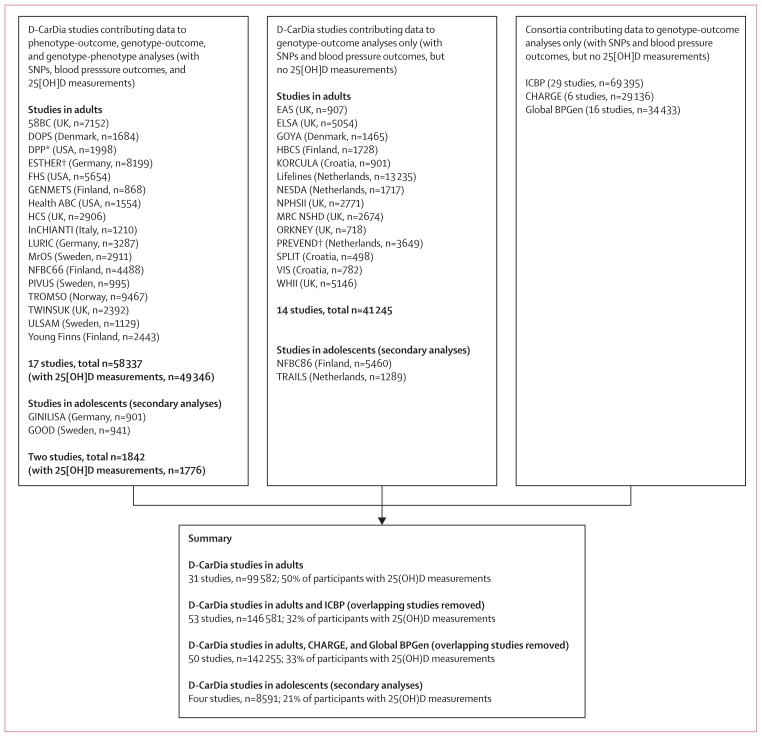
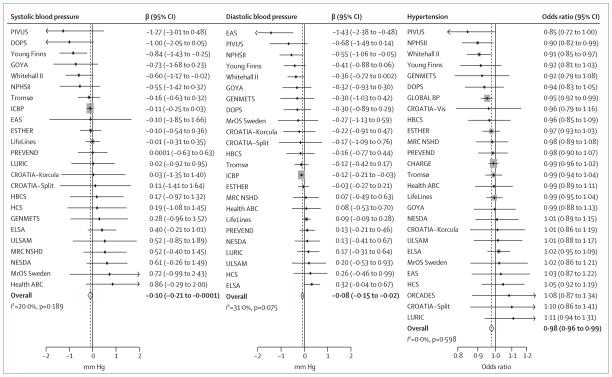
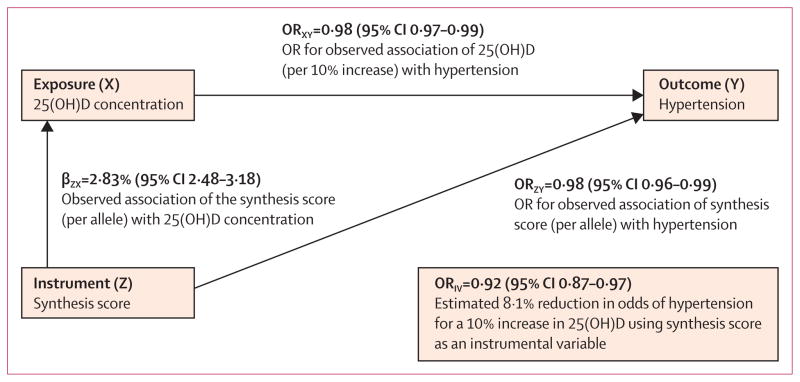
Karani S Vimaleswaran
Karani S Vimaleswaran, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,*,
Alana Cavadino
Alana Cavadino, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,*,
Diane J Berry
Diane J Berry, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1;
LifeLines Cohort Study investigators1,
Rolf Jorde
Prof Rolf Jorde, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Aida Karina Dieffenbach
Aida Karina Dieffenbach, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Chen Lu
Chen Lu, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Alexessander Couto Alves
Alexessander Couto Alves, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Hiddo J Lambers Heerspink
Hiddo J Lambers Heerspink, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Emmi Tikkanen
Emmi Tikkanen, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Joel Eriksson
Joel Eriksson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Andrew Wong
Andrew Wong, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Massimo Mangino
Massimo Mangino, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Kathleen A Jablonski
Kathleen A Jablonski, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ilja M Nolte
Ilja M Nolte, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Denise K Houston
Denise K Houston, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Tarunveer Singh Ahluwalia
Tarunveer Singh Ahluwalia, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Peter J van der Most
Peter J van der Most, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Dorota Pasko
Dorota Pasko, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Lina Zgaga
Lina Zgaga, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Elisabeth Thiering
Elisabeth Thiering, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Veronique Vitart
Veronique Vitart, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ross M Fraser
Ross M Fraser, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jennifer E Huffman
Jennifer E Huffman, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Rudolf A de Boer
Prof Rudolf A de Boer, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ben Schöttker
Ben Schöttker, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Kai-Uwe Saum
Kai-Uwe Saum, MPH
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Mark I McCarthy
Prof Mark I McCarthy, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Josée Dupuis
Prof Josée Dupuis, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Karl-Heinz Herzig
Prof Karl-Heinz Herzig, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Sylvain Sebert
Sylvain Sebert, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Anneli Pouta
Anneli Pouta, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jaana Laitinen
Jaana Laitinen, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Marcus E Kleber
Marcus E Kleber, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Gerjan Navis
Prof Gerjan Navis, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Mattias Lorentzon
Prof Mattias Lorentzon, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Karen Jameson
Karen Jameson, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Nigel Arden
Prof Nigel Arden, DM
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jackie A Cooper
Jackie A Cooper, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jayshree Acharya
Jayshree Acharya, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Rebecca Hardy
Prof Rebecca Hardy, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Olli Raitakari
Prof Olli Raitakari, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Samuli Ripatti
Prof Samuli Ripatti, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Liana K Billings
Liana K Billings, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jari Lahti
Jari Lahti, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Clive Osmond
Prof Clive Osmond, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Brenda W Penninx
Prof Brenda W Penninx, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Lars Rejnmark
Lars Rejnmark, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Kurt K Lohman
Kurt K Lohman, MSat
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Lavinia Paternoster
Lavinia Paternoster, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ronald P Stolk
Prof Ronald P Stolk, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Dena G Hernandez
Dena G Hernandez, MS
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Liisa Byberg
Liisa Byberg, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Emil Hagström
Emil Hagström, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Håkan Melhus
Prof Håkan Melhus, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Erik Ingelsson
Prof Erik Ingelsson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Dan Mellström
Prof Dan Mellström, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Östen Ljunggren
Prof Östen Ljunggren, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ioanna Tzoulaki
Ioanna Tzoulaki, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Stela McLachlan
Stela McLachlan, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Evropi Theodoratou
Evropi Theodoratou, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Carla M T Tiesler
Carla M T Tiesler, MSc
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Antti Jula
Antti Jula, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Pau Navarro
Pau Navarro, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Alan F Wright
Prof Alan F Wright, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Ozren Polasek
Ozren Polasek, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1;
International Consortium for Blood Pressure (ICBP), Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium, Global Blood Pressure Genetics (Global BPGen) consortium1,
Caroline Hayward
Caroline Hayward, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
James F Wilson
James F Wilson, DPhil
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Igor Rudan
Prof Igor Rudan, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Veikko Salomaa
Prof Veikko Salomaa, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Joachim Heinrich
Joachim Heinrich, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Harry Campbell
Prof Harry Campbell, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jacqueline F Price
Jacqueline F Price, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Magnus Karlsson
Prof Magnus Karlsson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Lars Lind
Lars Lind, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Karl Michaëlsson
Prof Karl Michaëlsson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Stefania Bandinelli
Stefania Bandinelli, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Timothy M Frayling
Prof Timothy M Frayling, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Catharina A Hartman
Catharina A Hartman, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Thorkild I A Sørensen
Prof Thorkild I A Sørensen, DrMedSci
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Stephen B Kritchevsky
Prof Stephen B Kritchevsky, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Bente Lomholt Langdahl
Prof Bente Lomholt Langdahl, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Johan G Eriksson
Prof Johan G Eriksson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Jose C Florez
Prof Jose C Florez, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Tim D Spector
Prof Tim D Spector, FRCP
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Terho Lehtimäki
Prof Terho Lehtimäki, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Diana Kuh
Prof Diana Kuh, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Steve E Humphries
Prof Steve E Humphries, FRCP
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Cyrus Cooper
Prof Cyrus Cooper, FMedSci
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Claes Ohlsson
Prof Claes Ohlsson, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Winfried März
Prof Winfried März, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Martin H de Borst
Martin H de Borst, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Meena Kumari
Prof Meena Kumari, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Mika Kivimaki
Prof Mika Kivimaki, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Thomas J Wang
Prof Thomas J Wang, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Chris Power
Prof Chris Power, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Hermann Brenner
Prof Hermann Brenner, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Guri Grimnes
Guri Grimnes, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Pim van der Harst
Pim van der Harst, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Harold Snieder
Prof Harold Snieder, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Aroon D Hingorani
Prof Aroon D Hingorani, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Stefan Pilz
Prof Stefan Pilz, MD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
John C Whittaker
Prof John C Whittaker, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Marjo-Riitta Järvelin
Prof Marjo-Riitta Järvelin, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1,
Elina Hyppönen
Prof Elina Hyppönen, PhD
1Population, Policy and Practice, UCL Institute of Child Health, London, UK (K S Vimaleswaran, PhD, A Cavadino, MSc, D J Berry, PhD, Prof. C Power, PhD, Prof. E Hyppönen, PhD); Hugh Sinclair Unit of Human Nutrition, Department of Food & Nutritional Sciences, School of Chemistry, Food & Pharmacy, University of Reading, Reading, UK (K S Vimaleswaran); Tromsø Endocrine Research Group, Department of Clinical Medicine, University of Tromsø, Tromsø, Norway (Prof. R Jorde, PhD, G Grimnes, MD); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (A K Dieffenbach, PhD, B Schöttker, PhD, K-U Saum, MPH, Prof. H Brenner, MD); Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA (C Lu, PhD, Prof. J Dupuis, PhD); Department of Epidemiology and Biostatistics (A C Alves, PhD, Prof. M-R Järvelin, PhD), Faculty of Medicine, School of Public Health (I Tzoulaki, PhD), and MRC-PHE Centre for Environment & Health (M-R Järvelin), Imperial College London, London, UK; Department of Clinical Pharmacology (H J L Heerspink, PhD), Department of Epidemiology (I M Nolte, PhD, Prof. H Snieder, PhD, P J van der Most, MSc, Prof. R P Stolk, PhD), Department of Psychiatry (C A Hartman, PhD), Department of Cardiology (Prof. R A de Boer, MD, P van der Harst, MD), and Department of Internal Medicine, Division of Nephrology (Prof. G Navis, MD, M H de Borst, MD), University Medical Center, University of Groningen, Groningen, Netherlands; Institute for Molecular Medicine Finland, Tukholmankatu, Finland (E Tikkanen, PhD); Centre for Bone and Arthritis Research, Institute of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (J Eriksson, MD, Prof. M Lorentzon, MD, Prof. D Mellström, MD, Prof. C Ohlsson, MD); MRC Unit for Lifelong Health and Ageing (A Wong, PhD, Prof. R Hardy, PhD, Prof. D Kuh, PhD), Cardiovascular Genetics, BHF Laboratories, Institute of Cardiovascular Science (J A Cooper, MSc, J Acharya, PhD, Prof. S E Humphries, FRCP), Genetic Epidemiology Group (Prof. A D Hingorani, PhD), and Department of Epidemiology and Public Health (Prof. M Kumari, PhD, Prof. M Kivimaki, PhD), University College London, London, UK; Department of Twin Research & Genetic Epidemiology, King’s College London, St Thomas’ Campus, London, UK (M Mangino, PhD, Prof. T D Spector, FRCP); Biostatistics Center, Department of Epidemiology and Biostatistics, School of Public Health, George Washington University, Rockville, MD, USA (K A Jablonski, PhD); Gerontology and Geriatric Medicine, Department of Internal Medicine, and J Paul Sticht Center on Aging (D K Houston, PhD, Prof. S B Kritchevsky, PhD), and Department of Biostatistical Sciences, Division of Public Health Sciences (K K Lohman, MStat), Wake Forest School of Medicine, Winston-Salem, NC, USA; Metabolic Genetics, Novo Nordisk Foundation Centre for Basic Metabolic Research (T S Ahluwalia, PhD, Prof. T I A Sørensen, DrMedSci), and Copenhagen Prospective Studies on Asthma in Childhood (T S Ahluwalia), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark (T I A Sørensen); Danish Pediatric Asthma Center, Gentofte Hospital, Copenhagen, Denmark (T S Ahluwalia); Genetics of Complex Traits, University of Exeter Medical School, Exeter, UK (Dorota Pasko, MSc, Prof. T M Frayling, PhD); Centre for Population Health Sciences (L Zgaga, PhD, Prof. H Campbell, MD, E Theodoratou, PhD, R M Fraser, PhD, J F Wilson, DPhil, Prof. I Rudan, MD, J F Price, MD, S McLachlan, MD), and MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine (V Vitart, PhD, P Navarro, PhD, J E Huffman, MSc, C Hayward, PhD, Prof. A F Wright, PhD), University of Edinburgh, Edinburgh, UK; Department of Public Health and Primary Care, Trinity College Dublin, Dublin, Ireland (L Zgaga); Institute of Epidemiology I, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (E Thiering, MSc, C M T Tiesler, MSc, J Heinrich, PhD); Division of Metabolic Diseases and Nutritional Medicine (E Thiering), and Institute of Medical Informatics, Biometry and Epidemiology (C M T Tiesler), Ludwig Maximilian University of Munich, Dr von Hauner Children’s Hospital, Munich, Germany; Oxford Centre for Diabetes, Endocrinology and Metabolism (Prof. M I McCarthy, MD), Wellcome Trust Centre for Human Genetics (M I McCarthy, Prof. E Ingelsson, MD), and NIHR Oxford Musculoskeletal Biomedical Research Unit (Prof. C Cooper, FMedSci, Prof. N Arden, DM), University of Oxford, Oxford, UK; Oxford NIHR Biomedical Research Centre, Oxford, UK (M I McCarthy); National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, MA, USA (J Dupuis); Institute of Biomedicine (Prof. K H Herzig, MD), Biocenter Oulu (K H Herzig, S Sebert, PhD, M-R Järvelin), and Institute of Health Sciences (M-R Järvelin, A C Alves, S Sebert), University of Oulu, Oulu, Finland; Medical Research Center Oulu (K H Herzig), and Obstetrics and Gynecology, Department of Clinical Sciences (A Pouta, MD), and Unit of Primary Care (M-R Järvelin), Oulu University Hospital, Oulu, Finland; National Institute for Health and Welfare, Oulu, Finland (A Pouta, M-R Järvelin); Finnish Institute of Occupational Health, Helsinki, Finland (J Laitinen, PhD); Medical Clinic V (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Mannheim Medical Faculty, University of Heidelberg, Mannheim, Germany (M E Kleber, PhD, Prof. W März, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK (K Jameson, MSc, C Cooper, N Arden, Prof. C Osmond, PhD); Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland (Prof. O Raitakari, MD); Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland (O Raitakari); Institute for Molecular Medicine Finland (Prof. S Ripatti, PhD, E Tikkanen), Hjelt Institute (S Ripatti, E Tikkanen), Institute of Behavioural Sciences (J Lahti, PhD), and Department of General Practice and Primary Health Care (Prof. J G Eriksson, MD), University of Helsinki, Helsinki, Finland; Department of Psychiatry, EMGO Institute, VU University Medical Centre, Amsterdam, Netherlands (Prof. B W Penninx, PhD); Center for Human Genetic Research and Diabetes Research Center (Diabetes Unit), Massachusetts General Hospital, Boston, MA, USA (L K Billings, MD, Prof. J C Florez, MD); Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA (L K Billings, J C Florez); Department of Medicine, Harvard Medical School, Boston, MA, USA (L K Billings, J C Florez); NorthShore University HealthSystem, Evanston, IL, USA (L K Billings); Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark (L Rejnmark, MD, Prof. B L Langdahl, MD); MRC Integrative Epidemiology Unit, School of Social and Community Medicine, University of Bristol, Bristol, UK (L Paternoster, PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, MS); Department of Surgical Sciences (L Byberg, PhD, Prof. K Michaëlsson, MD), Uppsala Clinical Research Centre, Department of Medical Sciences (E Hagström, MD, Prof. H Melhus, MD, E Ingelsson, Prof. Ö Ljunggren, MD, L Lind, MD), and Molecular Epidemiology and Science for Life Laboratory (E Ingelsson), Uppsala University, Uppsala, Sweden; Department of Chronic Disease Prevention, National Institute for Health and Welfare, Turku, Finland (A Jula, MD); Croatian Centre for Global Health, University of Split Medical School, Split, Croatia (O Polasek, MD); National Institute for Health and Welfare, Helsinki, Finland (Prof. V Salomaa, MD, J G Eriksson); Clinical and Molecular Osteoporosis Research Unit, Department of Clinical Sciences and Orthopaedic Surgery, Lund University, Skåne University Hospital, Malmö, Sweden (Prof. M Karlsson, MD); Geriatric Unit, Azienda Sanitaria Firenze, Florence, Italy (S Bandinelli, MD); Vasa Central Hospital, Vasa, Finland (J G Eriksson); Folkhälsan Research Centre, Helsinki, Finland (J G Eriksson); Unit of General Practice, Helsinki University Central Hospital, Helsinki, Finland (J G Eriksson); Department of Clinical Chemistry, Fimlab Laboratories and School of Medicine, University of Tampere, Tampere, Finland (Prof. T Lehtimäki, MD); Synlab Academy, Mannheim, Germany (W März); Division of Cardiovascular Medicine, Vanderbilt University, Nashville, TN, USA (Prof. T J Wang, MD); Department of Internal Medicine, Division of Endocrinology and Metabolism, and Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria (Prof. S Pilz, MD, W März); Quantitative Sciences, GlaxoSmithKline, Stevenage, UK (Prof. J C Whittaker, PhD); School of Population Health, Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia (E Hyppönen); and South Australian Health and Medical Research Institute, Adelaide, SA, Australia (E Hyppönen)
1