Abstract
The discovery of expression of the erythropoietin receptor (EPO-R) on neoplastic cells has led to concerns about the safety of treating anaemic cancer patients with EPO. In addition to its endocrine function, the receptor may play a role in tumour progression through an autocrine mechanism. In this study, the expression of EPO, EPO-R and platelet-derived growth factor BB (PDGF-BB) was analysed in five feline and 13 canine osteosarcomas using immunohistochemistry (IHC) and reverse transcription polymerase chain reaction (RT-PCR).
EPO expression was positive in all tumours by IHC, but EPO mRNA was only detected in 38% of the canine and 40% of the feline samples. EPO-R was expressed in all samples by quantitative RT-PCR (RT-qPCR) and IHC. EPO-R mRNA was expressed at higher levels in all feline tumours, tumour cell lines, and kidney when compared to canine tissues. PDGF-BB expression was variable by IHC, but mRNA was detected in all samples. To assess the functionality of the EPO-R on tumour cells, the proliferation of canine and feline osteosarcoma cell lines was evaluated after EPO administration using an alamarBlue assay and Ki67 immunostaining. All primary cell lines responded to EPO treatment in at least one of the performed assays, but the effect on proliferation was very low indicating only a weak responsiveness of EPO-R. In conclusion, since EPO and its receptor are expressed by canine and feline osteosarcomas, an autocrine or paracrine tumour progression mechanism cannot be excluded, although in vitro data suggest a minimal role of EPO-R in osteosarcoma cell proliferation.
Keywords: Cell culture, Erythropoietin, Erythropoietin receptor, Platelet-derived growth factor BB, Osteosarcoma, Dog, Cat
Introduction
Erythropoietin (EPO) is a small glycoprotein hormone which mostly promotes erythropoiesis, but has also non-haematopoietic, growth-promoting and apoptosis-inhibiting roles (Noguchi et al., 2008; Debeljak et al., 2014; Wan et al., 2014). Recently, it has been shown in a mouse model that angiogenesis in tumours is modulated by inducing EPO production via platelet-derived growth factor BB (PDGF-BB) in tumour stromal cells (Xue et al., 2012). This system is based on a complex paracrine mechanism involving tumour cells, stromal cells and, in particular, tumour blood vessels. In addition, high amounts of circulating EPO have been shown to stimulate proliferation, migration and cell sprouting of endothelial cells (Xue et al., 2012).
In adults, EPO is mainly produced in the kidneys. It binds to the erythropoietin receptor (EPO-R) and activates JAK-STAT-signalling (Noguchi et al., 2008). EPO-R is expressed predominately on erythrocyte progenitors and EPO can be used therapeutically in humans and animals to treat anaemia (Eschbach and Adamson, 1985). Human cancer patients with myelodysplastic disorders are frequently treated with EPO but there is an ongoing discussion about the effect of EPO on tumour cells. Positive effects of EPO for humans (Ludwig et al., 1994; de Campos et al., 1995; Demetri et al., 1998; Mittelman et al., 2001; Wallvik et al., 2002; Jädersten et al., 2008) and animals (Langston et al., 2003) have been reported. However, several clinical studies showed worse survival times and shorter disease-free intervals after EPO treatment in cancer patients (Henke et al., 2003, 2006; Leyland-Jones et al., 2005; Savonije et al., 2005; Bohlius et al., 2009).
The presence of EPO and EPO-R has been demonstrated in various tumours in humans and animals (Acs et al., 2001; Arcasoy et al., 2002; Batra et al., 2003; Sfacteria et al., 2005; Klopfleisch et al., 2012; Rózsás et al., 2013; Reinbothe et al., 2014) and EPO has been shown to promote angiogenesis, vessel maturity, drug resistance, proliferation and tumour progression (Morais et al., 2013; Asano et al., 2015). Others have questioned the functionality of EPO-R on tumour cells (Sinclair et al., 2007) due to its low level of expression (Swift et al., 2010). In addition, treatment of EPO-R-expressing cell lines with EPO did not enhance cell proliferation in vitro (Westphal et al., 2002; Farrell and Lee, 2004).
Osteosarcoma is considered to be the most frequent bone tumour in dogs and cats (Quigley and Leedale, 1983) but the metastatic behaviour differs between the two species, with frequent distant metastases in dogs (Spodnick et al., 1992) and a remarkably low metastatic rate in cats (Dimopoulou et al., 2008). To our knowledge, no studies about EPO and its receptor in bone tumours of cats or dogs have been reported. Recently, PDGFs and PDGF receptors (PDGF-R) were found to be overexpressed in canine osteosarcoma (Xue et al., 2012; Maniscalco et al., 2013). Moreover, PDGFs and PDGF-Rs were co-expressed, suggesting that an autocrine and/or paracrine loop could be involved (Xue et al., 2012; Maniscalco et al., 2013). No data about PDGFs exist in feline osteosarcomas.
We hypothesised that the expression pattern of EPO, EPO-R and PDGF-BB in canine and feline osteosarcoma cells differs between the two species and that this may influence tumour growth, angiogenesis and the different metastatic behaviour in the two species.
Materials and methods
Sample collection
Tumour samples from dogs (n = 13) and cats (n = 5) were collected after therapeutic limb amputation or euthanasia. Control samples (kidney, bone marrow, cerebellum) were obtained from animals euthanased for reasons other than osteosarcoma. Material was collected from surgery (with informed owner’s consent) or from cadaveric clinical waste and was conveyed to the Institute of Pathology of the University of Veterinary Medicine. The study was approved by the Ethical and Animal Welfare Committee of the University of Veterinary Medicine (15 December 2014) and conducted in accordance with the requirements of the Austrian Act on Animal Experiments.
Aliquots of samples were either fixed in 4% buffered formaldehyde or flash frozen in liquid nitrogen with or without RNA later solution (Life Technologies).
Immunohistochemistry
For immunohistochemistry (IHC), paraffin sections were used and stained with either an anti-EPO, (sc-7956, polyclonal, dilution 1:50; Santa Cruz Biotechnology), an anti-EPO-R (C-20, sc-696, polyclonal, dilution 1:50; Santa Cruz Biotechnology), an anti-EPO-R (M-20 sc-697, polyclonal, dilution 1:75; Santa Cruz Biotechnology) or an anti-PDGF-BB antibody (ab21234, polyclonal, dilution 1:500; Abcam). The evaluation score suggested by Detre et al. (1995) was used. Specificity of the assays was tested by immunoblotting (see Appendix: Supplementary material).
Cell culture
Commercially available canine osteosarcoma cells D-17 (LGC Standards) or primary tumour-derived neoplastic cells (canine: COS_1186w and COS_1189; feline: FOS_1077 and FOS_1140; Lonza) were used. Cells were grown in Dulbecco’s modified eagle’s medium (DMEM) low glucose with 10% foetal calf serum (FCS, Sigma-Aldrich) and 625 pg Amphotericin B (Bio&Sell), 2 nM glutamine (Biochrom) and 1% Pen/Strep/Fungi Mix (Bio&Sell).
AlamarBlue test
Cells were exposed to different concentrations of EPO and proliferation was determined with the alamarBlue test (Resazurin, Sigma Aldrich) to evaluate the functionality of EPO-R as suggested previously (Levine et al., 2005; LaMontagne et al., 2006; Laugsch et al., 2008). Briefly, 2000 cells/well were seeded in flat bottomed 96-well plates (Sarstedt) and supplemented with 0, 0.001, 0.01, 0.1, 1 or 10 U/mL of alpha erythropoietin (Epoietin, Binocrit 1000 IU/0.5 mL, Sandoz) in 5 μL PBS, or 5 μL PBS alone (control) with or without 10% FCS in the medium. Cells were incubated with EPO for 48 h before 10% alamarBlue was added, and the 570–600 nm ratio was measured on a plate reader (Sunrise, Tecan).
Ki67 immunocytochemistry
For Ki67 immunocytochemical staining, 23,000 (for D-17, FOS_1077 and FOS_1140) or 46,000 cells (for COS_1189 and COS_1186w) were seeded onto round glass cover slides, and supplemented with 0.001, 1 or 10 U/mL alpha erythropoietin (Epoietin, Binocrit, Sandoz) in 5 μL PBS or PBS alone with or without 10% FCS in the medium. After 48 h, cells were fixed in 4% formaldehyde and stained using an anti-Ki67 antibody (clone 7B11, Life Technologies). For the analysis of Ki67 staining, approximately 1000 cells/slide were counted and classified as stained or unstained to generate a percentage of positive cells.
PCR
About 30 mg of fresh frozen or RNAlater (Life Technologies)-preserved tissue or pellets of 105–106 cultured cells were used for RNA isolation using either the miRNeasy Mini Kit (Qiagen) or the RNeasy Fibrous Tissue Mini Kit (Qiagen). Concentration and the RNA Integrity Number (RIN) were determined with a 2100 Bioanalyzer (Agilent Technologies) (see Appendix: Supplementary material). Using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies), 500 ng RNA/sample was transcribed into cDNA. Feline and canine EPO and PDGF-B mRNA were detected by qualitative dye-based PCR. Qualitative assessment was performed by melting curve analysis. Canine EPO mRNA was measured with a qualitative hydrolysis-probe PCR assay. Quantification of EPO-R mRNA was performed by a conventional hydrolysis probe-based format. To compensate for technical variance, data were normalised with the reference genes OAZ1 (De Jonge et al., 2007; Kwon et al., 2009) and the canine and feline orthologues of human C12orf43 (Tsai and Breen, 2012).
Statistical evaluation
RT-qPCR data were analysed using REST 2009 (Qiagen). For the Ki67 and alamarBlue assays, significance was assessed by a linear regression model. A detailed description of the statistical methods is provided in the Appendix: Supplementary material.
Results
Immunohistochemistry
Total scores for EPO IHC ranged from 2 to 9 in feline tumours and from 3 to 9 in canine tumours. EPO immunostaining resulted either in a homogenous or heterogeneous pattern within the tumour area. Cytoplasmic and nuclear staining was present (Figs. 1a, b) with additional staining of giant cells and partial staining of endothelial cells within the tumour. In the canine and feline kidney (positive controls), tubules and collecting ducts were stained. Negative controls were negative.
Fig. 1.
Immunohistochemical staining of canine and feline osteosarcomas. (a) Canine osteosarcoma, erythropoietin (EPO); (b) feline osteosarcoma, EPO; tumour cells and stromal cells showed cytoplasmic and nuclear staining. (c) Canine osteosarcoma, erythropoietin receptor (EPO-R); (d) feline osteosarcoma, EPO-R; EPO-R was expressed in tumour cells and giant cells. (e) Canine osteosarcoma, platelet-derived growth factor BB (PDGF-BB); (f) feline osteosarcoma, PDGF-BB; osteosarcoma cells showed a cytoplasmic granular staining pattern. In some samples a perivascular distribution was observed. Scale bar = 100 μm.
EPO-R IHC resulted in a cytoplasmic staining pattern (total scores ranged from 2 to 9 in both species); a clear membrane staining was not observed (Figs. 1c, d). No differences were found when comparing the C-20 and the M-20 antibodies. All negative controls were negative.
The immunostaining pattern for PDGF-BB was considerably heterogeneous in the osteosarcomas of cats and dogs and total scores ranged from 0 to 9. The staining pattern in canine and feline tumour cells was cytoplasmic (Fig. 1e). In feline osteosarcomas a perivascular distribution of positive cells was partly observed (Fig. 1f). In addition to the tumour cells, giant cells and osteoblasts were positive for PDGF-BB immunostaining in both species. In the positive controls (feline and canine cerebellum), neurons were stained as expected and negative controls were unstained. IHC results are summarised in Table 1.
Table 1.
Distribution of immunohistological scores in canine and feline osteosarcomas.
| Total IHC score | EPO (n = 18) |
EPO-R (n = 18) |
PDGF-BB (n = 18) |
|||
|---|---|---|---|---|---|---|
| Dog | Cat | Dog | Cat | Dog | Cat | |
| 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1 | 0 | 0 | 0 | 0 | 2 | 0 |
| 2 | 0 | 1 | 1 | 1 | 5 | 1 |
| 3 | 2 | 0 | 4 | 0 | 3 | 1 |
| 4 | 1 | 2 | 1 | 1 | 0 | 0 |
| 6 | 7 | 0 | 5 | 1 | 3 | 0 |
| 9 | 3 | 2 | 2 | 2 | 0 | 2 |
EPO, erythropoietin; EPO-R, erythropoietin receptor; PDGF-BB, platelet-derived growth factor BB.
RNA expression analysis
PDGF-B mRNA was expressed in all feline (n = 5) and canine (n = 13) samples as well as in the kidney and the bone marrow (positive controls). In contrast, EPO mRNA was detected in 38% of the canine tumour samples and 40% of the feline osteosarcoma samples. Four canine samples had a near-threshold level of expression, but were graded as negative.
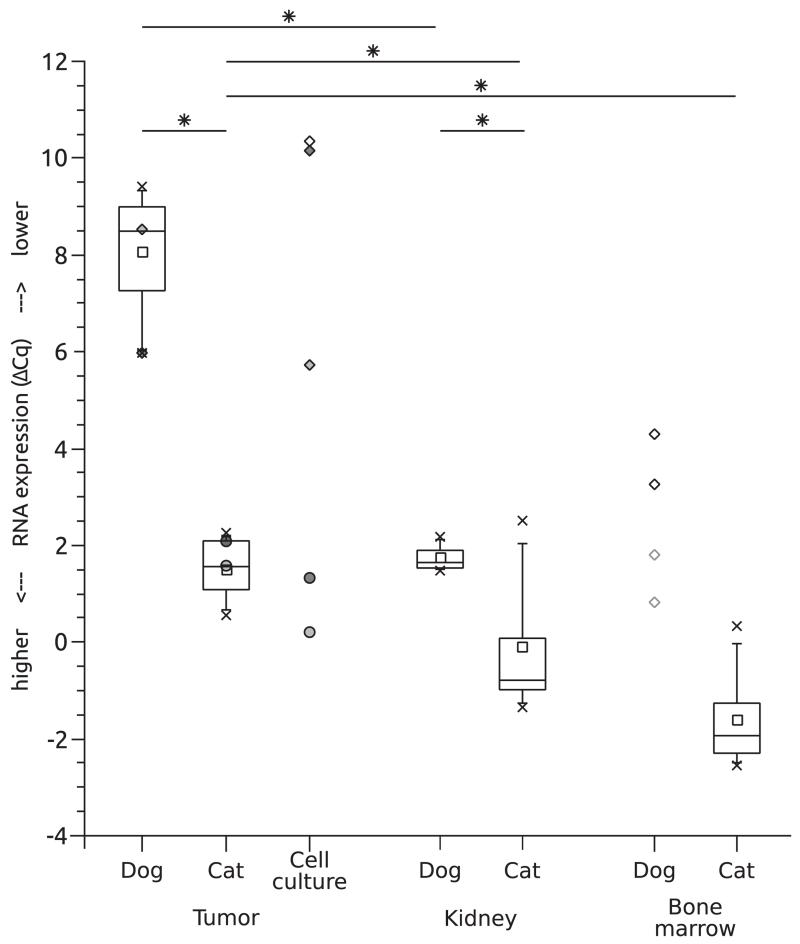
RT-qPCR showed that the average amount of EPO-R mRNA in feline osteosarcomas was higher than that of dogs. The average Cq value (±SD) for canine EPO-R mRNA (31.8 ± 1.4) was higher than that of the feline EPO-R mRNA (26.9 ± 0.5; P ≤ 0.001; 94-fold change) (Fig. 2). The tissue samples used as positive controls, kidney and bone marrow, also showed this different expression level of EPO-R mRNA between the two species. Cq values of canine EPO-R mRNA in kidney were 27.4 ± 0.5 compared to 24.8 ± 0.8 in feline kidney (P ≤ 0.001; 6.7-fold change). Canine bone marrow samples showed an average Cq of 31.2 ± 0.1, whereas feline samples showed an average Cq of 25.9 ± 1.6 (39-fold change).
Fig. 2.
Erythropoietin receptor (EPO-R) mRNA expression changes relative to the reference genes OAZ1 and C26H12orf43/CD3H12orf43 in canine and feline osteosarcomas and reference tissues as well as in osteosarcoma cell cultures, showing a significant difference between tumours and corresponding tissues, and between canine and feline osteosarcomas. Boxes depict 25%–75% interval, whiskers the 5%–95% interval. Highest and lowest values are indicated by x. Where boxplots were inappropriate, individual values are marked by circles (cat) or rectangles (dog). Tumours and corresponding cell lines are marked by the same shades of grey, and canine bone marrow samples showing inhibition are marked by light grey edging. Significant differences are marked by an asterisk.
Statistical analysis of bone marrow was not undertaken because of an observed RT-qPCR inhibition in 2/4 canine samples. For each species, the Cq values of tumour tissues were significantly lower compared to those in kidney and bone marrow (P ≤ 0.05). The expression patterns of primary cell cultures isolated from some of the original osteosarcomas correlated well with those of the original tumour tissue (Fig. 2).
In vitro EPO-R functionality assay
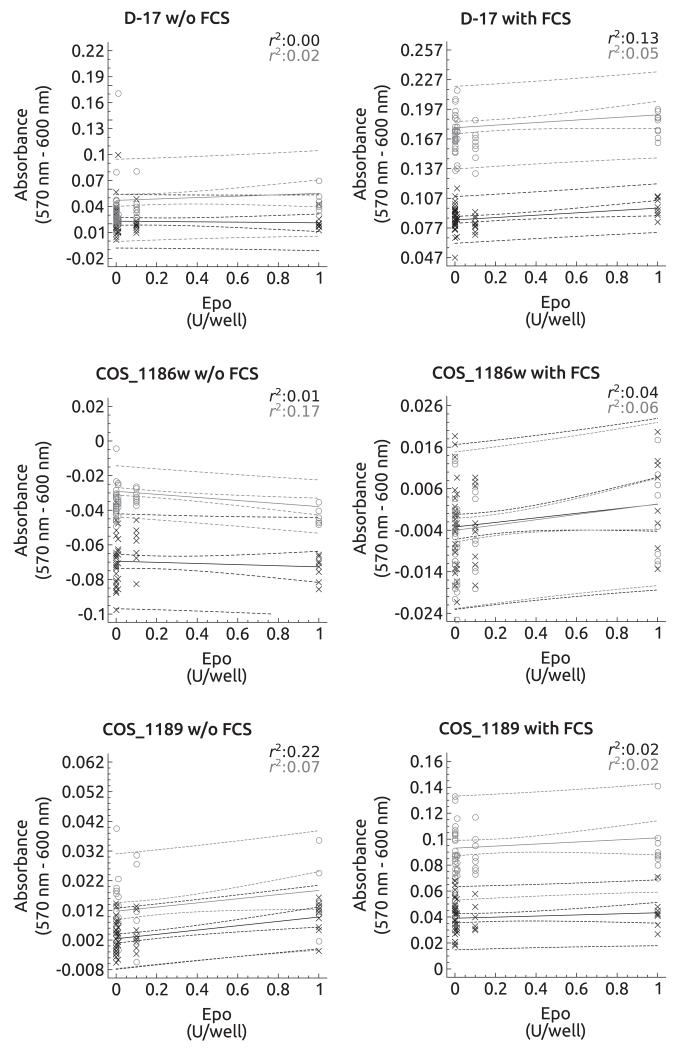
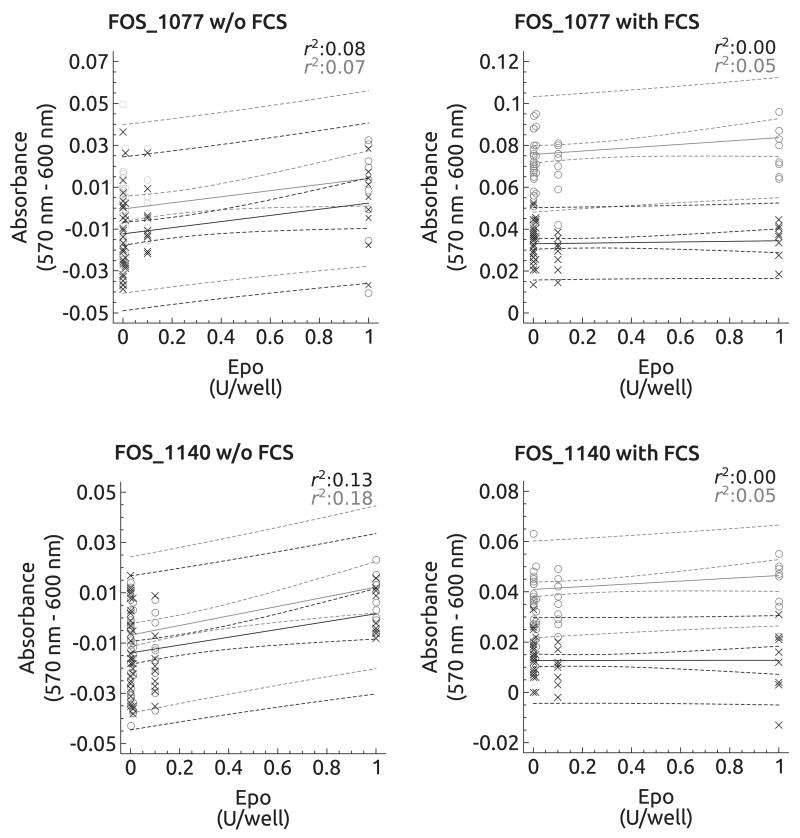
The effects of EPO in vitro are summarised in Figs. 3, 4. In the absence of FCS, the feline osteosarcoma cell lines and the canine cell line COS_1189 showed a significant dose-dependent increase in absorbance 2 h and 4 h after alamarBlue addition (FOS_1077: 2 h, P = 0.03 and 4 h, P = 0.05; FOS_1140: 2 h, P < 0.01 and 4 h, P = 0.01; COS_1189: 2 h, P < 0.01 and 4 h, P = 0.05) (Figs. 3, 4). However, the explained variance was low ranging from 7% to 22%. In COS_1186w cells, the absorbance decreased significantly after 4 h (P < 0.01) but not after 2 h (P = 0.52). In FCS supplemented medium, the D-17 cell line showed increased proliferation after 2 h, but not after 4 h. EPO treatment had no effect on all other cell lines.
Fig. 3.
Regression plots of canine cultured cells in the presence of erythropoietin (EPO) (0, 0.1, 0.01, 0.001, 0.0001 and 1 U/well) with or without foetal calf serum (FCS). Regression plots for the 2 h interval are depicted in black and individual values are marked as x. The 4 h interval is depicted in grey showing the individual values as ○. D-17 and COS_1186w cells showed a marked decrease in absorbance in the absence of FCS, but an increase in absorbance after 4 h for D-17 and after 2 h and nearing significance after 4 h in COS_1186w cells when FCS was added. COS_1189 cells showed an increase in absorbance after 4 h (without FCS) and 2 h and 4 h in the presence of FCS.
Fig. 4.
Regression plots of feline cultured cells in the presence of erythropoietin (EPO) (0, 0.1, 0.01, 0.001, 0.0001 and 1 U/well) with or without foetal calf serum (FCS). Regression plots for the 2 h interval are depicted in black and individual values are marked as x. The 4 h interval is depicted in grey showing the individual values as ○. FOS_1077 and FCS_1140 cells showed a significant increase in their absorbance in the presence of FCS, but not without FCS.
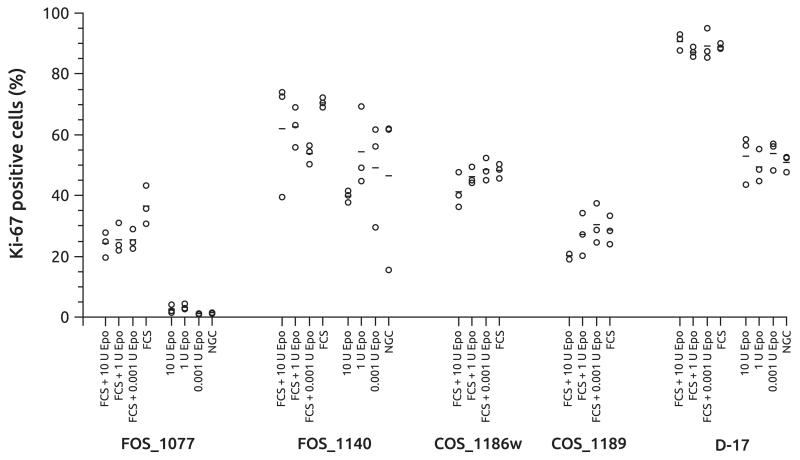
The relative number of Ki67 positive cells was used as an alternative way to determine the potential growth enhancing effects of EPO on osteosarcoma cells using EPO concentrations of 0.001 U/mL, 1 U/mL and 10 U/mL (Fig. 5). In the presence of FCS, two canine cell lines showed a significant increase of Ki67 positive cells (COS_1186w: P = 0.02; COS_1189: P = 0.02). Without stimulation by FCS, the numbers of cells for COS_1186w and COS_1189 were insufficient to determine a Ki67 index. All other set ups showed no impact of EPO treatment.
Fig. 5.
Percentage of Ki67 positive stained feline (FOS_1077, FOS_1140) and canine (COS_1186w, COS_1189, D-17) osteosarcoma cell lines after treatment with various concentrations of erythropoietin (EPO) with or without foetal calf serum (FCS). NGC, negative control samples, without FCS or EPO. Circles mark the individual values, bars depict the mean of the treatment group.
Discussion
The potential effect of EPO on tumours has been a longstanding debate. There are two primary mechanisms by which tumour growth could be affected: either via the direct action of EPO on tumour cells expressing a functional EPO-R or indirectly by induction of angiogenesis. Due to the low binding efficiency of EPO to its receptor, these effects should be greatly influenced by the amount of EPO present in the target tissue (Yan and Krzyzanski, 2013). Although it is generally assumed that the kidney is the only source of EPO (Krantz, 1991), several other organs (Fried, 1972; Digicaylioglu et al., 1995; Yasuda et al., 1998) and tumours (Suzuki et al., 2001; Batra et al., 2003; Pollio et al., 2005; Durno et al., 2011) also secrete EPO. Serum EPO concentrations are very similar in dogs and cats (Cook and Lothrop, 1994).
In the current study, EPO, EPO-R and PDGF-BB were detected by IHC in canine and feline osteosarcomas. The EPO and EPO-R immunostaining pattern was comparable between both species. The PDGF-BB results are in accordance with previous reports for dogs (Maniscalco et al., 2013). The distribution of PDGF-BB in the cat was partly perivascular in contrast to what was observed in canine tumours. Therefore, a paracrine or autocrine role for these factors is possible in canine and feline osteosarcomas.
The antibodies against EPO-R used in our study (M-20 and C-20) were not generated against the canine or feline EPO-R protein. Sequence similarities, positive controls, peptide blocking experiments, and their prior use on canine tissue by other groups (Sfacteria et al., 2005) convinced us of their suitability. It is noteworthy that the specificity of these commercial antibodies has been questioned (Brown et al., 2007; Jelkmann and Laugsch, 2007; Elliott et al., 2013). Western blotting indicated that only M-20 detects the correct band at 59 kD (Elliott et al., 2006) while C-20 detects three unspecific bands (Elliott et al., 2013), rendering them not suitable for IHC (Elliott et al., 2006). A novel EPO-R-specific antibody, A82, has been developed (Swift et al., 2010) but is currently not commercially available.
RT-PCR was used to support the IHC results. EPO-R and PDGF-B mRNA was present in all feline and canine samples supporting the protein data. Although EPO mRNA was only present in less than half of the feline and canine tumour samples, this difference could be explained by the presence of EPO originating from the serum. This finding supports the hypothesis that some of the sampled tumours are capable of autocrine stimulation as shown in other tumour types (Heldin, 2012).
Since Elliott et al. (2014) claimed that EPO-R mRNA concentration in tumours is too low to allow the receptor to be expressed on the cell surface, quantitative RT-PCR was used to determine EPO-R mRNA levels in canine and feline osteosarcomas. Interestingly, there was a significant difference in EPO-R mRNA expression between cat and dog samples. Generally higher amounts of EPO-R mRNA were detected in all samples (osteosarcomas, osteosarcoma-derived cells, kidney, and bone marrow) from cats compared with dogs. The primary feline and canine cell cultures maintained EPO-R mRNA expression and notably the expression level increased in three of four cell lines. These results confirm that osteosarcoma cells express EPO-R.
Consistent with results from previous studies (Swift et al., 2010; Elliott et al., 2013, 2014) the amount of EPO-R mRNA was lower in the tumour than in the reference tissues. To explain the lack of correlation between IHC results and RT-qPCR data, we should consider that proteins are synthesised much faster and have a longer half-life than mRNAs. Therefore, the correlation between mRNA and protein level is generally poor (Anderson and Seilhamer, 1997; Chen et al., 2002; Vogel and Marcotte, 2012).
Many tumours, in particular osteosarcomas, have a high level of intratumoural heterogeneity (Vogelstein et al., 2013). Therefore, using a small amount of homogenised tumour in PCR or Western blot might not reflect the whole neoplasm (Gerlinger et al., 2012; Raychaudhuri et al., 2012; Zhang et al., 2014), as structures other than tumour cells (blood vessels, stroma, immune cells) will also contribute to the quantitative results.
There is evidence that the expression of EPO-R mRNA alone is not sufficient to determine its effect on cells (Swift et al., 2010). Therefore, we used an alamarBlue assay and Ki67 immunohistochemistry to test the functional effect of EPO and functionality of EPO-R in an in vitro system. No general proliferation-enhancing effect of EPO on canine or feline osteosarcoma cells was observed. Minimal effects were seen in groups grown in medium without FCS supplementation in the alamarBlue test or in medium with FCS in the Ki67 assay, indicating influences of some undefined growth factors in serum. The anti-apoptotic properties of EPO, leading to longer cell survival in the absence of FCS, may result in higher turn-over measured in the alamarBlue assay, an effect possibly covered by the proliferation-enhancing effects of FCS. The Ki67 assay detected dividing cells, but not prolonged cell survival. Altogether, these results are in accordance with those of Shiozawa et al. (2013), who reported that EPO did not stimulate the growth of human prostate cells, but protected them from apoptosis.
Conclusions
EPO, EPO-R and PDGF-BB were expressed in cat and dog osteosarcomas, but no significant differences between the two species were observed, except for EPO-R mRNA expression. These results may suggest potential species-specific growth promoting or inhibiting effects of EPO on tumour cells.
Supplementary Material
Acknowledgements
This study was funded by the Austrian Science Fund (FWF), grant number P 23336-B11. We greatly acknowledge the technical support of Anne Fleming, Claudia Höchsmann, Martin Hofer, Hans Homola and Brigitte Machac.
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.tvjl.2015.06.003.
References
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Research. 2001;61:3561–3565. [PubMed] [Google Scholar]
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Amin K, Karayal AF, Chou S-C, Raleigh JA, Varia MA, Haroon ZA. Functional significance of erythropoietin receptor expression in breast cancer. Laboratory Investigation. 2002;82:911–918. doi: 10.1097/01.lab.0000020415.72863.40. [DOI] [PubMed] [Google Scholar]
- Asano R, Asai-Sato M, Miyagi Y, Mizushima T, Koyama-Sato M, Nagashima Y, Taguri M, Sakakibara H, Hirahara F, Miyagi E. Aberrant expression of erythropoietin in uterine leiomyoma: Implications in tumor growth. American Journal of Obstetrics and Gynecology. 2015 doi: 10.1016/j.ajog.2015.02.016. doi:10.1016/j.ajog.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Laboratory Investigation. 2003;83:1477–1487. doi: 10.1097/01.lab.0000090156.94795.48. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Brown WM, Maxwell P, Graham AN, Yakkundi A, Dunlop EA, Shi Z, Johnston PG, Lappin TR. Erythropoietin receptor expression in non-small cell lung carcinoma: A question of antibody specificity. Stem Cells. 2007;25:718–722. doi: 10.1634/stemcells.2006-0687. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang C-C, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Molecular and Cellular Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- Cook SM, Lothrop CD. Serum erythropoietin concentrations measured by radioimmunoassay in normal, polycythemic, and anemic dogs and cats. Journal of Veterinary Internal Medicine. 1994;8:18–25. doi: 10.1111/j.1939-1676.1994.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Debeljak N, Solár P, Sytkowski AJ. Erythropoietin and cancer: the unintended consequences of anemia correction. Frontiers in Immunology. 2014;5:563. doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos E, Radford J, Steward W, Milroy R, Dougal M, Swindell R, Testa N, Thatcher N. Clinical and in vitro effects of recombinant human erythropoietin in patients receiving intensive chemotherapy for small-cell lung cancer. Journal of Clinical Oncology. 1995;13:1623–1631. doi: 10.1200/JCO.1995.13.7.1623. [DOI] [PubMed] [Google Scholar]
- De Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te Meerman GJ, ter Elst A. Evidence based selection of housekeeping genes. PLoS ONE. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, Kris M, Wade J, Degos L, Cella D, Procrit Study Group Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: Results from a prospective community oncology study. Journal of Clinical Oncology. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. Journal of Clinical Pathology. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulou M, Kirpensteijn J, Moens H, Kik M. Histologic prognosticators in feline osteosarcoma: A comparison with phenotypically similar canine osteosarcoma. Veterinary Surgery. 2008;37:466–471. doi: 10.1111/j.1532-950X.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- Durno AS, Webb JA, Gauthier MJ, Bienzle D. Polycythemia and inappropriate erythropoietin concentrations in two dogs with renal and T-cell lymphoma. Journal of the Animal Hospital Association. 2011;47:122–128. doi: 10.5326/JAAHA-MS-5614. [DOI] [PubMed] [Google Scholar]
- Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, Spahr C, Um M, Van G, Begley CG. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- Elliott S, Swift S, Busse L, Scully S, Van G, Rossi J, Johnson C. Epo receptors are not detectable in primary human tumor tissue samples. PLoS ONE. 2013;8:e68083. doi: 10.1371/journal.pone.0068083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Sinclair A, Collins H, Rice L, Jelkmann W. Progress in detecting cell-surface protein receptors: The erythropoietin receptor example. Annals of Hematology. 2014;93:181–192. doi: 10.1007/s00277-013-1947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD) Kidney International. 1985;28:1–5. doi: 10.1038/ki.1985.109. [DOI] [PubMed] [Google Scholar]
- Farrell F, Lee A. The erythropoietin receptor and its expression in tumor cells and other tissues. The Oncologist. 2004;9:18–30. doi: 10.1634/theoncologist.9-90005-18. [DOI] [PubMed] [Google Scholar]
- Fried W. The liver as a source of extrarenal erythropoietin production. Blood. 1972;40:671–677. [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England Journal of Medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C-H. Autocrine PDGF stimulation in malignancies. Upsala Journal of Medical Sciences. 2012;117:83–91. doi: 10.3109/03009734.2012.658119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke M, Laszig R, Rübe C, Schäfer U, Haase K-D, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- Henke M, Mattern D, Pepe M, Bézay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? Journal of Clinical Oncology. 2006;24:4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- Jädersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, Pascutto C, Porwit A, Cazzola M, Hellström-Lindberg E. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. Journal of Clinical Oncology. 2008;26:3607–3613. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Laugsch M. Problems in identifying functional erythropoietin receptors in cancer tissue. Journal of Clinical Oncology. 2007;25:1627–1628. doi: 10.1200/JCO.2007.10.9728. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R, Meyer A, Schlieben P, Bondzio A, Weise C, Lenze D, Hummel M, Einspanier R, Gruber AD. Transcriptome and proteome analysis of tyrosine kinase inhibitor treated canine mast cell tumour cells identifies potentially kit signaling-dependent genes. BMC Veterinary Research. 2012;8:96–107. doi: 10.1186/1746-6148-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Kwon MJ, Oh E, Lee S, Roh MR, Kim SE, Lee Y, Choi Y-L, In Y-H, Park T, Koh SS, et al. Identification of novel reference genes using multiplatform expression data and their validation for quantitative gene expression analysis. PLoS ONE. 2009;4:e6162. doi: 10.1371/journal.pone.0006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne KR, Butler J, Marshall DJ, Tullai J, Gechtman Z, Hall C, Meshaw A, Farrell FX. Recombinant epoetins do not stimulate tumor growth in erythropoietin receptor-positive breast carcinoma models. Molecular Cancer Therapeutics. 2006;5:347–355. doi: 10.1158/1535-7163.MCT-05-0203. [DOI] [PubMed] [Google Scholar]
- Langston RD, Presley R, Flanders WD, McClellan WM. Renal insufficiency and anemia are independent risk factors for death among patients with acute myocardial infarction. Kidney International. 2003;64:1398–1405. doi: 10.1046/j.1523-1755.2003.00200.x. [DOI] [PubMed] [Google Scholar]
- Laugsch M, Metzen E, Svensson T, Depping R, Jelkmann W. Lack of functional erythropoietin receptors of cancer cell lines. International Journal of Cancer. 2008;122:1005–1011. doi: 10.1002/ijc.23201. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. Journal of Clinical Oncology. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Fritz E, Leitgeb C, Pecherstorfer M, Samonigg H, Schuster J. Prediction of response to erythropoietin treatment in chronic anemia of cancer. Blood. 1994;84:1056–1063. [PubMed] [Google Scholar]
- Maniscalco L, Iussich S, Morello E, Martano M, Biolatti B, Riondato F, Salda LD, Romanucci M, Malatesta D, Bongiovanni L, et al. PDGFs and PDGFRs in canine osteosarcoma: New targets for innovative therapeutic strategies in comparative oncology. The Veterinary Journal. 2013;195:41–47. doi: 10.1016/j.tvjl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais C, Johnson DW, Vesey DA, Gobe GC. Functional significance of erythropoietin in renal cell carcinoma. BMC Cancer. 2013;13:14. doi: 10.1186/1471-2407-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Reviews in Molecular Medicine. 2008;10:e36. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollio F, Staibano S, Mansueto G, De Rosa G, Persico F, De Falco M, Di Lieto A. Eryhtropoietin and erythropoietin receptor system in a large uterine myoma of a patient with myomatous erythrocytosis syndrome: Possible relationship with the pathogenesis of unusual tumor. Human Pathology. 2005;36:120–127. doi: 10.1016/j.humpath.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Quigley P, Leedale A. Tumors involving bone in the domestic cat: A review of fifty-eight cases. Veterinary Pathology. 1983;20:670–686. doi: 10.1177/030098588302000603. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri M, Schuster T, Buchner T, Malinowsky K, Bronger H, Schwarz-Boeger U, Höfler H, Avril S. Intratumoral heterogeneity of microRNA expression in breast cancer. The Journal of Molecular Diagnostics. 2012;14:376–384. doi: 10.1016/j.jmoldx.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Larsson AM, Vaapil M, Wigerup C, Sun J, Jögi A, Neumann D, Rönnstrand L, Pahlman S. EPO-independent functional EPO receptor in breast cancer enhances estrogen receptor activity and promotes cell proliferation. Biochemical and Biophysical Research Communications. 2014;445:163–169. doi: 10.1016/j.bbrc.2014.01.165. [DOI] [PubMed] [Google Scholar]
- Rózsás A, Berta J, Rojkó L, Horváth LZ, Keszthelyi M, Kenessey I, László V, Berger W, Grusch M, Hoda MA, et al. Erythropoietin receptor expression is a potential prognostic factor in human lung adenocarcinoma. PLoS ONE. 2013;8:e77459. doi: 10.1371/journal.pone.0077459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonije JH, van Groeningen CJ, van Bochove A, Honkoop AH, van Felius CL, Wormhoudt LW, Giaccone G. Effects of early intervention with epoetin alfa on transfusion requirement, hemoglobin level and survival during platinum-based chemotherapy: Results of a multicenter randomised controlled trial. European Journal of Cancer. 2005;41:1560–1569. doi: 10.1016/j.ejca.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Sfacteria A, Mazzullo G, Bertani C, Calabro P, De Vico G, Macri B. Erythropoietin receptor expression in canine mammary tumor: An immunohistochemical study. Veterinary Pathology. 2005;42:837–840. doi: 10.1354/vp.42-6-837. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, McGee S, Pienta MJ, McGregor N, Jung Y, Yumoto K, Wang J, Berry JE, Pienta KJ, Taichman RS. Erythropoietin supports the survival of prostate cancer, but not growth and bone metastasis. Journal of Cellular Biochemistry. 2013;114:2471–2478. doi: 10.1002/jcb.24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AM, Todd MD, Forsythe K, Knox SJ, Elliott S, Begley CG. Expression and function of erythropoietin receptors in tumors: Implications for the use of erythropoiesis-stimulating agents in cancer patients. Cancer. 2007;110:477–488. doi: 10.1002/cncr.22832. [DOI] [PubMed] [Google Scholar]
- Spodnick G, Berg J, Rand W, Schelling S, Couto G, Harvey H, Henderson R, MacEwen G, Mauldin N, McCaw D. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988) Journal of the American Veterinary Medical Association. 1992;200:995–999. [PubMed] [Google Scholar]
- Suzuki M, Takamizawa S, Nomaguchi K, Suzu S, Yamada M, Igarashi T, Sato I. Erythropoietin synthesis by tumour tissues in a pataient with uterine myoma and erythrocytosis. British Journal of Haematology. 2001;113:49–51. doi: 10.1046/j.1365-2141.2001.02682.x. [DOI] [PubMed] [Google Scholar]
- Swift S, Ellison AR, Kassner P, McCaffery I, Rossi J, Sinclair AM, Begley CG, Elliott S. Absence of functional EpoR expression in human tumor cell lines. Blood. 2010;115:4254–4263. doi: 10.1182/blood-2009-10-248674. [DOI] [PubMed] [Google Scholar]
- Tsai P-C, Breen M. Array-based comparative genomic hybridization-guided identification of reference genes for normalization of real-time quantitative polymerase chain reaction assay data for lymphomas, histiocytic sarcomas, and osteosarcomas of dogs. American Journal of Veterinary Research. 2012;73:1335–1343. doi: 10.2460/ajvr.73.9.1335. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Reviews. Genetics. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallvik J, Stenke L, Bernell P, Nordahl G, Hippe E, Hast R. Serum erythropoietin (EPO) levels correlate with survival and independently predict response to EPO treatment in patients with myelodysplastic syndromes. European Journal of Haematology. 2002;68:180–185. doi: 10.1034/j.1600-0609.2002.01530.x. [DOI] [PubMed] [Google Scholar]
- Wan L, Zhang F, He Q, Tsang WP, Lu L, Li Q, Wu Z, Qiu G, Zhou G, Wan C. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS ONE. 2014;9:e102010. doi: 10.1371/journal.pone.0102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal G, Niederberger E, Blum C, Wollman Y, Knoch TA, Rebel W, Debus J, Friedrich E. Erythropoietin and G-CSF receptors in human tumor cells: Expression and aspects regarding functionality. Tumori. 2002;88:150–159. doi: 10.1177/030089160208800214. [DOI] [PubMed] [Google Scholar]
- Xue Y, Lim S, Yang Y, Wang Z, Jensen LDE, Hedlund E-M, Andersson P, Sasahara M, Larsson O, Galter D, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nature Medicine. 2012;18:100–110. doi: 10.1038/nm.2575. [DOI] [PubMed] [Google Scholar]
- Yan X, Krzyzanski W. Quantitative assessment of minimal effective concentration of erythropoiesis-stimulating agents. CPT Pharmacometrics and Systems Pharmacology. 2013;2:e62. doi: 10.1038/psp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. The Journal of Biological Chemistry. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Weaver DL, Munjal K, Evans MF. Intratumoral DNA content heterogeneity in breast carcinomas demonstrated by core punch tissue sampling and flow cytometry. Journal of Clinical Pathology. 2014;67:821–824. doi: 10.1136/jclinpath-2014-202418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.