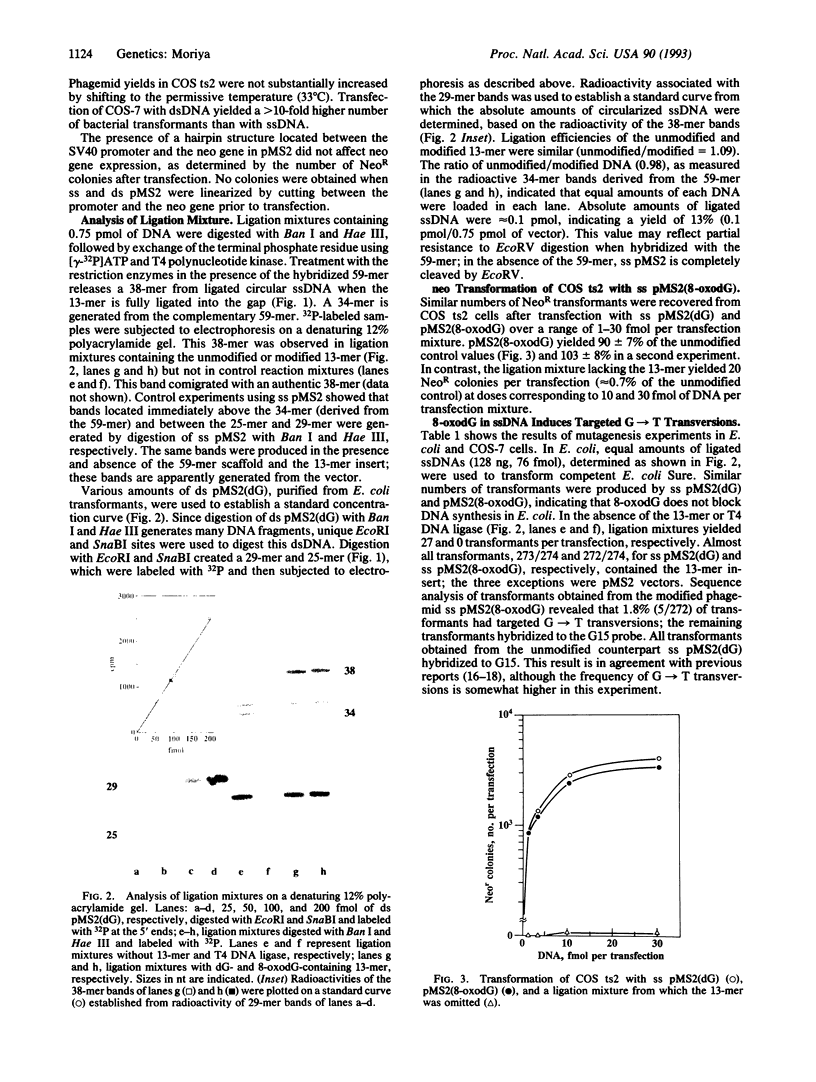
Abstract
A single-stranded shuttle vector has been developed for the purpose of investigating translesional events in mammalian cells. The vector is designed to permit site-specific introduction of defined DNA lesions between a gene for neomycin resistance and its promoter. Efficiencies of translesional synthesis in simian kidney cells (COS) and Escherichia coli are established by determining the number of neomycin- and ampicillin-resistant colonies recovered, respectively, after introduction of a modified vector. Fidelity of translesional synthesis is evaluated by analyzing the nucleotide sequence of progeny phagemid DNA in the region corresponding to the lesion site. This experimental system, capable of detecting mutagenic and nonmutagenic events at and adjacent to the lesion site, was used to establish the mutagenic potential of a single 8-oxoguanine residue in DNA. This modified base, produced by attack of reactive oxygen species on cellular DNA, did not cause a decrease in the number of transformants when single-stranded DNA containing the lesion replicated in COS cells or E. coli. The predominant mutations observed (> 78%) were G-->T transversions targeted to the site of the lesion. The mutation frequencies for this event were 2.5-4.8% in COS cells and 1.8% in E. coli. It is concluded that a single-stranded shuttle vector, utilized in conjunction with a site-specific approach, can be used to investigate translesional events in mammalian cells and in bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams P. J., Van der Eb A. J. Host-cell reactivation of ultraviolet-irradiated SV40 DNA in five complementation groups of xeroderma pigmentosum. Mutat Res. 1976 Apr;35(1):13–22. doi: 10.1016/0027-5107(76)90164-0. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991 Sep-Oct;250(1-2):3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- Au K. G., Clark S., Miller J. H., Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Borden A., Christensen R. B., LeClerc J. E., Lawrence C. W. SOS-dependent replication past a single trans-syn T-T cyclobutane dimer gives a different mutation spectrum and increased error rate compared with replication past this lesion in uninduced cells. J Bacteriol. 1990 Apr;172(4):2105–2112. doi: 10.1128/jb.172.4.2105-2112.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Christensen R. B., Lawrence C. W., LeClerc J. E. Frequency and spectrum of mutations produced by a single cis-syn thymine-thymine cyclobutane dimer in a single-stranded vector. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8141–8145. doi: 10.1073/pnas.85.21.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Nghiem Y., Miller J. H. mutM, a second mutator locus in Escherichia coli that generates G.C----T.A transversions. J Bacteriol. 1988 Nov;170(11):5405–5407. doi: 10.1128/jb.170.11.5405-5407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kim H. S., Ohtsuka E., Kasai H., Yamamoto F., Nishimura S. An endonuclease activity in human polymorphonuclear neutrophils that removes 8-hydroxyguanine residues from DNA+. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1472–1478. doi: 10.1016/0006-291x(91)91059-l. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madzak C., Menck C. F., Armier J., Sarasin A. Analysis of single-stranded DNA stability and damage-induced strand loss in mammalian cells using SV40-based shuttle vectors. J Mol Biol. 1989 Feb 5;205(3):501–509. doi: 10.1016/0022-2836(89)90221-0. [DOI] [PubMed] [Google Scholar]
- Madzak C., Sarasin A. Conversion of a single-stranded simian virus 40 (SV40)-based shuttle vector to its double-stranded form does not require the SV40 T antigen in monkey cells. J Gen Virol. 1991 Dec;72(Pt 12):3091–3093. doi: 10.1099/0022-1317-72-12-3091. [DOI] [PubMed] [Google Scholar]
- Madzak C., Sarasin A. Mutation spectrum following transfection of ultraviolet-irradiated single-stranded or double-stranded shuttle vector DNA into monkey cells. J Mol Biol. 1991 Apr 20;218(4):667–673. doi: 10.1016/0022-2836(91)90252-2. [DOI] [PubMed] [Google Scholar]
- Menck C. F., Madzak C., Renault G., Margot A., Sarasin A. SV40-based shuttle viruses. Mutat Res. 1989 Mar-May;220(2-3):101–106. doi: 10.1016/0165-1110(89)90015-8. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Cruz C., Grollman A. P., Miller J. H. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7022–7025. doi: 10.1073/pnas.89.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels M. L., Pham L., Cruz C., Miller J. H. MutM, a protein that prevents G.C----T.A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1991 Jul 11;19(13):3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsuk S. Novel eukaryotic expression vectors which permit single-stranded replication in Escherichia coli and in vitro translational analysis of cloned genes. Gene. 1988 Oct 30;70(2):313–319. doi: 10.1016/0378-1119(88)90203-x. [DOI] [PubMed] [Google Scholar]
- Moriya M., Ou C., Bodepudi V., Johnson F., Takeshita M., Grollman A. P. Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat Res. 1991 May;254(3):281–288. doi: 10.1016/0921-8777(91)90067-y. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Nghiem Y., Cabrera M., Cupples C. G., Miller J. H. The mutY gene: a mutator locus in Escherichia coli that generates G.C----T.A transversions. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Clark S. G., Tjian R. A mammalian host-vector system that regulates expression and amplification of transfected genes by temperature induction. Science. 1985 Jan 4;227(4682):23–28. doi: 10.1126/science.2981116. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Laithier M., Lando D., Ryhiner M. L. Infectious DNA from herpes simplex virus: infectivity of double-stranded and single-stranded molecules. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3621–3625. doi: 10.1073/pnas.70.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Essigmann J. M. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991 Jun;12(6):949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]