tables to compare the BCSC BBD model to the model without BBD. The addition of BBD to age, race/ethnicity, family history of breast cancer, breast density, and history of breast biopsy correctly reclassified 0.82% of women and incorrectly reclassified 0.54% of women (Appendix Table A2, online only).
DISCUSSION
We updated the BCSC risk prediction model with BBD diagnoses including atypical hyperplasia and LCIS. The addition of BBD provided minimal improvement in discrimination in the entire cohort, in part, because we did not have the pathology results for the majority of women who reported breast biopsies. However, for women reporting proliferative disease on breast biopsy, the addition of BBD to our model markedly increased the proportion of women identified as high risk for invasive breast cancer.
The BCSC BBD model calculates both the 5- and 10-year risk for breast cancer. The 5-year risk has been the standard used for decision making about chemoprevention because the BCRAT model, which reports a 5-year risk for invasive breast cancer, was the basis for enrollment onto the two major US prevention trials27,28 and forms the basis of the US Food and Drug Administration indication for both tamoxifen and raloxifene. The US Preventive Services Task Force recently changed their guidelines to state that for women with a 5-year risk ≥ 3% using models like the BCSC or BCRAT, a provider should discuss the use of selective estrogen receptor modulators for primary prevention.2 Using the BCSC BBD model, 27% of women with proliferative disease have an estimated 5-year risk ≥ 3%. There are three other models that include BBD; these are the BCRAT,6 the Tirer-Cuzick model,7 and the Mayo Clinic model.29 The BCRAT model risk factors include atypical hyperplasia but not other forms of BBD, such as LCIS, and the BCSC model has better risk discrimination than the BCRAT.10 The Tirer-Cuzick model was developed in women at high familial risk for breast cancer and overestimates a woman's risk by a factor of 2 in the general population and in women with atypical hyperplasia.7,30,31 The Gail model, however, markedly underestimates the risk of women with atypical hyperplasia.32 Thus, the BCSC BBD model improves on the models currently available for women with high-risk breast lesions and can be used to help guide their appropriate use of selective estrogen receptor modulators.
The estimation of a woman's 10-year risk for breast cancer is an additional useful estimate from breast cancer risk assessment models. The primary risk calculators for two other diseases important to women's health, cardiovascular disease (American College of Cardiology/American Heart Association model33 and Framingham model34) and osteoporotic fractures (FRAX model35), report 10-year risk for health outcomes. Calculating the 10-year risk for breast cancer allows clinicians and patients to compare all three health outcomes on the same time scale and can inform screening and prevention strategies.
A major strength of the BCSC model is that it integrates BI-RADS breast density to estimate a woman's future risk for breast cancer. The BI-RADS breast manual recommends that BI-RADS density be included in all mammography reports to providers in the United States. Breast density is used to identify women who must be told that they have dense breasts in accordance with laws passed in 22 states, including the four most populous states (California, New York, Florida, and Texas). The BCSC model allows clinicians and patients to use breast density to estimate a woman's risk when they are notified that a woman has dense breasts. Adding a continuous measure of breast density improved risk discrimination for both the Gail model36 and the Tirer-Cuzick model,37 but neither continuous measure is clinically available in the United States.
The BCSC BBD model uses SEER breast cancer data incidence. Thus, we did not expect perfect calibration in the sample of women in the BCSC, all of whom have had a mammogram. For example, women younger than age 40 years who are screened for breast cancer with mammography are likely to be at higher risk than the general population, because mammography is not recommended for this age group. The underestimation of risk by the BCSC BBD model (E/O ratio, 0.86) in this age group was expected. Similarly, the follow-up of women for incident cancer is likely incomplete because some women will move away from the regions captured by the cancer registries used to identify breast cancers. The observed incidence in the BCSC is likely lower for women age 40 to 74 years than the true incidence because women who undergo routine mammography tend to be healthier. Finally, the distribution of risk factors may differ in the SEER population and the BCSC population, which could influence model calibration. We have previously shown that the distribution of demographic factors in the BCSC is similar to that of the overall US population.12
The primary limitation of our analysis is the lack of external validation. The original BCSC model had nearly identical discrimination and calibration in the internal validation data set10 as well as in an external cohort.38 The model is based on a large set of nationally representative data, which should enhance its generalizability. However, it is still essential to validate the results in other cohort studies, as we did with the BCSC risk calculator.37
In summary, the addition of BBD to the BCSC model had minimal impact on risk discrimination overall but changed risk significantly for women with new biopsy results, particularly if the results showed proliferative disease. Initial results found that the addition of data from the combination of 76 single-nucleotide polymorphisms to the original BCSC significantly improved risk discrimination on a population basis.38 Future work will focus on additional validation of the updated BCSC BBD model with single-nucleotide polymorphisms and additional risk factors, such as serum hormone levels. The BCSC online calculator for the model described in this report can be found at https://tools.bcsc-scc.org/BC5yearRisk/intro.htm.
Acknowledgment
We thank the Breast Cancer Surveillance Consortium (BCSC) investigators, participating mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes is provided at: http://breastscreening.cancer.gov/.
Appendix
Let X be a woman's age and r be her race/ethnicity. We estimated the baseline age- and race/ethnicity-specific annual incidence of invasive breast cancer (per 100 women), IX,r, by fitting a third-order polynomial to the 2000 to 2010 SEER invasive breast cancer rates stratified by race/ethnicity as follows:
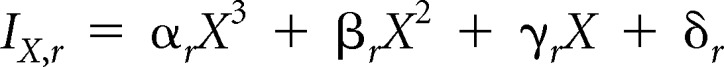 |
where the coefficients varied by race/ethnicity r as shown in Appendix Table A3.
To account for competing risks, after the first year, the number at risk for breast cancer was decreased by the number of women diagnosed with invasive breast cancer, the number of women diagnosed with ductal carcinoma in situ (DCIS), and the number of women who died from causes other than breast cancer in the prior year. The calculations for the competing risks of DCIS and non–breast cancer mortality are described here.
We estimated the baseline age- and race/ethnicity-specific DCIS rate (per 100 women), DX,r, by fitting a third-order polynomial to the 2000 to 2010 SEER DCIS rates stratified by race/ethnicity as follows:
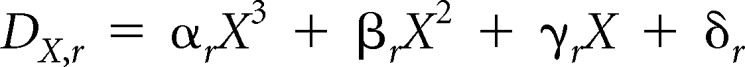 |
where the coefficients varied by race/ethnicity r as shown in Appendix Table A4.
We estimated the age- and race/ethnicity-specific mortality rate (per 100 women), MX,r, by fitting an exponential curve to the 2010 US Vital Statistics data (total mortality minus breast cancer mortality) stratified by race/ethnicity as follows:
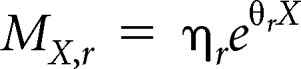 |
where the coefficients varied by race/ethnicity r as shown in Appendix Table A5.
To account for a woman's Breast Imaging Reporting and Data System (BI-RADS) density, family history of breast cancer, and benign breast disease, represented by the vector Z, we adjusted the baseline incidence of invasive breast cancer and DCIS using the hazard ratios estimated from this study (see Table 2 for select ages). The hazard ratios were standardized to be relative to average risk using age- and race/ethnicity-specific distributions of risk factors, estimated from a larger set of 4,610,085 mammograms from the Breast Cancer Surveillance Consortium.
We used logistic regression to model BI-RADS density, family history of breast cancer, and benign breast disease as a function of age (linear and quadratic terms), race/ethnicity, and an interaction between race/ethnicity and age (linear term) among women age 40 years and older. We used the model-predicted probabilities from the three models to estimate the proportion of women in each age, race/ethnicity, and risk factor category to adjust the hazard ratios to be relative to average risk. The standardized hazard ratios are contained in SAS format (SAS Institute, Cary, NC) files provided with the public-use macro, which is available at https://tools.bcsc-scc.org/BC5yearRisk/sourcecode.htm.
The risk factor–specific annual incidence of invasive breast cancer (per 100 women), IX,r,Z*, and risk factor–specific annual incidence of DCIS (per 100 women), DX,r,Z*, are given by:
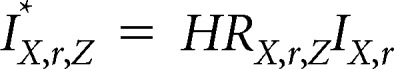 |
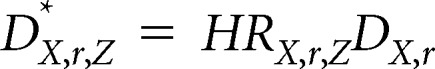 |
where HRX,r,Z is the combined standardized hazard ratio for a given age (X), race/ethnicity, and BI-RADS density, family history of breast cancer, and benign breast disease (Z).
Given IX,r,Z*, DX,r,Z*, and MX,r, we defined the proportion of women at risk for breast cancer by age, race/ethnicity, and other risk factors, PX,r,Z, as follows:
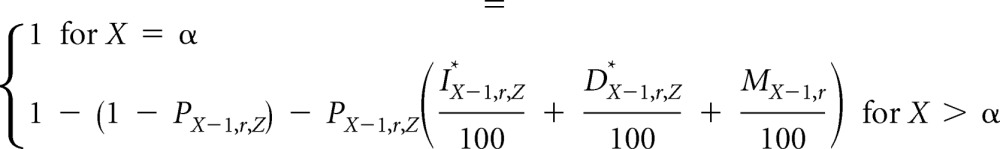 |
To calculate the 5- or 10-year absolute risk, ARX,r,Z, we summed the product of risk factor–specific annual incidence of invasive breast cancer and the risk factor–specific proportion still at risk over t = 5 or t = 10 consecutive years:
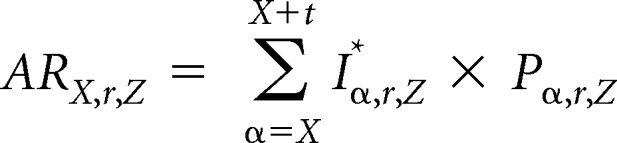 |
Table A1.
Overall Calibration and Discrimination of the BCSC BBD Model Compared With the Original BCSC Model
| Sample | No. of Women | Expected Rate | Observed Rate | E/O Ratio (95% CI) | AUC |
|---|---|---|---|---|---|
| Biopsy only | 1,135,977 | 1.20 | 1.15 | 1.04 (1.03 to 1.06) | 0.664 |
| BBD | 1,135,977 | 1.20 | 1.15 | 1.04 (1.03 to 1.06) | 0.665 |
NOTE. The observed rate is the actual rate per 500 woman-years (equivalent to 5 year risk) observed in the cohort. The expected rate is the average of the BCSC BBD model predicted risk for each woman in the sub-cohort. No additional adjustments were performed.
Abbreviations: AUC, area under the receiver operating characteristic curve; BBD, benign breast disease; BCSC, Breast Cancer Surveillance Consortium; E/O, expected rate divided by the observed rate.
Table A2.
Risk Reclassification Table Comparing the BCSC BD Model With the BCSC BBD Model in the Entire Cohort
| 5-Year Risk in BCSC BD Model* | 5-Year Risk in BCSC BBD Model* |
Total Correctly Reclassified† |
||||
|---|---|---|---|---|---|---|
| 0% to < 1.67% | 1.67% to < 3% | ≥ 3% | Total (row) | No. | % (95% CI) | |
| 0% to < 1.67% | 61 | 0.01 (0.00 to 0.01) | ||||
| No. of women | 987,470 | 2,844 | 385 | 990,699 | ||
| No. of events | 8,845 | 45 | 16 | 8,906 | ||
| No. of nonevents | 978,625 | 2,799 | 369 | 981,793 | ||
| 1.67% to < 3% | 5,453 | 2.77 (2.70 to 2.84) | ||||
| No. of women | 5,406 | 188,262 | 3,244 | 196,912 | ||
| No. of events | 84 | 4,012 | 131 | 4,227 | ||
| No. of nonevents | 5,322 | 184,250 | 3,113 | 192,685 | ||
| ≥ 3% | 4,437 | 17.74 (17.27 to 18.21) | ||||
| No. of women | 0 | 4,574 | 20,440 | 25,014 | ||
| No. of events | 0 | 137 | 786 | 922 | ||
| No. of nonevents | 0 | 4,437 | 19,654 | 24,092 | ||
| Total | 9,951 | 0.82 (0.80 to 0.84) | ||||
| No. of women | 992,876 | 195,680 | 24,069 | 1,212,625 | ||
| No. of events | 8,930 | 4,194 | 933 | 14,056 | ||
| No. of nonevents | 983,946 | 191,486 | 23,136 | 1,198,569 | ||
Abbreviations: BBD, benign breast disease; BCSC, Breast Cancer Surveillance Consortium; BD, breast density.
Based on the 5-year survivor function estimates from the respective Cox models to ensure accurate calibration.
Cases reassigned to higher risk categories, and noncases reassigned to lower risk categories.
Table A3.
Coefficients for the Third-Order Polynomial Fit of Invasive Breast Cancer by Race/Ethnicity
| Race/Ethnicity | αr | βr | γr | δr |
|---|---|---|---|---|
| White, non-Hispanic | −0.000007162 | 0.001111136 | −0.043528999 | 0.514780021 |
| Black, non-Hispanic | −0.000004332 | 0.000644475 | −0.021243209 | 0.195808387 |
| Asian | −0.000003002 | 0.000372751 | −0.007182055 | −0.025628846 |
| American Indian | −0.000005880 | 0.000861287 | −0.032530311 | 0.373493536 |
| Hispanic | −0.000004211 | 0.000628530 | −0.022484578 | 0.234323344 |
Table A4.
Coefficients for the Third-Order Polynomial Fit of Ductal Carcinoma in Situ by Race/Ethnicity
| Race/Ethnicity | αr | βr | γr | δr |
|---|---|---|---|---|
| White, non-Hispanic | −0.000002094 | 0.000284086 | −0.009292718 | 0.080659188 |
| Black, non-Hispanic | −0.000002706 | 0.000403385 | −0.016548963 | 0.207686538 |
| Asian | −0.000001496 | 0.000185804 | −0.004747568 | 0.016457265 |
| American Indian | 0.000000191 | −0.000049665 | 0.004730528 | −0.102520406 |
| Hispanic | −0.000001403 | 0.000195157 | −0.006832187 | 0.067237073 |
Table A5.
Coefficients for the Exponential Fit of Mortality by Race/Ethnicity
| Race/Ethnicity | ηr | θr |
|---|---|---|
| White, non-Hispanic | 0.004741239 | 0.082473361 |
| Black, non-Hispanic | 0.008976903 | 0.077540550 |
| Asian | 0.001324086 | 0.091167044 |
| American Indian | 0.016491843 | 0.067539660 |
| Hispanic | 0.002253560 | 0.088334802 |
Footnotes
Written on behalf of the Breast Cancer Surveillance Consortium.
Supported by the National Cancer Institute–funded Breast Cancer Surveillance Consortium (Grants No. P01 CA154292 and HHSN261201100031C) and Vermont Population-Based Research Optimizing Screening Through Personalized Regimens (Grant No. U54CA163303). The collection of cancer and vital status data used in this study were supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://breastscreening.cancer.gov/work/acknowledgment.html.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey A. Tice, Diana L. Miglioretti, Karla Kerlikowske
Financial support: Diana Miglioretti, Karla Kerlikowske
Administrative support: Karla Kerlikowske
Provision of study materials or patients: Diana Miglioretti, Karla Kerlikowske
Collection and assembly of data: Diana Miglioretti, Karla Kerlikowske
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Breast Density and Benign Breast Disease: Risk Assessment to Identify Women at High Risk of Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jeffrey A. Tice
No relationship to disclose
Diana L. Miglioretti
No relationship to disclose
Chin-Shang Li
No relationship to disclose
Celine M. Vachon
No relationship to disclose
Charlotte C. Gard
No relationship to disclose
Karla Kerlikowske
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA US Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: Recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:698–708. doi: 10.7326/0003-4819-159-10-201311190-00717. [DOI] [PubMed] [Google Scholar]
- 3.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 4.Wickerham DL, Vogel VG. Breast cancer chemoprevention: The saga of underuse continues. J Natl Cancer Inst. 2015;107:399. doi: 10.1093/jnci/dju399. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein M. Where's the outrage? J Am Coll Surg. 2009;208:78–79. doi: 10.1016/j.jamcollsurg.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 7.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 8.Tice JA, O'Meara ES, Weaver DL, et al. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105:1043–1049. doi: 10.1093/jnci/djt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sickles E, D'Orsi C, Bassett L, et al. Reston, VA: American College of Radiology; 2013. ACR BI-RADS Atlas® Mammography, ACR BI-RADS Atlas®, Breast Imaging Reporting and Data System. [Google Scholar]
- 10.Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: A national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 12.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235:775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
- 13.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 14.Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast: A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: A retrospective cohort study. Lancet. 2003;361:125–129. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Heagerty PJ. Partly conditional survival models for longitudinal data. Biometrics. 2005;61:379–391. doi: 10.1111/j.1541-0420.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee EO, Wei L, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 18.National Cancer Institute. SEER*Stat Database Version 8.1.2. http://seer.cancer.gov/seerstat/
- 19.Centers for Disease Control and Prevention. CDC WONDER Online Database, released 2012. http://wonder.cdc.gov/
- 20.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viallon V, Ragusa S, Clavel-Chapelon F, et al. How to evaluate the calibration of a disease risk prediction tool. Stat Med. 2009;28:901–916. doi: 10.1002/sim.3517. [DOI] [PubMed] [Google Scholar]
- 22.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Stone M. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc B. 1974;26:111–147. [Google Scholar]
- 24.Stone M. An asymptotic equivalence of choice of model by cross-validation and Akaike's criterion. J R Stat Soc B. 1977;39:44–47. [Google Scholar]
- 25.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: The role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;30:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 28.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 29.Pankratz VS, Degnim AC, Frank RD, et al. Model for individualized prediction of breast cancer risk after a benign breast biopsy. J Clin Oncol. 2015;33:923–929. doi: 10.1200/JCO.2014.55.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40:807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591–3596. doi: 10.1200/JCO.2010.28.0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374–5379. doi: 10.1200/JCO.2007.14.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, Johansson H, Oden A, et al. The effects of a FRAX revision for the USA. Osteoporos Int. 2010;21:35–40. doi: 10.1007/s00198-009-1033-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98:1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 37.Warwick J, Birke H, Stone J, et al. Mammographic breast density refines Tyrer-Cuzick estimates of breast cancer risk in high-risk women: Findings from the placebo arm of the International Breast Cancer Intervention Study I. Breast Cancer Res. 2014;16:451. doi: 10.1186/s13058-014-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;4:107. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]


