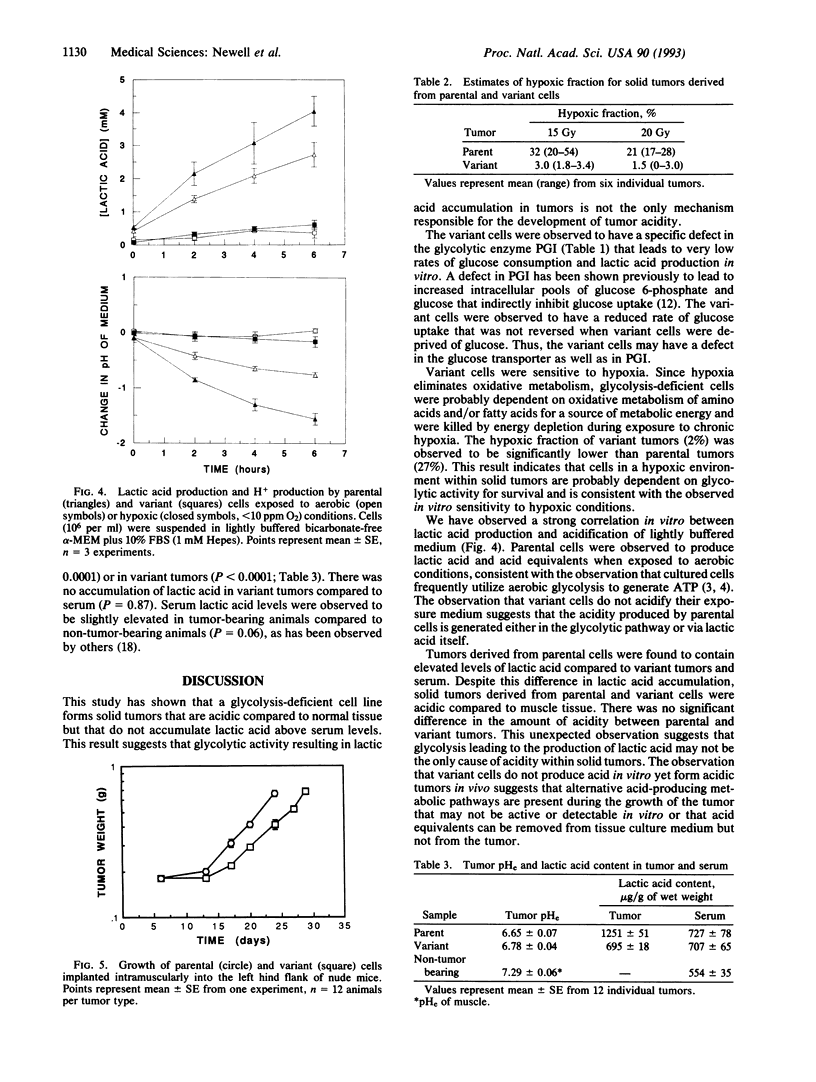
Abstract
Solid tumors have been observed to develop an acidic extracellular environment, which is believed to occur as a result of lactic acid accumulation produced during aerobic and anaerobic glycolysis. Experiments using glycolysis-deficient ras-transfected Chinese hamster lung fibroblasts have been performed to test the hypothesis that lactic acid production within solid tumors is responsible for the development of tumor acidity. The variant cells have defects in glucose transport and in the glycolytic enzyme phosphoglucose isomerase with 1% activity compared to parental cells. Consequently, the in vitro rate of lactic acid production by variant cells was < 4% compared to parental cells. An in vitro correlation between lactic acid production and acidification of exposure medium was observed for parental and variant cells. Implantation of both cell lines into nude mice led to tumors with minimal difference in growth rate. As expected, variant cells died when exposed to hypoxic conditions in culture, and parental tumors were observed to have a larger fraction of cells resistant to radiation due to hypoxia (27%) than variant tumors (2%). Using pH microelectrodes, parental (n = 12) and variant (n = 12) tumors were observed to have extracellular pH (pHe) values of 6.65 +/- 0.07 and 6.78 +/- 0.04 (mean +/- SE, P = 0.13), respectively, whereas normal muscle had a pHe of 7.29 +/- 0.06 (P < 0.0001 for both cell lines). The lactic acid content of variant tumors was found to be similar to that in serum, whereas parental tumors had lactic acid content that was higher than in serum (P < 0.0001). We conclude that there was no correlation between lactic acid content and acidosis for these tumors derived from ras-transfected fibroblasts. These results provide evidence that the production of lactic acid via glycolysis is not the only mechanism responsible for the development of an acidic environment within solid tumors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argilés J. M., López-Soriano F. J. The energy state of tumor-bearing rats. J Biol Chem. 1991 Feb 15;266(5):2978–2982. [PubMed] [Google Scholar]
- Dickson J. A., Calderwood S. K. Effects of hyperglycemia and hyperthermia on the pH, glycolysis, and respiration of the Yoshida sarcoma in vivo. J Natl Cancer Inst. 1979 Dec;63(6):1371–1381. [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Smith S. H., Haggerty A. C. Modifications of the acid-base status of the internal milieu of tumors. J Natl Cancer Inst. 1965 Jun;34(6):857–869. [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Tyler G., Mueller-Klieser W., Vaupel P. Growth-related changes of oxygen consumption rates of tumor cells grown in vitro and in vivo. J Cell Physiol. 1989 Jan;138(1):183–191. doi: 10.1002/jcp.1041380124. [DOI] [PubMed] [Google Scholar]
- Kalmus J., Okunieff P., Vaupel P. Effect of intraperitoneal versus intravenous glucose administration on laser Doppler flow in murine FSaII tumors and normal skin. Cancer Res. 1989 Nov 15;49(22):6313–6317. [PubMed] [Google Scholar]
- Meyerson L. R., Nesta D. [3H]acetazolamide binding to carbonic anhydrase in normal and transformed cells. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):995–1000. doi: 10.1016/0006-2952(91)90206-k. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Salomon J. C., Silvestre P. Isolation of a Chinese hamster fibroblast mutant defective in hexose transport and aerobic glycolysis: its use to dissect the malignant phenotype. Proc Natl Acad Sci U S A. 1980 May;77(5):2698–2701. doi: 10.1073/pnas.77.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistlethwaite A. J., Alexander G. A., Moylan D. J., 3rd, Leeper D. B. Modification of human tumor pH by elevation of blood glucose. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):603–610. doi: 10.1016/0360-3016(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Tobari C., Van Kersen I., Hahn G. M. Modification of pH of normal and malignant mouse tissue by hydralazine and glucose, with and without breathing of 5% CO2 and 95% air. Cancer Res. 1988 Mar 15;48(6):1543–1547. [PubMed] [Google Scholar]
- Varnes M. E., Tuttle S. W., Biaglow J. E. Nitroheterocycle metabolism in mammalian cells. Stimulation of the hexose monophosphate shunt. Biochem Pharmacol. 1984 May 15;33(10):1671–1677. doi: 10.1016/0006-2952(84)90290-9. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Weinberg M. J., Lapointe T. A., Rauth A. M. Growth delay in a murine squamous cell tumor after local radiation and concurrent infusional 5-fluorouracil treatment. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1449–1452. doi: 10.1016/0360-3016(86)90192-6. [DOI] [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]



