ABSTRACT
Many neuropeptides are members of peptide families, with multiple structurally similar isoforms frequently found even within a single species. This raises the question of whether the individual peptides serve common or distinct functions. In the accompanying paper, we found high isoform specificity in the responses of the lobster (Homarus americanus) cardiac neuromuscular system to members of the pyrokinin peptide family: only one of five crustacean isoforms showed any bioactivity in the cardiac system. Because previous studies in other species had found little isoform specificity in pyrokinin actions, we examined the effects of the same five crustacean pyrokinins on the lobster stomatogastric nervous system (STNS). In contrast to our findings in the cardiac system, the effects of the five pyrokinin isoforms on the STNS were indistinguishable: they all activated or enhanced the gastric mill motor pattern, but did not alter the pyloric pattern. These results, in combination with those from the cardiac ganglion, suggest that members of a peptide family in the same species can be both isoform specific and highly promiscuous in their modulatory capacity. The mechanisms that underlie these differences in specificity have not yet been elucidated; one possible explanation, which has yet to be tested, is the presence and differential distribution of multiple receptors for members of this peptide family.
KEY WORDS: Central pattern generator, Neuromodulation, Peptide
Summary: Crustaceans typically possess multiple pyrokinins; in the lobster, all isoforms tested similarly activated the gastric mill rhythm, suggesting that the pyrokinin receptor(s) in the stomatogastric nervous system is relatively promiscuous.
INTRODUCTION
It is now well established that central pattern generators, although they are anatomically ‘hard-wired’, are functionally flexible; they are capable of generating multiple motor outputs under the influence of a wide variety of neuromodulators (e.g. Blitz and Nusbaum, 2011, 2012; Dembrow and Johnston, 2014; Dickinson, 2006; Fénelon et al., 2003; Hooper and DiCaprio, 2004; LeBeau et al., 2005; LeBeau and Whittington, 2005; Marder and Bucher, 2001; Marder et al., 2014; Miles and Sillar, 2011; Mitchell and Johnson, 2003; Nusbaum and Beenhakker, 2002; Nusbaum et al., 2001; Rauscent et al., 2006; Selverston, 2005; Selverston and Ayers, 2006; Sillar et al., 2008; Stein, 2009). The compounds that serve as neuromodulators are highly diverse and include small-molecule transmitters (e.g. acetylcholine, glutamate and GABA), amines (e.g. dopamine and serotonin) and diffusible gases (e.g. nitric oxide), as well as a variety of different peptides, which represent the largest single class of neuroactive molecules. The number of different peptides that has been identified within the nervous system of a single species is remarkably high. Recent studies of the neuropeptidomes of several decapod crustaceans, for example, have shown that these species often contain upwards of 100 or more distinct peptides (e.g. Fu et al., 2005; Hui et al., 2013, 2012; Ma et al., 2009, 2010, 2008). Many of these peptides display high levels of amino acid similarity, suggesting that they evolved from a single common precursor (e.g. Christie et al., 2010). It is not known whether isoform diversity within a species is derived from ‘neutral changes’ or whether different peptides within a family have evolved to subsume different functions.
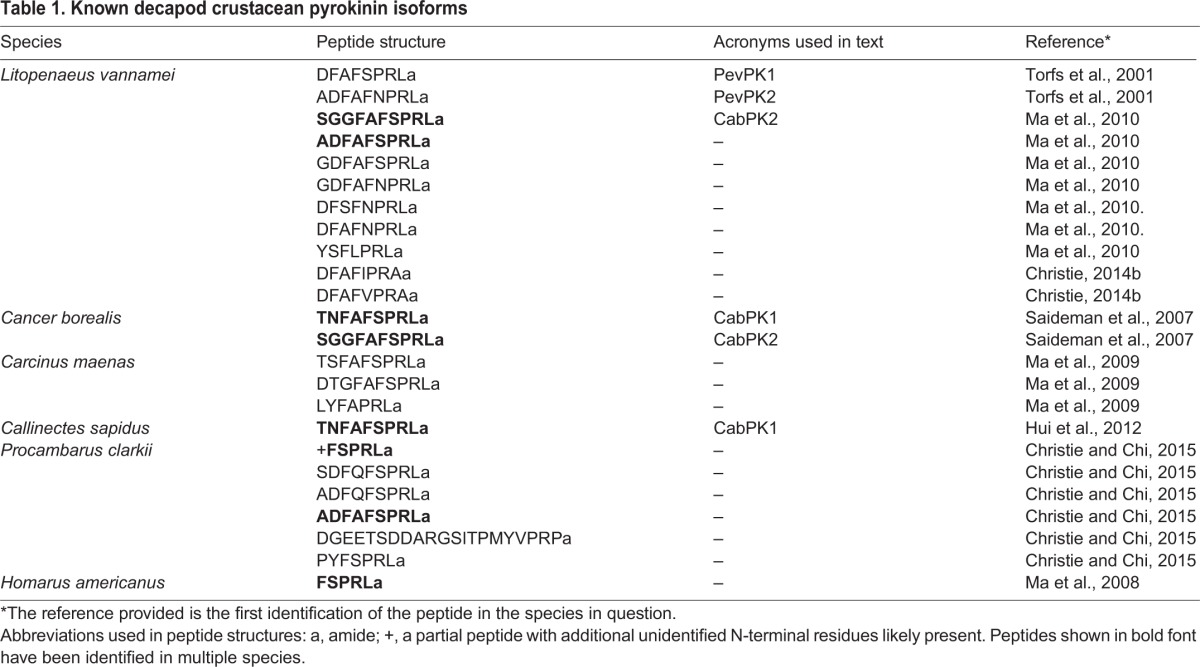
Originally identified in insects (Holman et al., 1986), members of the pyrokinin peptide family have recently been found in several crustacean species (Christie, 2014a,b; Christie et al., 2013; Hui et al., 2012; Ma et al., 2009, 2010, 2008; Saideman et al., 2007; Torfs et al., 2001; Christie and Chi, 2015). The pyrokinins are members of the pheromone biosynthesis activating neuropeptide (PBAN)/diapause hormone/pyrokinin/periviscerokinin superfamily of peptides, which typically possess the carboxy (C)-terminal sequence –FXPRLamide, or a close approximation thereof (e.g. Predel and Wegener, 2006; Rafaeli, 2009). Members of this peptide superfamily are well studied in insects and have been implicated in the control of a large number of biological processes, including, but not limited to, pheromone biosynthesis, ecdysone biosynthesis, visceral muscle contraction, cuticle melanization, acceleration of pupation and induction of diapause (e.g. Predel and Wegener, 2006; Rafaeli, 2009). In insects, members of the PBAN/diapause hormone/pyrokinin/periviscerokinin superfamily are encoded by two genes: capability and hugin (e.g. Baggerman et al., 2002; Hewes and Taghert, 2001; Kean et al., 2002; Meng et al., 2002; Vanden Broeck, 2001). Twenty different pyrokinins have been identified in six different decapod crustaceans (Table 1; Christie, 2014a; Hui et al., 2012; Ma et al., 2009, 2010, 2008; Saideman et al., 2007; Torfs et al., 2001; Christie and Chi, 2015), with each species having from one [the lobster Homarus americanus (Ma et al., 2008) and the crab Callinectes sapidus (Hui et al., 2012)] to 11 [the penaeid shrimp Litopenaeus vannamei (Christie, 2014a; Ma et al., 2010; Torfs et al., 2001)] different isoforms. However, only two studies to date (Saideman et al., 2007; and our accompanying paper, Dickinson et al., 2015) have examined the biological activity of these peptides in the crustaceans; both studies found that the pyrokinins are active neuromodulators.
Table 1.
Known decapod crustacean pyrokinin isoforms
The stomatogastric nervous system (STNS) contains the four ganglia that generate the rhythmic motor patterns that control the movements of the foregut (i.e. the oesophageal, cardiac sac, gastric mill and pyloric rhythms). A previous study conducted on the STNS of the crab (Cancer borealis) examined the modulatory effects of three different pyrokinins (Saideman et al., 2007), two native to C. borealis (Saideman et al., 2007) and one derived from the cockroach Rhyparobia (formerly Leucophaea) maderae (Holman et al., 1986). All three pyrokinins exerted virtually identical effects on the crab stomatogastric system, suggesting that the structural differences between these peptides are functionally unimportant, and that the pyrokinin receptor does not distinguish between these different isoforms (Saideman et al., 2007). In contrast, of five crustacean pyrokinins tested in the lobster (H. americanus) cardiac ganglion, which controls the rhythmic beating of the neurogenic heart in crustaceans, only the shrimp L. vannamei isoform (ADFAFNPRLamide; PevPK2), exerted any significant modulatory effects (Dickinson et al., 2015). This suggests that the pyrokinin receptor in the lobster cardiac ganglion is highly selective, and that small changes in the pyrokinin sequence have considerable functional consequences.
List of abbreviations
- CabPK1
Cancer borealis pyrokinin 1
- CabPK2
Cancer borealis pyrokinin 2
- coc
circumoesophageal connective
- CoG
commissural ganglion
- Dappu-ETH
Daphnia pulex ecdysis-triggering hormone
- dgn
dorsal gastric nerve
- dlvn
dorsal lateral ventricular nerve
- dpon
dorsal posterior oesophageal nerve
- dvn
dorsal ventricular nerve
- ETH
ecdysis-triggering hormone
- Homam-sNPF
Homarus americanus short neuropeptide F
- icn
inferior cardiac nerve
- ion
inferior oesophageal nerve
- ln
labral nerve
- LPG
lateral posterior gastric neuron
- lvn
lateral ventricular nerve
- mvn
medial ventricular nerve
- NDS
normal donkey serum
- on
oesophageal nerve
- PBAN
pheromone biosynthesis activating neuropeptide
- PevPK1
Penaeus vannamei pyrokinin 1
- PevPK2
Penaeus vannamei pyrokinin 2
- son
superior oesophageal nerve
- STG
stomatogastric ganglion
- STKR
Stomoxys calcitrans tachykinin-related peptide receptor
- stn
stomatogastric nerve
- STNS
stomatogastric nervous system
- vlvn
ventral lateral ventricular nerve
- vpdn
ventral pyloric dilator nerve
Despite the contrasting selectivity of the pyrokinin response in these two species and central pattern generators, immunohistochemical studies suggest that pyrokinin is in fact present within both neural networks. Pyrokinin-like immunoreactivity was found in the neuropil of the stomatogastric ganglion (STG) in the crab (Saideman et al., 2007); it was also found in neuroendocrine organs and throughout the H. americanus cardiac ganglion, including the terminals surrounding both the small pacemaker and large motor neurons of this ganglion (Dickinson et al., 2015). These contrasting results led us to ask: (1) whether pyrokinin is distributed similarly in the crab and the lobster STNS, and (2) whether the neural circuits in the STG of the lobster would respond broadly to pyrokinins, as they do in the C. borealis STG, or selectively, as they do in the H. americanus cardiac ganglion.
RESULTS
Distribution of pyrokinin-like immunoreactivity in the Homarus STNS
To determine the distribution of pyrokinin-like peptides in the STNS of H. americanus (N=15 animals), whole-mount immunohistochemistry was conducted using an antibody generated against the sequence FSPRLamide, the only pyrokinin thus far isolated from H. americanus (Ma et al., 2008). As in all decapod species, the STNS of H. americanus consists of the unpaired STG, the unpaired oesophageal ganglion and the paired commissural ganglia (CoGs), as well as a number of interconnecting and motor nerves (Fig. 1). Within all STGs examined (N=15 ganglia), pyrokinin-like labeling was restricted to the neuropile, with none of the approximately 30 intrinsic somata exhibiting pyrokinin immunoreactivity (Fig. 2A). The immunopositive STG neuropile originates from axons projecting into the ganglion from the stomatogastric nerve (stn) via the paired superior oesophageal nerves (sons). These axons are likely to be derived from somata present in the CoGs (Fig. 2B); in each CoG (N=29 ganglia) approximately 14 somata exhibit pyrokinin-like labeling (range, 6–30 somata per ganglion), as does an extensive neuropile, which appears to be composed of processes originating from both the intrinsic immunopositive cell bodies and from immunoreactive axons projecting into the ganglion from the circumoesophageal connective (coc), a nerve that links the STNS to the brain and fused thoracic and abdominal ganglia via each CoG. Because of the weak intensity and punctate nature of labeling of the axons in the stn and sons, as well as their tendency toward fasciculation, it was not possible to unambiguously count the number of axons that give rise to the pyrokinin-like immunoreactivity in the STG neuropile, although we estimate that there are approximately eight axons contributing to it.
Fig. 1.
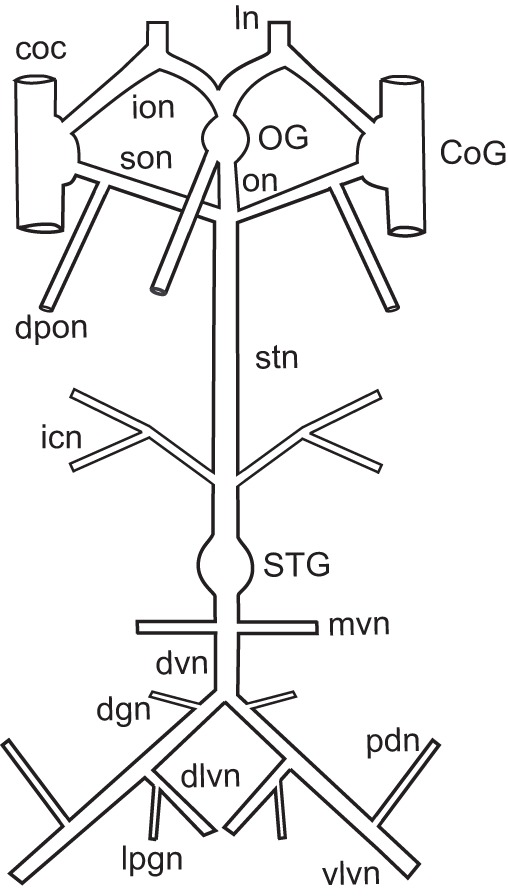
Schematic representation of the stomatogastric nervous system of Homarus americanus. The stomatogastric nervous system (STNS) consists of four ganglia as well as the nerves that connect them and motor nerves that innervate the muscles of the gastric mill and pylorus. coc, circumoesophageal connective; CoG, commissural ganglion; dgn, dorsal gastric nerve; dlvn, dorsal lateral ventricular nerve; dvn, dorsal ventricular nerve; dpon, dorsal posteror oesophageal nerve; icn, inferior cardiac nerve; ion, inferior oesophageal nerve; ln, labral nerve; mvn, medial ventricular nerve; OG, oesophageal ganglion; on, oesophageal nerve; pdn, pyloric dilator nerve; son, superior oesophageal nerve; stn, stomatogastric nerve; vlvn, ventral lateral ventricular nerve.
Fig. 2.
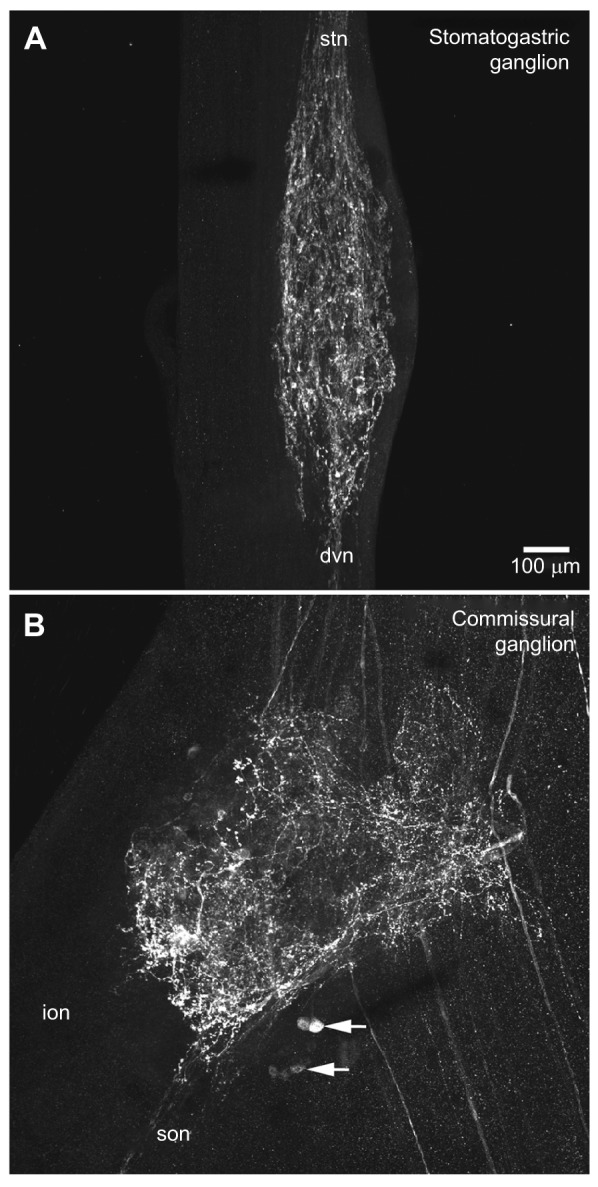
Distribution of pyrokinin-like labeling in the stomatogastric ganglion and commissural ganglion of Homarus americanus. (A) In the stomatogastric ganglion (STG), several fascicles of immunopositive axons projecting from the stomatogastric nerve (stn) arborize within the ganglion, producing a neuropile composed of a dense network of fine fibers studded with bead-like varicosities; most, if not all, of the axons producing this neuropile appear to exit the ganglion via the dorsal ventricular nerve (dvn). No pyrokinin-like immunoreactivity was seen in any of the 30 or so intrinsic STG somata. This image is a brightest pixel projection of 43 optical sections taken at approximately 2 μm intervals. (B) Pyrokinin-like labeling in the CoG consists of approximately 14 immunopositive somata (two groups indicated by arrows) and an extensive neuropile. This neuropile is likely to be derived from processes originating from both the intrinsic immunopositive cell bodies and from immunoreactive axons projecting into the ganglion from the circumoesophageal connective (coc). Immunopositive axons exiting the CoG via the superior oesophageal nerve (son) are the source of the pyrokinin labeling in the STG. No pyrokinin-like immunoreactive axons were seen exiting the CoG via the inferior oesophageal nerve (ion). This image is a brightest pixel projection of 38 optical sections taken at approximately 2 μm intervals, and is shown at the same scale as A.
At least a subset (and perhaps all) of the pyrokinin-immunopositive axons that produce the labeling in the STG exit the ganglion via the dorsal ventricular nerve (dvn), where they project approximately halfway to the branch point of the lateral ventricular nerves (lvns) and terminate in putative neuropile-like profiles. The axons that give rise to the pyrokinin-immunopositive STG neuropile are also likely to be the source of an extensive endocrine-like plexus that covers much of the anterior portion of the STNS (Fig. 3). This plexus is superficially located, with immunoreactivity within or just below the sheath. The immunoreactivity in the structure is distinctly bark-like in appearance, and extends from the branch point of the dorsal posterior oesophageal nerve (dpon) on each son to the junction of the sons and stn, down the stn approximately half way to the STG, and up the oesophageal nerve (on) and inferior oesophageal nerves (ions) to the branch point of the labral nerve (ln) on each ion. All of the pyrokinin-immunopositive structures reported here were seen in all preparations, although the intensity of labeling was quite variable between animals.
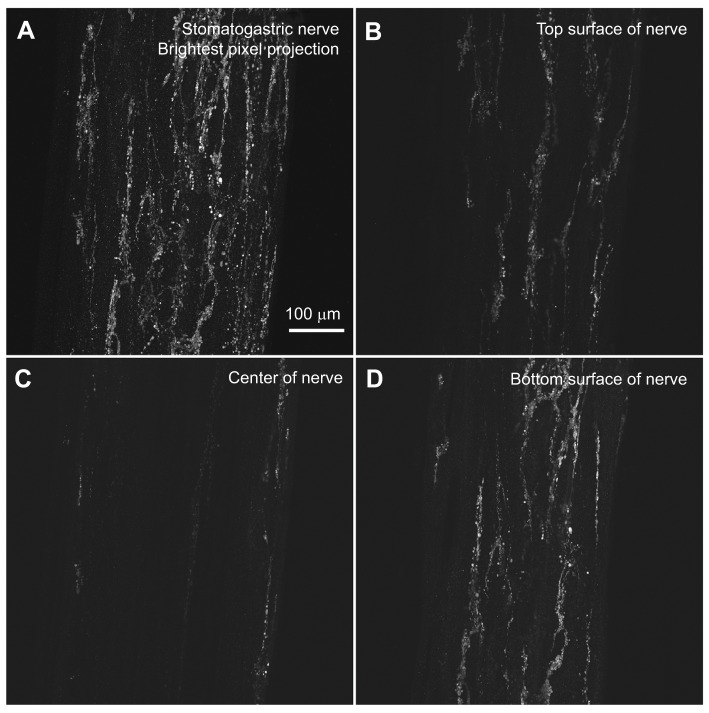
Fig. 3.
An extensive pyrokinin immunoreactive plexus covers the surface of much of the anterior portion of the Homarus americanus stomatogastric nervous system. An extensive neuroendocrine-like plexus covers much of the anterior portion of the STNS. This structure is located within or just below the sheath that covers the STNS and has a distinctly bark-like appearance. This putative neuroendocrine release structure extends from the branch point of the dorsal posterior oesophageal nerve on each superior oesophageal nerve (son) to the junction of the sons and stomatogastric nerve (stn), down the stn approximately half way to the stomatogastric ganglion, and up the oesophageal nerve and inferior oesophageal nerves (ions) to the branch point of the labral nerve on each ion. The images shown were collected from the stn, just below the son/stn junction (see Fig. 1). (A) A brightest pixel projection of 68 optical sections taken at approximately 1 μm intervals through this region. (B–D) Single optical sections taken at roughly the top (section 14), middle (section 35) and bottom (section 58) portions of the nerve, and illustrate the superficial nature of the staining. All images are shown at the same magnification.
Specificity of pyrokinin-like labeling
To strengthen our confidence that the pyrokinin-like immunoreactivity reported here is due to the presence of native pyrokinins, two sets of specificity controls were conducted. To ensure that the lobster ganglia did not react with non-specific components of the rabbit serum, CoGs incubated in pre-immune serum were compared with those incubated in serum R3-30 (N=3 CoG pairs). For all CoG pairs, only the ganglion incubated in immune serum showed immunopositive structures (data not shown).
To ensure that the antibodies we used interacted specifically with pyrokinins, and not with other lobster neuropeptides, we conducted a series of antibody adsorptions, in which serum R3-30 was incubated with a number of peptides, including both pyrokinins and other crustacean peptides, particularly those that have significant sequence similarity to the pyrokinins. For these experiments, serum R3-30 was incubated with a 10−3 mol l−1 concentration of FSPRLamide (the H. americanus peptide against which the antibody was directed; Ma et al., 2008), SGGFAFSPRLamide [Cancer borealis pyrokinin-2 (CabPK2); Saideman et al., 2007], DFAFSPRLamide [Litopenaeus (Penaeus) vannamei pyrokinin-1 (PevPK1); Torfs et al., 2001], DPSPEPFNPNYNRFRQKIPRIamide [an ecdysis-triggering hormone (ETH) predicted from the cladoceran crustacean Daphnia pulex (Dappu-ETH); Gard et al., 2009], DTSTPALRLRFamide [a short neuropeptide F isoform from H. americanus (Homam-sNPF); Ma et al., 2008] or SGRNFLRFamide (a native H. americanus FLRFamide; Ma et al., 2008) for 3 h at room temperature. Antiserum adsorption by FSPRLamide, CabPK2 or PevPK1 completely abolished all staining in the CoG (N=3 CoG pairs per peptide) whereas adsorption of R3-30 by Dappu-ETH, Homam-sNPF or SGRNFLRFamide (N=3 CoG pairs per peptide) had no effect on immunolabeling (data not shown).
Qualitatively, pyrokinins resulted in enhanced gastric mill activity
The only pyrokinin peptide that has been identified in H. americanus is FSPRLamide, the conserved sequence found in all pyrokinins (Ma et al., 2008), whereas all other crustaceans in which they have been identified generally include longer pyrokinins, as well as more pyrokinins. Moreover, only the shrimp pyrokinin ADFAFNPRLamide [Litopenaeus (Penaeus) vannamei pyrokinin-2 (PevPK2); Torfs et al., 2001] had any effect on the H. americanus heart (Dickinson et al., 2015), whereas the crab stomatogastric system responds equally to multiple pyrokinins (Saideman et al., 2007). Thus, we examined the effects of not only FSPRLamide, but also of four other identified crustacean pyrokinins (Table 1), two identified from the shrimp L. vannemei (PevPK1 and PevPK2) and two from the crab C. borealis [CabPK2 and TNFAFSPRLamide (Cancer borealis pyrokinin-1 or CabPK1); Saideman et al., 2007)] on the activity of the stomatogastric networks in the lobster.
In preparations in which the gastric mill pattern was weakly or moderately active, superfusion of FSPRLamide and the other crustacean pyrokinins at 10−6 mol l−1 (PevPK1 shown) enhanced gastric activity in gastric mill neurons. In such preparations (e.g. Fig. 4), most of the gastric motor neurons fired in patterns that included both gastric and pyloric components in control saline. Upon superfusion with the pyrokinins, activity became predominantly gastric in these neurons. For example, in the recording shown in Fig. 4A, the lateral posterior gastric (LPG) and dorsal gastric neurons fired primarily in shorter, pyloric-timed bursts, with gastric modulation, in saline; in response to PevPK1 superfusion, lateral gastric neuron bursts increased in duration and spike frequency within bursts increased as well. Similarly, bursts in the dorsal gastric neuron, which were not well defined and included weak pyloric characteristics in saline, were stronger, with a higher spike frequency and an increase in the gastric mill timing, in the presence of pyrokinin. The LPG neuron similarly began to fire strongly and primarily in gastric mill time. Additionally, the inferior cardiac neuron, which fired with weak pyloric rhythmicity in saline (Fig. 4B), often increased its firing frequency and began to fire more focused bursts in gastric time in the presence of pyrokinin (Fig. 4B).
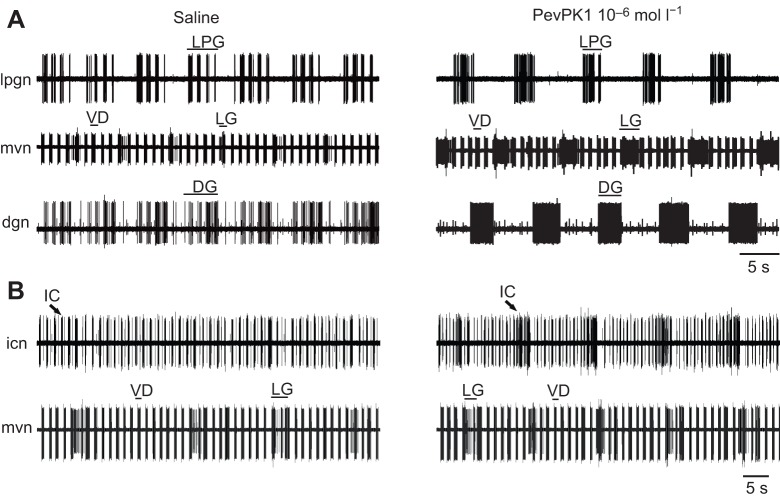
Fig. 4.
Superfusion of PevPK1 excites gastric activity within the STNS of the lobster. (A) Activity in three gastric neurons. (B) A fourth gastric neuron from another preparation. Pyloric activity was not altered by superfusion of 10−6 mol l−1 PevPK1. (A) The lateral posterior gastric (LPG), lateral gastric (LG) and dorsal gastric (DG) motor neurons, whose axons were recorded in extracellular recordings of activity in the lateral posterior gastric nerve (lpgn), the medial ventral nerve (mvn) and the dorsal gastric nerve (dgn), all fired with short, high-frequency bursts or modulation, which corresponds to the pyloric motor in saline (left panel). In the presence of pyrokinins (PevPK1 shown here), they began to fire with typical gastric timing (longer bursts at lower frequency; right panel). The pyloric ventricular dilator (VD) neuron (mvn) continued to fire in pyloric time in PevPK1. (B) In control saline (left panel), the inferior cardiac (IC) neuron (icn) fired largely in pyloric-timed bursts (similar to the pyloric-timed ventricular dilator bursts recorded on the mvn); in the presence of PevPK1 (10−6 mol l−1; right panel), the inferior cardiac neuron began to fire more strongly during gastric bursts, coordinated with the lateral gastric neuron (mvn).
All crustacean pyrokinins excite gastric mill neurons to a similar extent
Superfusion with any of the pyrokinins tested exerted excitatory effects on a number of gastric mill and gastropyloric neurons (Fig. 5). At least superficially, the effects exerted by the five pyrokinins were very similar: bursting in all of the gastric mill neurons we recorded from became more intense and the pattern appeared to be more robust. Moreover, all five pyrokinins appeared to have similar state-dependent effects on gastric mill cycle frequency, with increases in frequency when the pattern was slow in control saline, but decreases when the initial frequency was higher and the pattern was robust (Fig. 6). Mean residuals from the non-linear fit of percentage change in frequency as a function of baseline cycle frequency did not differ significantly among the five pyrokinins, nor did they vary systematically as a function of baseline conditions, indicating that all five pyrokinins had similar effects on the overall burst frequency of the gastric pattern.
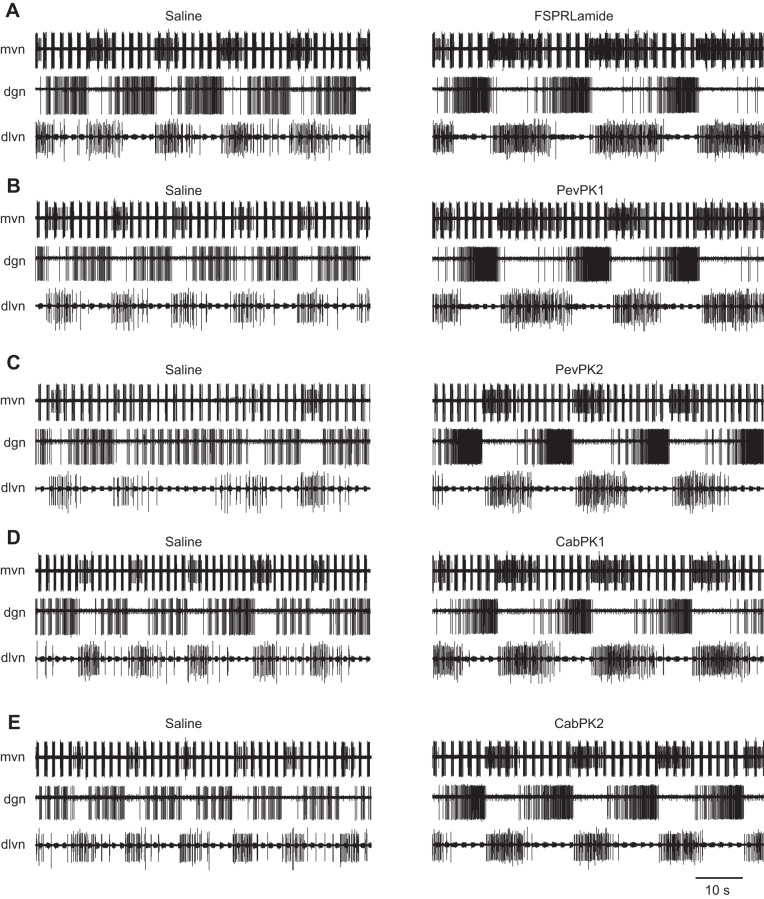
Fig. 5.
Superfusion of the five pyrokinins elicits similar effects on gastric activity in the STNS of the lobster, suggesting that receptors for pyrokinins within the STG bind to pyrokinins indiscriminately. (A) FSPRLamide, (B) PevPKI, (C) PevPK2, (D) CabPK1 and (E) CabPK2. In each panel, the top extracellular recording (mvn) shows the lateral gastric neuron becoming activated during pyrokinin application, whereas the ventricular dilator neuron continues to fire in pyloric time. The middle recording of the dgn shows dorsal gastric neurons changing from firing less-defined gastric bursts with some pyloric characteristics in saline to firing in defined gastric bursts during pyrokinin application. In the recording of the dorsal lateral ventricular nerve (dlvn), the bursts in the LPG neuron became more focused and intense. Recordings are from a single preparation in which the five pyrokinins were applied sequentially, with 1 h washes in saline between each peptide application.
Fig. 6.
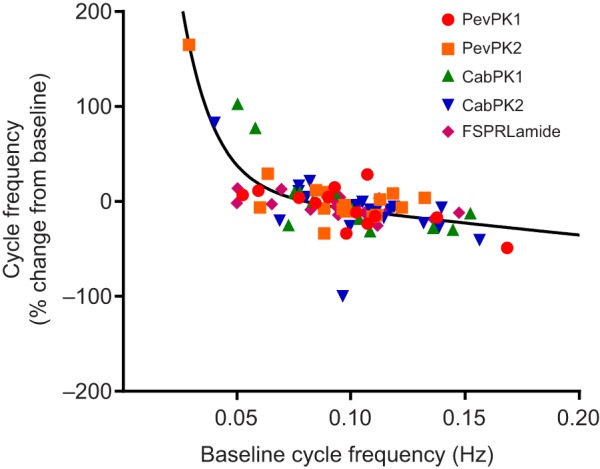
At 10−6 mol l−1, all crustacean pyrokinins alter gastric activity, with the extent of change dependent on the initial state of the preparation. All five pyrokinins elicit similar changes in the STNS of the lobster. Generally, change in cycle frequency recorded during pyrokinin application was inversely correlated with the gastric cycle frequency before application of the peptides, which fitted best to a two-phase exponential decay, R2=0.59. Data pooled from 20 lobsters.
Although the details of the patterns evoked by each peptide often differed slightly (Fig. 5), this was probably due to the fact that the control pattern changed slightly over time, and at least some of the effects of the peptides are state dependent, so that they differ depending on the initial pattern. Thus, to determine whether there were differences in the response of the STNS to the different pyrokinins that were not evident visually, we quantified the effects of the five pyrokinins on two gastric mill motor neurons, one lateral tooth motor neuron – the lateral gastric neuron – and one medial tooth motor neuron – the dorsal gastric neuron.
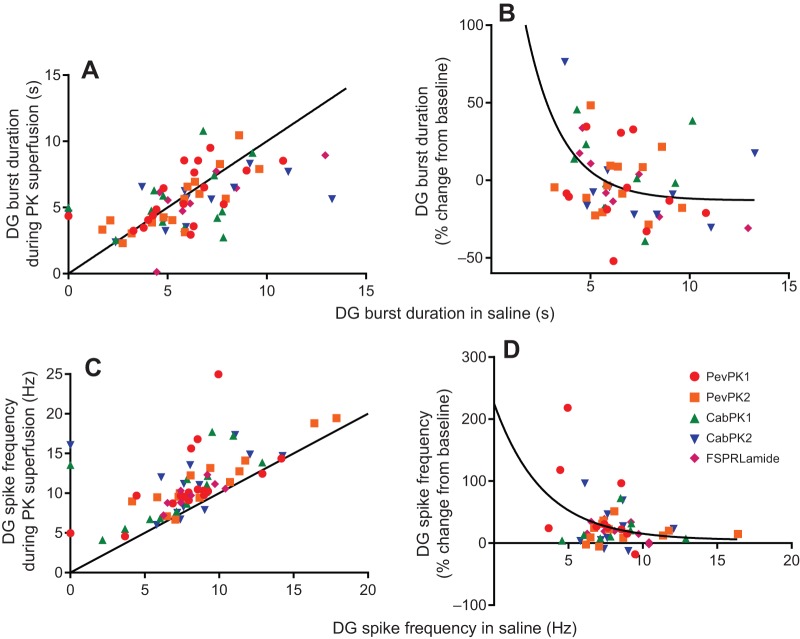
Regardless of the initial conditions, both burst duration and spike frequency in the lateral gastric neuron most often increased during pyrokinin application (Fig. 7A,B; pooled data, single sample t-test, P<0.0001, N=77). However, like cycle frequency, the percentage change in these values tended to be inversely correlated with the starting value (Fig. 7C,D), although these correlations were not strong, with R2 values of only 0.26 (spike frequency) to 0.46 (burst duration). Moreover, in some preparations, particularly those with very long initial durations, burst duration decreased in the presence of the pyrokinins. Nonetheless, mean residuals from the fitted curve did not differ significantly between the five peptides (ANOVA, P=0.31 for duration, P=0.84 for spike frequency), suggesting that they enhanced lateral gastric neuron activity similarly.
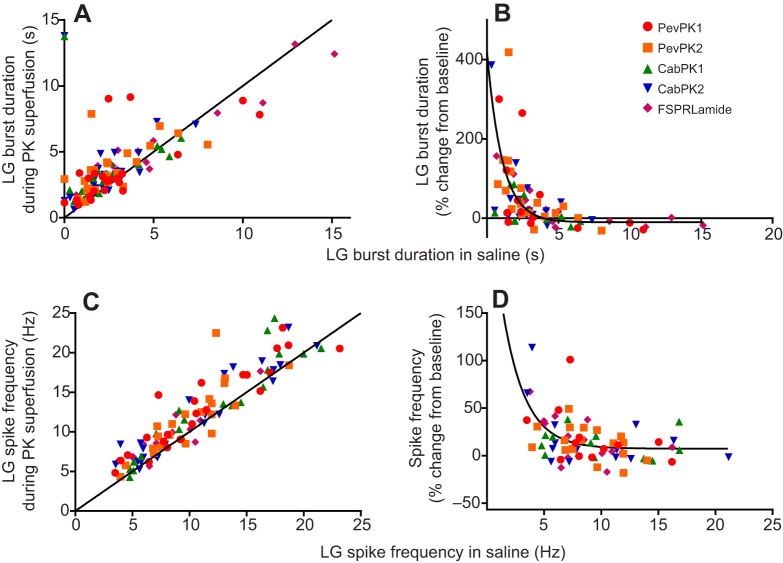
Fig. 7.
All five pyrokinins enhance activity in the lateral gastric neuron, with state-dependent increases in both burst duration and spike frequency within the bursts. Pyrokinins were superfused at 10−6 mol l−1. (A) lateral gastric neuron burst duration increased in most cases in all five pyrokinins, as seen by the fact that most of the points fall above the equality line. However, when burst durations were initially long (>∼5 s), burst duration often decreased. (B) To see the extent to which the effects of the pyrokinins were dependent on the initial parameters, we plotted the percentage change in pyrokinin as a function of the initial burst duration. Although there is some scatter, the percentage change clearly decreases as baseline duration increases, with R2=0.45. (C) Like burst duration, spike frequency in the lateral gastric neuron increased in the presence of all five pyrokinins. (D) Although changes in spike frequency were inversely correlated with initial spike frequency, there was considerable variability between preparations, with R2=0.26.
Interestingly, in the dorsal gastric neuron, while the pyrokinins clearly enhanced gastric activity in individual preparations, their effects on burst duration were not consistent, nor were they strongly dependent on the starting values (Fig. 8A,B). In fact, when all data were averaged, there was not a significant change in dorsal gastric burst duration in any of the peptides (pooled data from all peptides, single sample t-test, P=0.98, N=56). Nonetheless, in individual preparations, changes in dorsal gastric burst duration were often evident, with increases of over 90% in some preparations and decreases of as much as 65% in others. This variability was seen in all of the peptides, with no differences among peptides in their effects on this parameter (i.e. residuals did not differ significantly from one another). In contrast, all of the pyrokinins consistently increased the spike frequency within bursts (Fig. 8C,D; pooled % change from all peptides, single sample t-test, P<0.0001, N=53), regardless of the starting value. These differences were seen in all of the peptides, and there were again no differences among peptides in their effects on this parameter. This was borne out by comparisons of the residuals to a nonlinear fit of the change in duration as a function of starting duration; residuals did not differ significantly among peptides.
Fig. 8.
All five pyrokinins increased spike frequency but did not consistently alter burst duration within dorsal gastric neuron bursts. (A) Burst duration did not consistently change in the presence of the pyrokinins, as seen by the even distribution of points above and below the equality line. (B) Changes in burst duration were highly variable between preparations, as seen by the wide scatter of points; the mean change in burst duration was thus not significantly different from 0 (P=0.99, one sample t-test, N=56), although some individual changes were quite large. Consequently, the R2 values for a one-phase exponential decay or linear regression was only 0.19. However, the slope of a linear regression (not shown) was significantly non-zero (P=0.01), suggesting that the extent of the change is nonetheless dependent on the starting spike frequency. (C) Spike frequency consistently increased in all of the pyrokinins, with no obvious differences between isoforms. In nearly all cases, the points fall above the equality line. (D) As was the case with burst duration, the extent of the increase in dorsal gastric (DG) neuron spike frequency was highly variable between preparations. Nonetheless, a single value t-test for pooled data showed that mean spike frequency did increase (t-test, P<0.001, N=53). Although R2 for the non-linear regression was only 0.12, the slope of a linear fit was significantly different from 0 (P=0.02), indicating that the change was dependent on the starting conditions, but that other factors are likely also important in determining the extent of the response to pyrokinins in the dorsal gastric neuron. Moreover, there were no differences between peptides in the extent to which spike frequency increased (ANOVA, P=0.66, d.f.=4.48), nor in the residuals from the non-linear fit (P=0.9).
Pyrokinins do not alter pyloric activity
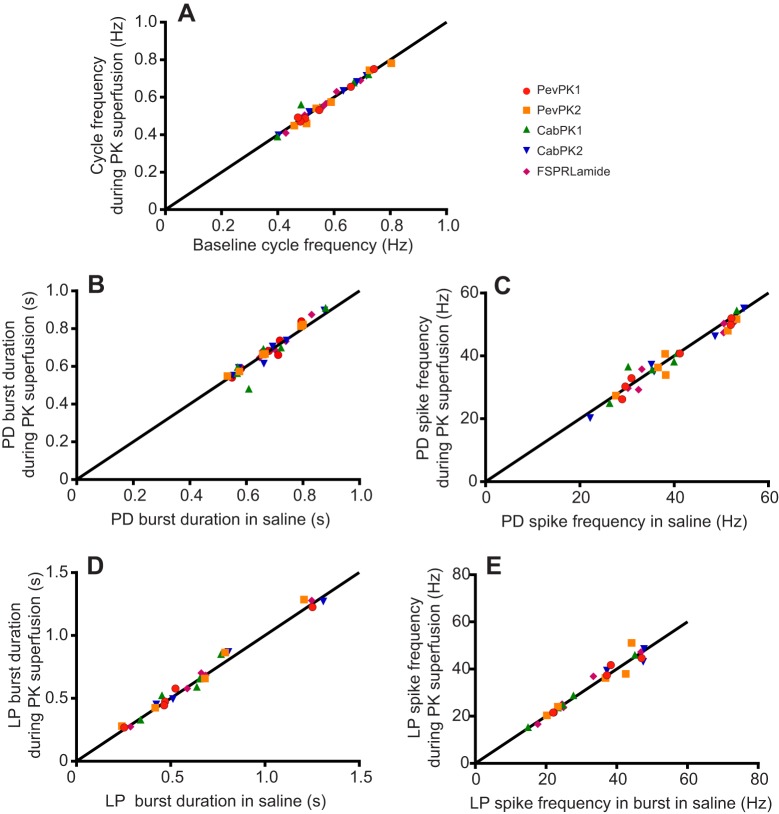
In contrast to the activation of the gastric pattern by all the crustacean pyrokinins, the pyloric motor pattern did not appear to be modulated by any of the pyrokinins (Fig. 9); we recorded no apparent changes in cycle frequency or in the activity of the pyloric dilator neurons, the ventricular dilator neuron, or the lateral pyloric neuron (Fig. 9). To ensure that the apparent lack of effect of the pyrokinins was not due to variability in the response to the peptides, as was the case for dorsal gastric burst duration and has been seen in other systems (e.g. Wiwatpanit et al., 2012), we plotted the effects of the peptides on pyloric cycle frequency (Fig. 10A) as well as on burst duration and spike frequency in the pyloric dilator neurons (Fig. 10B,C) and in the lateral pyloric neuron (Fig. 10D,E) for individual preparations. Mean values did not change significantly for any parameter, and changes in individual preparations were generally quite small, suggesting that the pyrokinins have little or no modulatory effect on the pyloric motor pattern.
Fig. 9.

Superfusion of 10−6 mol l−1 pevpyrokinin-1 over the STNS of the lobster did not alter the pyloric motor pattern. (A) Control recordings show the typical triphasic pyloric pattern, with bursts in the pyloric dilator (PD) neurons (pdn), followed by bursts in the lateral pyloric (LP) and pyloric (PY) motor neurons (vlvn). Pyloric-timed bursts in the ventricular dilator (VD) neuron (mvn) are largely in phase with the PY neuron bursts. A single burst in the lateral gastric (LG) motor neuron is also visible on the mvn recording. (B) When PevPK1 was superfused across the stomatogastric system, no changes in the firing of any of the pyloric neurons was evident, although the duration of the single burst seen here in the lateral gastric motor neuron increased.
Fig. 10.
Crustacean pyrokinins superfused over the stomatogastric ganglion did not alter the pyloric motor pattern. Pyrokinins were superfused at 10−6 mol l−1. Scatterplots show the effects of crustacean pyrokinins on (A) pyloric cycle frequency, (B) burst duration in the pyloric dilator neuron, (C) spike frequency in the pyloric dilator neuron, (D) burst duration in the lateral pyloric neuron and (E) spike frequency in the lateral pyloric neuron. Each data point represents the mean value of 10 consecutive cycles in a single preparation during both saline and pyrokinin superfusion. All points fell on or near the equality line, indicating that the pyrokinins did not quantitatively alter the pyloric pattern. Data are pooled from six preparations that were treated consecutively with the five pyrokinins, separated by 1 h saline washes.
DISCUSSION
The distribution of pyrokinin-like labeling in the crab and lobster STNS are remarkably similar
The distribution of pyrokinin-like immunoreactivity has now been mapped in the STNS of two decapods: the crab C. borealis (Saideman et al., 2007) and the lobster H. americanus (this study). Despite C. borealis and H. americanus being from different infraorders, Brachyura and Astacidea, respectively, the distribution of pyrokinins in their STNSs is remarkably similar. Specifically, in both species, labeling in the STG is restricted to the neuropile and is derived from a small number of axons (or fascicles of axons) projecting to the ganglion via the stn and sons. Within the CoGs, similar numbers of pyrokinin-immunopositive somata and an extensive region of neuropile were seen in both the crab and lobster; a subset of these labeled somata is proposed as the source of the STG neuropile in both species. Similarly, in neither species were any somata labeled in the oesophageal ganglion, nor were any immunopositive axons seen exiting the CoGs via the ions. In fact, the only major differences seen between crab and lobster staining patterns were the presence of an endocrine-like plexus covering the nerves of the anterior portion of the STNS in H. americanus – a structure not labeled in C. borealis – and a small region of neuropil in the dlvn, which was again seen in the lobster, but not in the crab. Thus, the high degree of conservation seen in the STNS distribution of pyrokinins in these two decapods is suggestive of conserved physiological roles being played by this peptide family in rather distantly related species, particularly in their local modulation of the neural circuits present in the STG.
Pyrokinins are likely to function as both local and hormonal modulators of the stomatogastric neural circuits
In the lobster, pyrokinin-like immunoreactivity was found in the STNS itself, as well as in several neuroendocrine organs, notably the pericardial organs and the sinus gland (Dickinson et al., 2015), suggesting that members of this peptide family might act as modulators that are delivered to the STNS both locally and hormonally. Here, we examined and compared the effects of the pyrokinins at concentrations of 10−6 mol l−1, which is generally considered to be a concentration that would mimic local rather than hormonal release (e.g. Christie et al., 1995). However, an earlier study in the crab (Saideman et al., 2007) found that the gastric mill pattern generator responded to concentrations as low as 10−8 mol l−1, which is in the range of peptide concentrations that can be considered as hormonal (e.g. Christie et al., 1995). For example, while measurements of some peptides (e.g. those in the FLRFamide family) in hemolymph are in the order of 10−9 mol l−1 in both insects and snails (Price et al., 1985; Robb and Evans, 1990), others, such as ETH and vitellogenin inhibiting hormone, have been measured at concentrations as high as 3×10−8–4×10−8 mol l−1, suggesting that the pyrokinins, consistent with their distribution, might well serve as both local and hormonal modulators.
Pyrokinins activate the gastric mill but not the pyloric motor pattern
Although the effects of pyrokinins in insects have been examined extensively and they have been shown to have pleiotropic effects, most of these studies have focused on their ability to activate pheromone synthesis (e.g. Predel and Wegener, 2006; Rafaeli, 2009). Nonetheless, pyrokinins have been found within the central nervous systems of many species (e.g. Predel and Wegener, 2006; Rafaeli, 2009), suggesting that they have direct effects on behavior as well as indirect effects via the activation of pheromone synthesis. In the only study of the functional effects of pyrokinins on the crustacean STNS, Saideman et al. (2007) found that pyrokinins excite gastric mill motor neurons and the gastric mill pattern as a whole, including gastro-pyloric neurons, but they do not alter the pyloric motor pattern in the crab C. borealis. Our results suggest that these peptides have effects in the lobster H. americanus that are strikingly parallel to those seen in the crab. As was the case in the studies of crab (Saideman et al., 2007), pyrokinins strengthened the gastric mill pattern, and enhanced the gastric-mill-timed firing of gastro-pyloric neurons (e.g. the LPG neuron), but had no effect on purely pyloric motor neurons such as the pyloric dilator and lateral pyloric neurons. Nonetheless, there were minor differences in the responses of the crab and lobster STNS to pyrokinins. Specifically, in the rare cases in which the gastric mill pattern was not active in control saline in the lobster, pyrokinin application was not able to activate the pattern, whereas it did so in the crab (data not shown). Additionally, while the dorsal gastric neuron in the crab most often fired in strong bursts that were independent of the remaining gastric mill neurons, the enhanced firing induced by pyrokinins in the dorsal gastric neuron in the lobster was always coordinated with the other gastric mill neurons. Despite these differences, the overall pattern of pyrokinin effects was remarkably similar between the crab (Saideman et al., 2007) and the lobster.
Multiple crustacean pyrokinins have similar effects on the gastric mill pattern
As was the case with the three pyrokinins, i.e. two native isoforms and the cockroach isoform Leucophaea maderae pyrokinin that were tested in the crab stomatogastric system (Saideman et al., 2007), the effects of the five pyrokinins examined in this study did not differ significantly from one another. The only pyrokinin that has been identified from Homarus is an amidated pentapeptide, FSPRLamide (Ma et al., 2008), corresponding to the conserved sequence that characterizes the pyrokinin family. This contrasts sharply with the case in most other crustaceans, as well as most insects. To date, pyrokinins have been identified in six decapod crustacean species, including a shrimp (L. vannamei), three species of crab (C. borealis, Carcinus maenas and C. sapidus), the crayfish Procambarus clarkii and the lobster H. americanus (Christie, 2014a; Hui et al., 2012; Ma et al., 2009, 2010, 2008; Saideman et al., 2007; Torfs et al., 2001; Christie and Chi, 2015); in all of these except H. americanus, pyrokinins with extended C-termini have been identified. In contrast to the case in insects, where many functions have been ascribed to pyrokinins (e.g. Predel and Wegener, 2006; Rafaeli, 2009), the function of these peptides in the crustaceans has been examined minimally. In one such study, the two shrimp pyrokinins (PevPK1 and PevPK2) were shown to increase the amplitude and frequency of hindgut contractions (Torfs et al., 2001); interestingly, these studies were done not on the shrimp from which the peptides were isolated, but instead on a crayfish (Astacus leptodactylas) and on the cockroach R. maderae, from which the first pyrokinins had been identified and shown to be myotropic (Holman et al., 1986). The effects of the two pyrokinins in this study were very similar, although the threshold for effects on the crayfish was lower than that on the cockroach (Torfs et al., 2001). The other previous study of pyrokinin function in the crustaceans showed that the two native crab pyrokinins and the cockroach pyrokinin had virtually indistinguishable effects on the crab (C. borealis) stomatogastric nervous system (Saideman et al., 2007). In the present study, five different crustacean pyrokinins had virtually identical effects, suggesting that the pyrokinin receptor is relatively promiscuous, and does not distinguish between the different pyrokinins. An earlier study in insects similarly reported that changes to the N-terminus of the pyrokinin peptide sequence, where the largest differences between the crustacean pyrokinins are found, had only minor effects on activity. However, while the pyrokinin receptor required only the conserved sequence, –FXPRLamide, for binding and activation, the response to the conserved sequence was only 30% of that recorded with the longer peptide sequences (Nachman et al., 1986), whereas in the lobster STNS, there was no apparent difference between the response to FSRPRLamide and the other crustacean pyrokinins. It should be noted, however, that the present study used suprathreshold concentrations of all of the pyrokinins, whereas Nachman and colleagues focused on identifying the threshold concentration. It is possible that quantitative differences in response might be seen if lower concentrations, mimicking hormonal rather than local release, were used in the lobster STNS.
The specificity of pyrokinin effects in the lobster STNS differs from that in the lobster cardiac ganglion
In contrast to the similarity of the responses of the STNS to the different crustacean pyrokinins, the lobster cardiac ganglion responds to only one of these peptides (Dickinson et al., 2015). Moreover, the peptide that is active on the cardiac system is not the short sequence that has been identified in the lobster nervous system using mass spectrometry (Ma et al., 2008). This suggests that there are multiple pyrokinins in H. americanus, including in the STNS. In most crustaceans thus far examined, this is the case (Christie, 2014a,b; Christie et al., 2013; Hui et al., 2012; Ma et al., 2009, 2010, 2008; Saideman et al., 2007; Torfs et al., 2001; Christie and Chi, 2015), with at least one species (the shrimp L. vannamei) possessing 11 distinct PBAN/diapause hormone/pyrokinin/periviscerokinin superfamily members (Christie, 2014a; Ma et al., 2010; Torfs et al., 2001). The differences in responses of the cardiac and stomatogastric ganglia to different pyrokinins would also be explained if there were at least two pyrokinin receptors, one of which was highly specific, while the other(s) was much more promiscuous in its binding properties – a situation that exists in at least some insects (e.g. Hariton-Shalev et al., 2013; Jiang et al., 2014). In this case, differential distribution of the receptors, with the specific receptor located in the cardiac ganglion and the less specific one(s) (or both) present in the STNS would account for the different specificities of the responses to pyrokinins. Similarly, there could be multiple pyrokinin receptors, all of which are relatively specific, with just one in the cardiac ganglion, but multiple receptors present in the STNS. In our opinion, the former seems more likely, because it seems less likely that multiple receptors would all have the same distribution and effects on the various neurons that constitute the gastric mill pattern generator.
Alternatively, it is possible that all of the pyrokinins bind to the same receptor, but that each pyrokinin alters that receptor differentially, thus activating different second messenger pathways. In this case, the pathways that are activated by most of the pyrokinins might be present in the STNS but not in the cardiac ganglion, or those pathways may not alter the parameters (contraction amplitude and frequency, burst duration) that were modified by PevPK2 in this study (Dickinson et al., 2015). Although it is not common for the same receptor to mediate different effects in response to activation by different ligands, such a situation exists in the responses to tachykinins in insects (Poels et al., 2005). Poels et al. (2005) found that four different tachykinins were able to activate the same receptor [Stomoxys calcitrans tachykinin-related peptide receptor (STKR), a neurokinin/tachykinin receptor identified from the stable fly]. STKR is a G-protein-coupled receptor that activates both a cyclic AMP pathway and a phospholipase C/Ca2+ pathway (Torfs et al., 2000), and can do so when expressed in an insect cell line. Strikingly, when challenged with four different tachykinins, this receptor responded with different patterns of increases in the two second messengers; some tachykinins had much greater effects on the cAMP pathway whereas others had larger effects on the Ca2+ pathway, leading Poels et al. (2005) to suggest that the receptor may be able to adopt one of two distinct functional states, which might then interact with different G proteins (reviewed in Nusbaum and Blitz, 2012). Similar reports of multiple states for neurokinin (Holst et al., 2001; Palanche et al., 2001; Sachon et al., 2002) and muscarinic (Gurwitz et al., 1994) receptors from mammals, as well as for aminergic receptors from Drosophila (Reale et al., 1997; Robb et al., 1994), have likewise been reported.
MATERIALS AND METHODS
Animals and tissue collection
Animals
American lobsters, Homarus americanus Milne-Edwards 1837, were purchased from local (Bar Harbor and Brunswick, ME, USA) seafood retailers. All animals were housed in flow-through or recirculating natural seawater aquaria at 10–12°C and were fed approximately weekly on a diet of chopped squid.
Dissections
For the isolation of the STNS (Fig. 1) for both immunohistochemistry and physiological experiments, animals were cold-anesthetized by packing in ice for approximately 20–30 min. After anesthetization, the foregut was removed and the STNS was manually dissected from the surrounding musculature in chilled (approximately 4°C) physiological saline (composition in mmol l−1: 479.12 NaCl, 12.74 KCl, 13.67 CaCl2, 20.00 MgSO4, 3.91 Na2SO4, 11.45 Trizma base and 4.82 maleic acid, pH=7.45).
Whole-mount immunohistochemistry
Development of pyrokinin antisera
In order to map the distribution of pyrokinin-like peptides in the lobster nervous system, custom antibodies were designed and generated against the only known H. americanus member of this peptide family [i.e. FSPRLamide (Ma et al., 2008)]; antibody production was contracted through LAMPIRE Biological Laboratories, Inc. (Pipersville, PA, USA). In brief, a synthetic peptide with the sequence CFSPRLamide was synthesized and conjugated to bovine serum albumin (BSA) via the N-terminal cysteine using m-maleimidobenzoyl-N-hydroxysuccinimide ester (GenScript Corporation, Piscataway, NJ, USA). New Zealand white rabbits [three in total; animal codes 15417 (rabbit 1, R1), 15418 (rabbit 2, R2) and 15419 (rabbit 3, R3)] were injected subcutaneously and intradermally at multiple locations with 500 mg of peptide–BSA conjugate emulsified in 500 ml of complete Freund's adjuvant. Rabbits were boosted with 500 mg of peptide–BSA conjugate emulsified in 500 ml of Freund's incomplete adjuvant at 3 week intervals.
Preimmune serum was drawn from each rabbit prior to the initial injection of immunogen, with post-injection bleeds taken 30, 50 and 57 days after the initial immunogen injection. Preimmune sera and sera produced from all post-injection bleeds were stored at −80°C. The immune sera from all three rabbits and all three bleeds produced apparently identical labeling in the STNS and neuroendocrine organs; however, all descriptions of immunoreactivity reported in this study were derived from tissues labeled using serum from the Day 30 bleed of rabbit 3 (serum code R3-30).
Immunoprocessing
Immunolabeling was done using whole-mount preparations. In brief, dissected tissues were pinned out in Sylgard-lined Petri dishes and fixed for 12–24 h in a solution of 4% paraformaldehyde (EM grade; Electron Microscopy Sciences; Hatfield, PA, USA; cat. no. 15710) in 0.1 mol l−1 sodium phosphate buffer, pH 7.4 (PB). Following fixation, tissues were rinsed five times at 1 h intervals in PB containing 0.3% Triton X-100 (PBT), after which they were incubated for approximately 72 h in pyrokinin antibody (see above) diluted to a final concentration of 1:10,000–1:15,000 in PBT containing 10% normal donkey serum (NDS; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; cat. no. 017-000-121). After incubation in primary antibody, tissues were rinsed five times at 1 h intervals in PBT and then incubated for 12–24 h in DyLight 488-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch; cat. no. 711-485-152) diluted to 1:300 in PBT containing 10% NDS. Following incubation in secondary antibody, tissues were rinsed five times at 1 h intervals in PB and then mounted between a glass microscope slide and coverslip in Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA; cat. no. H-1000). Fixation and incubations in both primary and secondary antibody were conducted at 4°C, while all rinses were conducted at room temperature (approximately 22°C). Incubation in secondary antibody and subsequent processing was conducted in the dark and slides were stored at 4°C in the dark until examined for labeling.
Specificity controls
To strengthen our confidence that the immunolabeling reported here is due to the presence of pyrokinin-related peptides, we conducted two sets of specificity controls. In the first set of experiments, the two CoGs from a lobster were isolated after fixation; one ganglion was then processed using R3 pre-immune serum (cocs left long), while the other CoG (cocs cut short) was processed with serum R3-30 as described above. After incubation in primary antibody, the two CoGs were rinsed separately and then incubated together in secondary antibody solution (see above). All subsequent processing and imaging were done simultaneously.
For the second set of specificity controls, we pre-adsorbed the antibody with a number of peptides, including both pyrokinins and other crustacean peptides, particularly those that have significant sequence similarity to the pyrokinins. For these experiments, serum R3-30 was incubated with a 10−3 mol l−1 concentration of FSPRLamide (the H. americanus peptide against which the antibody was directed; Ma et al., 2008), CabPK2 (Saideman et al., 2007), PevPK1 (Torfs et al., 2001), Dappu-ETH (Gard et al., 2009), Homam-sNPF (Ma et al., 2008) or SGRNFLRFamide (Ma et al., 2008) for 3 h at room temperature. Simultaneously, aliquots of serum R3-30 were held at room temperature for 3 h without peptide. As was the case for the preimmune controls, CoG pairs were used here, with one CoG from each lobster incubated with control antibody and the other incubated in pre-adsorbed antibody; they were processed simultaneously as described above.
Epifluorescence and confocal microscopy
Data were collected and digital images were generated using an Olympus BX-51 upright compound microscope (Olympus America, Center Valley, PA, USA) outfitted with epifluorescence and an OPTRONICS MacroFire digital camera (OPTRONICS, Goleta, CA, USA) or an Olympus Fluoview 1000 confocal system that utilizes an Olympus IX-81 inverted microscope and blue diode, multi-argon, green HeNe and red HeNe lasers. For the production of figures, digital images were exported from the Olympus confocal system as tiff files and then arranged using Photoshop software (v7.0; Adobe Systems Inc., San Jose, CA, USA). The contrast and brightness of final figures were adjusted as needed to optimize the clarity of the printed images.
Extracellular recordings
For recordings of neuronal activity, the STNS was pinned out in a clear Sylgard184 (KR Anderson, Santa Clara, CA, USA)-lined Petri dish. The STG was desheathed to provide the pyrokinins with direct access to the ganglion. Neuronal activity was recorded using monopolar pin electrodes inserted in petroleum jelly wells surrounding and isolating portions of each nerve of interest [medial ventricular nerve (mvn), inferior cardiac nerve (icn), lvn, dorsal gastric nerve (dgn), dorsal lateral ventricular nerve (dlvn), ventral lateral ventricular nerve (vlvn), ventral pyloric dilator nerve (vpdn)], with ground electrodes in the bath. Electrical activity was amplified using an A-M Systems Differential AC Amplifier (Model 1700) and a Brownlee Precision Instrumentation Amplifier (Model 210 A). A Cambridge Electronic Design Power 1401 and Spike2 were used for data recording into a computer with a sampling rate of 10 kHz. The STNS was constantly superfused with saline kept at 10–13°C with a Peltier cooling system (CL-100 bipolar temperature controller and SC-20 solution heater/cooler; Warner Instruments, Hamden, CT, USA) via a peristaltic pump, with a flow rate of approximately 5 ml min−1. Peptides were applied through the perfusion system.
Peptides
Five pyrokinins, FSPRLamide, PevPK1, PevPK2, CabPK1 and CabPK2, were used for physiological studies (Table 1). PevPK1, PevPK2 and FSPRLamide were custom synthesized by GenScript Corporation, while the initial CabPK1 and CabPK2 used here were gifts from Dr Michael Nusbaum (University of Pennsylvania School of Medicine, Philadelphia, PA, USA), with additional samples of these peptides custom synthesized by GenScript. Regardless of source, all peptides were stored at −20°C as a 10−3 mol l−1 solution in deionized water. Pyrokinins were diluted to 10−6 mol l−1 in saline immediately before use. The pyrokinin solution was then superfused over the STG for approximately 10 min, followed by a saline wash for at least 50 min to ensure that neuronal activity returned to baseline before another pyrokinin solution was applied.
Data analysis
Data were analyzed in Spike2 using the built-in functions and custom-written scripts available at http://stg.rutgers.edu/Resources.html. Quantitative analyses of extracellular recordings were used to examine cycle frequency, burst duration and spike frequency of two gastric neurons, the lateral gastric and dorsal gastric neurons and of two pyloric neurons, the lateral pyloric and pyloric dilator neurons. To compare the effects of the peptides to control activity, the mean of measurements taken from 10 bursts just before pyrokinin application was compared with the means of 10 bursts taken during the time at which the peptide had its maximum effect. Data were analyzed and statistical comparisons made using Prism 6 (GraphPad Software, San Diego, CA, USA). For some parameters, data were normalized by determining percent change from control; one-sample t-tests (two tailed) were used to determine whether these changes were significantly different from zero. Gastric cycle frequency, as well as burst parameters of individual neurons in saline versus percent change during pyrokinin superfusion, were fitted with a one-phase exponential decay function, from which the R2 was analyzed to assess the fit. A one-way ANOVA was run to test for differences in mean residuals between the different crustacean pyrokinins. The model residuals did not systematically vary across the independent variables for any peptide. Physiology figures were made from Spike2 files incorporated into CorelDraw (Corel, Inc., Mountain View, CA, USA).
Acknowledgements
The authors thank Greg Anderson for reading and commenting on preliminary drafts of this manuscript. We also thank the Mount Desert Island Biological Laboratory and Dr Patricia Hand (PI of the Maine INBRE grant) for providing space and funding for the initial experiments that led to this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
P.S.D. and A.E.C. conceived and designed this study. Experiments were executed and data were analyzed by P.S.D., S.C.K., X.Q., A.S., M.A.K., A.H.W., A.B.Y., B.P. and A.E.C. The manuscript was written by P.S.D., S.C.K. and A.E.C.
Funding
This work was supported by the National Science Foundation [IOS-1121973 to P.S.D., IOS-1354567 to P.S.D., ISO-1353023 to A.E.C.]; Maine Space Grant MERITS Program; and the National Institutes of Health [5P20RR016463-12 to Patricia Hand, 8P20GM103423-12 to Patricia Hand]. Deposited in PMC for release after 12 months.
References
- Baggerman G., Cerstiaens A., De Loof A. and Schoofs L. (2002). Peptidomics of the larval Drosophila melanogaster central nervous system. J. Biol. Chem. 277, 40368-40374. 10.1074/jbc.M206257200 [DOI] [PubMed] [Google Scholar]
- Blitz D. M. and Nusbaum M. P. (2011). Neural circuit flexibility in a small sensorimotor system. Curr. Opin. Neurobiol. 21, 544-552. 10.1016/j.conb.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz D. M. and Nusbaum M. P. (2012). Modulation of circuit feedback specifies motor circuit output. J. Neurosci. 32, 9182-9193. 10.1523/JNEUROSCI.1461-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A. E. (2014a). Expansion of the Litopenaeus vannamei and Penaeus monodon peptidomes using transcriptome shotgun assembly sequence data. Gen. Comp. Endocrinol. 206, 235-254. 10.1016/j.ygcen.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Christie A. E. (2014b). Prediction of the peptidomes of Tigriopus californicus and Lepeophtheirus salmonis (Copepoda, Crustacea). Gen. Comp. Endocrinol. 201, 87-106. 10.1016/j.ygcen.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Christie A. E. and Chi M. (2015). Prediction of the neuropeptidomes of members of the Astacidea (Crustacea, Decapoda) using publicly accessible transcriptome shotgun assembly (TSA) sequence data. Gen. Comp. Endocrinol. (in press). 10.1016/j.ygcen.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Skiebe P. and Marder E. (1995). Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J. Exp. Biol. 198, 2431-2439. [DOI] [PubMed] [Google Scholar]
- Christie A. E., Stemmler E. A. and Dickinson P. S. (2010). Crustacean neuropeptides. Cell. Mol. Life Sci. 67, 4135-4169. 10.1007/s00018-010-0482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A. E., Roncalli V., Wu L.-S., Ganote C. L., Doak T. and Lenz P. H. (2013). Peptidergic signaling in Calanus finmarchicus (Crustacea, Copepoda): in silico identification of putative peptide hormones and their receptors using a de novo assembled transcriptome. Gen. Comp. Endocrinol. 187, 117-135. 10.1016/j.ygcen.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Dembrow N. and Johnston D. (2014). Subcircuit-specific neuromodulation in the prefrontal cortex. Front. Neural Circuits 8, 54 10.3389/fncir.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson P. S. (2006). Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr. Opin. Neurobiol. 16, 604-614. 10.1016/j.conb.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Sreekrishnan A., Kwiatkowski M. A. and Christie A. E. (2015). Distinct or shared actions of peptide family isoforms: I. Peptide-specific actions of pyrokinins in the lobster cardiac neuromuscular system. J. Exp. Biol. 218, 2892-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénelon V., Le Feuvre Y., Bem T. and Meyrand P. (2003). Maturation of rhythmic neural network: role of central modulatory inputs. J. Physiol. Paris 97, 59-68. 10.1016/j.jphysparis.2003.10.007 [DOI] [PubMed] [Google Scholar]
- Fu Q., Kutz K. K., Schmidt J. J., Hsu Y.-W. A., Messinger D. I., Cain S. D., de la Iglesia H. O., Christie A. E. and Li L. (2005). Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J. Comp. Neurol. 493, 607-626. 10.1002/cne.20773 [DOI] [PubMed] [Google Scholar]
- Gard A. L., Lenz P. H., Shaw J. R. and Christie A. E. (2009). Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen. Comp. Endocrinol. 160, 271-287. 10.1016/j.ygcen.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Haring R., Heldman E., Fraser C. M., Manor D. and Fisher A. (1994). Discrete activation of transduction pathways associated with acetylcholine m1 receptor by several muscarinic ligands. Eur. J. Pharmacol. 267, 21-31. 10.1016/0922-4106(94)90220-8 [DOI] [PubMed] [Google Scholar]
- Hariton-Shalev A., Shalev M., Adir N., Belausov E. and Altstein M. (2013). Structural and functional differences between pheromonotropic and melanotropic PK/PBAN receptors. Biochim. Biophys. Acta 1830, 5036-5048. 10.1016/j.bbagen.2013.06.041 [DOI] [PubMed] [Google Scholar]
- Hewes R. S. and Taghert P. H. (2001). Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 11, 1126-1142. 10.1101/gr.169901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman G. M., Cook B. J. and Nachman R. J. (1986). Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp. Biochem. Physiol. C Comp. Pharmacol. 85, 219-224. 10.1016/0742-8413(86)90077-0 [DOI] [PubMed] [Google Scholar]
- Holst B., Hastrup H., Raffetseder U., Martini L. and Schwartz T. W. (2001). Two active molecular phenotypes of the tachykinin NK1 receptor revealed by G-protein fusions and mutagenesis. J. Biol. Chem. 276, 19793-19799. 10.1074/jbc.M100621200 [DOI] [PubMed] [Google Scholar]
- Hooper S. L. and DiCaprio R. A. (2004). Crustacean motor pattern generator networks. Neurosignals 13, 50-69. 10.1159/000076158 [DOI] [PubMed] [Google Scholar]
- Hui L., Xiang F., Zhang Y. and Li L. (2012). Mass spectrometric elucidation of the neuropeptidome of a crustacean neuroendocrine organ. Peptides 36, 230-239. 10.1016/j.peptides.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L., D'Andrea B. T., Jia C., Liang Z., Christie A. E. and Li L. (2013). Mass spectrometric characterization of the neuropeptidome of the ghost crab Ocypode ceratophthalma (Brachyura, Ocypodidae). Gen. Comp. Endocrinol. 184, 22-34. 10.1016/j.ygcen.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wei Z., Nachman R. J., Adams M. E. and Park Y. (2014). Functional phylogenetics reveals contributions of pleiotropic peptide action to ligand-receptor coevolution. Sci. Rep. 4, 6800 10.1038/srep06800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean L., Cazenave W., Costes L., Broderick K. E., Graham S., Pollock V. P., Davies S. A., Veenstra J. A. and Dow J. A. T. (2002). Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am. J. Physiol. 282, R1297-R1307. [DOI] [PubMed] [Google Scholar]
- LeBeau F. E. N. and Whittington M. A. (2005). Structure/function correlates of neuronal and network activity - an overview. J. Physiol. 562, 1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau F. E. N., El Manira A. and Griller S. (2005). Tuning the network: modulation of neuronal microcircuits in the spinal cord and hippocampus. Trends Neurosci. 28, 552-561. 10.1016/j.tins.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Ma M. M., Chen R., Sousa G. L., Bors E. K., Kwiatkowski M. A., Goiney C. C., Goy M. F., Christie A. E. and Li L. (2008). Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 156, 395-409. 10.1016/j.ygcen.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Bors E. K., Dickinson E. S., Kwiatkowski M. A., Sousa G. L., Henry R. P., Smith C. M., Towle D. W., Christie A. E. and Li L. (2009). Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen. Comp. Endocrinol. 161, 320-334. 10.1016/j.ygcen.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Gard A. L., Xiang F., Wang J., Davoodian N., Lenz P. H., Malecha S. R., Christie A. E. and Li L. (2010). Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei. Peptides 31, 27-43. 10.1016/j.peptides.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. and Bucher D. (2001). Central pattern generators and the control of rhythmic movements. Curr. Biol. 11, R986-R996. 10.1016/S0960-9822(01)00581-4 [DOI] [PubMed] [Google Scholar]
- Marder E., O'Leary T. and Shruti S. (2014). Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu. Rev. Neurosci. 37, 329-346. 10.1146/annurev-neuro-071013-013958 [DOI] [PubMed] [Google Scholar]
- Meng X., Wahlström G., Immonen T., Kolmer M., Tirronen M., Predel R., Kalkkinen N., Heino T. I., Sariola H. and Roos C. (2002). The Drosophila hugin gene codes for myostimulatory and ecdysis-modifying neuropeptides. Mech. Dev. 117, 5-13. 10.1016/S0925-4773(02)00175-2 [DOI] [PubMed] [Google Scholar]
- Miles G. B. and Sillar K. T. (2011). Neuromodulation of vertebrate locomotor control networks. Physiology 26, 393-411. 10.1152/physiol.00013.2011 [DOI] [PubMed] [Google Scholar]
- Mitchell G. S. and Johnson S. M. (2003). Invited review: neuroplasticity in respiratory motor control. J. Appl. Physiol. 94, 358-374. 10.1152/japplphysiol.00523.2002 [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M. and Cook B. J. (1986). Active fragments and analogs of the insect neuropeptide leucopyrokinin: structure-function studies. Biochem. Biophys. Res. Commun. 137, 936-942. 10.1016/0006-291X(86)90315-3 [DOI] [PubMed] [Google Scholar]
- Nusbaum M. P. and Beenhakker M. P. (2002). A small-systems approach to motor pattern generation. Nature 417, 343-350. 10.1038/417343a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum M. P. and Blitz D. M. (2012). Neuropeptide modulation of microcircuits. Curr. Opin. Neurobiol. 22, 592-601. 10.1016/j.conb.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum M. P., Blitz D. M., Swensen A. M., Wood D. and Marder E. (2001). The roles of co-transmission in neural network modulation. Trends Neurosci. 24, 146-154. 10.1016/S0166-2236(00)01723-9 [DOI] [PubMed] [Google Scholar]
- Palanche T., Ilien B., Zoffmann S., Reck M.-P., Bucher B., Edelstein S. J. and Galzi J.-L. (2001). The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J. Biol. Chem. 276, 34853-34861. 10.1074/jbc.M104363200 [DOI] [PubMed] [Google Scholar]
- Poels J., Nachman R. J., Åkerman K. E., Oonk H. B., Guerrero F., De Loof A., Janecka A. E., Torfs H. and Vanden Broeck J. (2005). Pharmacology of stomoxytachykinin receptor depends on second messenger system. Peptides 26, 109-114. 10.1016/j.peptides.2004.07.015 [DOI] [PubMed] [Google Scholar]
- Predel R. and Wegener C. (2006). Biology of the CAPA peptides in insects. Cell. Mol. Life Sci. 63, 2477-2490. 10.1007/s00018-006-6187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. A., Cottrell G. A., Doble K. E., Greenberg M. J., Jorenby W., Lehman H. K. and Riehm J. P. (1985). A novel FMRFamide-related peptide in Helix: pQDPFLRFamide. Biol. Bull. 169, 256-266. 10.2307/1541402 [DOI] [Google Scholar]
- Rafaeli A. (2009). Pheromone biosynthesis activating neuropeptide (PBAN): regulatory role and mode of action. Gen. Comp. Endocrinol. 162, 69-78. 10.1016/j.ygcen.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Rauscent A., Le Ray D., Cabirol-Pol M.-J., Sillar K. T., Simmers J. and Combes D. (2006). Development and neuromodulation of spinal locomotor networks in the metamorphosing frog. J. Physiol. Paris 100, 317-327. 10.1016/j.jphysparis.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Reale V., Hannan F., Hall L. M. and Evans P. D. (1997). Agonist-specific coupling of a cloned Drosophila melanogaster D1-like dopamine receptor to multiple second messenger pathways by synthetic agonists. J. Neurosci. 17, 6545-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb S. and Evans P. D. (1990). FMRFamide-like peptides in the locust: distribution, partial characterization and bioactivity. J. Exp. Biol. 149, 335-360. [DOI] [PubMed] [Google Scholar]
- Robb S., Cheek T. R., Hannan F. L., Hall L. M., Midgley J. M. and Evans P. D. (1994). Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 13, 1325-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachon E., Girault-Lagrange S., Chassaing G., Lavielle S. and Sagan S. (2002). Analogs of substance P modified at the C-terminus which are both agonist and antagonist of the NK-1 receptor depending on the second messenger pathway. J. Pept. Res. 59, 232-240. 10.1034/j.1399-3011.2002.01977.x [DOI] [PubMed] [Google Scholar]
- Saideman S. R., Ma M., Kutz-Naber K. K., Cook A., Torfs P., Schoofs L., Li L. and Nusbaum M. P. (2007). Modulation of rhythmic motor activity by pyrokinin peptides. J. Neurophysiol. 97, 579-595. 10.1152/jn.00772.2006 [DOI] [PubMed] [Google Scholar]
- Selverston A. I. (2005). A neural infrastructure for rhythmic motor patterns. Cell. Mol. Neurobiol. 25, 223-244. 10.1007/s10571-005-3154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston A. I. and Ayers J. (2006). Oscillations and oscillatory behavior in small neural circuits. Biol. Cybern 95, 537-554. 10.1007/s00422-006-0125-1 [DOI] [PubMed] [Google Scholar]
- Sillar K. T., Combes D., Ramanathan S., Molinari M. and Simmers J. (2008). Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res. Rev. 57, 94-102. 10.1016/j.brainresrev.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Stein W. (2009). Modulation of stomatogastric rhythms. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 195, 989-1009. 10.1007/s00359-009-0483-y [DOI] [PubMed] [Google Scholar]
- Torfs H., Shariatmadari R., Guerrero F., Parmentier M., Poels J., Van Poyer W., Swinnen E., De Loof A., Åkerman K. and Vanden Broeck J. (2000). Characterization of a receptor for insect tachykinin-like peptide agonists by functional expression in a stable Drosophila Schneider 2 cell line. J. Neurochem. 74, 2182-2189. 10.1046/j.1471-4159.2000.0742182.x [DOI] [PubMed] [Google Scholar]
- Torfs P., Nieto J., Cerstiaens A., Boon D., Baggerman G., Poulos C., Waelkens E., Derua R., Calderón J., De Loof A. et al. (2001). Pyrokinin neuropeptides in a crustacean: isolation and identification in the white shrimp Penaeus vannamei. Eur. J. Biochem. 268, 149-154. 10.1046/j.1432-1327.2001.01858.x [DOI] [PubMed] [Google Scholar]
- Vanden Broeck J. (2001). Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides 22, 241-254. 10.1016/S0196-9781(00)00376-4 [DOI] [PubMed] [Google Scholar]
- Wiwatpanit T., Powers B. and Dickinson P. S. (2012). Inter-animal variability in the effects of C-type allatostatin on the cardiac neuromuscular system in the lobster Homarus americanus. J. Exp. Biol. 215, 2308-2318. 10.1242/jeb.069989 [DOI] [PMC free article] [PubMed] [Google Scholar]