Abstract
Der p 23, a new, major house dust mite (HDM) allergen that is recognized by >70% of HDM-allergic patients, has high allergenic activity and, therefore, must be considered an important component for HDM-specific immunotherapy. We constructed and characterized a hypoallergenic Der p 23 vaccine for HDM immunotherapy. Three nonallergenic peptides from the C-terminal IgE epitope-containing part of Der p 23 (P4, P5) and P6, a mutant peptide containing serines instead of cysteines, were identified. Peptides were fused to the hepatitis B virus–derived PreS domain as recombinant fusion proteins (i.e., PreS-2XP4P5 and PreS-4XP6) that were expressed in Escherichia coli and purified to homogeneity. Compared with Der p 23, PreS-2XP4P5 and PreS-4XP6 showed no relevant IgE reactivity and exhibited considerably reduced allergenic activity in basophil activation tests using blood from HDM-allergic patients. Upon immunization of rabbits, only PreS-2XP4P5 induced high levels of Der p 23–specific IgG Abs that inhibited binding of patients’ IgE to Der p 23, comparable to IgG Abs induced with Der p 23, whereas Abs induced with PreS-4XP6 had only low blocking capacity. Additionally, IgG Abs induced with PreS-2XP4P5 inhibited Der p 23–induced basophil activation comparable to IgG Abs induced with Der p 23. Compared with Der p 23, PreS-2XP4P5 induced lower T cell proliferation but higher levels of the tolerogenic cytokine IL-10 and the Th1 cytokine IFN-γ in PBMCs from HDM-allergic patients, indicating an immunomodulatory capacity of the fusion protein. Therefore, PreS-2XP4P5 represents a promising candidate for immunotherapy of HDM-allergic patients.
House dust mites (HDMs) represent one of the most important allergen sources worldwide (1). Depending on geographic location, up to 50% of allergic patients are sensitized to HDM allergens (2). HDM-allergic patients mainly suffer from respiratory and cutaneous manifestations of allergy and have a high risk for progressing to severe disabling disease manifestations, such as asthma (3).
HDMs contain several potent allergens, as well as components that are not allergens per se but activate the immune system and facilitate allergic sensitization (1, 4). Studies (5–7) performed with purified natural and recombinant HDM allergens identified Der p 1 and Der p 2 as the two major HDM allergens, with IgE-binding frequencies > 80%; therefore, they must be considered as essential components in vaccines for immunotherapy of HDM allergy. Very recently, a new major HDM allergen, Der p 23, was identified that is recognized by >70% of HDM-allergic patients and exhibits high allergenic activity, as determined by its ability to induce IgE-dependent basophil activation (8). Der p 23 is an ~8-kDa protein that shows homology to chitin-binding domains and is found mainly in peritrophic membranes surrounding fecal pellets, which are a major source for other HDM allergens (8, 9). Although natural allergen extracts from HDMs used for current forms of diagnosis and immunotherapy contain varying amounts of Der p 1 and Der p 2, many other important HDM allergens, including Der p 23, are represented poorly or not at all in natural allergen extracts (8, 10, 11). Therefore, allergen-specific immunotherapy with HDM extracts is often less effective than specific immunotherapy (SIT) with other extracts (12, 13). Because of the limits set by technologies based on allergen extracts and the progress made in the field of allergen research, molecular approaches for SIT have been developed. In fact, the concept of molecular therapy is very promising, because several successful immunotherapy trials have been performed with recombinant allergens, as well as allergen derivatives, such as T cell peptides or recombinant hypoallergenic allergen derivatives, indicating that it should be possible to improve safety and efficacy of SIT with molecular treatment approaches and alternative routes of administration (14–19).
To improve safety and efficacy of HDM-SIT, several hypoallergenic derivatives of Der p 1 and Der p 2 have recently been developed, but no vaccine has been engineered for Der p 23 (20–22).
In this article, we report the conversion of the Der p 23 allergen into a hypoallergenic allergen derivative for SIT. For this purpose, we first identified Der p 23–derived peptides that lacked allergenic activity and showed reduced ability to activate allergen-specific T cells of HDM-allergic patients. To render these peptides immunogenic, they were fused with a carrier protein derived from hepatitis B (i.e., PreS) in the form of a recombinant fusion protein using genetic engineering (23–25). The recombinant Der p 23–derived PreS-fusion protein was characterized with regard to its allergenic activity and its ability to induce, upon immunization, IgG Abs that inhibit allergic patients’ IgE binding to the wild-type allergen and allergen-induced basophil degranulation.
Materials and Methods
HDM-allergic patients
Serum samples from HDM-allergic patients with IgE Abs specific for Der p 23 (n = 68) were used for the characterization of the hypoallergenic Der p 23 derivatives described in this study. The diagnosis of HDM allergy was based on a case history and indoor symptoms (rhinitis, conjunctivitis, and/or asthma) indicative of HDM allergy, a positive skin test for HDM, and/or the presence of Dermatophagoides pteronyssinus–specific IgE Abs > 0.7 kUA/l in serum, as determined with the ImmunoCAP System (Thermo Fisher Scientific/Phadia, Uppsala, Sweden). Sera from nonallergic individuals were used for control purposes. Anonymized serum samples were analyzed in all experiments. The study was approved by the ethics committee of the Medical University of Vienna. Blood samples were taken after informed consent was obtained from patients.
Peptide synthesis
Six peptides with a length between 29 and 39 aa, covering the sequence of Der p 23, were synthesized using the Fmoc (9-fluorenylmethoxycarbonyl) strategy, as described (26). Peptides were identified by mass spectrometry and purified by preparative HPLC, yielding a purity > 90%. Each of the Der p 23 peptides was coupled to keyhole limpet hemocyanin (KLH) and purified using a conjugation kit (Pierce, Thermo Fisher Scientific), as described (26, 27).
Expression and purification of the recombinant proteins
Two synthetic genes with codons optimized for expression in Escherichia coli were produced (Fig. 1A) (GenScript USA). Both genes contained the sequence coding for the major surface Ag from hepatitis B virus, subtype: adw2 large S (GenBank: AAT28735.1) and six His codons at the C terminus. One construct (PreS-2XP4P5) contained two copies of the DNA sequence coding for peptide 4 and 5 of Der p 23 (GenBank: EU4147510.1), and the other construct (PreS-4XP6) contained four copies of the DNA sequence coding for peptide 6 (Fig. 1A). The synthetic genes were subcloned into the expression vector pET-17b (Novagen, Madison, WI) using the restriction sites NdeI and EcoRI. The DNA sequences were confirmed by sequencing both DNA strands (GenScript USA). The two PreS-fusion proteins (PreS-2XP4P5 and PreS-4XP6) were expressed in E. coli BL21 (DE3) (Stratagene, Santa Clara, CA) in liquid culture, as described (21). Both proteins were found in the inclusion body fractions, which were solubilized by stirring overnight at room temperature in 6 mol/l guanidine hydrochloride, 100 mmol/l NaH2PO4, and 10 mmol/l Tris-HCl (pH 8). The two preS-fusion proteins were purified under denaturing conditions over Ni–nitrilotriacetic acid resin affinity columns (QIAGEN, Hilden, Germany) (21); finally, PreS-2XP4P5 was dialyzed against 5 mmol/l NaH2PO4 (pH 4.5), and PreS-4XP6 was dialyzed against 5 mmol/l NaH2PO4 (pH 8). The concentrations of both proteins were measured using the BCA Assay Kit (Pierce, Rockford, IL), and the proteins were frozen at −20°C until use. The recombinant fusion proteins were analyzed by means of SDS-PAGE under reducing and nonreducing conditions, using an SDS buffer, with or without 2-ME (62.8 mM Tris, 2.3% SDS, 10% glycerin, 1% bromophenol blue [pH 6.8]) and Coomassie brilliant blue staining (28). The identity of the PreS-fusion proteins was confirmed by MALDI mass spectrometry, as described (29). Recombinant Der p 23 and PreS were expressed and purified as described (8, 24).
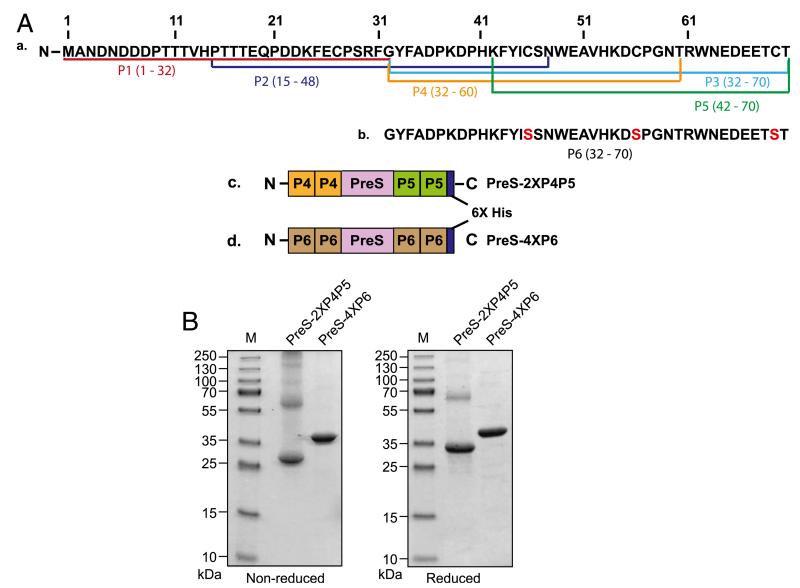
FIGURE 1.
Construction of PreS-fusion proteins and biochemical characterization. (A) Schematic representation of peptides and PreS-fusion proteins derived from Der p 23. (Aa) The position of the peptides (P1–P5) and their amino acids are indicated in the Der p 23 amino acid sequence. (Ab) The amino acid sequence of P6, in which cysteines were replaced by serine residues (red) is shown separately. The architecture of the two PreS-fusion proteins of Der p 23 containing two copies of P4 at the N terminus and two copies of P5 at the C terminus (PreS-2XP4P5) (Ac) or four copies of P6 at the N and C termini (PreS-4XP6) (Ad) and their C-terminal hexahistidine tags are shown. (B) The two PreS-fusion proteins, PreS-2XP4P5 and PreS-4XP6, and a molecular mass marker were separated by SDS-PAGE under nonreducing (left panel) and reducing (right panel) conditions and stained with Coomassie brilliant blue. Molecular masses are indicated on the left as kDa.
IgE reactivity of recombinant Der p 23 and the PreS-fusion proteins, as determined by radio-allergo-sorbent-test–based nondenaturing dot-blot assays
Aliquots of 2 μl containing 0.5 μg recombinant Der p 23, PreS-2XP4P5, or PreS-4XP6 were dotted onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). BSA and PreS (0.5 μg) were dotted for control purposes. Membranes were blocked with gold buffer (50 mM sodium phosphate [pH 7.4], 0.5% [v/v] Tween-20, 0.5% [w/v] BSA, and 0.05% [w/v] sodium azide), twice for 10 min and once for 30 min, and incubated with HDM-allergic patients’ sera (1:10 in gold buffer), serum from a nonallergic person (1:10 in gold buffer), or buffer alone overnight at 4°C. Bound IgE Abs were detected with 1:10 diluted 125I-labeled anti-human IgE Abs (Demeditec Diagnostics, Kiel, Germany) and visualized by autoradiography (Kodak XOMAT film).
Immunization of rabbits
Rabbit Abs specific for Der p 23 and Der p 23 derivatives were obtained by immunizing rabbits three times in monthly intervals with 200 μg of the purified recombinant proteins with aluminum hydroxide as adjuvant (SERVA Electrophoresis, Heidelberg, Germany), or with Freund’s adjuvant (once with CFA, twice with IFA), or with 200 μg KLH–coupled peptides adsorbed to Freund’s adjuvant (Charles River Breeding Laboratories, Kisslegg, Germany) (30). Der p 23–specific IgG responses were measured by ELISA, as described (31).
Determination of Der p 23–specific Ab concentrations in anti-sera
For the isolation of Der p 23-specific Abs, 2 ml recombinant Der p 23 (0.3 mg/ml) was dialyzed against coupling buffer (0.2 M NaHCO3, 0.5 M NaCl [pH 8]) and coupled to a HiTrap NHS-Activated HP column (GE Healthcare Bio-Science). Rabbit anti–Der p 23 Abs were purified by affinity chromatography to be used as a standard to determine Der p 23–specific Ab levels in anti-sera by ELISA.
For this purpose, ELISA plates (Nunc MaxiSorp, Roskilde, Denmark) were coated with 100 μl recombinant Der p 23 (5 μg/ml in PBS) overnight at 4°C. Plates were blocked with PBS, 0.05% Tween-20 (PBST), 3% (w/v) BSA. Using different concentrations (0.2–500 ng/ml) of purified Der p 23–specific Abs, a standard curve was established that allowed determination of the concentrations of Der p 23–specific Abs in the different rabbit antisera. The rabbit anti-sera against Der p 23, PreS-2XP4P5, and PreS-4XP6 were added at dilutions from 1:1,000 to 1:1,000,000 in PBST, 0.5% BSA, and the plates were incubated overnight at 4°C. Bound IgG Abs were detected with 1:2000 diluted HRP-labeled donkey anti-rabbit IgG (GE Healthcare, UK Limited, Chalfant St Giles, U.K.) for 1 h at 37°C and for 1 h at 4°C. After washing with PBST (five times), the color development was performed by addition of staining solution [2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt] (100 μl/well; Sigma-Aldrich, St. Louis, MO). OD measurements were carried out at 405 and 490 nm in an ELISA reader (SpectraMax Plus, Molecular Devices, Sunnyvale, CA). The concentration of Der p 23–specific Abs in the rabbit antisera was calculated in comparison with the standard curve.
Inhibition of allergic patients’ IgE binding to recombinant Der p 23 by specific rabbit Abs
IgE inhibition ELISAs were performed as described (31), using purified recombinant Der p 23 on the solid phase. The plates were preincubated with rabbit anti-recombinant Der p 23, anti–PreS-2XP4P5, and anti–PreS-4XP6 antisera or the corresponding preimmune sera overnight at 4°C. Sera from CFA-immunized rabbits were diluted 1:20, whereas sera from alumimmunized rabbits were diluted 1:5. Analysis of the concentrations of Der p 23–specific Abs in the diluted anti-sera used for competition showed them to be similar (i.e., 3.02–5.32 μg Der p 23–specific Abs/well).
After washing, the plates were incubated with sera from HDM-allergic patients (1:5 in PBST, 0.5% [w/v] BSA) overnight at 4°C. Bound human IgE Abs were detected with 1:2500 diluted HRP-coupled goat anti-human IgE Abs (KPL, Gaithersburg, MD), as described (32). The percentage inhibition of IgE binding was calculated as follows: Percentage of inhibition (%) = 100 − (ODs/ODp) × 100, where ODp and ODs represent the optical densities after preincubation with the preimmune and immune serum, respectively.
Lymphoproliferation assays and cytokine measurements
To reduce endotoxin concentrations in proteins used for T cell assays, each of the Ags was subjected to affinity chromatography using a polymyxin matrix (Bio-Rad, Hercules, California). The endotoxin contents in the protein solutions were determined by the Limulus Amebocyte Lysate assay (Lonza, Basel, Switzerland) and were found to be in the range of 12.4–54 ng/mg of protein. Heparinized venous blood samples were collected from six patients with HDM allergy. PBMCs were isolated by Ficoll (Amersham Bioscience, Uppsala, Sweden) and incubated in control medium or 1.25 μM recombinant Der p 23, PreS-2XP4P5, PreS, peptide 4 (P4), or peptide 5 (P5) for 6 d (37°C). Proliferation assays were performed as previously described, and results were expressed as stimulation index (33). The cultured cell supernatants from the lymphocyte proliferation assays were used to determine the concentrations of cytokines with the Bio-Plex Pro Human Cytokine 17-Plex Panel (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions, as described (25).
In vitro basophil-activation tests and inhibition of CD203c expression with specific rabbit Abs
In vitro basophil-activation tests were performed with heparinized peripheral blood from six HDM-allergic patients with IgE Abs specific for Der p 23 and, for control purposes, with blood from one nonallergic individual. Recombinant Der p 23, P4, P5, PreS-2XP4P5, and PreS were diluted in equimolar concentrations (0.012–1200 nM) and incubated for 15 min at 37°C with the blood samples (90 μl). CD203c expression on basophils was measured as described (34). For the inhibition of recombinant Der p 23–induced CD203c upregulation on basophils, we used blood samples from five HDM-allergic patients with Der p 23–specific IgE Abs and from one nonallergic individual. Rabbit antisera induced with recombinant Der p 23 or PreS-2XP4P5 using alum as adjuvant, as well as the corresponding preimmune sera, were heat inactivated at 56°C for 1 h and preincubated with increasing amounts of recombinant Der p 23 (0.012–1200 nM) at 4°C overnight at a 1:10 dilution. Blood samples from the HDM-allergic patients and from the nonallergic individual were incubated with the preincubated mixes for 15 min at 37°C. Upregulation of CD203c expression on basophils was determined by FACS analysis and expressed as stimulation index, as described (34).
Results
Identification of Der p 23–derived peptides for the construction of PreS-fusion proteins
Three overlapping peptides (peptide 1 [P1], peptide 2 [P2], and peptide 3 [P3]), with a length between 33 and 39 aa, were synthesized; they span the complete sequence of Der p 23 (Fig. 1A). Dot-blot assays showed that P1 and P2 have lost their IgE reactivity, whereas the IgE reactivity of P3 was comparable to that of Der p 23 (data not shown). This indicates that major IgE epitopes of Der p 23 are present at the C-terminal part, which shows homology with chitin-binding domain type 2 and chitin binding peritrophin-A domains (8). Only the KLH-coupled P3 induced high Der p 23–specific IgG titers upon immunization of rabbits, whereas KLH-coupled P1 and P2 were not immunogenic (data not shown). Considering these results, three additional peptides were made to render the P3-defined region hypoallergenic (i.e., P4 [aa 32–60], P5 [aa 42–70], and peptide 6 [P6; aa 32–70]) (Fig. 1A). P4 and P5 were truncated forms of P3, whereas cysteines were replaced by serine residues in P6. P4, P5, and P6 induced Der p 23–specific IgG Abs in rabbits comparable to P3, but they did not show IgE reactivity with HDM-allergic patients’ sera (data not shown). Furthermore, P4, P5, and P6 showed lower induction of T cell proliferation in PBMCs from mite-allergic patients compared with complete Der p 23 (data not shown). Therefore, P4, P5, and P6 fulfilled requirements of a vaccine that should avoid IgE- and T cell–mediated side effects and, upon coupling to carriers, they induced Der p 23–specific IgG Abs that inhibited mite-allergic patients’ IgE binding to Der p 23 (24, 25, 35, 36). Thus, these three peptides were used for the construction of hypoallergenic vaccines for Der p 23 in the form of PreS-fusion proteins (24, 25) (Fig. 1A). Fusion proteins consisting of the peptides and PreS from the major surface Ag of the hepatitis B virus should induce allergen-specific blocking IgG, with T cell help from PreS as a carrier (24, 25, 37).
Expression, purification, and biochemical characterization of the PreS-fusion proteins
As shown in Fig. 1A, two His-tagged PreS-fusion proteins were made, containing either two copies of P4 at the N-terminus and two copies of P5 at the C terminus (PreS-2XP4P5) or four copies of P6, two at the N terminus and two at the C terminus. Both proteins were expressed in E. coli BL21 (DE3) and purified by Ni-affinity chromatography, yielding high amounts of the recombinant proteins (~15–20 mg/l E. coli culture) at laboratory scale conditions. SDS-PAGE analysis indicated that PreS-4XP6 lacking cysteines migrated under reducing and nonreducing conditions as a monomer at ~37.5 kDa, whereas PreS-2XP4P5 migrated mainly as a monomer at ~33 kDa but also showed dimers and larger aggregates (Fig. 1B). Migration of the proteins in SDS-PAGE corresponded to the molecular masses determined by MALDI-TOF mass spectrometry (32.5 and 37.1 kDa) and to the masses calculated from the deduced amino acid sequences (32.5 and 37.1 kDa). Circular dichroism analysis indicated a predominantly random coil structure of both recombinant fusion proteins (data not shown).
PreS-fusion proteins lack relevant IgE reactivity
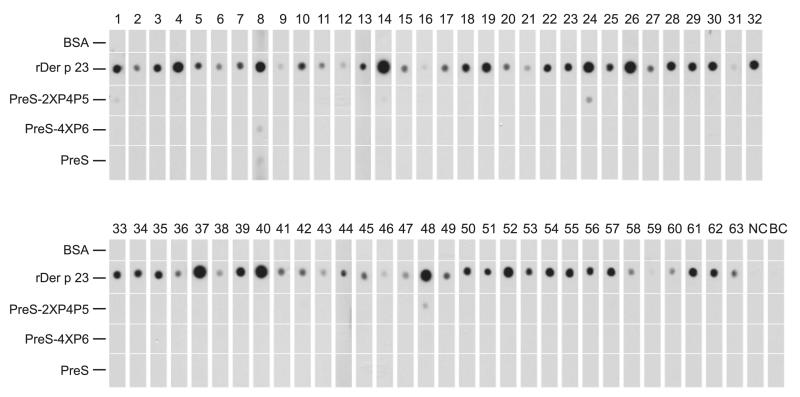
To compare the IgE reactivity of the two PreS-fusion proteins with that of Der p 23, nondenaturing, radio-allergo-sorbent-test–based dot-blot assays were performed using sera from 63 HDM-allergic patients with Der p 23–specific IgE Abs. Each of the patients showed IgE reactivity to recombinant Der p 23, whereas only four patients (patients 1, 14, 24, and 48) showed weak IgE reactivity to PreS-2XP4P5 (Fig. 2). Only one patient (patient 8) showed very weak IgE reactivity to PreS-4XP6, but this patient even reacted with the carrier protein PreS (Fig. 2). None of the patients reacted to BSA. Serum from a nonallergic individual and the buffer control did not show IgE reactivity to any of the dotted proteins (Fig. 2).
FIGURE 2.
IgE reactivity of recombinant Der p 23 and the PreS-fusion proteins. Dot blotted recombinant Der p 23, PreS-2XP4P5, PreS-4XP6, PreS, and BSA were tested for IgE reactivity with sera from HDM-allergic patients (1–63), serum from a nonallergic individual (NC), and buffer alone (BC).
PreS-2XP4P5 induces high levels of Der p 23–specific IgG Abs in rabbits that inhibit patients’ IgE binding to Der p 23
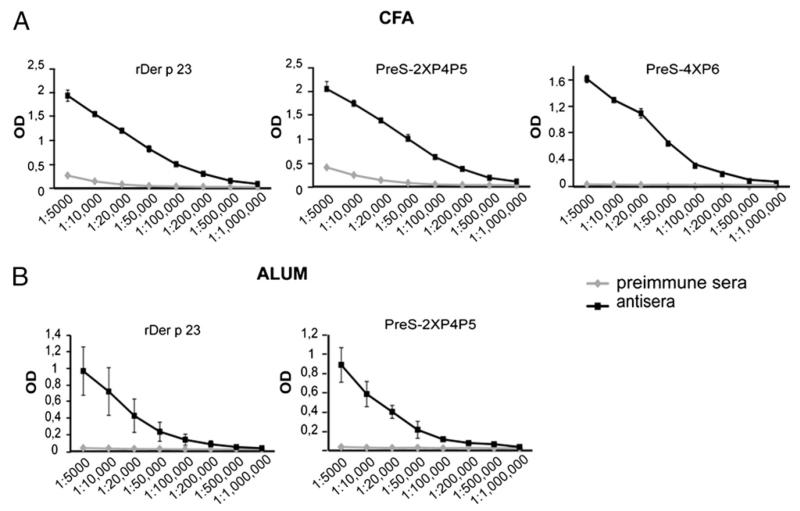
Rabbit sera obtained after immunization with recombinant Der p 23 or the PreS-fusion proteins (PreS-2XP4P5 and PreS-4XP6) using Freund’s adjuvant were tested for IgG reactivity to Der p 23 by ELISA (Fig. 3A). Both PreS-fusion proteins induced high levels of Der p 23–specific IgG Abs comparable to Der p 23, whereas the rabbit preimmune sera did not show relevant IgG reactivity to Der p 23 (Fig. 3A). We then tested whether IgG Abs induced by immunization with the PreS-fusion proteins could inhibit mite-allergic patients’ IgE binding to Der p 23 in ELISA competition assays, using sera from 18 mite-allergic patients (Table I). Rabbit anti–Der p 23 Abs inhibited patients’ IgE binding to Der p 23 between 42.8 and 93.2% (mean: 70.7%). The inhibition obtained with rabbit Abs raised against PreS-2XP4P5 was comparable to the inhibition obtained with rabbit anti–Der p 23 Abs (43.4–93.4%, mean: 67.1%), whereas the inhibition of patients’ IgE binding to Der p 23 with rabbit anti-PreS-4XP6 Abs was considerably lower (19.6–68.4%, mean: 42.2%) (Table I). Therefore, the PreS-fusion protein PreS-4XP6 was not studied further as a candidate vaccine for immunotherapy; additional experiments were conducted only with PreS-2XP4P5.
FIGURE 3.
Der p 23–specific IgG responses of rabbit anti–recombinant Der p 23 and anti–PreS-fusion protein antisera. Der p 23–specific IgG levels (y-axes: OD values) of different dilutions (1:5,000–1:1,000,000) (x-axes) of antisera from rabbits that were immunized with recombinant Der p 23, PreS-2XP4P5, or PreS-4XP6 adsorbed to CFA (A) and recombinant Der p 23 or PreS-2XP4P5 adsorbed to alum (B) are shown in comparison with the corresponding preimmune sera. Results are displayed as mean values ± SD of three determinations of one rabbit (A) or of three determinations performed with sera from three rabbits (B).
Table I.
Inhibition of HDM-allergic patients’ IgE binding to recombinant Der p 23 with rabbit antisera induced by immunization with recombinant Der p 23 or PreS-fusion proteins in CFA
| Recombinant Der p 23 |
PreS-2XP4P5 |
PreS-4XP6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Preimmune | Antiserum | % of Inhibition | Preimmune | Antiserum | % of Inhibition | Preimmune | Antiserum | % of Inhibition |
| 1 | 0.36 | 0.16 | 54.97 | 0.36 | 0.18 | 49.96 | 0.31 | 0.25 | 19.58 |
| 3 | 0.32 | 0.10 | 70.48 | 0.32 | 0.09 | 71.04 | 0.30 | 0.14 | 51.77 |
| 4 | 1.20 | 0.10 | 91.84 | 1.18 | 0.12 | 89.69 | 1.07 | 0.51 | 52.16 |
| 5 | 0.73 | 0.09 | 88.13 | 0.70 | 0.12 | 82.99 | 0.65 | 0.20 | 68.41 |
| 6 | 0.58 | 0.25 | 56.29 | 0.56 | 0.30 | 46.15 | 0.55 | 0.39 | 28.62 |
| 9 | 0.57 | 0.33 | 42.84 | 0.65 | 0.34 | 47.61 | 0.50 | 0.30 | 39.02 |
| 10 | 0.70 | 0.13 | 82.09 | 0.71 | 0.20 | 71.72 | 0.69 | 0.43 | 37.48 |
| 11 | 1.86 | 0.37 | 80.19 | 1.81 | 0.59 | 67.38 | 1.78 | 0.98 | 45.18 |
| 13 | 1.44 | 0.10 | 93.18 | 1.41 | 0.09 | 93.41 | 1.31 | 0.60 | 54.46 |
| 17 | 0.32 | 0.07 | 78.63 | 0.32 | 0.08 | 76.49 | 0.28 | 0.13 | 55.06 |
| 18 | 0.62 | 0.11 | 81.76 | 0.65 | 0.09 | 86.21 | 0.63 | 0.28 | 55.99 |
| 19 | 0.72 | 0.17 | 77.11 | 0.69 | 0.17 | 74.92 | 0.62 | 0.44 | 29.17 |
| 20 | 0.51 | 0.19 | 62.55 | 0.52 | 0.20 | 62.03 | 0.47 | 0.37 | 22.46 |
| 21 | 0.39 | 0.18 | 55.54 | 0.38 | 0.16 | 57.51 | 0.36 | 0.22 | 39.89 |
| 64 | 0.25 | 0.13 | 45.28 | 0.25 | 0.14 | 46.02 | 0.23 | 0.15 | 34.16 |
| 65 | 1.82 | 0.13 | 92.81 | 1.81 | 0.17 | 90.65 | 1.76 | 0.71 | 59.61 |
| 66 | 0.30 | 0.12 | 59.50 | 0.31 | 0.15 | 51.44 | 0.30 | 0.21 | 30.13 |
| 67 | 0.26 | 0.11 | 59.57 | 0.27 | 0.15 | 43.37 | 0.26 | 0.16 | 36.79 |
| Mean | 0.72 | 0.16 | 70.71 | 0.72 | 0.19 | 67.14 | 0.67 | 0.36 | 42.22 |
The optical densities, corresponding to the amount of bound Abs, are shown after preincubation with the preimmune sera or antiserum.
To investigate whether PreS-2XP4P5 also can induce blocking IgG Abs in rabbits when aluminum hydroxide, the most common adjuvant in allergy vaccines, is used, three rabbits were immunized with alum-adsorbed Der p 23 or PreS-2XP4P5. Again, PreS-2XP4P5 induced Der p 23–specific IgG responses comparable to Der p 23, whereas the preimmune sera did not show IgG reactivity to Der p 23 (Fig. 3B). Table II shows the percentage inhibition of patients’ IgE binding to Der p 23 by rabbit IgG Abs induced by immunization with the alum-adsorbed proteins. Rabbit anti–Der p 23 Abs inhibited patients’ IgE binding to Der p 23 between 60.9 and 93.8% (mean: 81.9%), and a comparable inhibition was obtained with rabbit anti–PreS-2XP4P5 Abs (52.3–88.5%, mean: 70.1%) (Table II).
Table II.
Percentage of inhibition of patients’ IgE binding to recombinant Der p 23 by rabbit IgG Abs induced by immunization with recombinant Der p 23 or PreS-2XP4P5 adsorbed to aluminum hydroxide
| Recombinant Der 23 |
PreS-2XP4P5 |
|||||
|---|---|---|---|---|---|---|
| Patient | Preimmune Serum | Antiserum | % of Inhibition | Preimmune Serum | Antiserum | % of Inhibition |
| 1 | 0.81 | 0.13 | 83.63 | 0.79 | 0.20 | 75.10 |
| 2 | 0.23 | 0.05 | 76.86 | 0.26 | 0.07 | 71.03 |
| 3 | 0.30 | 0.09 | 70.80 | 0.28 | 0.11 | 59.92 |
| 4 | 1.72 | 0.11 | 93.84 | 1.55 | 0.19 | 87.89 |
| 6 | 0.30 | 0.11 | 62.15 | 0.31 | 0.12 | 60.88 |
| 10 | 0.33 | 0.04 | 86.90 | 0.34 | 0.06 | 81.07 |
| 11 | 0.69 | 0.27 | 60.89 | 0.86 | 0.34 | 60.09 |
| 13 | 2.09 | 0.14 | 93.49 | 2.06 | 0.46 | 77.64 |
| 14 | 0.88 | 0.12 | 85.97 | 0.85 | 0.22 | 74.64 |
| 17 | 0.39 | 0.05 | 86.34 | 0.42 | 0.09 | 79.14 |
| 18 | 0.69 | 0.09 | 86.58 | 0.64 | 0.08 | 86.99 |
| 19 | 0.83 | 0.11 | 86.46 | 0.86 | 0.31 | 63.47 |
| 20 | 0.34 | 0.06 | 83.12 | 0.34 | 0.07 | 78.46 |
| 21 | 0.20 | 0.07 | 67.21 | 0.22 | 0.08 | 65.35 |
| 22 | 0.57 | 0.18 | 67.92 | 0.62 | 0.26 | 57.60 |
| 23 | 0.98 | 0.20 | 79.65 | 0.92 | 0.33 | 64.09 |
| 24 | 3.23 | 0.29 | 90.88 | 3.22 | 1.02 | 68.23 |
| 25 | 0.65 | 0.22 | 65.42 | 0.68 | 0.32 | 52.28 |
| 26 | 2.12 | 0.24 | 88.72 | 2.02 | 0.61 | 69.91 |
| 28 | 0.86 | 0.08 | 90.12 | 0.81 | 0.24 | 70.25 |
| 29 | 0.30 | 0.06 | 78.52 | 0.31 | 0.12 | 61.17 |
| 32 | 1.33 | 0.13 | 90.57 | 1.37 | 0.21 | 84.48 |
| 34 | 0.42 | 0.13 | 69.81 | 0.42 | 0.17 | 58.58 |
| 39 | 2.16 | 0.16 | 92.40 | 2.19 | 0.62 | 71.83 |
| 40 | 2.99 | 0.29 | 90.16 | 3.15 | 1.22 | 61.36 |
| 57 | 2.00 | 0.30 | 85.12 | 1.89 | 0.88 | 53.37 |
| 64 | 0.70 | 0.07 | 90.32 | 0.70 | 0.08 | 88.54 |
| 65 | 3.09 | 0.29 | 90.49 | 3.09 | 0.64 | 79.18 |
| Mean | 1.11 | 0.15 | 81.94 | 1.11 | 0.33 | 70.09 |
The optical densities, corresponding to the amount of bound Abs, are shown after preincubation with the preimmune sera or antiserum.
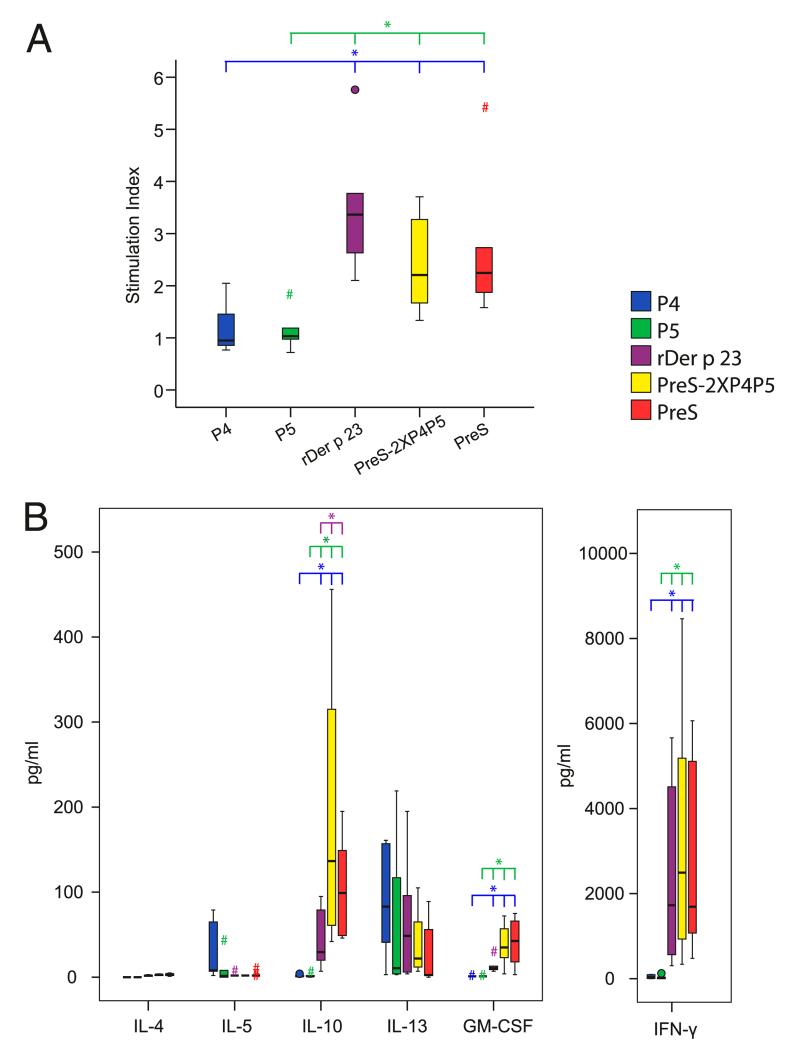
PreS-2XP4P5 induces a tolerogenic and Th1 cytokine profile in PBMCs from HDM-allergic patients
To investigate whether the PreS-fusion protein lacks the important T cell epitopes of Der p 23, PBMCs from six patients with HDM allergy were stimulated with the PreS-fusion protein (PreS-2XP4P5), Der p 23, the individual peptides P4 and P5, and the carrier protein, PreS (Fig. 4A). Der p 23 induced much stronger T cell proliferation in PBMCs from mite-allergic patients than did the two peptides (P4 and P5). The PreS-fusion protein induced lower proliferative responses than Der p 23, which was comparable to that induced by PreS, indicating that most of the proliferation induced by the PreS-fusion protein was caused by carrier-specific T cells (Fig. 4A). Next, we investigated the cytokine production in PBMCs from the same six patients after stimulation with Der p 23, the peptides, PreS-2XP4P5, or PreS (Fig. 4B). PreS-2XP4P5 induced significantly higher levels of IL-10 and IFN-γ in PBMCs from HDM-allergic patients than did Der p 23 and the individual Der p 23 peptides. In contrast, the Der p 23–derived fusion protein induced lower levels of the Th2 cytokine IL-13 than did Der p 23, whereas the levels of IL-4 and IL-5 were low for all tested proteins (Fig. 4B). The production of the proinflammatory cytokines related to innate immunity (e.g., GM-CSF) was higher in cultures stimulated with the PreS-fusion protein than in cultures exposed to Der p 23 (Fig. 4B). Statistically significant differences (p < 0.05) among the different proteins are denoted by asterisks in Fig. 4.
FIGURE 4.
Lymphoproliferative and cytokine responses to Der p 23, Der p 23 peptides, PreS, and PreS-fusion proteins. PBMCs from six patients with HDM allergy were stimulated with 1.25 μM of each Ag. Mean stimulation indexes (A) and cytokine responses (B) (y-axes) of triplicate cultures are shown in box and whiskers plots, where 50% of the values are within the boxes and nonoutliers between the bars. Extremes and outliers are indicated by hashtags and circles, respectively. Vertical lines at the top indicate the molecules that show statistically significant differences from the reference molecule of the same color as the bar. *p < 0.05, Mann–Whitney U test.
PreS-2XP4P5 lacks allergenic activity
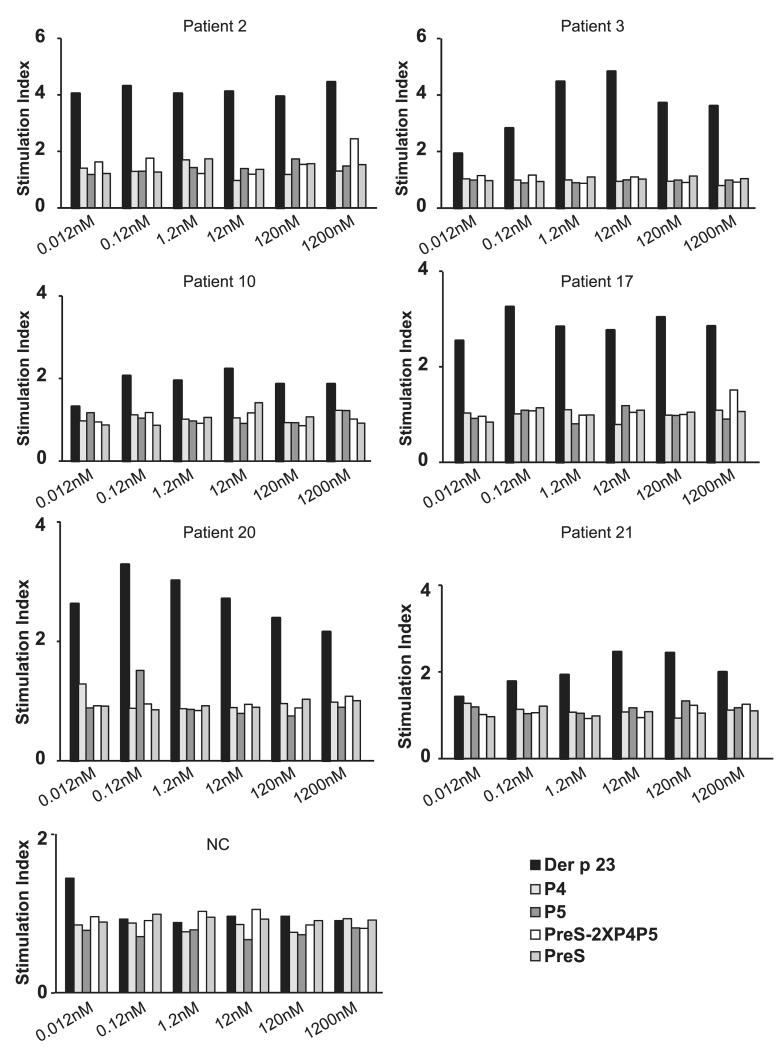
To determine the allergenic activity of PreS-2XP4P5, basophils from six HDM-allergic patients containing Der p 23–specific IgE and from one nonallergic individual were incubated with equimolar concentrations of Der p 23, PreS-2XP4P5, P4 and P5, or PreS (Fig. 5). Recombinant Der p 23 induced upregulation of CD203c expression at very low concentrations (0.012–0.12 nM), whereas the individual peptides (P4 and P5) and PreS did not induce CD203c upregulation up to a concentration of 1200 nM in the HDM-allergic patients examined (Fig. 5). No upregulation of CD203c expression was obtained with PreS-2XP4P5 up to a concentration of 120 nM, and a slight CD203c upregulation was seen at the highest concentration (1200 nM) in only two patients (patients 2 and 17). None of the proteins induced CD203c upregulation in the nonallergic individual, up to a concentration of 1200 nM (Fig. 5). Anti-human IgE Abs were used as positive control and induced upregulation of CD203c expression on basophils in all donors (data not shown).
FIGURE 5.
Allergenic activity of recombinant Der p 23, peptides, PreS, and PreS-fusion proteins. Blood samples from six mite-allergic patients and one nonallergic individual (NC) were exposed to different equimolar concentrations (1200–0.012 nM) of the Ags (x-axes). Upregulation of CD203c expression on basophils is displayed as stimulation index (y-axes).
PreS-2XP4P5–specific rabbit Abs inhibit Der p 23–induced basophil activation
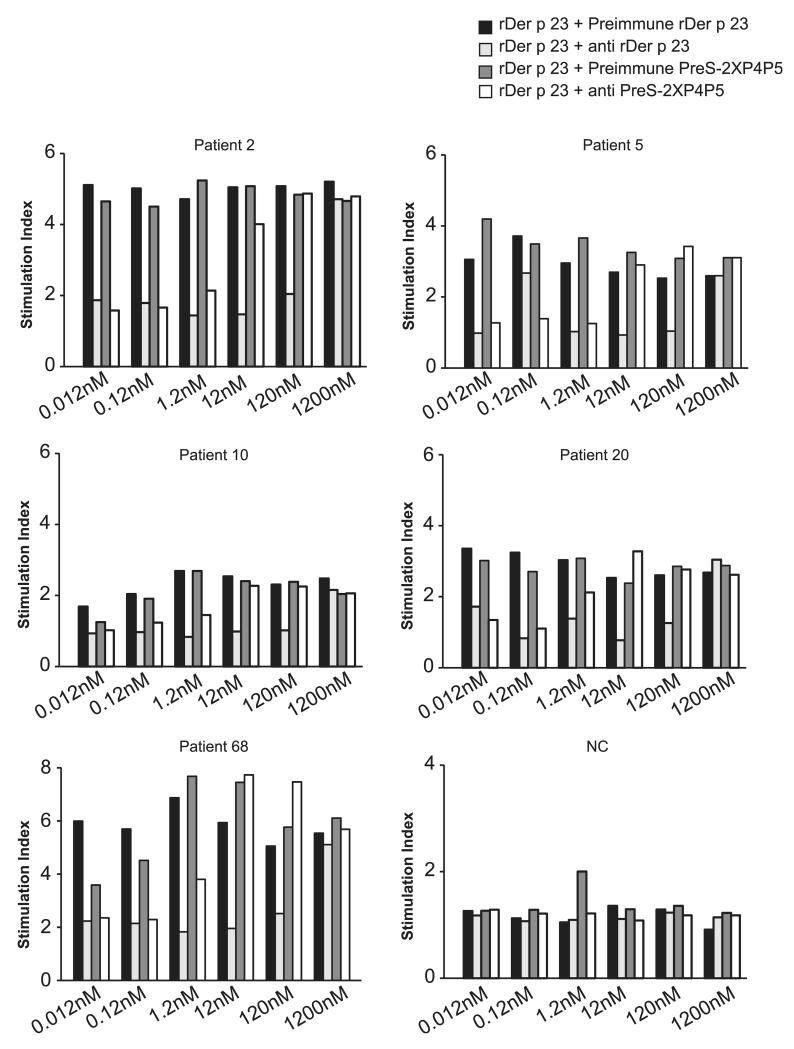
Alum-adsorbed PreS-2XP4P5 induced high levels of Der p 23–specific IgG Abs in rabbits, which inhibited patients’ IgE binding to Der p 23 to a similar extent as did anti–Der p 23 Abs (Table II). Therefore, we studied whether PreS-2XP4P5–specific Abs also could inhibit Der p 23–induced basophil activation. Different concentrations of Der p 23 were preincubated with anti–PreS-2XP4P5 Abs, Der p 23–specific Abs, or the corresponding preimmune sera. Blood samples from five HDM-allergic patients with Der p 23–specific IgE reactivity and from one nonallergic individual were incubated with the mixes, and CD203c upregulation was determined by FACS analysis (Fig. 6). PreS-2XP4P5–specific Abs inhibited Der p 23–induced upregulation of CD203c expression up to a Der p 23 concentration of 1.2 nM, whereas Der p 23 induced strong CD203c upregulation at concentrations of 0.012 nM when preimmune serum was used (Fig. 6). Anti–Der p 23 Abs inhibited Der p 23–induced basophil activation up to a Der p 23 concentration of 120 nM (Fig. 6). No relevant upregulation of CD203c expression was observed with basophils from the nonallergic individual (Fig. 6).
FIGURE 6.
Inhibition of recombinant Der p 23–induced CD203c upregulation by specific Abs. Different concentrations of recombinant Der p 23 (1200–0.012 nM) were preincubated with rabbit anti–recombinant Der p 23, rabbit anti–PreS-2XP4P5 Abs, or the corresponding preimmune sera (x-axes). Blood samples from five patients with HDM allergy and one nonallergic individual (NC) were exposed to the mixes. Upregulation of CD203c expression on basophils is displayed as stimulation index (y-axes).
Discussion
HDM allergy is one of the most frequent forms of respiratory allergies, but immunotherapy with HDM extracts is often less effective than immunotherapy with grass pollen extracts and is associated with high rates of severe side effects (38, 39). To improve HDM SIT, molecular approaches including the major HDM allergens, Der p 1 and Der p 2, have been developed. Recently, a new major HDM allergen (i.e., Der p 23) with high allergenic activity was identified. Because of its high frequency of IgE recognition and allergenic activity, Der p 23 must be considered an important component to be included in an HDM allergy vaccine. In this study, we used the peptide-carrier fusion protein approach to convert Der p 23 into a hypoallergenic vaccine. This approach is based on the identification of peptides derived from the IgE binding sites of allergens, which, per se, lack IgE reactivity and allergenic activity, as well as exhibit reduced ability to activate allergen-specific T cells (40). The allergen-derived peptides are fused to a nonallergenic carrier protein that provides T cell help upon immunization, with the goal to induce IgG Abs against the IgE binding areas on the allergen with reduced IgE- or T cell–mediated side effects. For this purpose, we synthesized three overlapping peptides (P1–P3) covering Der p 23 to destroy IgE epitopes that are involved in mast cell activation and T cell epitopes and, hence, may induce T cell–mediated inflammation. Only the C-terminal P3 induced high Der p 23–specific IgG titers upon immunization of rabbits, whereas P1 and P2 were not immunogenic and failed to induce allergen-specific blocking IgG Abs. However, P3 reacted with IgE Abs from HDM-allergic patients, indicating that the C-terminal part of Der p 23 contains major IgE epitopes (8). To reduce IgE reactivity, we produced two truncated forms of P3 (P4 [aa 32–60] and P5 [aa 42–70]), as well as P3 as a peptide in which the cysteines were replaced by serines (P6 [aa 32–70]). The new peptides, in particular P4 and P5, lacked relevant IgE-binding capacity and allergenic activity, and they induced Der p 23–specific IgG Abs in rabbits when they were coupled to the carrier molecule, KLH (41) (data not shown). Compared with complete Der p 23, P4 and P5 induced less allergen-specific T cell activation. For the construction of the fusion protein, we chose the PreS domain of the major surface Ag of hepatitis B virus, which was used recently for the engineering of a vaccine for cat and birch pollen allergy (24, 25). PreS appears to have several possible advantages when used as a carrier protein. First, it has been safely used for vaccination against hepatitis B infections and was shown to induce protective Abs (42). Thus, it is quite possible that PreS-containing allergy vaccines also will induce protective immunity against hepatitis B. Furthermore, it seems that recombinant PreS fusion proteins can be well expressed in E. coli and purified in good yields and quality (24, 25). Finally, our data indicate that PreS is a good carrier for increasing the immunogenicity of allergy vaccines, and it exhibits a beneficial modulation of immune responses toward a tolerogenic and/or Th1 phenotype (25).
We then engineered two PreS fusion proteins, containing P4 at the N terminus and P5 at the C terminus (PreS-2XP4P5) or P6 at both the N and C termini (PreS-4XP6), which could be expressed in E. coli in large amounts and were purified to homogeneity. The fusion proteins lacked relevant IgE reactivity and allergenic activity and induced the tolerogenic cytokine IL-10 and the Th1 cytokine IFN-γ in cultured PBMCs from HDM-allergic patients, similar to what was observed for a PreS-based birch vaccine, indicating that PreS has an immunomodulatory capacity. Upon immunization of rabbits with PreS-fusion proteins, PreS-2XP4P5 induced higher levels of blocking IgG than did PreS-4XP6. IgG Abs induced in rabbits with alum-adsorbed PreS-2XP4P5 inhibited allergic patients’ IgE binding to Der p 23, comparable to anti–Der p 23 Abs, as well as inhibited Der p 23–induced activation of HDM allergic patients’ basophils. The inhibition obtained with PreS-2XP4P5–specific Abs was lower than that obtained with Der p 23–specific Abs, probably because the hypoallergenic construct did not contain the full sequence of Der p 23 and, thus, induced Abs with lower coverage of Der p 23 epitopes and/or lower avidity. However, the >100-fold reduced allergenic activity of PreS-2XP4P5 compared with Der p 23 should make it possible to define a dose of PreS-2XP4P5 that is more safe and effective for treatment than administration of the wild-type allergen.
Based on the in vitro and in vivo characterization of PreS-2XP4P5, we foresee that the protein can be used for immunotherapy of mite-allergic patients similar to natural allergens; however, because of the hypoallergenic nature of the protein, it should be possible to vaccinate safely with a few high-dose administrations, avoiding the long updosing regimens based on multiple injections. Thus, the vaccine should be safer and more convenient than those based on natural allergens. As a mode of action, it may be anticipated that the vaccine will have beneficial effects by inducing high levels of allergenspecific IgG Abs that inhibit IgE-mediated mast cell degranulation and, thus, immediate allergic inflammation. Furthermore, it is expected that IgE-facilitated allergen presentation will be inhibited; thus, there should be a tolerogenic effect on T cells that also may be mediated by PreS-induced IL-10. Finally, if the vaccine is given over a prolonged period > 2 y, it is possible that the presence of allergenspecific IgG will suppress allergen-induced boosts of IgE production and, thus, induce long-term tolerance to allergens, even after discontinuation of treatment.
In summary, the PreS-fusion protein, PreS-2XP4P5, represents a promising candidate for immunotherapy of mite-allergic patients because of its reduced allergenic activity and its ability to induce blocking Abs.
Acknowledgments
R.V. has been supported by grants from the Austria Science Fund, Biomay AG, and Thermo Fisher Scientific and has received consulting fees or honorarium from Biomay AG and Thermo Fisher Scientific.
This work was supported by Austrian Science Fund Grants F4602, F4605, and F4611; Thermo Fisher Scientific; Biomay AG; and the Christian Doppler Research Association, Austria.
Abbreviations used in this article
- HDM
house dust mite
- KLH
keyhole limpet hemocyanin
- P1
peptide 1
- P2
peptide 2
- P3
peptide 3
- P4
peptide 4
- P5
peptide 5
- P6
peptide 6
- PBST
PBS, 0.05% Tween-20
- SIT
specific immunotherapy
Footnotes
Disclosures
The other authors have no financial conflicts of interest.
References
- 1.Vrtala S, Huber H, Thomas WR. Recombinant house dust mite allergens. Methods. 2014;66:67–74. doi: 10.1016/j.ymeth.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzerling L, Frew AJ, Bindslev-Jensen C, Bonini S, Bousquet J, Bresciani M, Carlsen KH, van Cauwenberge P, Darsow U, Fokkens WJ, et al. Standard skin prick testing and sensitization to inhalant allergens across Europe—a survey from the GALEN network. Allergy. 2005;60:1287–1300. doi: 10.1111/j.1398-9995.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- 3.Platts-Mills TA, Rakes G, Heymann PW. The relevance of allergen exposure to the development of asthma in childhood. J. Allergy Clin. Immunol. 2000;105:S503–S508. doi: 10.1016/S0091-6749(00)90051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacquet A. The role of the house dust mite-induced innate immunity in development of allergic response. Int. Arch. Allergy Immunol. 2011;155:95–105. doi: 10.1159/000320375. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien RM, Thomas WR. Immune reactivity to Der p I and Der p II in house dust mite sensitive patients attending paediatric and adult allergy clinics. Clin. Exp. Allergy. 1994;24:737–742. doi: 10.1111/j.1365-2222.1994.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 6.Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, Kraft D, Valenta R. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin. Exp. Allergy. 2004;34:597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- 7.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, Horak F, Grönlund H, van Hage M, Valenta R, Vrtala S. Variability of IgE reactivity profiles among European mite allergic patients. Eur. J. Clin. Invest. 2008;38:959–965. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 8.Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, Thomas WR, Fernández-Caldas E, Kabesch M, Ferrara R, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J. Immunol. 2013;190:3059–3067. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 10.Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int. Arch. Allergy Immunol. 2012;159:253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–190. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J. Allergy Clin. Immunol. 2001;107:87–93. doi: 10.1067/mai.2001.112027. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet J, Michel FB. Specific immunotherapy in asthma. Allergy Proc. 1994;15:329–333. doi: 10.2500/108854194778816562. [DOI] [PubMed] [Google Scholar]
- 14.Larché M. T cell epitope-based allergy vaccines. Curr. Top. Microbiol. Immunol. 2011;352:107–119. doi: 10.1007/82_2011_131. [DOI] [PubMed] [Google Scholar]
- 15.Cromwell O, Häfner D, Nandy A. Recombinant allergens for specific immunotherapy. J. Allergy Clin. Immunol. 2011;127:865–872. doi: 10.1016/j.jaci.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 17.Rancitelli P, Hofmann A, Burks AW. Vaccine approaches for food allergy. Curr. Top. Microbiol. Immunol. 2011;352:55–69. doi: 10.1007/82_2011_126. [DOI] [PubMed] [Google Scholar]
- 18.Mascarell L, Zimmer A, Van Overtvelt L, Tourdot S, Moingeon P. Induction of allergen-specific tolerance via mucosal routes. Curr. Top. Microbiol. Immunol. 2011;352:85–105. doi: 10.1007/82_2011_132. [DOI] [PubMed] [Google Scholar]
- 19.Senti G, Johansen P, Kündig TM. Intralymphatic immunotherapy: from the rationale to human applications. Curr. Top. Microbiol. Immunol. 2011;352:71–84. doi: 10.1007/82_2011_133. [DOI] [PubMed] [Google Scholar]
- 20.Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, Viñuela JE, Sanz ML, Andreu C, Martínez A. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin. Exp. Allergy. 2009;39:1088–1098. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen KW, Blatt K, Thomas WR, Swoboda I, Valent P, Valenta R, Vrtala S. Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J. Allergy. Clin. Immunol. 2012;130:435–443.e4. doi: 10.1016/j.jaci.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walgraffe D, Mattéotti C, El Bakkoury M, Garcia L, Marchand C, Bullens D, Vandenbranden M, Jacquet A. A hypoallergenic variant of Der p 1 as a candidate for mite allergy vaccines. J. Allergy Clin. Immunol. 2009;123:1150–1156. doi: 10.1016/j.jaci.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Neurath AR, Kent SB. The pre-S region of hepadnavirus envelope proteins. Adv. Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- 24.Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, van Hage M, Gronlund H, Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J. Allergy Clin. Immunol. 2011;127:1562–1570.e6. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, Gieras A, Swoboda I, Zafred D, Keller W, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J. Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, Kraft D, Valenta R. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 27.Westritschnig K, Focke M, Verdino P, Goessler W, Keller W, Twardosz A, Mari A, Horak F, Wiedermann U, Hartl A, et al. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, Phl p 7. J. Immunol. 2004;172:5684–5692. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- 28.Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal. Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 29.Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, Swoboda I, Thomas WR, Thalhamer J, Valenta R, Vrtala S. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin. Exp. Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen KW, Fuchs G, Sonneck K, Gieras A, Swoboda I, Douladiris N, Linhart B, Jankovic M, Pavkov T, Keller W, et al. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol. Immunol. 2008;45:2486–2498. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, Focke-Tejkl M, Blatt K, Gieras A, Zafred D, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J. Allergy Clin. Immunol. 2010;126:1024–1031. 1031.e1–8. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, Valenta R, Spitzauer S. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J. Immunol. 2002;168:4576–4584. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 33.Vrtala S, Fohr M, Campana R, Baumgartner C, Valent P, Valenta R. Genetic engineering of trimers of hypoallergenic fragments of the major birch pollen allergen, Bet v 1, for allergy vaccination. Vaccine. 2011;29:2140–2148. doi: 10.1016/j.vaccine.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, Bühring HJ, Valenta R, Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 35.Twaroch TE, Focke M, Fleischmann K, Balic N, Lupinek C, Blatt K, Ferrara R, Mari A, Ebner C, Valent P, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin. Exp. Allergy. 2012;42:966–975. doi: 10.1111/j.1365-2222.2012.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, Ferrara R, Quirce S, Spitzauer S, Swoboda I, Valenta R. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J. Allergy Clin. Immunol. 2011;128:178–184.e7. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Muttil P, Prego C, Garcia-Contreras L, Pulliam B, Fallon JK, Wang C, Hickey AJ, Edwards D. Immunization of guinea pigs with novel hepatitis B antigen as nanoparticle aggregate powders administered by the pulmonary route. AAPS J. 2010;12:330–337. doi: 10.1208/s12248-010-9192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akçakaya N, Hassanzadeh A, Camcioğlu Y, Cokuğraş H. Local and systemic reactions during immunotherapy with adsorbed extracts of house dust mite in children. Ann. Allergy Asthma Immunol. 2000;85:317–321. doi: 10.1016/S1081-1206(10)62536-7. [DOI] [PubMed] [Google Scholar]
- 39.Mellerup MT, Hahn GW, Poulsen LK, Malling H. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin. Exp. Allergy. 2000;30:1423–1429. doi: 10.1046/j.1365-2222.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- 40.Focke-Tejkl M, Valenta R. Safety of engineered allergenspecific immunotherapy vaccines. Curr. Opin. Allergy Clin. Immunol. 2012;12:555–563. doi: 10.1097/ACI.0b013e328357ca53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen KW, Focke-Tejkl M, Blatt K, Kneidinger M, Gieras A, Dall’Antonia F, Faé I, Fischer G, Keller W, Valent P, et al. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients. Allergy. 2012;67:609–621. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rendi-Wagner P, Shouval D, Genton B, Lurie Y, Rümke H, Boland G, Cerny A, Heim M, Bach D, Schroeder M, Kollaritsch H. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine. 2006;24:2781–2789. doi: 10.1016/j.vaccine.2006.01.007. [DOI] [PubMed] [Google Scholar]