Key Points
MDSCs are potent suppressors of alloimmune responses; however, efficacy is limited in the context of acute GVHD due to inflammasome induction.
Abstract
Myeloid-derived suppressor cells (MDSCs) are a naturally occurring immune regulatory population associated with inhibition of ongoing inflammatory responses. In vitro generation of MDSCs from bone marrow has been shown to enhance survival in an acute model of lethal graft-versus-host disease (GVHD). However, donor MDSC infusion only partially ameliorates GVHD lethality. In order to improve the potential therapeutic benefit and ultimately survival outcomes, we set out to investigate the fate of MDSCs after transfer in the setting of acute GVHD (aGVHD). MDSCs transferred to lethally irradiated recipients of allogeneic donor hematopoietic grafts are exposed to an intense inflammatory environment associated with aGVHD, which we now show directly undermines their suppressive capacity. Under a conditioning regimen and GVHD inflammatory settings, MDSCs rapidly lose suppressor function and their potential to inhibit GVHD lethality, which is associated with their induced conversion toward a mature inflammasome-activated state. We find even brief in vitro exposure to inflammasome-activating mediators negates the suppressive potential of cultured murine and human-derived MDSCs. Consistent with a role for the inflammasome, donor MDSCs deficient in the adaptor ASC (apoptosis-associated speck-like protein containing a CARD), which assembles inflammasome complexes, conferred improved survival of mice developing GVHD compared with wild-type donor MDSCs. These data suggest the use of MDSCs as a therapeutic approach for preventing GVHD and other systemic inflammatory conditions will be more effective when combined with approaches limiting in vivo MDSC inflammasome activation, empowering MDSCs to maintain their suppressive potential.
Introduction
Allogeneic hematopoietic cell transplantation is a potentially curative therapy for a variety of hematologic diseases including leukemias and lymphomas. However, the risk of morbidity and mortality from graft-versus-host disease (GVHD) remains an obstacle to widespread use.1 Immune-suppressive cell therapy has the potential to control GVHD while reducing conditioning-regimen side effects. Myeloid-derived suppressor cells (MDSCs), defined broadly as myeloid lineage cells with suppressive capacity, emerge coincident with pathologies such as tumors, trauma, and infection.2 De novo MDSC production from the bone marrow (BM) occurs in response to inflammation and growth factor release (ie, granulocyte-macrophage colony-stimulating factor [GM-CSF] and granulocyte colony-stimulating factor [G-CSF]). Recent interest in the clinical application of MDSC therapy has grown in response to basic research and a better understanding of MDSC biology; however, questions remain regarding in vivo viability, trafficking, and expansion.3-5
Much like regulatory T cells (Tregs), which have shown promise in preclinical and early-phase clinical trials for GVHD,6 MDSCs can suppress systemic immune pathology via unique mechanisms including local amino acid deprivation, nitric oxide, prostaglandin E2, anti-inflammatory cytokines, reactive oxygen species, and promotion of Tregs.7 We have shown that BM-derived cultures of MDSCs incubated with interleukin-13 (IL-13) suppress acute GVHD (aGVHD) via an arginase 1 (Arg1)-dependent depletion of l-arginine, which in turn inhibits allogenic T-cell responses.8 Translation requires a thorough understanding of cell fate and function with attention paid to potentially adverse effects.
aGVHD has 3 phases: (1) transplant conditioning, (2) donor T-cell priming, and (3) effector-phase tissue apoptosis.9-11 The conditioning regimen and rapid priming of a high frequency of allospecific donor T cells leads to intense systemic inflammation. Because aGVHD expands donor T cells that have the capacity to attack the recipient, the most effective approaches to prevent lethality are those that dampen T-cell responses early posttransplant, when inflammation is mounting and alloreactive T cells are contributing to organ injury, amplifying the inflammatory response.12 For donor MDSCs to be optimally effective, these cells must maintain their viability and function for a sufficiently long period of time to impede alloreactive T-cell priming and expansion.
Factors associated with inflammasome activation are produced during allogeneic hematopoietic cell transplantation, including gut-associated leakage of bacterial products and danger-associated molecules from dead and dying cells.1 The inflammasome is a multimolecular complex, which acts as an important downstream component of innate immune sensing pathways.13,14 For inflammasome activation to occur, initiating signals converge and lead to adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD)-mediated pro–caspase-1 autocatalytic cleavage and cleavage and export of active IL-1β or IL-18. Molecules involved in the different inflammasomes vary depending on the source of activating signals and the “upstream” molecule that coalesce with ASC; for example, AIM2-like receptor family inflammasomes can be initiated by binding cytosolic double-stranded DNA (dsDNA) to AIM2 (absent in myeloma 2), while the NLRP3 (NOD-like receptor family, pyrin domain containing-3) inflammasome is activated by microbial and host danger signals, as well as changes in extracellular adenosine triphosphate (ATP) content.15
Here, we demonstrate that in a murine GVHD model, a single early-posttransplant MDSC infusion transiently suppresses but does not eliminate GVHD. Our data establish that the inflammation of GVHD drives MDSCs toward a state of inflammasome activation, which is counterproductive to MDSC suppressive function in GVHD mice. However, genetic alteration of donor MDSCs to disable inflammasome activation results in increased GVHD survival relative to control MDSC therapy. Furthermore, we find supporting evidence that the same pathways are active in human MDSCs. Taken together, this new information should be used to augment ongoing and proposed studies of MDSCs and their potential therapeutic application.
Methods
MDSC generation
Murine MDSCs were generated in complete Dulbecco’s modified Eagle medium plus 10% fetal calf serum, supplemented with 100 ng/mL G-CSF and 2.5 ng/mL mouse GM-CSF for 4 days. On day 3, 40 ng/mL recombinant murine IL-13 was added for Arg1 induction; alternatively, 40 ng/mL interferon-γ was added. MDSCs were harvested on day 4 using trypsin/EDTA and light scraping to recover adherent cells resulting in >92% CD11b+ recovery.8 For inflammasome induction, lipopolysaccharide (LPS) (0.2 µg/mL) was added to prime cultures. After 3 hours, to stimulate the NLRP3 inflammasome, 2 mM ATP was added for 1 hour; alternatively, for AIM2 inflammasome activation, poly(dT) was added using Lipofectamine 2000 reagent.
All other methods are described in detail in the supplemental files (available on the Blood Web site).
Results
MDSCs undergo rapid differentiation to CD11c+ activated phenotype in the context of GVHD
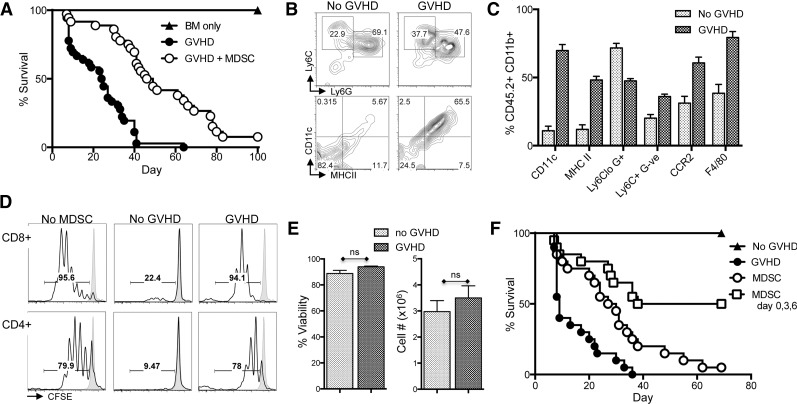
MDSCs were generated from cultured fresh BM with GM-CSF and G-CSF in 4 days. Previously, we demonstrated that activation of cultured MDSCs 1 day prior to harvest with IL-4 or IL-13 stimulates expression of Arg1 and promotes functional suppression of T cells both in vitro and in the context of GVHD.8 To demonstrate that IL-13–activated MDSCs (MDSC-IL13) are effective at prolonging survival in a murine aGVHD model, lethally irradiated BALB/c recipients were given allogeneic C57Bl/6 BM plus CD25-depleted T cells to induce aGVHD, in the presence or absence of MDSC-IL13 cellular therapy, at day 0. T cells induce aGVHD, resulting in a mean survival time of 24.5 days. However, animals receiving MDSC-IL13 therapy had extended survival to 47.5 days (Figure 1A). Despite an improved clinical outcome, a majority of treated animals succumbed to GVHD-induced death by day 100, suggesting pathology was reduced or delayed, but not eliminated. To investigate how conditions associated with aGVHD might directly alter MDSC function, BM or BM plus T cell (GVHD conditions) with congenic CD45.2+ MDSC-IL13 were given to CD45.1+ animals that were then sacrificed at day 5 posttransplant to examine the phenotype, function, and fate of recovered MDSCs.
Figure 1.
BM-derived MDSC-IL13 enhance GVHD survival, but suppression is compromised after 5 days in vivo. (A) Lethally irradiated BALB/c recipients were given 1 × 107 C57Bl/6 BM (BM only), BM plus 2 × 106 CD25-depleted T-cells (GVHD), or BM, T cells, and 6 × 106 MDSC-IL13 (GVHD + MDSC) as indicated. Kaplan-Meier survival curve represents 4 pooled and independent experiments (n = 40 animals/group). GVHD vs GVHD + MDSC, P < .0001. (B-C) Surface expression of congenic (CD45.2+) MDSC-IL13 recovered from spleens 5 days after transfer to irradiated animals receiving BM only (no GVHD) or BM plus T cells (GVHD). Data represent 3 replicates per group with P < .001 for all markers shown. (D) Representative histograms indicating responding T-cell proliferation as denoted by CFSE dilution. Purified MDSC-IL13 from pooled spleens 5 days after transplant were plated at 5 × 105/mL with an equal number of CFSE-labeled responder T cells, 0.25 μg/mL anti-CD3ε mAb, and 2.5 × 105/mL irradiated T-cell–depleted splenocytes in specially formulated 150 μM l-arginine RPMI media. Shaded histogram indicates proliferation of unstimulated controls. Data are representative of 3 samples per group and a total of 3 independent experiments. (E) Summary data of recovered MDSCs showing viability and total cell numbers recovered, gated CD11b+ CD45.2+. Data represent 3 samples per group and are representative of 3 independent experiments. (F) Lethally irradiated BALB/c recipients transplanted as above or given 3 consecutive infusions of MDSC-IL13 as indicated on days 0, 3, and 6. All mice receiving MDSCs demonstrated increased survival vs GVHD (P < .001). MDSCs vs MDSCs on days 0, 3, and 6 (P < .0001). Survival curve represents 20 animals per group from 2 independent experiments and is representative of an additional experiment giving multiple infusions on days 0, 7, and 14. ns, not significant.
Phenotypically, MDSCs recovered from transplanted animals receiving only conditioning plus BM maintained an immature CD11clo, MHCIIlo, F4/80int appearance (Figure 1B-C). However, MDSC-IL13 transferred to animals undergoing aGVHD conditions (CD8+CD4+CD25− T cells) upregulated CD11c and major histocompatibility complex class II, hallmarks of activated myeloid cells. F4/80 expression was also increased, suggesting activation and differentiation occurred rapidly in response to the ongoing inflammatory environment promoted by alloreactive T cells (Figure 1B-C). Other markers of costimulation and activation remained unchanged (supplemental Figure 1). To measure the functional status of recovered MDSCs, we cocultured ex vivo isolated MDSCs (supplemental Figure 2) with anti-CD3ε monoclonal antibody (mAb)-activated, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled T cells. MDSC-IL13 from day 5 animals transplanted with BM only was highly suppressive (Figure 1D, see “no GVHD”). In contrast, T-cell responses in the presence of MDSC-IL13 recovered from GVHD animals showed only a slight reduction in overall proliferation relative to no-MDSC controls (Figure 1D), indicating a loss of suppressor cell function of MDSCs in GVHD recipients.
One explanation for the loss of suppression by MDSCs in vivo could be compromised survival of the suppressive cells. MDSC viability is maintained by continuous suppression of the extrinsic caspase-8 and intrinsic mitochondrial death pathways.5 Manipulation of either of these pathways causes a rapid decline in MDSC viability and a concomitant decrease in suppression. Therefore, we investigated whether the number and viability of transferred cells was compromised in recipient spleens posttransfer. No significant differences were observed between BM-only and GVHD conditions when looking at the total CD45.2+CD11b+ cell number or viability at day 5 (Figure 1E), though a differential fate might have been evident at later time points. Taken together, these data suggest that MDSC-IL13 reduce aGVHD for a limited period of time posttransplant and that MDSC-IL13 subsequently lose suppressor function under GVHD conditions, resulting in a failure to sustain a therapeutic benefit.
Multiple MDSC-IL13 infusions improve GVHD long-term survival
To further investigate the limited suppressive function of MDSC in our model of aGVHD, we first set out to determine if increasing the dose of MDSC-IL13 could enhance survival. Increasing the ratio of MDSC-IL13 to T cells during GVHD induction from 3:1 to 6:1 (2×) or 9:1 (3×) at BM transplant did not significantly increase survival (supplemental Figure 3). We hypothesized that suppressor function was limited by GVHD-induced inflammation. To address this, we investigated whether repetitive doses of freshly cultured MDSC-IL13 might promote or maintain a suppressive environment during the crucial peritransplant period of T-cell priming during aGVHD. Three infusions of cultured MDSC-IL13 were given on days 0, 3, and 6 posttransplant, totaling an aggregate MDSC:T-cell ratio of 9:1. Multiple MDSC-IL13 infusions promoted a cohort (50%) of long-term survivors vs <10% long-term survivors when given a single day 0 MDSC-IL13 infusion (Figure 1F). The observation that repeated infusions of freshly cultured MDSC-IL13 augment survival supports the hypothesis that the intense inflammatory environment found during the induction of aGVHD does not affect MDSC persistence or viability but instead changes the host environment so that transferred MDSC-IL13 are effectively less suppressive with time.
Associated conversion to IL-1β production and inflammasome activation
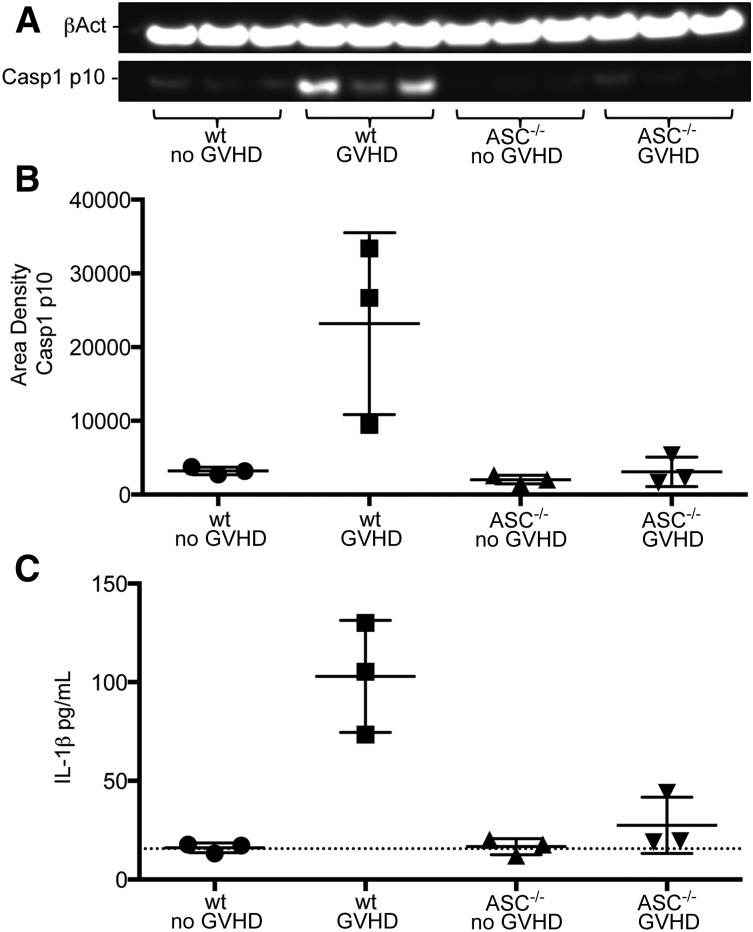
Recent data have shown host NLRP3 inflammasome activity exacerbates GVHD in a murine model and that GVHD patients exhibit increased levels of inflammasome-associated serum caspase-1 and IL-1β.16 Bruchard et al found that MDSCs produce IL-1β when exposed to certain chemotherapeutic agents, resulting in an altered antitumor response.17 To investigate the pathway-dependent mechanisms causing the diminished suppressive activity of transferred MDSC-IL13 in aGVHD, we focused on the MDSC-intrinsic activation of inflammasomes. Cell lysates from MDSC-IL13 recovered from GVHD conditions on day 5 were probed for the processed p10 form of caspase-1, an upstream mediator of inflammasome activity. Western blot analyses showed increased amounts of caspase-1 p10 in MDSC-IL13 recovered from GVHD conditions relative to MDSC-IL13 recovered from BM controls (Figure 2A-B). Further evidence for MDSC conversion to inflammasome activation was found when recovered MDSC-IL13 were placed in complete media for an overnight culture; analysis of supernatants demonstrate increased IL-1β from GVHD conditions (Figure 2C). These data establish a correlation between MDSC-IL13 in the context of aGVHD conditions and inflammasome activity.
Figure 2.
Inflammasome activity evident in recovered MDSCs. (A) Western blot of cell lysates from recovered wild-type or ASC−/− MDSC-IL13 probed for the active p10 form of caspase-1 and β-actin. ImageJ software was used to convert to grayscale and straighten and crop the gel image to highlight lanes of interest according to size. (B) Caspase-1 p10 blot quantification relative to β-actin; GVHD vs all other groups (P < .05). Quantification was carried out on scanned blots by densitometric analysis from ImageJ software (National Institutes of Health). (C) IL-1β enzyme-linked immunosorbent assay (ELISA) of supernatants after day 5–recovered MDSC-IL13 were plated in complete RPMI media overnight; GVHD vs all other groups (P < .05). Dotted line indicates limit of ELISA detection. All data are representative of 2 independent experiments. wt, wild-type.
MDSC-IL13 in vitro induction of IL-1β requires ASC
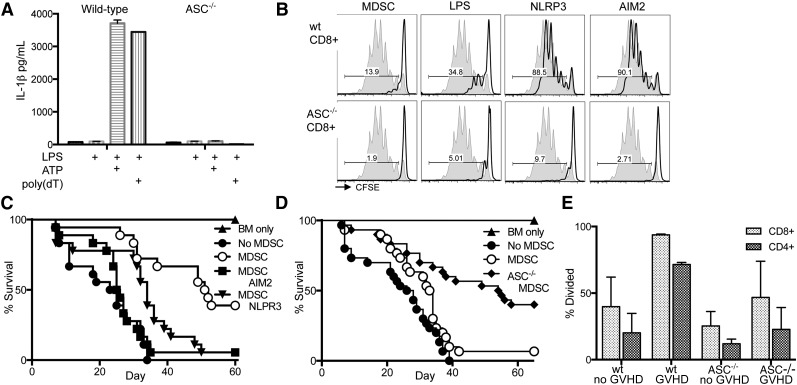
IL-1β can directly interfere with Treg-mediated suppression and promotion of T-effector function.18 To investigate inflammasome activation and IL-1β production in our system, we examined in vitro inflammasome induction of cultured MDSC-IL13. NLRP3 and AIM2 represent 2 families of inflammasomes in which various signals can potentiate the inflammasome cascade. However, reports suggest a common component required for full activation is the adaptor protein ASC.14 Wild-type and ASC−/− (Pycard−/−) BM was used to generate MDSC-IL13 to test for inflammasome activity.19 At transfer, ASC−/− MDSC-IL13 had a surface phenotype similar to wild-type MDSC-IL13 (supplemental Figure 4). The NLRP3 inflammasome was tested by incubating MDSC-IL13 with LPS for 3 hours followed by ATP, whereas the AIM2 inflammasome was engaged using LPS followed by poly(dT) transfection. In as little as 1 hour after secondary stimuli, IL-1β was detected in culture supernatants from wild-type MDSC-IL13 (Figure 3A). However, ASC−/− MDSC-IL13 produced no detectable IL-1β, establishing that MDSC-IL13 are capable of rapidly responding to changes in their environment to produce the proinflammatory mediator IL-1β in an ASC-dependent fashion. We next determined if inflammasome induction of MDSC-IL13 might alter their suppressive capacity. Following the same procedure used to induce inflammasomes in just 4 hours, MDSC-IL13 were then washed extensively and plated in an in vitro suppression assay of T-cell proliferation to anti-CD3ε stimulation. We observed that in vitro inflammasome induction reduces MDSC-IL13’s ability to functionally suppress responding CD8+ (Figure 3B) and CD4+ (supplemental Figure 5) T cells. Furthermore, this effect was also dependent on ASC, supporting our hypothesis that inflammasome activation and not compromised viability or other maturation factors are associated with a loss of suppressive function. To determine if ASC deficiency was intrinsically associated with suppression of T-cell responses independent of inflammasome activity, BM-derived dendritic cells were generated from wild-type and ASC−/− donors and used as stimulators in an allogeneic mixed lymphocyte reaction assay. BALB/c T effectors responded equivalently to dendritic cells regardless of genotype (supplemental Figure 6), supporting a link between ASC-associated inflammasome-mediated loss of function.
Figure 3.
In vitro inflammasome induction in MDSCs leads to loss of suppressor function. Inflammasome induction in freshly cultured wild-type and ASC−/− MDSC-IL13 was carried out by adding 0.2 µg/mL LPS for 3 hours, followed by addition of 2 mM ATP or 0.8 µg/mL poly(dT) transfection. (A) Culture supernatants were harvested after an additional 1 hour and assayed for IL-1β production by ELISA. Data are representative of 3 independent experiments. (B) Inflammasome-induced MDSC-IL13 were washed extensively and plated in a CFSE suppression assay at a 1:1 ratio; data are representative of gated CFSE-labeled CD8+ responder T cells (n = 6 samples/group from 2 independent experiments). NLRP3 indicates LPS + ATP treatment, and AIM2 indicates LPS + poly(dT) treatment; gray histogram represents the no-MDSC proliferation control. Gated CD4+ responder T cells shown in supplemental Figure 5. (C) Kaplan-Meier survival curve of the C57Bl/6 → BALB/c GVHD model using inflammasome-induced MDSC-IL13, treated as above. MDSC v no MDSC P < .0001, MDSC vs MDSC AIM2 P < .0001, MDSC v MDSC NLRP3 P = .0029. Data represent n = 18 animals per group, combined from 2 independent experiments. (D) Kaplan-Meier survival curve of GVHD using MDSC-IL13 generated from either wild-type or ASC−/− mice as indicated. Data represent n = 30 animals per group in 3 independent experiments. MDSC vs no MDSC P = .0399, MDSC vs ASC−/− MDSC P = .0006. (E) Histograms represent % divided CFSE-labeled responding T-cells when plated against recovered wild-type or ASC−/− MDSC-IL13 from day 5 posttransplant at a ratio of 1:1 and collected on day 3. Significant P values (< .05) were found when comparing any single group to wild-type MDSC-IL13 recovered from GVHD mice. Data are representative of 2 independent experiments. wt, wild-type.
Inflammasome induction reduces efficacy of MDSC-IL13 in GVHD
Having demonstrated that MDSC-IL13 respond to in vitro inflammasome activation via either LPS plus ATP or LPS plus poly(dT) transfection by rapidly producing IL-1β and that in vitro suppression appears to be compromised by inflammasome activation, we sought to determine if in vitro inflammasome-activated MDSC-IL13 also had a diminished ability to promote survival in the setting of aGVHD. In vitro cultured MDSC-IL13 were treated as above for activation of the NLRP3 or AIM2 inflammasome, followed by extensive washing prior to transfer into our aGVHD model. As seen for in vitro suppression, inflammasome activation of MDSC-IL13 caused a diminution in the GVHD survival benefit compared with control MDSC-IL13 therapy, which significantly increased GVHD survival (P < .0001; Figure 3C).
Having implicated MDSC-IL13 conversion to a mature, inflammasome-activated state after therapeutic transfer in the setting of aGVHD, we hypothesized that using MDSC-IL13 genetically incapable of inflammasome activation would better maintain function and further enhance GVHD survival. Indeed, survival of recipients of ASC−/− MDSC-IL13 was further improved relative to wild-type MDSC-IL13 (P = .0006), and both had significantly better survival than the no-MDSC group (Figure 3D). Furthermore, ASC−/− MDSC-IL13 recovered from GVHD animals 5 days posttransfer had increased T-cell–suppressive capacity compared with wild-type MDSC-IL13 (Figure 3E). These findings together directly implicate MDSC-IL13 intrinsic inflammasome activation under GVHD conditions as playing a role in limiting the efficacy of MDSC cellular therapy.
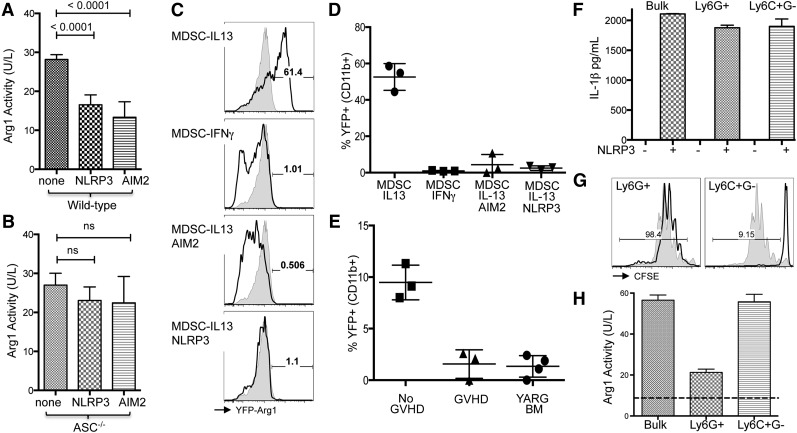
Previously, we have demonstrated that the mechanism for MDSC-IL13–mediated suppression of GVHD is Arg1 activity, which directly undermines T-cell responsiveness and promotes GVHD survival.8 To determine whether arginase activity was reduced in concert with inflammasome induction we measured enzymatic bioactivity from MDSC-IL13 after inflammasome induction. A marked drop in arginase activity as measured by either mRNA (not shown) or bio-enzymatic activity was found when either NLRP3 or AIM2 inflammasomes were induced (Figure 4A-B). A similar drop was not evident in ASC−/− MDSCs, though there was a trend, suggesting that the pathways may not be directly linked. To further investigate Arg1 activity specifically, we used Arg1 reporter mice (YARG), in which Arg1 expression is linked to yellow fluorescent protein (YFP) expression, allowing us to quickly assay for an associated loss of expression.20 Using this system, we found IL-13 readily upregulated YFP fluorescence as expected, whereas interferon-γ (which induces inducible nitric oxide synthase–expressing MDSCs) did not increase YFP fluorescence (Figure 4C-D). As above, inflammasome induction via NLRP3 or AIM2 pathways resulted in a concomitant loss of YFP fluorescence, indicating Arg1 expression had been arrested in association with inflammasome inducing conditions. Finally, MDSC-IL13 generated from YARG transgenic animals were applied to our transplant model, given either BM only or BM plus whole T cells for GVHD conditions. YFP fluorescence from MDSCs recovered from BM-only animals on day 5 was reduced relative to expression immediately after culture but still readily detected above background (Figure 4E). However, in MDSC-IL13 recovered from GVHD animals, YFP was no longer detectable, being reduced to background levels seen in unstimulated YARG transgenic BM. These results further support our conclusions and link an associated loss of Arg1 expression to inflammasome activity during GVHD.
Figure 4.
Inflammasome induction in MDSC associated with loss of Arg1 expression. MDSC-IL13 were induced for NLRP3 or AIM2 inflammasomes as indicated, washed extensively, and replated in complete media overnight. (A) Enzymatic activity of cell-associated arginase for wild-type or (B) ASC−/− MDSCs, normalized to total cell number. Data are pooled from 2 independent experiments. MDSCs generated from YARG mice, followed by in vitro induction of inflammasomes (as previously discussed) and replating in complete media. (C) YFP fluorescence for CD11b-gated MDSCs after 2 additional days in culture indicated as representative histograms (shaded histogram represents unstimulated BM from YARG donor) and (D) summary data of % YFP+. (E) YFP detection for MDSC-IL13 recovered from day 5–transplanted animals with BM only (no GVHD) or BM plus whole T cells (GVHD). YARG BM (YFP-Arg1 bone marrow) indicates baseline YFP fluorescence. (F) IL-1β production before and after NLRP3 (ATP + LPS) inflammasome activation for bulk MDSC-IL13 or sorted granulocytic Ly6G+C+ (Ly6G+) and monocytic Ly6C+ subsets. (G) CFSE proliferation of anti-CD3ε driven CD8+ B6 T cell responses in the presence of sorted granulocytic (Ly6G+) or monocytic (Ly6C+) subsets of MDSC-IL13 at a 1:1 ratio. (H) Cell-associated arginase bioactivity for bulk MDSC-IL13 and sorted granulocytic (Ly6G+) or monocytic (Ly6C+) subsets. The dashed line indicates background activity for Arg1-deficient splenocytes. Data regarding MDSC subsets are representative of 3 independent experiments.
MDSC are often defined as heterogeneous, with 2 major subsets described in murine systems being granulocytic (Ly6G+) and monocytic (Ly6C+G−).7 At the time of transfer, we noted that while >90% are Ly6C+, a majority of our cultured MDSC-IL13 have an Ly6Gdim phenotype, with a minor subset (<30%) exhibiting increased Ly6G+ staining (supplemental Figure 4). To determine whether each subset had a similar capacity for inflammasome activation and how suppressor capacity might be differentially affected, MDSC-IL13 were sorted based on Ly6G expression (supplemental Figure 7). NLRP3 inflammasome-activating conditions (LPS + ATP) lead to equivalent levels of IL-1β production for both Ly6C+G− monocytic and Ly6G+ granulocytic subsets (Figure 4F). However, when applied to an in vitro suppression assay independent of inflammasome activation, we found that suppressor capacity was contained virtually in its entirety within the Ly6C+G− monocytic subset (Figure 4G), which is consistent with our recent report of MDSCs generated with IL-6 and GM-CSF5; furthermore, Arg1 bioactivity was associated with the monocytic subset (Figure 4H). Thus, although each subset appears to have an equal capacity for inflammasome-mediated production of IL-1β, arginase activity and suppressive capacity are primarily associated with the monocytic (Ly6C+G−) subset. These results suggest that further enrichment of the monocytic product would likely enhance overall therapeutic potential.
Human cultured MDSCs lose function when their inflammasome is activated
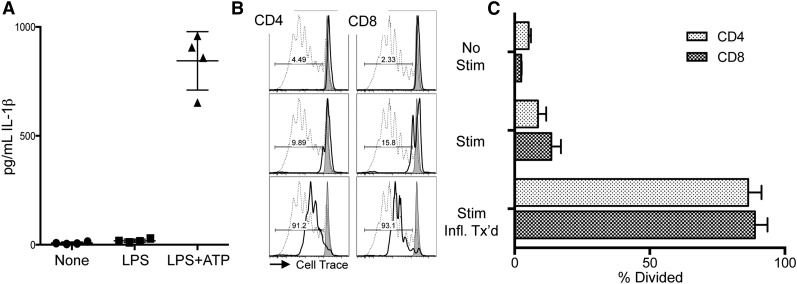
To investigate whether human MDSC might have a similar predisposition toward inflammasome-activated loss of function as found with murine MDSCs, we generated MDSCs from peripheral blood mononuclear cells (PBMCs) using a modified method from a published protocol.21 Human PBMCs, enriched for the myeloid marker CD33, were cultured for 7 days with GM-CSF and IL-6. Under these conditions, human MDSCs suppressed anti-CD3ε mAb-driven proliferation of unrelated PBMC responders.22 We confirmed that human MDSCs are capable of responding to inflammasome-activating conditions by adding LPS followed by ATP to engage the NLRP3 inflammasome. As expected, both LPS and ATP are needed to drive MDSC production of IL-1β (Figure 5A). Next, inflammasome-activated MDSCs were added to the anti-CD3ε mAb-driven PBMC proliferation assay. As in the murine system, MDSCs exposed to inflammasome-activating components (Stim + Infl. Tx’d) lost suppression concomitant with IL-1β production (Figure 5B-C).
Figure 5.
Inflammasome induction in human MDSC interferes with their suppressor function. Human MDSCs were generated from donor PBMCs. (A) IL-1β ELISA of supernatants from MDSCs treated with 0.2 µg/mL LPS for 3 hours followed by 2 mM ATP for 1 hour prior to harvest. Data are representative of 2 independent experiments. (B) Representative histograms of responder PBMCs labeled with CellTrace Violet (Life Technologies) in the presence of cultured human MDSCs from unrelated donors indicated by solid line. Dotted line indicates no-MDSC proliferation control, gray histogram indicates PBMCs alone (no CD3ε or MDSCs). No Stim represents the alloresponse against MDSCs with no anti-CD3ε. Stim indicates addition of anti-CD3ε microbeads (2:1) + IL-2 (100 U/mL) to demonstrate MDSC suppression of T-cell activation. Stim + Infl. Tx’d indicates MDSCs have been treated for inflammasome activation prior to plating with anti-CD3ε microbeads + IL-2. (C) Aggregate data show percent division of responding CD8 and CD4 T cells. Data represent responses from 3 unrelated PBMC donors and are representative of 2 independent experiments.
Discussion
Alloreactive T cells are a major contributing factor to morbidity and mortality in clinical GVHD, and the use of regulatory cell therapy is gaining traction as a viable means to bring them under control. MDSCs can be generated from normal BM in a relatively short amount of time and have been shown to effectively suppress GVHD8,23 as well as autoimmunity24 and allograft rejection.25,26 In our studies, we have found that MDSCs activated by the cytokine IL-13 produce Arg1 that is in turn critical to their ability to suppress GVHD.8 However, in a stringent GVHD model with full major histocompatibility complex mismatching, MDSC therapy promotes extended survival but fails to ultimately protect a majority of animals from lethal GVHD. Here, we demonstrate that intrinsic inflammasome activation of adoptively transferred MDSCs limits their efficacy in vivo. We establish that cultured MDSCs are capable of rapidly responding in an ASC-dependent fashion to produce significant amounts of IL-1β, resulting in an associated loss of suppression in vitro. Furthermore, shortly after in vivo transfer, MDSCs in the context of aGVHD convert to a mature CD11c+ phenotype and demonstrate a loss of ex vivo suppressive capacity. These cells have increased amounts of an important indicator of inflammasome activation, caspase-1 p10, and secrete IL-1β when placed in culture overnight, unlike controls under non-GVHD conditions. Finally, when inflammasome activation is genetically ablated using ASC−/− MDSC-IL13, GVHD survival is further improved over wild-type MDSC-IL13 transplant recipients and recovered ASC−/− MDSC-IL13 maintain better ex vivo suppressive capacity.
Myeloid cells play a critical role in initiating and shaping immune responses in both pro- and anti-inflammatory directions and demonstrate remarkable plasticity.27-29 This adaptability may be both a blessing and a curse, in that it allows us to rapidly generate highly suppressive cells from normal BM in vitro yet permits the transient efficacy seen upon transfer to a severe inflammatory environment such as aGVHD. Although MDSCs are described as heterogeneous in nature and phenotypic markers do not always translate between disease models or species,28,30 inflammasome-activation pathways appear to be highly conserved, and in this study, we find that MDSCs generated from mouse BM or human PBMCs readily activate inflammasomes, resulting in IL-1β production that then correlates to a loss of suppressor function. Our finding that the monocytic (Ly6C+G−) subset is primarily responsible for suppressive capacity of cultured MDSC-IL13 has implications for future studies aimed at improving the therapeutic potential of cultured MDSCs. Interestingly, the ratio of monocytic to granulocytic cells at the time of transfer (roughly 2:1; supplemental Figure 4) is inverted by day +5 posttransplant (Figure 1B). Due to the limited recovery, it was not feasible to further sort these cells; however, future studies will be aimed at addressing the tempo of phenotypic and functional changes for MDSCs in the context of GVHD as well as varied conditioning regimens.
Reports have demonstrated cleaved caspase-1 and increased IL-1β in patients with aGVHD, further supporting the conclusion that GVHD is associated with inflammasome activation.16 MDSCs are nearly ubiquitously associated with established tumors and actively perturb immune therapy interventions.3,25 An important distinction between tumor- and GVHD-induced inflammasome activation of MDSCs is that tumor-associated MDSC development occurs in the setting of chronic localized inflammation,3 in contrast to the intense, systemic inflammatory response of GVHD. It will be of interest to better understand the inciting signals for these divergent effects that are dependent upon the milieu in which MDSCs reside.
Because GVHD-activated MDSCs secrete IL-1β, it is possible that such MDSCs contributed to the GVHD lethality process. Earlier studies examining the IL-1 pathway in GVHD found an important role for IL-1 in the initiation of aGVHD.31 IL-1β has pleiotropic effects dependent on the cell producing it, the state of the surrounding microenvironment, and temporal expression, but it is generally understood to be proinflammatory and, in some instances, counterregulatory.18,32 When MDSC-IL13 recovered from day 5 GVHD transplant recipients are applied to an in vitro suppression assay, the proportion of T cells proliferating was not different from no-MDSC controls, although proliferating T cells underwent fewer cell divisions, suggesting some suppressive capacity remained. These data suggest that GVHD-activated MDSC-IL13 did not directly drive GVHD lethality, consistent with the finding that survival curves in MDSC-IL13–treated recipients paralleled those of no-MDSC controls after a 2- to 3-week delay. Although IL-18 can also be produced by activated inflammasomes33,34 and is also produced during clinical GVHD, several reports have found IL-18 actually attenuates GVHD.35,36 However, we have been unable to detect active IL-18 secretion from in vitro or ex vivo inflammasome-activated MDSCs (not shown). Thus, we do not favor a role of IL-18 in GVHD suppression by inflammasome-activated MDSCs. In contrast, Arg1 expression, which we have previously shown is critical for the survival benefit conferred by MDSC-IL13 during adoptive transfer,8 was inhibited in inflammasome-activated MDSCs, potentially accounting for the loss of suppression by donor MDSCs.
Members of the NLR family of inflammasome mediators, such as NLRP3, are likely candidates for promoting inflammasome conversion under GVHD conditions, as both ATP37 and associated danger signals are found after conditioning and are known to play a role in enhancing GVHD.16,38,39 In a preliminary study using a nonconditioning model (BALB/c Rag2−/−γ/c−/− recipients) of aGVHD, we found that wild-type and ASC−/− MDSC-IL13 performed similarly in enhancing survival (supplemental Figure 8). These findings implicate the conditioning regimen in concert with allo–T effectors as mediators of inflammasome-mediated loss of function, and future studies will be aimed at alternative induction protocols. NLRP3 inflammasome activation in the context of GVHD has been demonstrated for both radiotherapy and chemotherapy induction protocols, resulting in tissue damage and release of danger-associated molecular patterns.16,37 Our findings show that adoptively transferred MDSCs are also susceptible to inflammasome induction, and the same mediators are likely to contribute to in vivo activation. Reagents selectively targeting the NLRP3 inflammasome may be worth exploring for the dual purpose of inhibiting inflammasome activation in the host and infused donor MDSCs. MCC950,40 a small-molecule inhibitor, and β-hydroxybutyrate,41 a ketone produced under metabolic stress, have demonstrated specificity toward suppressed NLPR3 activation and NLRP3-mediated diseases. Alternatively, viral and bacterial products, such as dsDNA, can also be potent drivers of both GVHD and the AIM2-like receptor family of inflammasomes. Although GVHD development is not dependent on host MyD88/TRIF pathway activity,42 the release of dsDNA by dead/dying cells from radiation and GVHD-induced injury may amplify lethality under some conditions.42,43
Taken together, our data indicate that for the translational potential of donor MDSCs to be fully realized, inhibition of intrinsic inflammasome activation that occurs during the intense inflammatory environment of GVHD should be addressed. Until such a time as in vivo approaches for inhibiting inflammasome activation are available, multiple MDSC doses given during crucial early stages of allo–T-cell priming can be used to provide more continuous suppression by “replacing” MDSCs that have already experienced inflammasome activation.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood grants R01 HL56067 and HL1181879 (B.R.B.); National Institute of Allergy and Infectious Diseases grants AI34495 (B.R.B.), R37-AI029564 and U19-AI067798 (J.P.-Y.T.); National Cancer Institute grants CA156330 (J.P.-Y.T.) and RO1 CA166794 (J.S.S.); and the DFG, Heisenberg Professorship ZE 872/3 (R.Z.).
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.H.K., J.S.M., J.T., D.H.M., W.J.M., J.S.S., D.I.G., V.B., P.J.M., J.P.-Y.T, R.Z., and B.R.B. designed research; B.H.K., V.M., W.J.B., J.M.H., and P.A. performed experiments; J.S.M., D.I.G., P.J.M., and J.P.-Y.T. contributed analytic tools; and B.H.K., P.J.M., and B.R.B. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Mayo Mail Code 109, 420 Delaware St SE, University of Minnesota, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
- 1.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25(1):139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Yu Y, Haarberg K, et al. Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant. 2013;19(5):692–702. doi: 10.1016/j.bbmt.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haverkamp JM, Smith AM, Weinlich R, et al. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity. 2014;41(6):947–959. doi: 10.1016/j.immuni.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markey KA, MacDonald KPA, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124(3):354–362. doi: 10.1182/blood-2014-02-514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124(3):363–373. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic D, Ganesan J, Bscheider M, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210(10):1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruchard M, Mignot G, Derangère V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan BJ, Thomas HE, Pai S, et al. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176(12):7278-7287. [DOI] [PubMed]
- 19.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 20.Reese TA, Liang HE, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, French DL, Ma G, et al. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28(3):620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Tu Z, Qian S, et al. Myeloid-derived suppressor cells as a potential therapy for experimental autoimmune myasthenia gravis. J Immunol. 2014;193(5):2127–2134. doi: 10.4049/jimmunol.1400857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53(3):1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76(3):509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy PL, Jr, Abhyankar S, Neben S, et al. Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood. 1991;78(8):1915–1918. [PubMed] [Google Scholar]
- 32.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131(2):298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 2011;23(5):591–597. doi: 10.1016/j.coi.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P, Teshima T, Hildebrandt G, et al. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101(7):2877–2885. doi: 10.1182/blood-2002-08-2566. [DOI] [PubMed] [Google Scholar]
- 36.Reddy P, Ferrara JLM. Role of interleukin-18 in acute graft-vs-host disease. J Lab Clin Med. 2003;141(6):365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm K, Ganesan J, Müller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16(12):1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 38.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–3213. [PubMed] [Google Scholar]
- 39.Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107(12):1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Matte-Martone C, Tan HS, et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol. 2011;186(1):230–241. doi: 10.4049/jimmunol.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor PA, Ehrhardt MJ, Lees CJ, et al. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;112(8):3508–3516. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]