ABSTRACT
Small non-coding RNAs (microRNAs) are important regulators of gene expression that modulate many physiological processes; however, their role in regulating intracellular transport remains largely unknown. Intriguingly, we found that the dynamin (DNM) genes, a GTPase family of proteins responsible for endocytosis in eukaryotic cells, encode the conserved miR-199a and miR-199b family of miRNAs within their intronic sequences. Here, we demonstrate that miR-199a and miR-199b regulate endocytic transport by controlling the expression of important mediators of endocytosis such as clathrin heavy chain (CLTC), Rab5A, low-density lipoprotein receptor (LDLR) and caveolin-1 (Cav-1). Importantly, miR-199a-5p and miR-199b-5p overexpression markedly inhibits CLTC, Rab5A, LDLR and Cav-1 expression, thus preventing receptor-mediated endocytosis in human cell lines (Huh7 and HeLa). Of note, miR-199a-5p inhibition increases target gene expression and receptor-mediated endocytosis. Taken together, our work identifies a new mechanism by which microRNAs regulate intracellular trafficking. In particular, we demonstrate that the DNM, miR-199a-5p and miR-199b-5p genes act as a bifunctional locus that regulates endocytosis, thus adding an unexpected layer of complexity in the regulation of intracellular trafficking.
KEY WORDS: miRNA, miR-199, Endocytosis, LDLR
Summary: A characterization of how an intronic miRNA encoded in the dynamin genes controls cellular endocytosis by targeting multiple components of the endocytic machinery.
INTRODUCTION
Endocytosis is an essential process in cell physiology by which eukaryotic cells take up macromolecules and particles from the surrounding medium. Many physiological processes, including cell migration, angiogenesis, metabolism and development, depend on proper functioning of endocytosis and thus cells have developed multiple mechanisms to ensure proper intracellular trafficking and endocytosis (Fernández-Rojo et al., 2012; Gu et al., 2011; Lee et al., 2014; Mousley et al., 2012; Parachoniak et al., 2011). There are multiple pathways of endocytosis into cells, including, clathrin-dependent, caveolin-dependent and clathrin- and caveolin-independent internalization. In all of them, the material to be internalized is surrounded by an area of plasma membrane, which then buds off inside the cell to form a vesicle containing the ingested material that is delivered to intracellular organelles and cytosol (McMahon and Boucrot, 2011). The best-characterized form of this process is receptor-mediated endocytosis (RME), which provides a mechanism for the selective uptake of essential nutrients such as low-density lipoprotein (LDL), through the LDL receptor (LDLR) (Brown and Goldstein, 1986), or iron, through transferrin receptor (TfR) (Harding et al., 1983). Thus, factors that affect RME have a direct effect on these receptors, and, in the case of LDLR, to regulate intracellular cholesterol levels. In both the LDLR and TfR internalization processes, clathrin plays a key role during the formation of coated vesicles (Moore et al., 1987). Once vesicles are internalized, their passage through a broad endosomal compartment system is required; first they are rapidly transported into early endosomes, where Rab5A is a key regulator (Nielsen et al., 1999), and subsequently to late endosomes and lysosomes. Whichever route of entry is used, a crucial step in endocytosis and intracellular transport is the formation of endocytic vesicles, which specifically requires the participation of the dynamin (DNM) GTPase family to promote their scission and fission from the plasma membrane (Ferguson and De Camilli, 2012; Jones et al., 1998; Roux et al., 2006; Takei et al., 1995). DNM proteins are also involved in other membrane remodeling processes, such as fission of clathrin-coated vesicles from the trans-Golgi network (TGN) (Cao et al., 2000) and membrane ruffling through the interaction with actin nucleators (Gu et al., 2010). In mammals, the DNM gene family is encoded by three separate genes: DNM1, DNM2 and DNM3. Although all three DNM genes share a high degree of sequence similarity, thus resulting in proteins containing similar domains, they differ in their tissue-specific expression (Urrutia et al., 1997).
It is presently accepted, that small non-coding RNAs, known as microRNAs (miRNAs), are key regulators of several cellular processes (Bushati and Cohen, 2007). This regulatory control is carried out through repression of gene expression at the post-transcriptional level by base pairing with complementary regions mainly within the 3′ untranslated regions (3′UTRs) of target mRNAs, thus promoting mRNA degradation, translational repression, or both (Ambros, 2004; Bartel, 2009; Filipowicz et al., 2008). Most miRNAs are first transcribed into long transcripts of primary miRNAs (pri-miRNAs) that are then processed sequentially in the nucleus by Drosha and DGCR8 to generate pre-miRNAs, and by Dicer in the cytoplasm to generate the mature ∼22 nt miRNA duplex (Lee et al., 2003) that, after strand selection, mediates the targeting activity when incorporated into the RISC complex (Chendrimada et al., 2007). There is mounting evidence that suggests that both strands, the guide or ‘5p’ strand as well as the miRNA* (also known as the passenger or ‘3p’ strand) have important regulatory activity (Chamorro-Jorganes et al., 2014; Goedeke et al., 2013). Approximately half of the miRNA genes can be found in intergenic regions, whereas the intragenic miRNAs are predominantly located inside introns and usually oriented on the same DNA strand of the host gene (Saini et al., 2007). Intergenic miRNA genes have their own promoter region and, thus, their expression is regulated by the same molecular mechanisms that control the expression of protein-coding genes. By contrast, same-strand intronic miRNAs are co-transcribed with their host gene (Rodriguez et al., 2004) and then processed to finally become mature functional miRNAs. A number of studies have shown that intronic miRNAs localized in the same orientation as their host genes usually cooperate with them to regulate similar cellular functions (Rayner et al., 2010; van Rooij et al., 2009). However, exceptions to this common scheme of co-transcription have been reported. In fact, ∼26% of intronic miRNAs are antisense orientated and transcribed independently of their host genes (Rodriguez et al., 2004; Siegel et al., 2009). Despite this, intronic miRNA can support the function of its host gene by silencing genes that are functionally antagonistic to the host or act synergistically with the host by coordinating the expression of genes with related functions.
Interestingly, the miR-199a and miR-199b (hereafter denoted miR-199a/b) family members are encoded within introns of the DNM genes in the opposite orientation to the host gene. The miR-199a/b family is composed of three members, miR-199a1, miR-199a2 and miR-199b located within the DNM2, DNM3 and DNM1 genes, respectively. MiR-199a/b gene sequences exhibit high conservation across species and share the same seed sequence, thereby potentially targeting the same group of genes. Interestingly, predicted target genes for miR-199a/b-5p (guide) strands are broadly conserved among species compared to the miR-199a/b-3p (passenger) strand. Therefore, here, we investigated potential miR-199a/b-5p target genes using several miRNA target bioinformatic algorithms. Importantly, we identified putative binding sites for miR-199a/b-5p in the 3′UTR of genes involved in vesicle-mediated transport and endocytosis. Of note, our present findings indicate that miR-199a/b-5p regulates the expression of multiple genes participating in clathrin-dependent endocytosis (Cltc, Rab5A, Rab21 and Ldlr) and clathrin-independent endocytosis (Cav-1). Furthermore, we demonstrate that miR-199a/b-5p inhibits clathrin-mediated endocytosis through the regulation of CLTC, Rab5A and Rab21 expression, affecting the normal function of receptors located in the plasma membrane such as LDLR and TfR. Taken together, our work shows a new mechanism by which miRNAs regulate intracellular trafficking. In particular, we describe that the DNM genes along with miR-199 act as a bifunctional locus encoding the DNM, a GTPase that is a crucial mediator of endocytosis, and miR-199a/b, which also regulates intracellular trafficking, thus adding an unexpected layer of complexity in the regulation of endocytosis.
RESULTS
miR-199a/b-5p are potential regulators of transport and vesicle-mediated trafficking processes
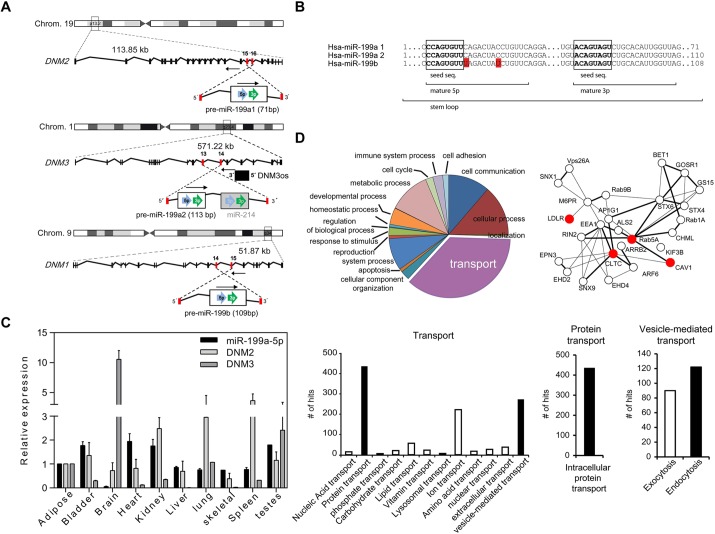
While investigating the genomic location of miRNAs encoded in the human genome, we noted the intriguing presence of a highly conserved miRNA family, miR-199a/b, embedded within the intronic sequences of the DNM genes (Fig. 1A). The miR-199a/b family consists of three members, miR-199a-1, miR-199a-2 and miR-199b, which are transcribed from conserved antisense intronic transcripts of the DNM2 locus (human chromosome 19), DNM3 locus (human chromosome 1) and DNM1 locus (human chromosome 9), respectively (Fig. 1A). Human miR-199a1-5p and miR-199a2-5p have identical mature sequences, but the miR-199b-5p mature sequence differs in two nucleotides outside of the seed sequence (Fig. 1B). The miR-199a-5p mature sequences show higher conservation among vertebrate species than miR-199b-5p (supplementary material Fig. S1A), indicating that miR-199a1 and miR-199a2 are evolutionarily conserved. Given this seed sequence conservation, we focused our study on miR-199a-5p.
Fig. 1.
miR-199 and DNM loci genomic location, human tissue expression and bioinformatic analysis of predicted miR-199a/b target genes. (A) Schematic representation of genomic location of DNM genes and their miR-199a/b intronic family members. Intronic miR-199a2-5p is co-transcribed in a cluster with miR-214. Note that the three members of miR-199a/b are encoded on the opposite strand to the DNM host genes. (B) Sequence alignment between human miR-199 family members. Seed sequences are indicated in boxes. The red color in the miR-199b sequence indicates those nucleotides that have diverged with respect to miR-199a. Stem loop, mature 5p and 3p forms are indicated. (C) Gene expression analyses (qRT-PCR) of miR-199a-5p, DNM2 and DNM3 in different human tissues normalized to snoRD68 for miR-199a-5p and GAPDH, for the DNM2 and DNM3 genes. Data are expressed relative to the amount of miR-199a-5p transcripts expressed in adipose tissue. Results are mean±s.e.m. for three experiments. (D) Gene ontology analysis of the predicted miR-199a/b target genes using Panther software (upper left and bottom panels). A protein–protein interaction analysis scheme of selected predicted miR-199a-5p target genes using String 9.1 software and Navigator 2.2. is shown in the upper right panel.
Mammalian miRNAs are present in the genome either as independent transcriptional units or embedded within the introns of protein-coding genes. To determine whether the expression of the miR-199a/b family members and DNM genes are co-regulated, we measured their expression in different human tissues. As seen in Fig. 1C and supplementary material Fig. S1B, we observed that the mature miR-199a-5p (miR-199a1-5p and miR-199a2-5p), miR-199b-5p and their respective precursors (pre-miR-199a-1, pre-miR-199a-2 and pre-miR-199b) (supplementary material Fig. S1C) were widely expressed in most tissues. Remarkably, compared with other tissues, mature miR-199a-5p was expressed at very low levels in the brain, which expresses high levels of DNM3 (Fig. 1C). Similarly, the expression of miR-199b-5p in the brain is markedly reduced compared with other tissues (supplementary material Fig. S1B). Interestingly, miR-199b-5p levels were inversely correlated with DNM1 expression (supplementary material Fig. S1B), suggesting that miR-199b-5p is regulated independently of its host gene.
We next sought to ascertain the potential function of miR-199a/b-5p. To this end, we employed a combination of bioinformatic algorithms [Targetscan (http://www.targetscan.org) and miRanda (http://www.microrna.org)] that predict miRNA targets largely based on the ability of the miRNA sequence to undergo specific base-pairing within the putative 3′UTR target. The predicted miR-199a/b-5p target genes were assigned to several functional annotation clusters and networks as shown in Fig. 1D. Interestingly, using gene ontology software analysis (Panther, http://www.pantherdb.org/) (Thomas et al., 2003), and the protein–protein interaction database, String (http://string-db.org/) (Szklarczyk et al., 2011), we observed that the most represented cluster was associated with genes involved in cellular transport (Fig. 1D). Among them specifically, miR-199a/b-5p was predicted to target a vast network of genes associated with endocytic functions, including CLTC, Cav-1, Rab5A, LDLR and Rab21 (Fig. 1D) (Bucci et al., 1994; Dorsey et al., 2007; Doyon et al., 2011; Pellinen et al., 2008; Simpson et al., 2004; Singh et al., 2003). This intriguing observation led us to investigate the biological role of miR-199a/b-5p in controlling receptor-mediated endocytosis.
MiR-199a-5p regulates the expression of endocytosis mediators
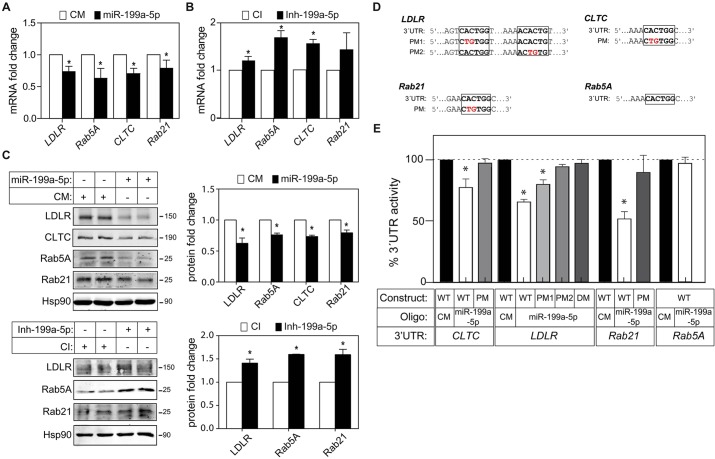
To evaluate the effect of miR-199a/b-5p on CLTC, LDLR, Rab5A and Rab21 expression, we transfected human hepatic Huh7 cells with synthetic miR-199a-5p mimics. As seen in Fig. 2A, CLTC, LDLR, Rab5A and Rab21 mRNA expression was inhibited in cells overexpressing miR-199a-5p compared to cells transfected with a non-targeting control miRNA mimic. Conversely, Huh7 cells transfected with antisense oligonucleotides directed against miR-199a-5p (inh-199a-5p) had significantly increased CLTC, LDLR and Rab5A mRNA expression compared to cells transfected with a non-targeting control inhibitor (Fig. 2B). Consistent with this, overexpression of miR-199a-5p markedly reduced CLTC, LDLR, Rab5A and Rab21 protein expression (Fig. 2C, upper panels). Moreover, inhibition of endogenous miR-199a-5p increased LDLR, Rab5A and Rab21 protein in Huh7 cells, suggesting that miR-199a/b-5p plays a physiological role in regulating cellular endocytosis (Fig. 2C, lower panels).
Fig. 2.
miR-199a/b regulates LDLR, CLTC, Rab5A and Rab21 expression in Huh7 cells. (A,B) qRT-PCR analysis of LDLR, CLTC, Rab5A and Rab21 expression in Huh7 cells transfected with non-targeting control mimic (CM), miR-199a-5p mimic, or control inhibitor (CI) and miR-199a-5p inhibitor. (C) Western blot analysis of LDLR, CLTC, Rab5A and Rab21 in Huh7 cells transfected with control mimic or miR-199a-5p mimic (upper panels) and control inhibitor or inh-199a-5p (lower panels). A densitometry analysis is shown in the corresponding histograms; Hsp90 was used as a loading control. (D) Human LDLR, CLTC, Rab21 and Rab5a 3′UTR containing the indicated point mutations (PM) in the miR-199a/b-5p target sites. (E) Luciferase reporter activity in COS7 cells transfected with control mimic or miR-199a-5p mimic and the indicated human 3′UTR containing or not (wild-type, WT) the indicated point mutation in the target miR-199a-5p-binding sites. In A and B, data are expressed as mean±s.e.m. and representative of ≥3 experiments in triplicate. In A–C, data are expressed as mean±s.e.m. and representative of ≥3 experiments performed in duplicate. In E, data are expressed as a percentage of 3′UTR activity of control mimic (±s.e.m.) and are representative of ≥3 experiments performed in triplicate. *P≤0.05.
CLTC, LDLR, Rab5A and Rab21 have one or more predicted miR-199a/b-5p-binding sites that are conserved across mammals (Fig. 2D; supplementary material Fig. S1D). To determine whether miR-199a-5p specifically targets the 3′UTR of CLTC, LDLR, Rab5A and Rab21, we cloned the entire 3′UTR of the aforementioned genes into a luciferase reporter vector and assessed whether miR-199a-5p overexpression could reduce luciferase reporter activity. As seen in Fig. 2E, miR-199a-5p markedly repressed CLTC, LDLR and Rab21 3′UTR activity. Surprisingly, Rab5A 3′UTR activity was not influenced by miR-199a-5p overexpression (Fig. 2E), despite its inhibitory effect on Rab5A mRNA and protein expression (Fig. 2A,C). As expected, mutation of the miR-199a-5p target sites relieved miR-199a-5p repression of CLTC, LDLR and Rab21 3′UTR activity, consistent with a direct interaction of miR-199a-5p with these sites (Fig. 2E). In addition to these genes, we also identified Cav-1 as a predicted miR-199a-5p target gene. As Cav-1 is expressed at very low levels in hepatic cell lines, we analyzed the role of miR-199a-5p in regulating Cav-1 expression in HeLa cells, which express significantly higher levels of this protein. The results show that miR-199a-5p overexpression markedly reduced Cav-1 mRNA and protein expression compared with cells transfected with control mimic (supplementary material Fig. S2A,C, left panels). A similar effect was observed when we analyzed the expression of Cav-1 by immunofluorescence (supplementary material Fig. S2D). Importantly, inhibition of endogenous miR-199a-5p led to an increase in Cav-1 expression (supplementary material Fig. S2A,C, right panels; supplementary material Fig. S2D). We also confirmed that miR-199a-5p directly targets Cav-1 by assessing the Cav-1 3′UTR luciferase activity in cells transfected with miR-199a-5p mimics (supplementary material Fig. S2B). Taken together, these results identify CLTC, LDLR, Rab21 and Cav-1 as direct targets of miR-199a-5p.
MiR-199a-5p inhibits RME
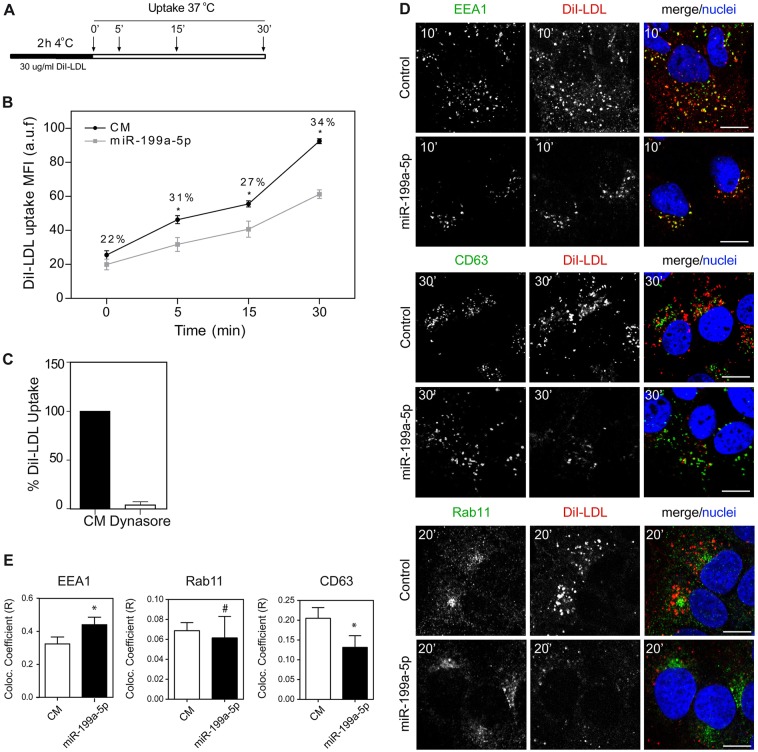
CLTC, Rab5A and Rab21 are essential components of RME, a process by which cells internalize molecules (de Hoop et al., 1994; Doyon et al., 2011; Simpson et al., 2004). RME is widely used for the specific uptake of substances required by the cell, including LDL through LDLR (Kang and Folsch, 2011; Mettlen et al., 2010), and iron, through the TfR (Gan et al., 2002; Tosoni et al., 2005). The LDLR binds to LDL particles and mediates their endocytosis together with CLTC, which is necessary for coated vesicle formation, and the Rab5A and Rab21 GTPases, which are involved in regulating vesicle trafficking in the early endosomal compartment (Semerdjieva et al., 2008; Simpson et al., 2004). Our previous results suggest that miR-199a-5p controls LDLR activity by directly targeting the LDLR (Fig. 2) but also by regulating its endocytosis through repression of Rab5A, Rab21 and CLTC (Fig. 2). Therefore, to functionally assess the role of miR-199a-5p in regulating LDLR activity in human hepatic cells, we overexpressed or inhibited miR-199a-5p and examined fluorescence-labeled LDL (DiI–LDL) binding (4°C for 2 h) and uptake (37°C for 5, 15 and 30 min) by flow cytometry (Fig. 3A). Transfection of Huh7 cells with miR-199a-5p mimics markedly reduced DiI–LDL-specific uptake at different time points. Importantly, the reduction in DiI–LDL internalization at 30 min was significantly greater (34%) than the DiI–LDL binding before incubating cells at 37°C (22%) (Fig. 3B). These results suggest that both DiI–LDL binding and uptake are regulated by miR-199a-5p. As expected, DiI–LDL uptake was significantly reduced in Huh7 cells transfected with miR-199a-5p at longer time points (2 and 4 h) and in cells treated with Dynasore, a widely used DNM inhibitor (Macia et al., 2006) (Fig. 3C; supplementary material Fig. S3A,C). By contrast, cells treated with the miR-199a-5p inhibitor (inh-199a-5p) had increased DiI–LDL uptake after 2 and 4 h (supplementary material Fig. S3B,C). Treatment of Huh7 cells with U18666A, a compound that blocks cholesterol trafficking from late endosomes and lysosomes to the endoplasmic reticulum (ER), resulting in enhanced processing of sterol regulatory binding protein 2 (SREBP2), and increased expression of LDLR (Liscum and Faust, 1989) was used as a positive control (supplementary material Fig. S3B, right panel; supplementary material Fig. S3D). To further confirm the effect of miR-199a-5p in regulating LDLR expression and activity, we next assessed LDLR–antibody internalization by immunofluorescence. As seen in supplementary material Fig. S4, we found a marked reduction in LDLR internalization as well as a concomitant decrease in CLTC staining in cells transfected with miR-199a-5p mimics compared to that in control mimic.
Fig. 3.
miR-199a/b-5p regulates the LDLR activity. (A) Schematic diagram showing timecourse experiment followed in B. (B) Flow cytometry analysis of DiI–LDL uptake in Huh7 cells transfected with a control mimic (CM) or miR-199a-5p mimic and incubated with 30 μg/ml DiI–LDL for the indicated times at 37°C. Data correspond to the MFI (mean±s.e.m.) of three experiments. a.u.f., arbitrary units of fluorescence. (C) DiI–LDL uptake in Huh7 cells treated or not with Dynasore for 2 h. Data are expressed as a percentage of the control (in the absence of Dynasore). Data correspond to the mean±s.e.m. of three experiments. (D) Representative confocal immunofluorescence images of Huh7 cells transfected as indicated and subjected to 10–30 min of DiI–LDL uptake at 37°C, before being fixed and stained for the early endosome antigen-1 (EEA1), Rab11 and the lysosomal marker CD63, and with TOPRO for the nuclei. Scale bars: 10 μm. (E) Histograms showing colocalization analysis of indicated markers and DiI–LDL in Huh7 cells transfected as indicated, by measuring the Mander's coefficient. Data are representative of ≥3 experiments. *P≤0.05; #P>0.05.
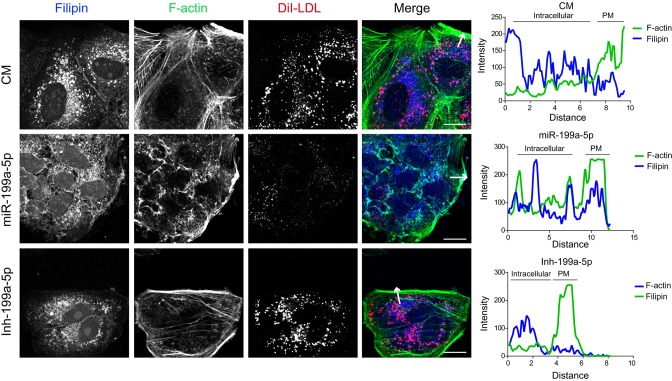
Upon internalization, LDL is delivered first to early endosomes and then to lysosomes where LDL-derived cholesteryl esters are hydrolyzed to unesterified cholesterol (Brown and Goldstein, 1986). To ascertain whether miR-199a-5p influences intracellular trafficking of LDL-derived cholesterol, we transfected Huh7 cells with a control mimic or a miR-199a-5p mimic and assessed DiI–LDL uptake and subcellular localization by immunofluorescence. Interestingly, we observed that overexpression of miR-199a-5p resulted in accumulation of DiI–LDL particles in the early endosomal compartment as seen by co-staining with the EEA1 marker at early time points (Fig. 3D,E). We also analyzed the intracellular colocalization of DiI–LDL with Rab11, a recycling endosomal marker, and with the late endosome and lysosomal compartment marker CD63 during the timecourse experiment. Although miR-199a-5p levels did not influence the colocalization of Rab11 and DiI–LDL after culturing the cells in the presence of DiI–LDL for 20 min, we found a little colocalization of DiI–LDL with CD63 in cells transfected with miR-199-5p after 30 min of incubation with DiI–LDL at 37°C (Fig. 3D,E). We also observed a marked reduction in DiI–LDL and CD63 colocalization in cells transfected with miR-199a-5p at 4 h after DiI–LDL incubation (supplementary material Fig. S3C). As expected, cells treated with U18666A accumulated DiI–LDL in the lysosome compartments, whereas Dynasore significantly reduced LDL internalization and colocalization with lysosomal CD63 protein (supplementary material Fig. S3D). Transfection of cells with Inh-199a-5p had no significant effect on the DiI–LDL and CD63 colocalization (supplementary material Fig. S3C, right panels). Given that the cholesterol uptake in the cells is mediated mainly by the internalization of LDL through LDLR, we next assessed the intracellular location of cholesterol using Filipin, a dye that stains unesterified cholesterol. Consistent with the diminished LDL uptake observed in miR-199a-5p-transfected cells, we found a striking accumulation of free cholesterol at the plasma membrane compared with that in cells treated with control mimic (Fig. 4, middle panel and intensity plot). Inhibition of endogenous miR-199a-5p expression showed a similar free cholesterol distribution to that in Huh7 cells transfected with control mimic (Fig. 4). Taken together, these results suggest that cells overexpressing miR-199a-5p have a trafficking defect that causes missorting of LDL particles after LDLR internalization.
Fig. 4.
MiR-199a-5p regulates free cholesterol intracellular localization. Representative immunofluorescence analysis of free cholesterol (Filipin), F-actin and DiI–LDL in Huh7 cells transfected with non-targeting control miRNA (CM), miR-199a-5p mimic or inh-199a-5p and incubated with 30 μg/ml DiI–LDL for 1 h at 37°C. Fluorescence intensity plots are shown in the right panels. PM, plasma membrane. White arrows indicate the region of the cell subjected to image analysis. Scale bars: 10 μm.
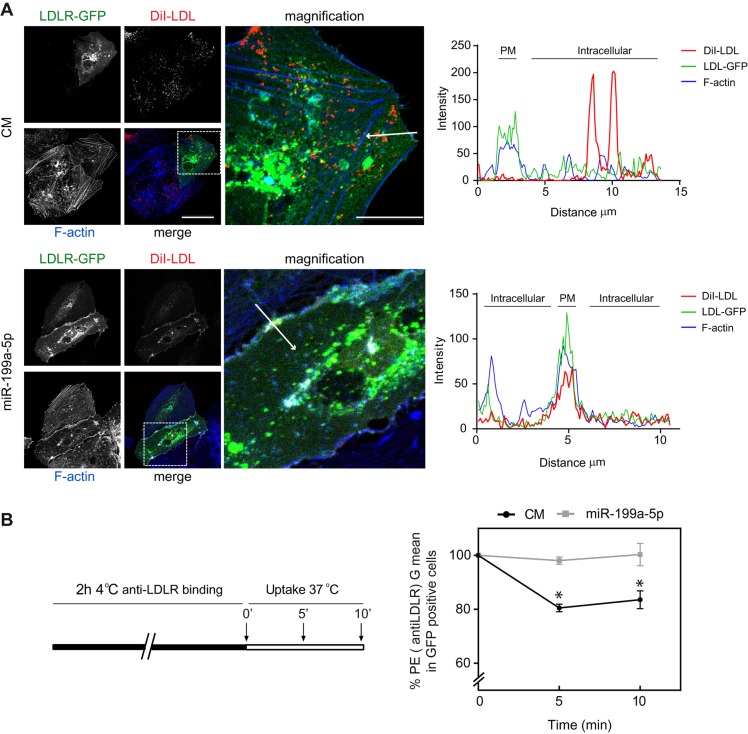
Because miR-199a-5p inhibits numerous components of the endocytic pathway as well as LDLR, the contribution of miR-199a-5p in regulating RME by assessing DiI–LDL uptake could be influenced by the significant reduction in LDLR expression observed in miR-199a-5p-overexpressing cells. To avoid this caveat, we transfected Huh7 cells with a LDLR–GFP cDNA construct that lacked the 3′UTR, thereby making it resistant to the inhibitory action of miR-199a-5p, and assessed DiI–LDL cellular localization in cells transfected with miR-199a-5p mimics. The results show that miR-199a-5p overexpression caused a marked retention of LDLR–GFP in the plasma membrane as revealed by actin co-staining, compared to cells transfected with a control mimic (Fig. 5A, intensity plots in right panels). We further analyzed LDLR internalization by flow cytometry. To this end, Huh7 cells were transfected with control mimic or miR-199a-5p and LDLR–GFP construct and incubated with an anti-LDLR antibody (labeled with phycoerythrin) for 2 h at 4°C. Then, cells were incubated at 37°C for different times to allow LDLR–antibody internalization. Importantly, we found that miR-199a-5p overexpression in LDLR–GFP-positive cells impaired surface internalization of LDLR upon engagement with antibodies and incubation at 37°C (Fig. 5B). These results suggest that the reduction of DiI–LDL uptake is mediated by two mechanisms; direct inhibition of LDLR expression and the repression of numerous components of the endocytic machinery.
Fig. 5.
MiR-199a-5p regulates internalization of LDLR. (A) Representative confocal images of DiI–LDL, LDLR–GFP and F-actin expression in Huh7 cells co-transfected with LDLR–GFP and control mimic (CM) or miR-199a-5p mimic and incubated with 30 μg/ml DiI–LDL for 1 h at 37°C. Scale bars: 10 μm (main images); 2.5 µm (magnification images). Fluorescence intensity plots for LDLR–GFP (green), F-actin (blue) and DiI–LDL (red) signals along the arrow in the image are shown in the right panels. PM, plasma membrane. (B) Schematic representation showing the timecourse experiment followed in the right panel. Huh7 cells transfected with LDLR–GFP and control mimic or miR-199a-5p was incubated with anti-LDLR antibodies at 4°C for 2 h, washed and then switched to 37°C to allow internalization for 5 and 10 min. Cells were then stained with anti-phycoerythrin secondary antibodies, washed, fixed with PFA and analyzed by FACS. The graph shows the level of LDLR internalization in gated LDLR–GFP-positive cells as the percentage of phycoerythrin fluorescence. Data are expressed as the geometrical mean (G mean) percentage compared with that for the control mimic at time 0 min (±s.e.m.) and are representative of ≥3 experiments in triplicate. *P≤0.05.
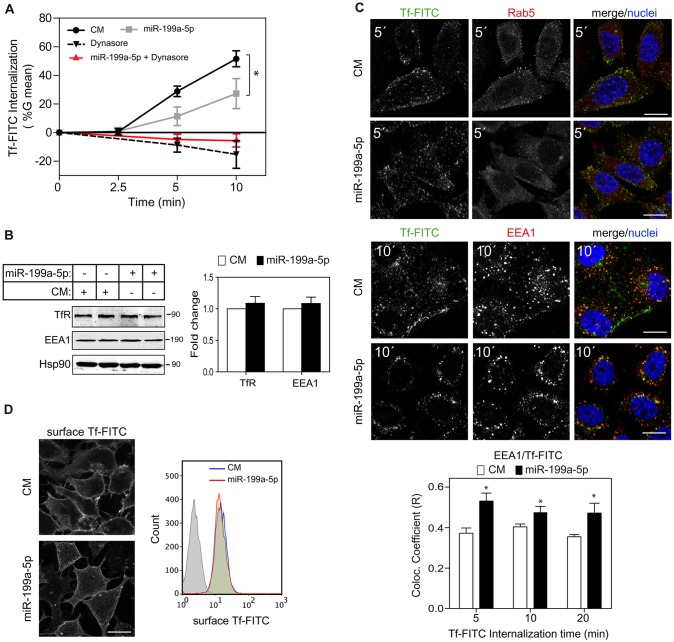
Given the direct effect of miR-199a-5p on LDLR expression and activity, we wondered whether or not other receptor-mediated processes would be affected by miR-199a-5p. Therefore we next assessed the effect of miR-199a-5p on TfR endocytosis. TfR regulates the import of the transferrin–iron complex through clathrin-mediated endocytosis (Killisch et al., 1992). To this end, we transfected human epithelial HeLa cells with miR-199a-5p and incubated them with FITC-conjugated transferrin at various time points. As shown in Fig. 6A, miR-199a-5p significantly inhibited transferrin internalization as assessed on a single-cell basis by flow cytometry. As expected, Dynasore treatment also inhibited transferrin internalization (Fig. 6A). This effect was independent of the total and surface TfR expression levels (Fig. 6B,D), suggesting that the observed internalization defect is due to other endocytic proteins rather than the absence of TfR. To rule out the possibility that miR-199a-5p might also be affecting exocytosis, we transfected HeLa cells with miR-199a-5p mimics for 48 h and then treated them with Dynasore. As seen in Fig. 6A, transferrin–FITC internalization under these conditions did not change compared to cells treated with Dynasore only, suggesting that exocytosis was not affected by miR-199a-5p. We next analyzed the intracellular localization of transferrin–FITC after miR-199a-5p transfection using confocal microscopy. As expected, miR-199a-5p-overexpressing cells showed a reduced Rab5 staining compared with cells transfected with the control mimic (Fig. 6C). Internalized transferrin–FITC in cells transfected with the control mimic showed a partial colocalization with EEA1 at 10 min after the start of the incubation (Fig. 6C). Most importantly, miR-199a-5p overexpression increased the colocalization of transferrin–FITC with EEA1, suggesting that there is a defect in the endocytic process (Fig. 6C). This effect was not due to changes in EEA1 protein levels as measured in cells transfected with miR-199a-5p mimics (Fig. 6B), suggesting that miR-199a-5p overexpression in cells triggers malfunctioning of the endosome compartment. Taken together, these results suggest that miR-199a-5p influences endocytic compartment functioning.
Fig. 6.
MiR-199a-5p regulates transferrin uptake. (A) Flow cytometry analysis of the transferrin–FITC (Tf-FITC) internalization assay, as described in the Materials and Methods, in HeLa cells transfected with negative control miRNA (CM) or miR-199a-5p mimic and treated as indicated. Data are expressed as the geometrical mean (G mean) of the difference of fluorescence of each time point minus the geometrical mean fluorescence at time 0 min (±s.e.m.) and are representative of ≥3 experiments. (B) Western blot analysis of TfR and EEA1 in HeLa cells transfected with control mimic or miR-199a-5p mimic. Hsp90 was used as a loading control. Quantification of protein fold change is shown in the histogram. (C) Representative confocal images of transferrin–FITC, Rab5 and EEA1 expression in HeLa cells transfected with control mimic or miR-199a-5p mimic, incubated for different times with transferrin–FITC. Histograms at the bottom show a colocalization analysis between EEA1 and transferrin–FITC. Data are representative of ≥3 experiments. *P≤0.05. (D) Left panel, representative confocal images of membrane transferrin–FITC localization in HeLa cells treated as C. Right panel, flow cytometry analysis of surface transferrin–FITC binding in HeLa cells transfected with control mimic and miR-199a-5p.
DISCUSSION
Although a large number of studies have shown that several miRNAs participate in cancer and disease development (Cuk et al., 2013; Pignot et al., 2013; Srivastava et al., 2013), little is known about the role of miRNAs in the central cell biology process of intracellular trafficking on which other cellular functions depend on for proper operation. The most salient finding of this study is the identification of miR-199a-5p as an important regulator of endocytosis. In particular, our study expands the current understanding of how miRNAs, specifically miR-199a/b-5p, contribute to intracellular trafficking control. Remarkably, the miR-199a/b family is encoded within the DNM genes, which are crucial components of the endocytic machinery, suggesting that DNM genes and miR-199a/b form a genomic locus that controls intracellular transport pathways (Fig. 7). Similar to other intronic miRNAs, such as miR-33a, miR-33b and miR-208 (Callis et al., 2009; Rayner et al., 2010), we found that miR-199a/b-5p regulates related physiological processes to those controlled by the host genes in which they are encoded. This finding prompted us to identify and further characterize target genes associated with cellular trafficking. Interestingly, we found that a significant number of predicted target genes for miR-199a/b-5p were associated with cellular transport.
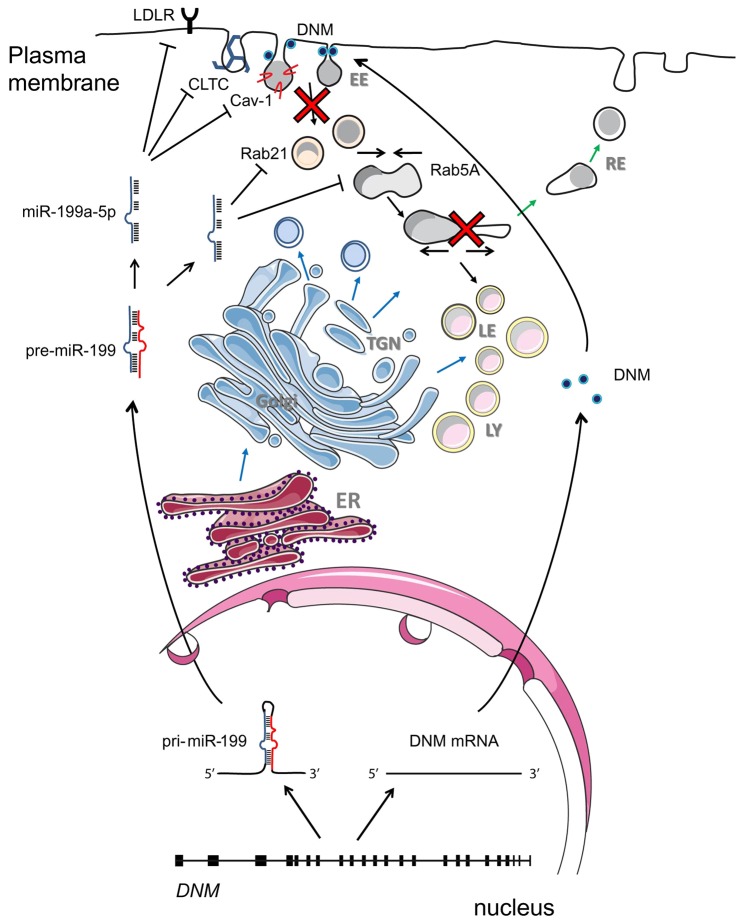
Fig. 7.
Proposed model of regulation of RME by DNM and miR-199a/b. Sense strands of the DNM genes are transcribed and translated to synthetize DNM proteins that are involved in endosome trafficking. miR-199a-5p is transcribed in the nucleus from the antisense strand of introns in the DNM2 and DNM3 gene and regulates receptor-mediated endocytosis and intracellular cholesterol levels by balancing the post-transcriptional levels of genes involved in endocytosis such as LDLR, CLTC, Cav-1, Rab5A and Rab21.
Our results also demonstrate that miR-199a-5p plays an opposite role to DNM in controlling endocytosis. DNM1 is selectively expressed at very high levels in neurons, where it is crucial for synapses to efficiently recycle synaptic vesicles during intense activity (Baurfend et al., 1995). DNM2 is ubiquitously expressed and DNM3 is found most prominently in the brain and in the testis (Cao et al., 1998). For instance, although DNM2 is required for receptor-mediated endocytosis, miR-199a-5p inhibits the expression of numerous genes associated with this process including CLTC and Rab GTPases. Our expression profile analysis is in agreement with the results obtained in other studies, which indicate that miR-199a/b-5p strands are moderately expressed in several tissues (Gu and Chan, 2012; Sakurai et al., 2011). As expected by their opposing roles, the expression of miR-199 members and their respective DNM host genes were inversely correlated in most human tissues. This is particularly remarkable in the case of the brain, where the expression of DNM1 is markedly high (Cao et al., 1998) compared with its intronic miRNA, miR-199b-5p, which is expressed at low levels (supplementary material Fig. S2A). Opposing expression levels are also observed in the heart, where the expression of miR-199a-5p and miR-199b-5p (da Costa Martins et al., 2010) is high, in contrast with the moderate expression of DNM2 and almost null expression of DNM1 and DNM3 (Fig. 1C). This observation suggests that organs that require active trafficking, such as the brain (Faire et al., 1992), express substantial amounts of DNM and very low levels of miR-199a/b.
So far, only a very few studies have shown the participation of miRNAs in receptor-mediated internalization or trafficking (Lin et al., 2014; Serva et al., 2012; Yang et al., 2013). In the case of miR-199a/b family, both strands of miR-199a-1 and miR-199a-2, namely miR-199a-5p and miR-199a-3p, are expressed in human tissues (Shatseva et al., 2011; Shen et al., 2010), and their expression originates from the DNM2 and DNM3 introns. Interestingly, miR-199a-3p, which is highly expressed in some tumor cells, has been reported to target caveolin-2 (Shatseva et al., 2011), a key structural protein regulating endocytosis. In addition to miR-199a, the same DNM3 intron also contains miR-214, which is expressed as a cluster together with miR-199a and subsequently processed to render mature forms. Of note, miR-214 is known to target phosphatase and tensin homolog (PTEN), which interacts with DNM and regulates receptor recycling (Yang et al., 2008). More interesting, the mirror miRNA miR-3120, which is fully the complement of miR-214 in the DNM3 intron, is co-expressed with its host gene mRNA and regulates uncoating of clathrin-coated vesicles by targeting Hsc70 and auxilin (Scott et al., 2012). These studies are in agreement with our hypothesis that DNM intronic miRNAs regulate important aspects of cellular functions that are similar to those regulated by its host gene.
Aside from using bioinformatic prediction tools, we performed several experiments to dissect the role of miR-199a-5p in regulating endocytosis, including the identification of putative regulators by mRNA expression profiling and analysis of protein level changes upon expression and/or inhibition of miR-199a-5p levels together with a fluorescence confocal microscopy-based assay and rescue of function analysis. In this study, we have characterized a prominent role of miR-199a-5p in regulating endocytosis that mechanistically is facilitated by the direct downregulation of multiple genes involved in the endocytic pathway (Fig. 7). As a result, overexpression of miR-199a-5p inhibited clathrin-mediated endocytosis as shown by our in vitro LDL and transferrin internalization assays. This observation suggests that the ability of miR-199a-5p to comprehensively control the internalization at different levels (LDLR and CLTC) of essential nutrients, such as cholesterol, present in LDL, and iron. Interestingly, antagonism of miR-199a-5p enhances LDLR, Rab21 and Rab5A protein expression levels significantly. This observation suggests that miR-199a-5p plays a role in regulating constitutive levels of these proteins, increasing LDL uptake and binding in Huh7 cells. In accordance with this, we observed an increase in the intracellular pool of LDL when inhibiting endogenous expression of miR-199a-5p, suggesting a physiological role in vivo for miR-199a-5p in controlling internalization pathways in the cell. These results also suggest that antagonism of endogenous miR-199a/b-5p might have a potential therapeutic effect for increasing levels of LDLR expression. It is also interesting to note that the DNM proteins encoded by the miR-199a/b-5p host genes associate with CTLC and mediate the formation of vesicles after endocytosis (Takei et al., 1999). Disruption of DNM function results in reduced internalization of receptors (Girard et al., 2011; Gray et al., 2003; Shajahan et al., 2004), such as LDLR. We had hypothesized that miR-199a-5p has a major role in endocytosis, and indeed, our rescue of function experiment with ectopic LDLR–GFP did not completely restore intracellular levels of DiI–LDL, as expected; these results are consistent with the finding that many other endocytic components are affected when miR-199a-5p levels are elevated. In this regard, a recent study has reported that miR-199a-5p targets the well-known endocytosis regulator Cav-1 (Lino Cardenas et al., 2013). We also confirmed that miR-199a-5p overexpression inhibits Cav-1 expression, but further studies will assess the contribution of this miRNA to Cav-1 functions.
One important question to address further is whether or not changes in miR-199a/b-5p endogenous levels influence endocytosis. The documented observation that in most of cancers miR-199a-5p expression is downregulated supports the idea that cancer cells can exploit this fact to ensure the intracellular trafficking necessary for growth, therefore enabling cancer progression (Ramsay et al., 2007; Xu et al., 2012). Intriguingly, miR-199a-5p overexpression inhibits tumor cell migration without affecting cellular proliferation and viability (Cheung et al., 2011; Duan et al., 2011). To do that, we speculate that miR-199a-5p might regulate key endocytic intermediates as described here to fine-tune intracellular trafficking routes that are implicated in cancer progression. As discussed previously, miRNAs are known to regulate many aspects of cancer cell biology (Iorio and Croce, 2012) and individual miRNAs could be differentially expressed under different stimuli. Because of their different genomic location, future experiments will further characterize the specific roles of miR-199a1-5p, miR-199a2-5p and miR-199b-5p in regulating endocytosis in this pathological context. In light of our findings, we can assume that miR-199a/b-5p function in the opposite way to DNM host genes, but additional work is needed to clarify whether or not the miR-199 and DNM locus coordinates a unique biological functional response. In summary, our study uncovers an elegant mechanism by which the miR-199 and DNM genomic locus coordinately regulates cellular endocytosis.
MATERIALS AND METHODS
Materials
Chemicals were obtained from Sigma-Aldrich unless otherwise noted. 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was purchased from Molecular Probes (Invitrogen). A rabbit polyclonal antibody against LDLR was obtained from Cayman Chemical and mouse monoclonal antibodies against HSP90, Rab5A, Rab21 and EEA1 were purchased from BD Biosciences. The mouse monoclonal antibody against LDLR and the goat antibody for TfR were obtained from Santa Cruz Biotechnology. The rabbit polyclonal antibodies to CLTC and Rab11 were purchased from Cell Signaling Technology. Transferrin–FITC and secondary fluorescently labeled antibodies were from Molecular Probes (Invitrogen). miRNA mimics and inhibitors were obtained from Dharmacon. The LDLR–GFP plasmid was kindly provided by Peter Tontonoz (UCLA, Los Angeles, CA).
Cell culture
Human hepatic (Huh7), cervix carcinoma (HeLa) and monkey kidney fibroblast (COS7) cells were obtained from the American Type Tissue Collection. Huh7, HeLa and COS7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 2% penicillin-streptomycin in 10 cm2 dishes at 37°C and 5% CO2. For DiI–LDL uptake and binding experiments, Huh7 cells were incubated in DMEM containing 10% lipoprotein-deficient serum (LPDS) supplemented with 30 μg/ml DiI–LDL cholesterol.
Bioinformatic analysis of miRNA target genes
Target genes for hsa-miR-199a/b were identified and compared using the online target prediction algorithm, miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/), which provides target interaction information from eight different prediction algorithms. Specifically, the programs miRanda, miRWalk and TargetScan were used. The putative targets produced by all three of these algorithms for miR-199a were uploaded into the gene classification system, PANTHER v8.0 (http://www.pantherdb.org) to identify gene targets that were mapped to the transport process (GO 0006810). The functional interactions of these predicted targets for miR-199a/b-5p described in STRING v9.05 (http://string-db.org) were then combined with the functional annotation groups described in DAVID. MATLAB and Cytoscape v2.8.3 were used to create the visualization networks, as previously described (Huang et al., 2008). STRING interactions with a confidence score of 0.4 or higher were added and highlighted in bold. Smaller annotation clusters and unconnected genes were left out of the visualization due to space constraints.
miRNA mimic and inhibitor transfections
For mimic and inhibitor transfections, Huh7 and HeLa cells were transfected with 40 nM miRIDIAN miRNA mimics (miR-199a-5p) or with 60 nM miRIDIAN miRNA inhibitors (Inh-199a-5p) (Dharmacon) using RNAimax (Invitrogen), or Lipofectamine 2000 (Invitrogen) for co-transfection experiments with the LDLR–GFP plasmid. All experimental control samples were treated with an equal concentration of a non-targeting control mimic sequence or inhibitor negative control sequence for use as controls for non-sequence-specific effects in miRNA experiments. Verification of miR-199a-5p overexpression and inhibition was determined using quantitative real-time PCR (qRT-PCR), as described below.
RNA isolation and qRT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. For mRNA quantification, cDNA was synthesized using iScript RT Supermix (Bio-Rad), following the manufacturer's protocol. qRT-PCR analysis was performed in triplicate using iQ SYBR green Supermix (BioRad) on an iCycler Real-Time Detection System (Eppendorf). The mRNA level was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a housekeeping gene. The human primer sequences used were: GAPDH, 5′-TTGATTTTGGAGGGATCTCG-3′ and 5′-CAATGACCCCTTCATTGACC-3′; LDLR, 5′-TGATGGGTTCATCTGACCAGT-3′ and 5′-AGTTGGCTGCGTTAATGTGAC-3′; CLTC, 5′-TGAGGCGACTGGGCGGAGTT-3′ and 5′-CCGGGGACGCAGGAAACTGG-3′; Cav-1, 5′-AGTGCATCAGCCGTGTCTATTCCA-3′ and 5′-TCTGCAAGTTGATGCGGACATTGC-3′; Rab5A, 5′-GGGGCTGCTTTTCTAACCCA-3′ and 5′-TTTGCTAGGTCGGCCTTGTT-3′; Rab21, 5′-CCTCCGGTGCCTGACGTGGT-3′ and 5′-CAGCCTTCCCCCAGCAGCAC-3′; DNM1, 5′-CACCGTTAGACAGTGCACCA-3′ and 5′-CCCTTGCGGATGACCAGAAT-3′; DNM2, 5′-CACAGCCCCACTCCACAGCG-3′ and 5′-CCTGGGGGAATCCCTGGGGG-3′; DNM3, 5′-CCCCCACTCTGGGGCTCCTC-3′ and 5′-GATGGGGGTGGTCTCCGGCT-3′; and TfR, 5′-GAACTACACCGACCCTCGTG-3′ and 5′-TGCCACACAGAAGAACCTGC-3′. For miRNA quantification, total RNA was reverse transcribed using the miScript II RT Kit (Qiagen). Primers specific for human pre-miR-199a1, pre-miR-199a2, pre-miR-199b, miR-199a-5p and miR-199a-3p (Qiagen) were used and values were normalized to SNORD68 (Qiagen) as a housekeeping gene.
Western blot analysis
Cells were lysed in ice-cold buffer containing 50 mM Tris-HCl pH 7.5, 125 mM NaCl, 1% NP-40, 5.3 mM NaF, 1.5 mM NaP, 1 mM orthovanadate and 1 mg/ml of protease inhibitor cocktail (Roche) and 0.25 mg/ml AEBSF (Roche). Cell lysates were rotated at 4°C for 1 h before the insoluble material was removed by centrifugation at 12,000 g for 10 min. After normalizing for equal protein concentration, cell lysates were resuspended in SDS sample buffer before separation by SDS-PAGE. Following overnight transfer of the proteins onto nitrocellulose membranes, the membranes were probed with antibodies against LDLR (1:500), Rab5 (1:1000), CLTC (1:1000), Rab21 (1:500), EEA-1 (1:1000), TfR (1:1000) or HSP90 (1:1000). Protein bands were visualized using the Odyssey Infrared Imaging System (LI-COR Biotechnology). Densitometry analysis of the gels was carried out using ImageJ software.
LDL receptor and TfR activity assays
Human LDL was isolated and labeled with the fluorescent probe DiI as previously reported (Calvo et al., 1998). Huh7 cells were transfected in 6- or 12-well plates with miRNA mimics and inhibitors in DMEM containing 10% LPDS for 48 h. Then, cells were washed once in 1× PBS and incubated in fresh medium containing DiI–LDL (30 µg cholesterol/ml). Non-specific uptake was determined in extra wells containing a 50-fold excess of unlabeled native LDL (nLDL). Cells were incubated for 5 min to 4 h at 37°C to allow for DiI–LDL uptake. In other instances, cells were incubated for 120 min at 4°C to assess anti-LDLR antibody binding. At the end of the incubation period, cells were washed, resuspended in 1 ml of PBS and analyzed by flow cytometry (FACScalibur, Becton Dickinson), as previously described (Suarez et al., 2004). The results are expressed in terms of the percentage of specific DiI–LDL uptake after subtracting autofluorescence of cells incubated in the absence of DiI–LDL, which was calculated from median fluorescence intensity (MFI) relative to 2 h time point. For timecourse experiments, DiI–LDL uptake is represented as MFI in arbitrary units.
For analysis of transferrin internalization cells were incubated for 45 min at 37°C in serum-free DMEM. Cells were first incubated on ice for 20 min followed by addition of 50 µg/ml transferrin–FITC (Sigma) in serum-free medium. After 30 min on ice cells were washed with iced cold PBS and transferred to a 37°C and 5% CO2 incubator in the presence of unlabeled transferrin for the indicated times. Cells were acid washed to remove surface-bound transferrin and analyzed by FACS or fixed in 4% paraformaldehyde (PFA) for 15 min for fluorescence microscopy analysis.
Fluorescence microscopy
For LDLR–antibody internalization and DiI–LDL uptake assays, Huh7 cells were grown on coverslips and transfected with a miR-199a-5p mimic and a negative control mimic in DMEM containing 10% LPDS. At 48 h post transfection, cells were cooled to 4°C for 20 min to stop membrane internalization. Cells were then incubated with anti-LDLR monoclonal antibody (C7, Santa Cruz Biotechnology) and 30 µg/ml DiI–LDL for 40 min at 4°C. Following incubation, cells were gently washed twice with cold medium and shifted to 37°C to allow for internalization of both LDLR–antibody complexes and DiI–LDL for the indicated times, and were then acid stripped and fixed with 4% PFA. After 5 min of 0.2% Triton X-100 permeabilization and 15 min of blocking (PBS with 3% BSA), cells were stained with anti-mouse-IgG conjugated to Alexa Fluor 488 (Molecular Probes) and TO-PRO 3 (Life Technologies) for 1 h at room temperature. After this, cells were washed twice with 1× PBS and mounted on glass slides with Prolong-Gold (Life Technologies).
For LDLR–GFP rescue experiments, Huh7 cells were grown on coverslips and co-transfected with 1 µg LDLR–GFP plasmid and 40 nM of a control mimic or miR-199a-5p mimic. At 48 h post transfection cells were incubated with 30 µg/ml DiI–LDL for 1 h at 37°C (uptake). Then, cells were washed twice with 1× PBS, fixed with 4% PFA and blocked (3% BSA in 1× PBS) for 15 min. Following this, cells were washed twice, stained with phalloidin to visualize F-actin and mounted on glass slides with Prolong-Gold (Life Technologies). All images were analyzed using a confocal microscope (Leica SP5 II) equipped with a 63× Plan Apo Lenses. All gains for the acquisition of comparable images were maintained at a constant level. Analysis of different images was performed using ImageJ (NIH) and Adobe Photoshop CS5.
3′UTR luciferase reporter assays
cDNA fragments corresponding to the entire 3′UTR of human LDLR, Cav-1, Rab5A, Rab21 and CLTC were amplified by RT-PCR from total RNA extracted from Huh7 cells with XhoI and NotI linkers. The PCR product was directionally cloned downstream of the Renilla luciferase open reading frame in the psiCHECK2™ vector (Promega), which also contains a constitutively expressed firefly luciferase gene, which is used to normalize transfections. Point mutations in the seed region of the predicted miR-199a binding sites within all the above 3′UTR were generated using the Multisite Quikchange kit (Stratagene), according to the manufacturer's protocol. All constructs were confirmed by sequencing. COS7 cells were plated into 12-well plates (Costar) and co-transfected with 1 μg of the indicated 3′UTR luciferase reporter vectors and miR-199a-5p mimics or control mimics (Dharmacon) using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured using the Dual-Glo luciferase assay system (Promega). Renilla luciferase activity was normalized to the corresponding firefly luciferase activity and plotted as a percentage of the control (cells co-transfected with the corresponding concentration of control mimic). Experiments were performed in triplicate wells of a 12-well plate and repeated at least three times.
Statistics
All data are expressed as mean±s.e.m. Statistical differences were measured using an unpaired Student's t-test. A value of P≤0.05 was considered statistically significant. Data analysis was performed using GraphPad Prism Software Version 6.03 (GraphPad, San Diego, CA). *P≤0.05.
Supplementary Material
Acknowledgements
We thank Dr Peter Tontonoz for generously providing the LDLR–GFP plasmid.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.F.A. and C.F.-H. conceived and designed the study. J.F.A., A.C.-D. and L.G. performed the experiments. Y.S. and C.F.H. assisted with experimental design and data interpretation. J.F.A. and C.F.-H. wrote the manuscript, which was commented on by all authors.
Funding
This work was supported by grants from the National Institutes of Health [grant numbers R01HL107953, R01HL106063 to C.F.-H., and 1F31AG043318 to L.G.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.165233/-/DC1
References
- Ambros V. (2004). The functions of animal microRNAs. Nature 431, 350-355. 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- Bartel D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215-233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurfend R., David C., Galli T., McPherson P. S., Takei K. and De Camilli P. (1995). Molecular mechanisms in synaptic vesicle endocytosis. Cold Spring Harb. Symp. Quant. Biol. 60, 397-404. 10.1101/SQB.1995.060.01.044 [DOI] [PubMed] [Google Scholar]
- Brown M. S. and Goldstein J. L. (1986). A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34-47. 10.1126/science.3513311 [DOI] [PubMed] [Google Scholar]
- Bucci C., Wandinger-Ness A., Lutcke A., Chiariello M., Bruni C. B. and Zerial M. (1994). Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 91, 5061-5065. 10.1073/pnas.91.11.5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N. and Cohen S. M. (2007). microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175-205. 10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- Callis T. E., Pandya K., Seok H. Y., Tang R.-H., Tatsuguchi M., Huang Z.-P., Chen J.-F., Deng Z., Gunn B., Shumate J. et al. (2009). MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Invest. 119, 2772-2786. 10.1172/JCI36154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo D., Gomez-Coronado D., Suarez Y., Lasuncion M. A. and Vega M. A. (1998). Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 39, 777-788. [PubMed] [Google Scholar]
- Cao H., Garcia F. and McNiven M. A. (1998). Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9, 2595-2609. 10.1091/mbc.9.9.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Thompson H. M., Krueger E. W. and McNiven M. A. (2000). Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J. Cell Sci. 113, 1993-2002. [DOI] [PubMed] [Google Scholar]
- Chamorro-Jorganes A., Araldi E., Rotllan N., Cirera-Salinas D. and Suarez Y. (2014). Autoregulation of glypican-1 by intronic microRNA-149 fine tunes the angiogenic response to FGF2 in human endothelial cells. J. Cell Sci. 127, 1169-1178. 10.1242/jcs.130518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada T. P., Finn K. J., Ji X., Baillat D., Gregory R. I., Liebhaber S. A., Pasquinelli A. E. and Shiekhattar R. (2007). MicroRNA silencing through RISC recruitment of eIF6. Nature 447, 823-828. 10.1038/nature05841 [DOI] [PubMed] [Google Scholar]
- Cheung H.-H., Davis A. J., Lee T.-L., Pang A. L., Nagrani S., Rennert O. M. and Chan W.-Y. (2011). Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene 30, 3404-3415. 10.1038/onc.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuk K., Zucknick M., Madhavan D., Schott S., Golatta M., Heil J., Marme F., Turchinovich A., Sinn P., Sohn C. et al. (2013). Plasma microRNA panel for minimally invasive detection of breast cancer. PLoS ONE 8, e76729 10.1371/journal.pone.0076729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins P. A., Salic K., Gladka M. M., Armand A.-S., Leptidis S., el Azzouzi H., Hansen A., Coenen-de Roo C. J., Bierhuizen M. F., van der Nagel R. et al. (2010). MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 12, 1220-1227. 10.1038/ncb2126 [DOI] [PubMed] [Google Scholar]
- de Hoop M. J., Huber L. A., Stenmark H., Williamson E., Zerial M., Parton R. G. and Dotti C. G. (1994). The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron 13, 11-22. 10.1016/0896-6273(94)90456-1 [DOI] [PubMed] [Google Scholar]
- Dorsey F. C., Muthusamy T., Whitt M. A. and Cox J. V. (2007). A novel role for a YXXPhi motif in directing the caveolin-dependent sorting of membrane-spanning proteins. J. Cell Sci. 120, 2544-2554. 10.1242/jcs.002493 [DOI] [PubMed] [Google Scholar]
- Doyon J. B., Zeitler B., Cheng J., Cheng A. T., Cherone J. M., Santiago Y., Lee A. H., Vo T. D., Doyon Y., Miller J. C. et al. (2011). Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat. Cell Biol. 13, 331-337. 10.1038/ncb2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Choy E., Harmon D., Liu X., Susa M., Mankin H. and Hornicek F. (2011). MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol. Cancer Ther. 10, 1337-1345. 10.1158/1535-7163.MCT-11-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faire K., Trent F., Tepper J. M. and Bonder E. M. (1992). Analysis of dynamin isoforms in mammalian brain: dynamin-1 expression is spatially and temporally regulated during postnatal development. Proc. Natl. Acad. Sci. USA 89, 8376-8380. 10.1073/pnas.89.17.8376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M. and De Camilli P. (2012). Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75-88. 10.1038/nrm3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rojo M. A., Restall C., Ferguson C., Martel N., Martin S., Bosch M., Kassan A., Leong G. M., Martin S. D., McGee S. L. et al. (2012). Caveolin-1 orchestrates the balance between glucose and lipid-dependent energy metabolism: implications for liver regeneration. Hepatology 55, 1574-1584. 10.1002/hep.24810 [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Bhattacharyya S. N. and Sonenberg N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102-114. 10.1038/nrg2290 [DOI] [PubMed] [Google Scholar]
- Gan Y., McGraw T. E. and Rodriguez-Boulan E. (2002). The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 4, 605-609. 10.1038/ncb827 [DOI] [PubMed] [Google Scholar]
- Girard E., Paul J. L., Fournier N., Beaune P., Johannes L., Lamaze C. and Védie B. (2011). The dynamin chemical inhibitor dynasore impairs cholesterol trafficking and sterol-sensitive genes transcription in human HeLa cells and macrophages. PLoS ONE 6, e29042 10.1371/journal.pone.0029042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedeke L., Vales-Lara F. M., Fenstermaker M., Cirera-Salinas D., Chamorro-Jorganes A., Ramirez C. M., Mattison J. A., de Cabo R., Suarez Y. and Fernandez-Hernando C. (2013). A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol. Cell. Biol. 33, 2339-2352. 10.1128/MCB.01714-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. W., Fourgeaud L., Huang B., Chen J., Cao H., Oswald B. J., Hémar A. and McNiven M. A. (2003). Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr. Biol. 13, 510-515. 10.1016/S0960-9822(03)00136-2 [DOI] [PubMed] [Google Scholar]
- Gu S. and Chan W.-Y. (2012). Flexible and versatile as a chameleon-sophisticated functions of microRNA-199a. Int. J. Mol. Sci. 13, 8449-8466. 10.3390/ijms13078449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Yaddanapudi S., Weins A., Osborn T., Reiser J., Pollak M., Hartwig J. and Sever S. (2010). Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 29, 3593-3606. 10.1038/emboj.2010.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Noss E. H., Hsu V. W. and Brenner M. B. (2011). Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 193, 61-70. 10.1083/jcb.201007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J. and Stahl P. (1983). Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329-339. 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T. and Lempicki R. A. (2008). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44-57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Iorio M. V. and Croce C. M. (2012). microRNA involvement in human cancer. Carcinogenesis 33, 1126-1133. 10.1093/carcin/bgs140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M., Howell K. E., Henley J. R., Cao H. and McNiven M. A. (1998). Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science 279, 573-577. 10.1126/science.279.5350.573 [DOI] [PubMed] [Google Scholar]
- Kang R. S. and Folsch H. (2011). ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J. Cell Biol. 193, 51-60. 10.1083/jcb.201012121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killisch I., Steinlein P., Romisch K., Hollinshead R., Beug H. and Griffiths G. (1992). Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J. Cell Sci. 103, 211-232. [DOI] [PubMed] [Google Scholar]
- Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S. et al. (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415-419. 10.1038/nature01957 [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Skoura A., Park E. J., Landskroner-Eiger S., Jozsef L., Luciano A. K., Murata T., Pasula S., Dong Y., Bouaouina M. et al. (2014). Dynamin 2 regulation of integrin endocytosis, but not VEGF signaling, is crucial for developmental angiogenesis. Development 141, 1465-1472. 10.1242/dev.104539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.-H., Yue P., Zhang C. and Wang W.-H. (2014). MicroRNA-194 (miR-194) regulates ROMK channel activity by targeting intersectin 1. Am. J. Physiol. Renal Physiol. 306, F53-F60. 10.1152/ajprenal.00349.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino Cardenas C. L., Henaoui I. S., Courcot E., Roderburg C., Cauffiez C., Aubert S., Copin M.-C., Wallaert B., Glowacki F., Dewaeles E. et al. (2013). miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet. 9, e1003291 10.1371/journal.pgen.1003291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L. and Faust J. R. (1989). The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J. Biol. Chem. 264, 11796-11806. [PubMed] [Google Scholar]
- Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C. and Kirchhausen T. (2006). Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839-850. 10.1016/j.devcel.2006.04.002 [DOI] [PubMed] [Google Scholar]
- McMahon H. T. and Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517-533. 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- Mettlen M., Loerke D., Yarar D., Danuser G. and Schmid S. L. (2010). Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J. Cell Biol. 188, 919-933. 10.1083/jcb.200908078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Mahaffey D. T., Brodsky F. M. and Anderson R. G. (1987). Assembly of clathrin-coated pits onto purified plasma membranes. Science 236, 558-563. 10.1126/science.2883727 [DOI] [PubMed] [Google Scholar]
- Mousley C. J., Yuan P., Gaur N. A., Trettin K. D., Nile A. H., Deminoff S. J., Dewar B. J., Wolpert M., Macdonald J. M., Herman P. K. et al. (2012). A sterol-binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell 148, 702-715. 10.1016/j.cell.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J. M., Hyman A. A. and Zerial M. (1999). Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1, 376-382. 10.1038/14075 [DOI] [PubMed] [Google Scholar]
- Parachoniak C. A., Luo Y., Abella J. V., Keen J. H. and Park M. (2011). GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev. Cell 20, 751-763. 10.1016/j.devcel.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J. K., Kallioniemi O. et al. (2008). Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371-385. 10.1016/j.devcel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Pignot G., Cizeron-Clairac G., Vacher S., Susini A., Tozlu S., Vieillefond A., Zerbib M., Lidereau R., Debre B., Amsellem-Ouazana D. et al. (2013). microRNA expression profile in a large series of bladder tumors: identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int. J. Cancer 132, 2479-2491. 10.1002/ijc.27949 [DOI] [PubMed] [Google Scholar]
- Ramsay A. G., Keppler M. D., Jazayeri M., Thomas G. J., Parsons M., Violette S., Weinreb P., Hart I. R. and Marshall J. F. (2007). HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 67, 5275-5284. 10.1158/0008-5472.CAN-07-0318 [DOI] [PubMed] [Google Scholar]
- Rayner K. J., Suarez Y., Davalos A., Parathath S., Fitzgerald M. L., Tamehiro N., Fisher E. A., Moore K. J. and Fernandez-Hernando C. (2010). MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570-1573. 10.1126/science.1189862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Griffiths-Jones S., Ashurst J. L. and Bradley A. (2004). Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902-1910. 10.1101/gr.2722704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A., Uyhazi K., Frost A. and De Camilli P. (2006). GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528-531. 10.1038/nature04718 [DOI] [PubMed] [Google Scholar]
- Saini H. K., Griffiths-Jones S. and Enright A. J. (2007). Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA 104, 17719-17724. 10.1073/pnas.0703890104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K., Furukawa C., Haraguchi T., Inada K.-I., Shiogama K., Tagawa T., Fujita S., Ueno Y., Ogata A., Ito M. et al. (2011). MicroRNAs miR-199a-5p and -3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 71, 1680-1689. 10.1158/0008-5472.CAN-10-2345 [DOI] [PubMed] [Google Scholar]
- Scott H., Howarth J., Lee Y. B., Wong L.-F., Bantounas I., Phylactou L., Verkade P. and Uney J. B. (2012). MiR-3120 is a mirror microRNA that targets heat shock cognate protein 70 and auxilin messenger RNAs and regulates clathrin vesicle uncoating. J. Biol. Chem. 287, 14726-14733. 10.1074/jbc.M111.326041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerdjieva S., Shortt B., Maxwell E., Singh S., Fonarev P., Hansen J., Schiavo G., Grant B. D. and Smythe E. (2008). Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J. Cell Biol. 183, 499-511. 10.1083/jcb.200806016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serva A., Knapp B., Tsai Y.-T., Claas C., Lisauskas T., Matula P., Harder N., Kaderali L., Rohr K., Erfle H. et al. (2012). miR-17–5p regulates endocytic trafficking through targeting TBC1D2/Armus. PLoS ONE 7, e52555 10.1371/journal.pone.0052555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A. N., Timblin B. K., Sandoval R., Tiruppathi C., Malik A. B. and Minshall R. D. (2004). Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279, 20392-20400. 10.1074/jbc.M308710200 [DOI] [PubMed] [Google Scholar]
- Shatseva T., Lee D. Y., Deng Z. and Yang B. B. (2011). MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell Sci. 124, 2826-2836. 10.1242/jcs.077529 [DOI] [PubMed] [Google Scholar]
- Shen Q., Cicinnati V. R., Zhang X., Iacob S., Weber F., Sotiropoulos G. C., Radtke A., Lu M., Paul A., Gerken G. et al. (2010). Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol. Cancer 9, 227 10.1186/1476-4598-9-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P. F., Busch C. J. L., Kane C. et al. (2009). A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 11, 705-716. 10.1038/ncb1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. C., Griffiths G., Wessling-Resnick M., Fransen J. A. M., Bennett H. and Jones A. T. (2004). A role for the small GTPase Rab21 in the early endocytic pathway. J. Cell Sci. 117, 6297-6311. 10.1242/jcs.01560 [DOI] [PubMed] [Google Scholar]
- Singh R. D., Puri V., Valiyaveettil J. T., Marks D. L., Bittman R. and Pagano R. E. (2003). Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254-3265. 10.1091/mbc.E02-12-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Goldberger H., Dimtchev A., Ramalinga M., Chijioke J., Marian C., Oermann E. K., Uhm S., Kim J. S., Chen L. N. et al. (2013). MicroRNA profiling in prostate cancer--the diagnostic potential of urinary miR-205 and miR-214. PLoS ONE 8, e76994 10.1371/journal.pone.0076994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Y., Fernandez C., Gomez-Coronado D., Ferruelo A. J., Davalos A., Martinez-Botas J. and Lasuncion M. A. (2004). Synergistic upregulation of low-density lipoprotein receptor activity by tamoxifen and lovastatin. Cardiovasc. Res. 64, 346-355. 10.1016/j.cardiores.2004.06.024 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P. et al. (2011). The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561-D568. 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., McPherson P. S., Schmid S. L. and De Camilli P. (1995). Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature 374, 186-190. 10.1038/374186a0 [DOI] [PubMed] [Google Scholar]
- Takei K., Slepnev V. I., Haucke V. and De Camilli P. (1999). Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1, 33-39. 10.1038/9004 [DOI] [PubMed] [Google Scholar]
- Thomas P. D., Campbell M. J., Kejariwal A., Mi H., Karlak B., Daverman R., Diemer K., Muruganujan A. and Narechania A. (2003). PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 13, 2129-2141. 10.1101/gr.772403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni D., Puri C., Confalonieri S., Salcini A. E., De Camilli P., Tacchetti C. and Di Fiore P. P. (2005). TTP specifically regulates the internalization of the transferrin receptor. Cell 123, 875-888. 10.1016/j.cell.2005.10.021 [DOI] [PubMed] [Google Scholar]
- Urrutia R., Henley J. R., Cook T. and McNiven M. A. (1997). The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc. Natl. Acad. Sci. USA 94, 377-384. 10.1073/pnas.94.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E., Quiat D., Johnson B. A., Sutherland L. B., Qi X., Richardson J. A., Kelm R. J. Jr and Olson E. N. (2009). A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 17, 662-673. 10.1016/j.devcel.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Zhang J., Shen C., Luo Y., Xia L., Xue F. and Xia Q. (2012). Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem. Biophys. Res. Commun. 423, 826-831. 10.1016/j.bbrc.2012.06.048 [DOI] [PubMed] [Google Scholar]
- Yang H., Kong W., He L., Zhao J.-J., O'Donnell J. D., Wang J., Wenham R. M., Coppola D., Kruk P. A., Nicosia S. V. et al. (2008). MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425-433. 10.1158/0008-5472.CAN-07-2488 [DOI] [PubMed] [Google Scholar]
- Yang S., Liu X., Li X., Sun S., Sun F., Fan B. and Zhao S. (2013). MicroRNA-124 reduces caveolar density by targeting caveolin-1 in porcine kidney epithelial PK15 cells. Mol. Cell. Biochem. 384, 213-219. 10.1007/s11010-013-1800-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.