Abstract
Background
Many studies have examined the relationship between physical activity and metabolic disorders. However, few have focused on specific associations between these disorders and muscular strengthening activity (MSA) patterns. The aim of the present study was to examine the association(s) for each metabolic syndrome criterion and MSA patterns.
Methods
The study sample (n = 5618) consisted of adults ≥20 years of age who participated in the 1999–2004 National Health and Nutrition Examination Survey. Cut-off points for metabolic syndrome criteria were derived from the American Heart Association ⁄ National Heart, Lung, and Blood Institute definition. The aggregate of data on weight lifting, push-ups, and sit-ups was used to establish patterns of MSA. Participants reporting ≥2 days/week MSA were coded as meeting current US MSA guidelines.
Results
Following adjustments, participants reporting ≥2 days/week MSA were found to be 28% (OR 0.72; 95% CI 0.62, 0.83) less likely to have dyslipidemia, 29% (OR 0.71; 95% CI 0.54, 0.93) less likely to have impaired fasting glucose, 19% (OR 0.81; 95% CI 0.65, 0.99) less likely to have prehypertension, and 43% (OR 0.57; 95% CI 0.46, 0.72) less likely to have augmented waist circumference compared with those reporting engaging in no MSA. No association was found for hypertension and MSA.
Conclusion
Engaging in ≥2 days/week MSA as part of an overall physical activity regimen may be prudent in preserving metabolic health. These findings strengthen the relationship between MSA and metabolic health; thus, clinicians should include MSA when discussing lifestyle approaches to better health.
Keywords: abdominal obesity, dyslipidemia, impaired fasting glucose, prehypertension, strength training
Introduction
Cardiovascular disease (CVD) continues to be the leading cause of death in the US.1 The metabolic abnormalities that can lead to an increased risk of CVD that have been the most heavily investigated include poor glucose control (i.e. impaired fasting glucose) or frank diabetes, overweight/obesity (particularly abdominal obesity), hypertriglyceridemia and low high-density lipoprotein–cholesterol (HDL-C; a type of dyslipidemia), and hypertension. An individual who possesses three or more of these CVD risk factors would be classified as having metabolic syndrome.2 Physical activity and exercise have been shown to play a favorable role in the prevention and treatment of these metabolic abnormalities on an individual level3–6 and when they aggregate (i.e. metabolic syndrome).7 The impact of muscular strengthening activities (MSA), either as part of a comprehensive exercise program or as an effective intervention independent of additional exercise modalities, is gaining recognition.8–10 The most recent physical activity guidelines from the Department of Health and Human Services (DHHS) recommend that all US adults should engage in at least 2 days/week MSA.11
Cauza et al.3 reported that 4 months of resistance training improved both glycemic control and lipid profiles better than endurance training in individuals with type 2 diabetes. Following a 2-week period in which the subjects were taught how to perform the exercises, the resistance exercise protocol included 10 different exercises performed three times a week on non-consecutive days. Exercises for all major muscle groups were performed, with sets per exercise being graduated from three to six during the study period. Participants were asked to perform 10–15 repetitions per set, with increases in intensity being made once a subject could easily perform 15 repetitions. The endurance protocol required the subjects to exercise on a cycle ergometer three times a week on non-consecutive days, starting with 15 min per session and gradually reaching 30 min per session during the last 4 weeks of the study. In a cross-sectional analysis, using data from the 2003–2006 National Health and Nutrition Examination Survey (NHANES), Ford et al.5 reported a significant positive association between television viewing time (a proxy for sedentary behavior) and insulin concentrations in US adults. In contrast, that study also illustrated a significant inverse association between leisure time physical activity (LTPA),5 which may include MSA, and insulin concentrations.
Hagerman et al.6 reported a significant reduction in body fat percentage and a trend towards improved lipids in the intervention group compared with controls in older (60–75 years) men following a 16-week high-intensity resistance training program, thus illustrating that significant favorable metabolic changes can occur in older individuals. Following one repetition maximum (1-RM; the amount of weight an individual can lift one time) testing, subjects assigned to the intervention engaged in a resistance exercise protocol that consisted of a brief warm-up, followed by the subjects performing double leg extension, double leg press and half-squat exercises using 85–90% of their 1-RM. The exercise sessions were performed twice a week separated by at least 48 h, and the subjects completed six to eight repetitions. In a recent cross-sectional study using data from the 2007 Behavioral Risk Factor Surveillance Survey, Churilla and Ford4 reported that hypertensive US adults were 15% less likely to report a level of physical activity that would meet the current physical activity recommendation compared with their non-hypertensive counterparts, thus suggesting that engaging in regular physical activity (which may include MSA) should help reduce the burden of hypertension.
The aims of the present study were to: (i) compare the odds specific to having each metabolic syndrome component among individuals reporting engaging in a level of MSA that would meet the current US government guideline of ≥2 days/week to those reporting no MSA; and (ii) examine the potential dose–response relationship between increasing levels of MSA and metabolic health outcomes.
Methods
Sample
The present cross-sectional study used 6 years of data from the most recent 1999–2004 NHANES, a continuous survey conducted by the National Center for Health Statistics.12 The NHANES was designed to provide national estimates of the health and nutritional status of non-institutionalized US civilians over the age of 2 months. Data were collected on a total of 31 126 participants between 1999 and 2004. The final sample for the present study consisted of 5618 US adults ≥20 years of age who met the following criteria: (i) adult men and women who gave informed consent; (ii) attended a mobile examination center examination (MEC) following an overnight fast (minimum of 8 h); (iii) if female, not pregnant; and (iv) had complete data for all variables of interest. The NHANES uses trained staff members to conduct in-home interview-administered questionnaires. Physicians and other health care professionals in the MEC performed standardized medical examinations.
The questionnaires collected demographic information and information regarding physical activity, diet, and current medical conditions. Physician-conducted examinations provided information regarding anthropometrics, blood pressure, and complete blood profiles. Lipid values were determined under the direction of the Lipoprotein Analytical Laboratory at John Hopkins University (Baltimore, MD, USA). Plasma glucose was measured using the hexokinase enzyme reaction at the Diabetes Diagnostic Laboratory at the University of Missouri (Columbia, MO, USA).
Blood pressure readings were obtained after the subject had been seated quietly for 5 min. Three to four consecutive measurements were taken on the same arm using a mercury sphygmomanometer and a Littman Classic stethoscope (3M; St Paul, MN, USA). Waist circumference (WC) was measured using a steel tape at the level of the uppermost lateral borders of the right and left ilium, wrapping the tape around the trunk horizontally. The Institutional Review Board of the University of North Florida approved the use of the 1999–2004 NHANES data.
American Heart Association/National Heart Lung and Blood Institute metabolic syndrome criteria
The dependent variables in the present study were a positive independent diagnosis for each of the criteria defining metabolic syndrome based on the American Heart Association and National Heart Lung and Blood Institute (AHA/NHLBI) definition.13 The AHA/NHLBI defines the following five CVD risk factors: (i) impaired fasting glucose (IFG) ≥100 mg/dL or undergoing pharmacological treatment to lower blood glucose; (ii) triglycerides ≥150 mg/dL; (iii) HDL-C < 40 mg/dL in men or <50 mg/dL in women or undergoing pharmacological treatment for abnormal HDL-C levels; (iv) WC ≥102 cm in men or ≥88 cm in women; and (v) blood pressure ≥130/85 mmHg or undergoing pharmacological treatment for hypertension. In addition, we also examined prehypertension (systolic blood pressure [SBP] 120–139 mmHg or diastolic blood pressure [DBP] 80–89 mmHg) independently.
Muscular strengthening activity
The following questions were used to identify those who reported engaging in MSA and to quantify the amount of MSA that was reported.
Over the past 30 days, did you do any physical activities specifically designed to strengthen your muscles, such as lifting weights, push-ups or sit-ups?
Over the past 30 days, how often did you do these physical activities designed to strengthen your muscles, such as lifting weights, push-ups or sit-ups?
Three levels of MSA were created: (i) no MSA; (ii) MSA <2 days/week; and (iii) MSA ≥2 days/week.
Covariates
Age was divided into seven 10-year age groups: 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years. Four levels of race or ethnicity were used: non-Hispanic White, non-Hispanic Black, Mexican American, and Other race or ethnicity. Education and the family poverty income ratio were both used as measures of socioeconomic status (SES). Education was divided into three categories (completing <12th grade, completing 12th grade, or education beyond 12th grade), whereas family poverty income ratio was divided into five levels (<100%, 100–199%, 200–299%, 300–399%, ≥400%). The standardized poverty threshold represents dollar amounts that define poverty status while accounting for family size. Falling below a poverty threshold of 100% demarcates living in poverty. Alcohol intake in moderation has been shown to have favorable effects in people with metabolic syndrome.14 In the present study, moderate alcohol intake was classified as one drink or less per day in women and two drinks or less per day in men. A three-level alcohol variable was created: (i) above moderate intake; (ii) at or below moderate intake; and (iii) no alcohol intake. Smoking is a primary risk factor for coronary heart disease;15,16 however, the effects of smoking on metabolic syndrome have been equivocal.17,18 In the present study, smoking status was divided into three categories: (i) current smoker; (ii) previous smoker; and (iii) non-smoker. Reported family histories of heart disease and diabetes were also covariates in the present study. The initial metabolic syndrome definition put forward by the National Cholesterol Education Program19 and the updated AHA/NHLBI13 definition were created to help identify those at high-risk for heart disease and diabetes. For a subject to be classified as having a positive family history of heart disease or diabetes, they must have reported either of their parents and/or siblings having the condition. These two variables were dichotomized.
Statistical analysis
Data from 1999–2004 NHANES was analyzed to determine whether there was a difference in the prevalence and risk estimates of individual metabolic syndrome criteria among US adults who reported engaging in various levels of MSA. The data in the present study were managed using SAS 9.1 (SAS Institute, Cary, NC, USA).20 SAS-callable SUDAAN21 was used to conduct the analysis, incorporating sampling weights within the context of the correlated multistage complex sampling design inherent to NHANES. Age-adjusted prevalence estimates were calculated using the Year 2000 US population. For prevalence estimates, non-overlapping 95% confidence intervals (CI) indicate significance. Multivariate logistic regression models were used to estimate OR and 95% CI for each of the prior to metabolic syndrome criteria. Two sets of logistic regression models were developed. The first model adjusted for age, gender, race or ethnicity, SES, smoking status, alcohol consumption, family history of diabetes, and family history of CVD. The second model added LTPA volume.
Results
The characteristics of the study sample are summarized in Table 1.
Table 1.
Characteristics of study sample, NationalHealth and Nutrition Examination Survey 1999-2004
| n | Weighted % (SE) | |
|---|---|---|
| Age (years) | ||
| 20-29 | 871 | 17.9 (1.0) |
| 30-39 | 917 | 21.1 (0.8) |
| 40-49 | 1007 | 22.0 (0.8) |
| 50-59 | 763 | 16.7 (0.8) |
| 60-69 | 949 | 11.2 (0.6) |
| 70-79 | 645 | 7.3 (0.3) |
| ≥80 | 466 | 3.8 (0.2) |
| Men | 2836 | 49.2 (0.5) |
| Women | 2782 | 50.8 (0.5) |
| Race or ethnicity | ||
| White | 2908 | 72.3 (1.8) |
| African American | 1002 | 10.5 (1.0) |
| Mexican American | 1290 | 7.2 (0.9) |
| Other | 418 | 10.0 (1.4) |
| Education | ||
| <High school | 1791 | 20.2 (0.8) |
| High school/GED | 1302 | 26.1 (0.9) |
| >High school | 2515 | 53.7 (1.1) |
| Family PIR | ||
| <0 to <100 | 873 | 12.8 (0.8) |
| >100 to <200 | 1342 | 21.2 (1.0) |
| >200 to <300 | 846 | 15.7 (0.8) |
| >300 to <400 | 628 | 13.8 (0.7) |
| >400 | 1470 | 36.5 (1.4) |
| Smoking status | ||
| Never smoked | 2822 | 49.5 (1.2) |
| Former smoker | 1551 | 26.1 (0.9) |
| Current smoker | 1239 | 24.4 (0.9) |
| Alcohol use | ||
| None | 1857 | 29.7 (1.7) |
| Moderate | 3088 | 62.0 (1.6) |
| Excessive | 381 | 8.3 (0.6) |
| Family history of CVD | 451 | 6.8 (0.3) |
| Family history of diabetes | 449 | 5.7 (0.4) |
GED, General Education Development; PIR, poverty income ratio; CVD, cardiovascular disease.
Dyslipidemia
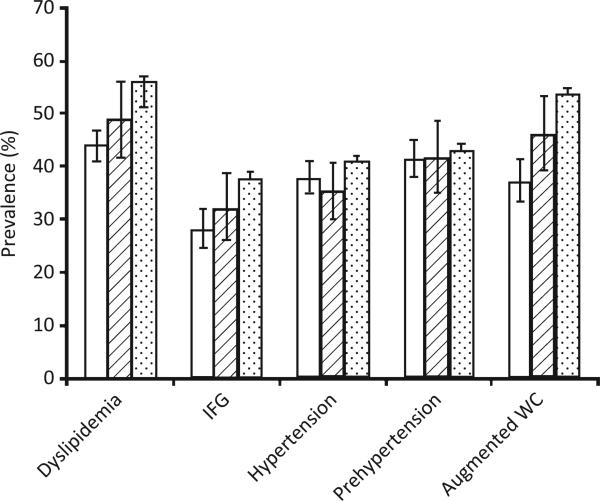
The age-adjusted prevalence estimates of dyslipidemia in US adults reporting engaging in ≥2 days/week MSA (meeting current DHHS recommendation for strength training) and those reporting no MSA were found to be 43.9% (95% CI 41.0, 46.7) and 56.4% (95% CI 54.5, 58.3), respectively (Fig. 1). Individuals reporting engaging in ≥2 days/week MSA were found to be 28% less likely to have dyslipidemia (triglycerides ≥150 mg/dL or HDL-C < 40 mg/dL in men or <50 mg/dL in women) compared with those reporting no MSA (Table 2). Additional significant protective covariates using the full model (including LTPA) were being non-Hispanic Black (referent = non-Hispanic White; OR 0.53; 95% CI 0.45, 0.63), being a former (OR 0.69; 95% CI 0.56, 0.86) or never smoker (referent = current smoker; OR 0.79; 95% CI 0.64, 0.97), reporting moderate (OR 0.76; 95% CI 0.63, 0.91) or above moderate alcohol consumption (referent = no alcohol consumption; OR 0.57; 95% CI 0.42, 0.77), and meeting the DHHS physical activity recommendation (OR 0.83; 95% CI 0.70, 0.99). Significant predictive covariates using the same model were being in an age group >29 years of age but <80 years of age (referent = 20–29 years; OR range 1.34, 1.93; P = 0.0006) and having a family history of CVD (referent = no family history of CVD; OR 2.07; 95% CI 1.53, 2.83).
Figure 1.
Age-adjusted prevalence estimates of metabolic syndrome criteria and prehypertension in US adults aged ≥20 years, according to level of muscular strengthening activity (MSA), National Health and Nutrition Examination Survey 1999–2004. (□), ≥2 days/week MSA; ( ), <2 days/week MSA; (
), <2 days/week MSA; ( ), no MSA. IFG, impaired fasting glucose; WC, waist circumference.
), no MSA. IFG, impaired fasting glucose; WC, waist circumference.
Table 2.
Odds ratios for having each metabolic syndrome criteria and prehypertension among US adults reporting engaging in ≥2 days/week muscular strengthening activity (MSA), the Department of Health and Human Services recommendation, compared with those reporting no MSA, National Health and Nutrition Examination Survey 1999-2004
| OR† | 95% CI | OR‡ | 95% CI | |
|---|---|---|---|---|
| Dyslipidemia | ||||
| Yes | 0.68† | 0.59, 0.79 | 0.72‡ | 0.62, 0.83 |
| No | 1.00 | |||
| Impaired fasting glucose | ||||
| Yes | 0.67† | 0.51, 0.88 | 0.71† | 0.54, 0.93 |
| No | 1.00 | |||
| Hypertension | ||||
| Yes | 0.82 | 0.67, 1.02 | 0.88 | 0.71, 1.09 |
| No | 1.00 | |||
| Prehypertension | ||||
| Yes | 0.80† | 0.65, 0.98 | 0.81† | 0.65, 0.99 |
| No | 1.00 | |||
| Augmented WC | ||||
| Yes | 0.53‡ | 0.43, 0.65 | 0.57‡ | 0.46, 0.72 |
| No | 1.00 | |||
P < 0.05
P < 0.01.
Model 1, adjusted for age, gender, alcohol consumption, family history of diabetes, family history of cardiovascular disease, smoking status, race, and socioeconomic status.
Model 1 plus total leisure time physical activity volume.
WC, waist circumference.
Impaired fasting glucose
The age-adjusted prevalence estimates of IFG in US adults reporting engaging in ≥2 days/week MSA (meeting current DHHS recommendation for strength training) and those reporting no MSA were found to be 28.3% (95% CI 24.8, 32.1) and 38.0% (95% CI 35.2, 40.9), respectively (Fig. 1). Individuals reporting engaging in ≥2 days/week MSA were found to be 29% less likely to have IFG (fasting blood glucose ≥100 mg/dL) compared with those reporting no MSA (Table 2). Other significant protective covariates using the full model (including LTPA) were being female (OR 0.41; 95% CI 0.36, 0.45), having an education greater than a high school (OR 0.75; 95% CI 0.59, 0.95), and having an annual income ≥400% of poverty (referent = <100% of poverty; OR 0.75; 95% CI 0.60, 0.93). Significant predictive covariates using the same model were being in an age group >29 years of age (referent = 20–29 years; OR range 1.84, 9.45; P < 0.0001), being of a race/ethnicity other than non-Hispanic White, non-Hispanic Black or Mexican American (referent = non-Hispanic White; OR 1.39; 95% CI 1.01, 1.93), and having a family history of diabetes (OR 1.81; 95% CI 1.28, 2.56).
Hypertension and prehypertension
The age-adjusted prevalence estimates of hypertension in US adults reporting engaging in ≥2 days/week MSA (meeting current DHHS recommendation for strength training) and those reporting no MSA were found to be 37.9% (95% CI 34.8, 41.0) and 41.1% (95% CI 39.2, 43.1), respectively (Fig. 1). No protection was found for individuals reporting engaging in ≥2 days/week MSA (Table 2). Using the full model, the significant protective covariates were being female (OR 0.69; 95% CI 0.58, 0.81) and being Mexican American (OR 0.64; 95% CI 0.48, 0.86). Significant predictive covariates were being in an age group >29 years of age (referent = 20–29 years; OR range 1.64, 27.71; P < 0.0001), being non-Hispanic black (OR 1.62; 95% CI 1.29–2.03), and being a former smoker (OR 1.46; 95% CI 1.12, 1.90) or current smoker (OR 1.70; 95% CI 1.35, 2.13).
The age-adjusted prevalence estimates of prehypertension in US adults reporting engaging in ≥2 days/week MSA and those reporting no MSA were found to be similar at 41.5% (95% CI 38.0, 45.0) and 43.2% (95% CI 41.0, 45.3), respectively. However, individuals reporting engaging in ≥2 days/week MSA were found to be 19% less likely to have prehypertension (SBP 120–139 mmHg or DBP 80–89 mmHg) compared with those reporting no MSA (Table 2).
Augmented WC
The age-adjusted prevalence estimates of US adults with augmented WC reporting engaging in ≥2 days/week MSA (meeting current DHHS recommendation for strength training) and those reporting no MSA were found to be 37.3% (95% CI 33.4, 41.4) and 53.7% (95% CI 51.8, 55.6), respectively (Fig. 1). Individuals reporting engaging in ≥2 days/week MSA were found to be 43% less likely to have an augmented WC (≥102 cm in men and ≥88 cm in women) compared with those reporting no MSA (Table 2). From the full model, other significant protective covariates were being of a race/ethnicity other than non-Hispanic White, non-Hispanic Black or Mexican American (referent = non-Hispanic white; OR 0.64; 95% CI 0.48, 0.85), moderate alcohol consumption (referent = no alcohol consumption; OR 0.72; 95% CI 0.60, 0.87) and meeting the DHHS physical activity recommendation (OR 0.80; 95% CI 0.65, 0.97). Using the same model, significant predictive covariates were being in an age group >29 years of age (referent = 20–29 years; OR range 1.48–3.69; P < 0.0001), being female (OR 2.00; 95% CI 1.67, 2.41), having a high school education (referent =<high school; OR 1.23; 95% CI 1.02, 1.49), and having a family history of diabetes (OR 1.53; 95% CI 1.08, 2.16).
Dose–response
Figure 1 illustrates a consistent inverse dose–response relationship for having dyslipidemia (Ptrend < 0.01), IFG (Ptrend < 0.05), and an augmented WC (Ptrend < 0.01) for those reporting engaging in MSA ≥2 days/week, some MSA (<2 days/week), and engaging in no MSA, respectively. No dose–response relationship between MSA and prehypertension or hypertension was found.
Discussion
To our knowledge this is one of the first studies to examine the association(s) between individual metabolic syndrome criteria and engaging in a level of MSA that meets the most recent DHHS physical activity recommendation in US adults.11 Our findings illustrate a potential inverse dose–response relationship between dyslipidemia, IFG, and having an augmented WC for various levels of MSA. In contrast, our study found no relationship between MSA and hypertension. However, individuals reporting engaging in MSA ≥2 days/week were found to be less likely to have prehypertension compared with those reporting engaging in no MSA.
The findings from MSA studies examining the impact or association on lipids have been equivocal.22,23 Kokkinos et al.22 reported significant increases in both upper (50%) and lower body (37%) muscular strength, but no changes in any lipid parameters following a 20-week resistance training program in middle-aged men who began the study with abnormal lipid values. Prabhakaran et al.23 reported significant improvements in lipid profiles and body fat percentage following a 14-week high-intensity (85% 1-RM) resistance-training program in premenopausal women compared with sedentary controls. Our study suggests potential protection from dyslipidemia for increasing levels of MSA.
Our findings also illustrate favorable associations between IFG and MSA. Castaneda et al.24 reported improved HbA1c (an indicator of long-term glucose control), increased muscle glycogen storage, and an attenuation in necessary pharmacotherapy following a 16-week progressive resistance-training program in individuals with type 2 diabetes compared with controls. Holten et al.25 reported increases in insulin action in the skeletal muscle of both euglycemic and individuals with type 2 diabetes following 6 weeks of strength training beginning with intensities of 50% 1-RM (initial 2 weeks) and ending with intensities between 70% and 80% 1-RM (latter 3–6 weeks). Significant increases in skeletal muscle levels of the primary glucose transporter protein GLUT-4 were also reported. This increase was attributed to skeletal muscle contractions performed three times a week for 30 min.
Engaging in MSA will lead to favorable changes in lean body mass, thus favorably impacting insulin sensitivity as long as body fat is maintained at a desirable level. This is due to the inverse relationship between subcutaneous fat GLUT-4 expression and body mass index.26 Some studies that have examined the association between MSA and body fat percentage have illustrated attenuations in percent body fat similar to those for aerobic-type activities; however, the reductions in body fat are suggested to be mediated by increases in fat-free mass, not directly from energy expenditure from resistance training.27 In addition, basal metabolic rates following acute MSA programs have been shown to be greater than after aerobic-type exercise programs, suggesting that MSA may play an important role in maintaining energy balance and a desirable body weight.28 Our findings suggest that engaging in a level of MSA that would meet the current DHHS recommendation may result in the attenuation of central obesity (i.e. abdominal obesity).
Whereas our study found no relationship between MSA and hypertension in US adults, we did find reduced odds of prehypertension in those meeting the MSA recommendation, which may have clinical significance in primary prevention. A meta-analysis of MSA interventions of both normotensive and hypertensive adults has shown a modest but significant reduction in SBP and DBP of approximately 3 mmHg.29 Modest reductions in blood pressure of this magnitude have been estimated to reduce coronary heart disease by 5–9%, stroke by 8–14%, and all-cause mortality by 4%, yet may not be sufficient to produce normotensive results in hypertensive individuals.30,31 It may be prudent to mention here that aerobic exercise has been more efficacious than resistance exercise as a blood pressure intervention and is the recommended type of exercise to be implemented in the prevention and treatment of hypertension.32 However, MSA provides modest additional or concomitant benefit.
Including MSA as part of a structured exercise program is one way to potentially improve and manage metabolic health. However, various lifestyle modifications have been shown to impact favorably on metabolic health risk. A loss of 5–10% of body weight in obese individuals has been shown to attenuate triglycerides by 20% and augment HDL-C by approximately 8–10%.33 Individuals with metabolic syndrome in the PREMIER Lifestyle Interventions for Blood Pressure Control trial demonstrated drops in blood pressure following the adoption of the dietary approaches to stop hypertension (DASH) dietary pattern.34 Increasing steps per day35 and increasing non-exercise activity thermogenesis (NEAT),36 for example using a walking workstation in the workplace, are a few lifestyle methods that have been shown to improve metabolic health profiles. Additional areas of research that have shown potential in ameliorating metabolic health risk are taking breaks from sedentary time (e.g. prolonged sitting)37,38 and limiting leisure time sedentary behavior (LTSB) to 1 h or less per day.39 More research needs to be performed in the area of reducing sedentary behavior.
Several limitations of the present study merit consideration. First, the cross-sectional nature of the study precludes establishing directionality of the associations. Second, MSA was derived from questions that were answered by the respondents and is subject to some degree of error (social desirability bias). Third, we used logistic regression analysis to calculate ORs. Because of the high prevalence of metabolic syndrome, the ORs should be viewed as measures of association rather than approximating prevalence ratios. Finally, we used definitions of the components of the metabolic syndrome according to the AHA/NHLBI criteria, which include the use of medications as part of the definitions. It is unclear whether excluding participants who used various medications to treat abnormal levels of metabolic syndrome components may have meaningfully affected the estimated OR.
Conclusions
Increasing levels of physical activity and exercise have been shown consistently to favorably impact metabolic health and improve fitness. The majority of people who engage in regular physical activity or exercise participate in aerobic-type activities, with walking being the most popular form of physical activity. Engaging in ≥2 days/week MSA as part of an overall physical activity regimen can increase lean body mass and reduce adiposity, augment skeletal muscle and adipose tissue insulin sensitivity, and may favorably alter lipid profiles. Our findings suggest that participating in regular MSA may be prudent in preserving metabolic health.
Significant findings of the study: US adults reporting engaging in ≥2 days/week MSA may be significantly less likely to have impaired fasting glucose, dyslipidemia, abdominal obesity, and prehypertension.
What this study adds: A novel perspective to the area of strength training and metabolic health. The present study is one of the first to report on the favorable dose–response effects of MSA and various metabolic markers in a representative sample of the US adult population.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure
None declared.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Churilla JR, Fitzhugh EC, Thompson DL. The metabolic syndrome: How definition impacts the prevalence and risk in US adults: 1999–2004 NHANES. Metab Syndr Relat Disord. 2007;5:331–42. doi: 10.1089/met.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cauza E, Hanusch-Enserer U, Strasser B, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–33. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Churilla JR, Ford ES. Comparing physical activity patterns of hypertensive and nonhypertensive US adults. Am J Hypertens. 2010;23:987–93. doi: 10.1038/ajh.2010.88. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism. 2010;59:1268–75. doi: 10.1016/j.metabol.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Hagerman FC, Walsh SJ, Staron RS, et al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55:B336–46. doi: 10.1093/gerona/55.7.b336. [DOI] [PubMed] [Google Scholar]
- 7.Churilla JR, Fitzhugh EC. Relationship between leisure-time physical activity and metabolic syndrome using varying definitions: 1999–2004 NHANES. Diab Vasc Dis Res. 2009;6:100–9. doi: 10.1177/1479164109336040. [DOI] [PubMed] [Google Scholar]
- 8.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–22. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 10.Jurca R, Lamonte MJ, Church TS, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–7. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services . 2008 2008 physical activity guidelines for Americans. US department of health and human services; 2008. [27 April 2011]. Available at http://www.health.gov/paguidelines.pdf. [Google Scholar]
- 12.National Center for Health Statistics Centers for Disease Control and Prevention [27 April 2011];NHANES Analytic and Reporting Guidelines June 2004 Version. Available at http:// www.cdc.gov/nchs/nhanes.htm.
- 13.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Dixon JB, Dixon ME, O'Brien PE. Alcohol consumption in the severely obese: Relationship with the metabolic syndrome. Obes Res. 2002;10:245–52. doi: 10.1038/oby.2002.33. [DOI] [PubMed] [Google Scholar]
- 15.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: A statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96:3243–7. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 16.Tonstad S, Andrew Johnston J. Cardiovascular risks associated with smoking: A review for clinicians. Eur J Cardiovasc Prev Rehabil. 2006;13:507–14. doi: 10.1097/01.hjr.0000214609.06738.62. [DOI] [PubMed] [Google Scholar]
- 17.Masulli M, Riccardi G, Galasso R, Vaccaro O. Relationship between smoking habits and the features of the metabolic syndrome in a non-diabetic population. Nutr Metab Cardiovasc Dis. 2006;16:364–70. doi: 10.1016/j.numecd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Onat A, Ozhan H, Esen AM, et al. Prospective epidemiologic evidence of a “protective” effect of smoking on metabolic syndrome and diabetes among Turkish women-without associated overall health benefit. Atherosclerosis. 2007;193:380–8. doi: 10.1016/j.atherosclerosis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program (NCEP) Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute . Base SAS 9.1.3 Procedures Guide. 2nd edn 1–4. SAS Institute; Cary: 2006. [Google Scholar]
- 21.Research Triangle Institute. SUDAAN Language Training Manual 9. Research Triangle Institute; Research Triangle Park, NC: 2004. [Google Scholar]
- 22.Kokkinos PF, Hurley BF, Smutok MA, et al. Strength training does not improve lipoprotein-lipid profiles in men at risk for CHD. Med Sci Sports Exerc. 1991;23:1134–9. [PubMed] [Google Scholar]
- 23.Prabhakaran B, Dowling EA, Branch JD, Swain DP, Leutholtz BC. Effect of 14 weeks of resistance training on lipid profile and body fat percentage in premenopausal women. Br J Sports Med. 1999;33:190–5. doi: 10.1136/bjsm.33.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–41. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 25.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 26.Trischitta V, Frittitta L, Vigneri R. Early molecular defects in human insulin resistance: Studies in healthy subjects with low insulin sensitivity. Diabetes Metab Rev. 1997;13:147–62. doi: 10.1002/(sici)1099-0895(199709)13:3<147::aid-dmr180>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Poehlman ET, Melby C. Resistance training and energy balance. Int J Sport Nutr. 1998;8:143–59. doi: 10.1123/ijsn.8.2.143. [DOI] [PubMed] [Google Scholar]
- 28.Melby C, Scholl C, Edwards G, Bullough R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J Appl Physiol. 1993;75:1847–53. doi: 10.1152/jappl.1993.75.4.1847. [DOI] [PubMed] [Google Scholar]
- 29.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure : A meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–43. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 30.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14:570–7. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- 31.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: Clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–8. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 32.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American college of sports medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 33.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–6. [PubMed] [Google Scholar]
- 34.Lien LF, Brown AJ, Ard JD, et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension. 2007;50:609–16. doi: 10.1161/HYPERTENSIONAHA.107.089458. [DOI] [PubMed] [Google Scholar]
- 35.Schneider PL, Bassett DR, Jr, Thompson DL, Pronk NP, Bielak KM. Effects of a 10 000 steps per day goal in overweight adults. Am J Health Promot. 2006;21:85–9. doi: 10.4278/0890-1171-21.2.85. [DOI] [PubMed] [Google Scholar]
- 36.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr. 2000;72:1451–4. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- 37.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 38.Swartz AM, Squires L, Strath SJ. Energy expenditure of interruptions to sedentary behavior. Int J Behav Nutr Phys Act. 2011;8:69. doi: 10.1186/1479-5868-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in US men and women. Metab Syndr Relat Disord. 2009;7:529–36. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]