Abstract
Background
Hemorrhagic shock (HS)-induce microvascular hyperpermeability involves disruption of endothelial cell adherens junctions leading to increase in paracellular permeability. β-Catenin, an integral component of the adherens junctional complex and Wnt pathway, and caspase-3 via its apoptotic signaling regulate endothelial cell barrier integrity. We have hypothesized that inhibiting phosphorylation of β-catenin and caspase-3 activity using glycogen synthase kinase-3 (GSK-3) specific inhibitor SB216763, would attenuate microvascular hyperpermeability following HS.
Methods
In Sprague-Dawley rats, HS was induced by withdrawing blood to reduce mean arterial pressure to 40 mmHg for 60 minutes followed by resuscitation. Rats were given SB216763 (600 μg/kg) intravenously 10 minutes prior to shock. To study microvascular permeability, the rats were injected intravenously with FITC-albumin (50 mg/kg) and its flux across the mesenteric post-capillary venules was determined using intravital microscopy. In cell-culture studies, rat lung microvascular endothelial cell (RLMEC) monolayers grown on Transwell plates were pre-treated with SB216763 (5 μM) followed by BAK (5 μg/mL) and caspase-3 (5 μg/mL) protein transfection. FITC-albumin (5 mg/mL) flux across cell monolayers indicates change in monolayer permeability. Activity of canonical Wnt pathway was determined by luciferase assay. Caspase-3 enzyme activity was assayed fluorometrically.
Results
The HS group showed significant increase in FITC-albumin extravasation (p<0.05) compared with sham. SB216763 significantly decrease HS-induced FITC-albumin extravasation (p<0.05). Pre-treatment with SB216763, protected against a BAK-induced increase in RLMEC monolayer permeability and caspase-3 activity, but failed to show similar results with a caspase-3-induced increase in monolayer permeability. Wnt3a treatment showed an increase in β-catenin dependent TCF-mediated transcription.
Conclusion
Inhibiting phosphorylation of β-catenin and caspase-3 activity using GSK-3 specific inhibitor SB216763, help regulate HS-induced microvascular hyperpermeability.
Introduction
Traumatic injuries are the leading cause of mortality with 5 million deaths annually worldwide and that number may increase up to 8 million by the year 2020, making it a major global health concern.1-3 Traumatic injuries leading to hemorrhagic shock (HS)-induced microvascular hyperpermeability can cause multiple organ failure, one of the most serious clinical complications, resulting in an increased number of deaths in trauma patients.4 HS followed by resuscitation leads to ischemia-reperfusion injury, which in turn may give rise to an abnormality called microvascular hyperpermeability.4-6 Microvascular hyperpermeability is a result of an increase in paracellular endothelial cell permeability due to endothelial cell barrier dysfunction.5-7 The endothelial cell barrier integrity is regulated by the adherens junctional complexes at the endothelial cell-cell junctions.7,8 The adherens junctional protein complex is made up of transmembrane vascular endothelial cadherin (VE-cadherin), that extracellularly attaches to other VE-cadherin protein molecules from adjacent endothelial cells through an ectodomain. Intracellularly, VE-cadherin attaches itself to the actin cytoskeleton through actin binding proteins such as α-actinin, vinculin and catenins including α-catenin, plakoglobin or γ-catenin, and β-catenin.7-11
β-Catenin, an integral component of the adherens junctional complex, plays an important role in preserving endothelial cell barrier integrity.12,13 The paracellular endothelial cell permeability can be regulated through stabilizing the adherens junctional complex either by supplementing β-catenin via gene or protein transfection.12 Recent studies have shown that post-translational β-catenin gene silencing can disrupt the adherens junctional complex resulting in endothelial cell hyperpermeability.12 Similarly, we have also illustrated the intracellular dynamics of β-catenin during disruption and recovery of the adherens junctional complex. Our laboratory has demonstrated that ß-catenin may be recycled back to the cell membrane following disruption of the endothelial adherens junction.13 Apart from being an adhesion molecule, β-catenin also acts as a signaling molecule involved in the canonical Wnt pathway.14,15 The Wnt signaling pathways are differentiated into a canonical or ß-catenin dependent and non-canonical or ß-catenin independent Wnt pathway.15-17 The non-canonical Wnt signaling is mediated by Wnt ligands such as Wnt5a and regulated through the intracellular calcium and JNK pathway.15.16,18 Whereas, the canonical Wnt pathway includes several Wnt ligands namely Wnt3a, Frizzled receptors and glycogen synthase kinase-3 (GSK-3).15-19
GSK-3 was identified and named after its role in phosphorylating glycogen synthase and rendering its inactivity to maintain blood glucose levels.20,21 GSK-3 is a well conserved, ubiquitously expressed serine/threonine kinase, known to have an important role in the Wnt signaling pathway and affects stability of several proteins through their phosphorylation.20,21 In the presence of active canonical Wnt signaling pathway, GSK-3 is inactive to target β-catenin. When β-catenin is not degraded, it is stabilized in the cytoplasm and subsequently translocates into the nucleus to stimulate target gene transcription.15-17,22 In the absence of the active signal transduction in the canonical Wnt pathway, degradation of β-catenin takes place by phosphorylation of β-catenin’s amino terminus by active GSK-3.15 The phosphorylation of amino acids promotes ubiquitination of β-catenin which leads to degradation of β-catenin through the proteosomal pathway.15 Therefore, one of the hypothesis for this study was that arresting β-catenin phosphorylation via inhibition of GSK-3 would protect against HS-induced microvascular hyperpermeability by preventing degradation of β-catenin. In this study, we have used a pharmacological agent, SB216763, as a selective, cell permeable small molecule that potently inhibits the activity of GSK-3 in several cell types such as neuronal, liver, kidney, and endothelial cells.23-25 The SB216763 selectivity to inhibit GSK-3 has been rigorously tested against several other protein kinases, and it has been demonstrated that no other protein kinases are inhibited at a concentration where a significant inhibition of GSK-3 occurs.26,27 Previous results from our laboratory have also shown an increase in ß-catenin mediated transcription activity in endothelial cells pretreated with SB216763 via luciferase assay.13
Recent studies from our laboratory have also shown that HS-induced microvascular hyperpermeability is regulated by mitochondria-mediated or intrinsic apoptotic signaling. HS-induced microvascular hypermeability initiates intrinsic apoptotic signaling through an up-regulation of a pro-apoptotic protein of Bcl-2 family, BAK.28 The intrinsic apoptotic signaling occurs when apoptotic signal directly converge on mitochondria resulting in activation of downstream effector caspase-3.28,29 The active caspase-3 subsequently disrupts the adherens junctional protein complexes resulting in microvascular endothelial cell hyperpermeability.28,30 Recent studies have shown GSK-3 activity in regulating apoptotic signaling.31 So, we have postulated that inhibition of GSK-3, that phosphorylates β-catenin, would also decrease BAK induced caspase-3 enzyme activity and attenuate microvascular hyperpermeability. Thus, our main objective was to test GSK-3 specific inhibitor SB216763 against microvascular hyperpermeability following HS.
Material and Methods
Animals
Sprague-Dawley male rats, weighing about 275–325 gms, were obtained from Charles River Laboratories, Wilmington, MA. These animals were lodged in the institutional animal facility of the Texas A&M Health Science Center College of Medicine at Scott and White Health care, Temple, TX. The institutional animal facility was approved by the American Association for Accreditation of Laboratory Animal Care, in accordance with the National Institutes of Health guidelines. In the animal facility, room temperature was maintained at 25°C ± 2°C and humidity was adjusted to 55%. The rats were kept on a 12:12 hour dark/light cycle, with free access to food and water. The rats were fasted for 18 hours and administered water ad libitum a night prior to every experiment. The in-vivo animal experiments were performed after getting proper consent from the Institutional Animal Care and Use Committee.
Cell-Culture
Rat lung microvascular endothelial cells (RLMECs), grown in the MCDB-131 complete media were obtained from VEC-technologies, Rensselaer, NY. RLMECs were grown as monolayer on Corning Transwell plates coated with fibronectin solution (0.1%) obtained from Sigma-Aldrich, St Louis, MO. Trypsin–EDTA solution (0.25%), used to detach the cells was obtained from Invitrogen-Gibco, Grand Island, NY.
Reagents
The pro-apoptotic BH3 peptide, BAK was obtained from R&D Systems, Minneapolis, MN. A polyamine protein transfection reagent, TransIT-LT1 was obtained from Mirus Bio Corporation, Madison, WI. Fluorescein isothiocyanate bovine albumin (FITC-albumin) was obtained from Sigma, St Louis, MO. The test solution was prepared by dissolving the FITC-albumin in 1 ml of saline and administered to the rats at 50 mg/kg. A fluorometric caspase-3 assay kit was obtained from R&D Systems, Minneapolis, MN. Luminometer Luciferase assay kits along with TOP-flash and FOP-flash vectors were obtained from Millipore, Billerica, MA. The transfection reagent, Transpass, was obtained from New England Biolab, Ipswich, MA. The GSK-3 specific inhibitor SB216763 (3-(2,4-Dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione) was obtained from Sigma-Aldrich Corp, St Louis, MO. The test solution was prepared by dissolving the SB216763 in dimethylsulfoxide (DMSO, < 1%) and was administered in following doses; in vivo at 600μg/kg IV and in vitro at 5 μM.32,33,34 The DMSO at this low concentration does not have an effect on microvascular endothelial cell cultures nor does it have an effect on permeability either in-vivo or in-vitro experimental settings.33-38
In-Vivo Studies
HS Sprague-Dawley rat model and Intravital Microscopy
To study HS-induced microvascular hyperpermeability, we have used a Sprague-Dawley rat model for HS using intravital microscopy. As described in our previous published work, Tharakan B et al. male Sprague-Dawley rats were anesthetized with a single intramuscular injection of 50% urethane (1.5g/kg).39 Polyethylene tubing (PE-50) of 0.58 mm internal diameter was used to cannulate the right internal jugular vein for continuous intravenous (IV) administration of normal saline (3 ml/hr) through an infusion pump obtained from Harvard Apparatus, South Natick, MA. The right carotid artery was cannulated for blood withdrawal, while the mean arterial pressure was monitored continuously through left femoral artery via a PE-50 cannula connected to a blood pressure monitor obtained from Dig-Med, BPA 400A, Micromed, Louisville, KY. The rats were then subjected to a level IV HS like condition by withdrawing blood from the right carotid artery into a syringe containing 100 units of heparin until the mean arterial pressure (MAP) was decreased to 40 mm Hg which corresponds to loss of approximately 50% to 60% of the animal’s blood volume. This process was followed by resuscitation with re-infusion of the shed blood plus two times the volume of normal saline to maintain a MAP at or above 90 mm Hg (T60), since the normal baseline MAP of the animal was around 90 to 100 mmHg. At 10-minute intervals for 60 minutes, corresponding parameters were recorded. A midline laparotomy incision was performed to exteriorize a section of mesentery from the proximal ileum for examination over a temperature controlled Plexiglas stage at 37°C. The rats were placed in a lateral decubitus position on a temperature controlled Plexiglas platform mounted on an intravital upright microscope obtained from Nikon E600, Tokyo, Japan. The mesentery was superfused with normal saline at 2ml/min and was covered with plastic wrap to reduce evaporation. The intravital microscope with the Nikon 20× objective, 0.45 to 2.16 mm working distance obtained from Nikon Instruments, Inc., Natick, MA, was used to examine venules with diameters of 20 to 30 μm. The images were generated using with a Photometric Cascade Camera obtained from Roper Scientific, Tucson, AZ. These images were then projected onto a computer monitor, and captured digitally on a computer disc. The analysis of the data was performed using MetaMorph 4.5/4.6 obtained from Universal Imaging Corp., Downington, PA.39
Effect of GSK-3 specific inhibitor SB216763 on HS-induced microvascular hyperpermeability
The rats were divided into three groups; a sham (control) group, a HS group (T60), and a HS group pretreated with GSK-3 specific inhibitor SB216763 (600μg/kg). Each experimental group consisted of five rats. A recovery time of 30 minutes was given to the animal to recover from surgical manipulation before the start of all experiments. During this period, FITC-albumin (50 mg/kg) was administered IV and baseline-integrated optical intensities were obtained intravascularly and extravascularly with two sites respectively along the same computed areas. The mean values were used for calculations. This was followed by the recording of baseline parameters such as MAP, red cell centerline velocity, and vessel diameter. SB216763 (600μg/kg) was administered IV, 10 minutes prior to inducing HS. The rats were subjected to HS as described above for 60 minutes at (T0). This was followed by resuscitation phase for another 60 minutes as described above. All the parameters were recorded post-shock at 10-minute intervals for 60 minutes. A trans-illuminated segment of small intestine containing mesenteric post-capillary venules was examined to quantify changes in albumin flux. The light intensity was calculated using following formula ΔI = Ii - Io/Ii, where ΔI change in the light intensity, Ii is the light intensity inside the vessel, and Io is the light intensity outside the vessel.39
In-Vitro Studies
Monolayer Permeability Assay
RLMECs were grown as monolayers on fibronectin coated luminal (upper) chamber of the Transwell plates using MCDB-131 complete media. The monolayers were exposed to fresh media without phenol red 60 minutes prior to the start of the experiments in order to avoid any interference with FITC-albumin during fluorometric reading. All of the treatments and pretreatments were carried out for 60 minutes each. During the experiment, the treatment of the monolayers was followed by adding FITC-albumin (5 mg/mL) to the luminal chamber of the Transwell plate, and the mixture was allowed to equilibrate for 30 minutes. Subsequently, 100 μl of samples were collected from the abluminal (lower) chambers for analyzing FITC-albumin fluorescent intensity using excitation/emission wavelength of 494 and 520 nm respectively, on a fluorometric plate reader.40
Luciferase Reporter Assay
In presence of an active canonical Wnt signaling pathway, the ß-catenin that is freely available in the cytoplasm is known to translocate into the nucleus for T-cell factor (TCF)-mediated gene transcription.15-17 The activity of ß-catenin dependent Wnt canonical pathway was demonstrated by using luciferase activity. The luciferase reporter assay consists of TOP-flash reporter construct containing TCF binding site upstream of a thymidine kinase promoter and the FOP-flash construct with a mutated site for TCF was used as a negative control. The freely available ß-catenin on binding to TCF site on TOP-flash activates transcription of luciferase reporter gene, whereas the FOP-flash reporter construct fails to show similar effect on binding with ß-catenin. The luciferase that is secreted in the cell medium has a luminescence signal which was measured on a luminometer.
Caspase-3 Assay
RLMECs were grown on fibronectin coated cell culture dishes in MCDB-131 complete media. After performing the experimental treatments, the RLMECs were lysed by adding caspase-3 sample lysis buffer provided in the assay kit and the homogenates were obtained for protein estimation. The homogenates were then treated with the substrate conjugate labeled with a fluorescent probe 7-amino-4-trifluoromethyl coumarin for the caspase-3 assay. The resulting fluorescent intensity was analyzed using excitation/emission wavelength of 400 and 505 nm respectively, on a fluorescent plate reader.30,40
Effect of Wnt3a and Wnt5a on TCF-mediated transcriptional activity
To determine the presence of an active canonical Wnt pathway in RLMECs, we have used a luciferase reporter assay to demonstrate ß-catenin dependent TCF-mediated transcriptional activity. In order to differentiate between Wnt canonical and non-canonical pathways, we have used Wnt 3a and Wnt 5a ligands respectively to determine the corresponding active signaling pathway in the endothelial cells. RLMECs grown on fibronectin coated cell culture dishes in MCDB-131 complete media were transfected with TOP-flash vector and with FOP-flash construct for 24 hours using a transfection reagent, Transpass. Following transfection, RLMECs were treated with Wnt3a and Wnt5a proteins for 60 minutes. Each experimental group was divided in to 4 replicates. Upon treatment with Wnt 3a and 5a ligand protein respectively, TCF-mediated transcriptional activity in RLMEC was determined using luciferase reporter assay as described above. The change in luminescence intensity due to luciferase signal between different experimental groups as determined by the luminometer was consider as an indicator for ß-catenin dependent TCF-mediated transcriptional activity.
Effect of GSK-3 specific inhibitor SB216763 on BAK-induced microvascular endothelial cell hyperpermeability
RLMEC monolayers grown on Transwell plates were used. The following experimental groups were studied: a control or untreated group, TransIT treated group, BAK peptide (5 μg/mL) treated group, BAK treated group pretreated with SB216763 (5 μM), and SB216763 alone group. The monolayers were treated with SB216763 for 60 min followed by treatment with BAK for 60 minutes.40 RLMEC monolayer permeability was assayed as described above.
Effect of GSK-3 specific inhibitor SB216763 on caspase-3-induced microvascular endothelial cell hyperpermeability
RLMEC monolayers grown on Transwell plates were used. The following experimental groups were studied: a control or untreated group, caspase-3 peptide (5 μg/mL) treated group, caspase-3 treated group pretreated with SB216763 (5 μM), and SB216763 alone group. The monolayers were treated with SB216763 for 60 min followed by treatment with caspase-3 for 60 minutes.40 RLMEC monolayer permeability was assayed as described above.
Effect of GSK-3 specific inhibitor SB216763 on BAK-induced caspase-3 activity
RLMECs grown on cell culture dishes were used. The following experimental groups were studied: a control or untreated group, BAK peptide (5 μg/mL) treated group, BAK treated group pretreated with SB216763 (5 μM), and SB216763 alone group. The following treatments were carried out for 60 minutes each. In RLMECs, caspase-3 enzyme activity was assayed as described above.
Statistical Analysis
During statistical analysis, the comparisons between experimental groups were made using analysis of variance /ANOVA followed by Bonferroni post-test for multiple comparisons. Student’s t test was also used to differentiate between two groups during luciferase activity measurement. A p value of ≤ 0.05 was considered to indicate statistically significance. All the experimental data are expressed as mean ± SEM. Statistical analysis was performed using Prism GraphPad software obtained from GraphPad Software, Inc. La Jolla, CA. In microvascular hyperpermeability in-vivo studies, to avoid bias between animals because of red blood cell accumulation and changes in room lighting, and in-vitro monolayer permeability studies, each experimental value was compared with initial baseline value and expressed as a percentage change.
Results
In-Vivo Studies
GSK-3 specific inhibitor SB216763 inhibits HS-induced microvascular hyperpermeability
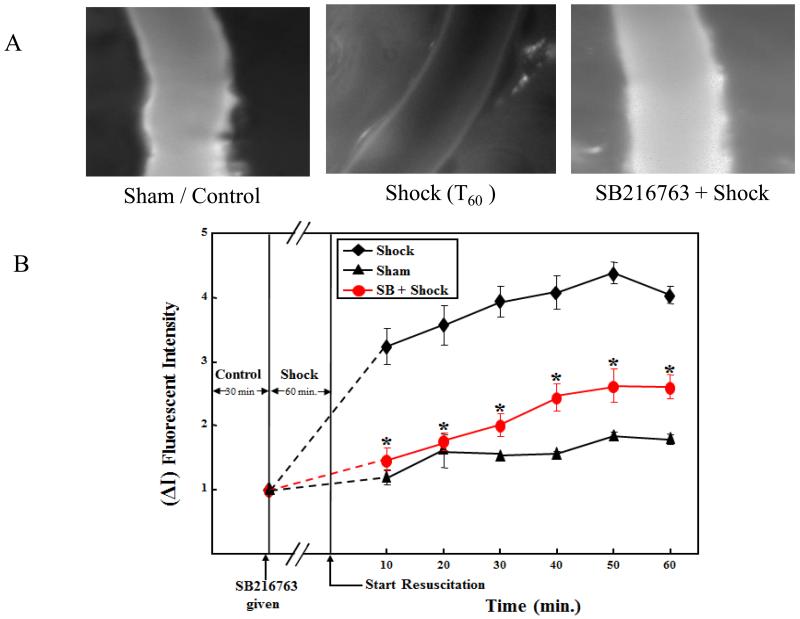
The rats subjected to HS showed microvascular hyperpermeability as demonstrated by the significant increase in FITC-albumin fluorescent intensity in the extravascular space, compared with the sham or control group (p < 0.05; Figures 1A and 1B). The similar results were also documented in the earlier studies from our laboratory.28,39,41 SB216763 pre-treatment in the HS group showed a decrease in microvascular hyperpermeability compared with the HS group alone without pre-treatment (p < 0.05; Figures 1A and 1B). Pre-treatment with SB216763 in the HS group showed statistically no change in permeability compared with the control group. Figure 1A shows a composite image of a rat mesenteric post-capillary venule. The first image which is from a control group demonstrates minimal extravasation of FITC-albumin into the extravascular space. The second image is from a group (T60) subjected to 60 minutes of HS followed by 60 minutes of resuscitation, demonstrates significant extravasation of FITC-albumin into the extravascular space. The third image is from treatment group showing SB216763 treatment before shock demonstrates protection against HS induced microvascular hyperpermeability. Figure 1B is a graphical representation of the changes in microvascular permeability in the sham or control, HS, and SB216763 treatment groups.
Figure 1.
GSK-3 specific inhibitor SB216763 protect against microvascular hyperpermeability following hemorrhagic shock (HS). Fig 1A; shows images of rat mesenteric post-capillary venules from control or sham treatment, HS for 60 minutes followed by resuscitation for another 60 min (T60), and SB216763 treatment in HS are shown. FITC-albumin extravasation into the extravascular space after HS (T60) is significant compare to the control or sham treatment. However, SB216763 treatment prevented FITC-albumin extravasation in rat mesenteric post-capillary venules. Fig 1B; shows graphical representation of increase in microvascular permeability following HS compared to control or sham group. SB216763 treatment decreases microvascular hyperpermeability following HS (*P < 0.05; n = 5).
In-Vitro Studies
Wnt3a show increase in TCF-mediated transcriptional activity
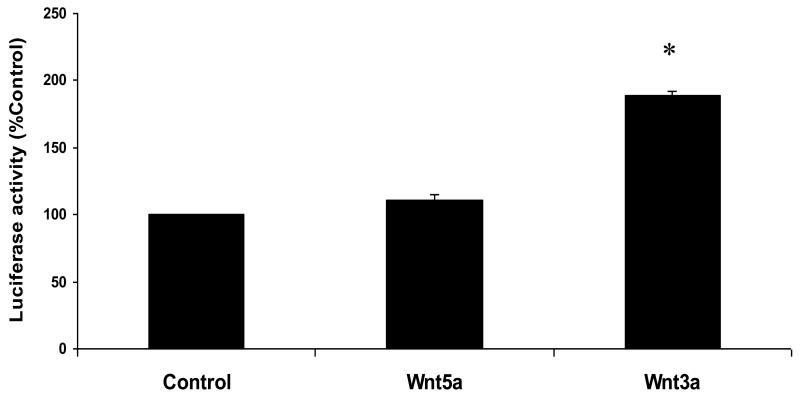
The control RLMECs transfected with TOP-flash alone showed minimal luciferase activity. Wnt3a treatment in TOP-flash transfected RLMECs showed significant increase in luciferase activity compared with the Wnt5a treatment and TOP-flash alone groups (p < 0.05; Figure 2). The luciferase activity of the basal control group is expressed as 100%, and the luciferase activity of the remaining groups is expressed as a percentage of the control group.
Figure 2.
The untreated control rat lung microvascular endothelial cells (RLMECs) transfected with TOPflash vector showed basal luciferase activity, which is expressed as 100%. Wnt3a treatment in RLMECs transfected with TOPflash vector showed significant increase in luciferase activity (*p<0.05). However, Wnt5a failed to show similar increase in luciferase activity.
GSK-3 specific inhibitor SB216763 inhibits BAK-induced microvascular endothelial cell hyperpermeability
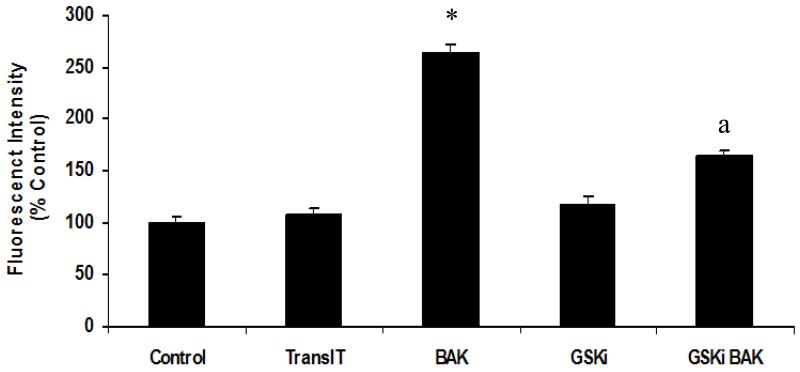
RLMECs showed increased monolayer permeability following BAK transfection compared with the control or untreated cells. The FITC-albumin fluorescent intensity in the media from abluminal chamber was significantly higher in RLMEC transfected with BAK compared with the control or untreated group, suggesting an increase in endothelial cell monolayer permeability. However, the monolayers pretreated with SB216763 showed significantly less fluorescent intensity of FITC-albumin in the media from the abluminal chamber (p < 0.05; Figure 3).
Figure 3.
Rat lung microvascular endothelial cell (RLMEC) monolayers transfected with BAK peptide showed significant increase in permeability compared to the untreated control or transfection reagent TransIT treated monolayers (*p<0.05). BAK transfected monolayers when pre-treated with SB216763 showed significant decrease in permeability compared to the BAK transfected RLMEC monolayers (a p<0.05).
GSK-3 specific inhibitor SB216763 show no effect on caspase-3-induced microvascular endothelial cell hyperpermeability
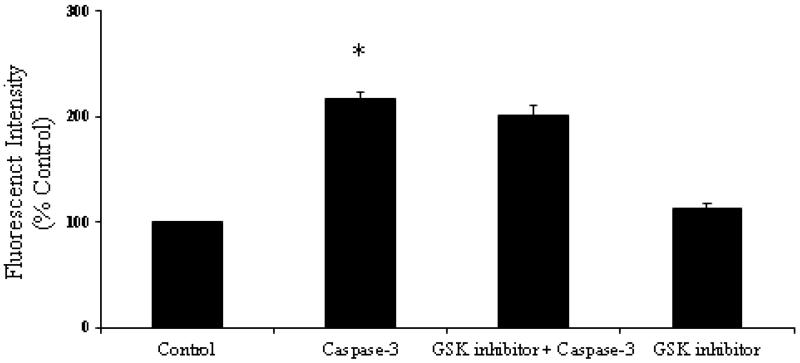
RLMECs showed increased monolayer permeability following caspase-3 transfection compared with the control or untreated cells. The FITC-albumin fluorescent intensity in the media from abluminal chamber was significantly higher in RLMEC transfected with caspase-3 compared with the control or untreated group, suggesting an increase in endothelial cell monolayer permeability (p < 0.05; Figure 4). However, the monolayers pretreated with SB216763 showed no significant change in the fluorescent intensity of FITC-albumin in the media from the abluminal chamber.
Figure 4.
Rat lung microvascular endothelial cell (RLMEC) monolayers transfected with active caspase-3 peptide showed significant increase in permeability compared to the untreated control monolayers (*p<0.05). Active caspase-3 transfected monolayers when pre-treated with SB216763 showed no significant effect in decreasing the hyperpermeability induced by active caspase-3 transfected RLMEC monolayers.
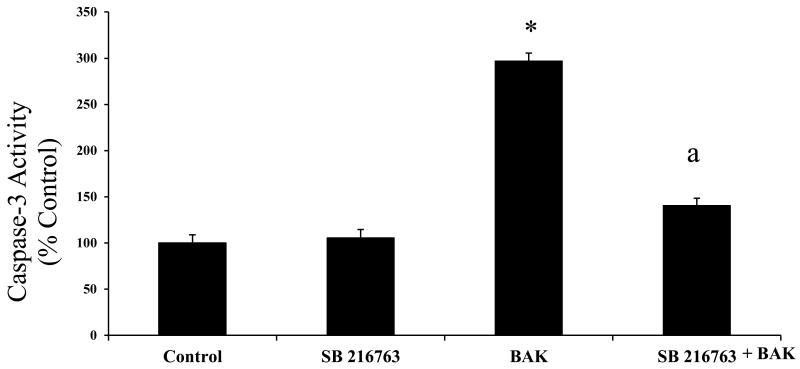
GSK-3 specific inhibitor SB216763 inhibits BAK-induced caspase-3 activity
RLMECs transfected with BAK showed significant increase in caspase-3 enzyme activity compared with control or untreated cells. Pre-treatment with SB216763, decreases BAK-induced increase in caspase-3 activity in RLMECs (p < 0.05; Figure 5).
Figure 5.
Rat lung microvascular endothelial cells (RLMECs) transfected with BAK peptide showed significant increase in caspase-3 activity (* p<0.05). SB216763 pre-treatment, decreases BAK-induced increase in caspase-3 activity significantly in RLMECs (a p<0.05).
Discussion
The results of this study have demonstrated that an active canonical Wnt signaling pathway is present in RLMECs, and pharmacological inhibition of GSK-3 activity via SB216763 can decrease HS-induced microvascular hyperpermeability in Sprague-Dawley rats. HS followed by resuscitation often leads to a microvascular hyperpermeability giving rise to various fatal clinical sequelae such as multiple organ dysfunction syndrome that often leads to an increase in morbidity and mortality in patients.4,6 Thus, the overall goal in managing such patients is to develop clinical and therapeutic strategies to reduce microvascular hyperpermeability. The increase in paracellular permeability leading to microvascular hyperpermeability is due to loss of endothelial cell adherens junctional protein complex integrity.6-8 ß-Catenin forms an essential part of the protein complex that helps in stabilizing adherens junctions and in turn regulate endothelial cell permeability.12,13 ß-Catenin is freely available in the cytoplasm in the presence of active canonical Wnt signaling.15-17
The canonical Wnt pathway is regulated by a group of proteins such as axin, adenomatous polyposis coli, casein kinase-1α and GSK-3 forming a destruction complex that keeps the level of β-catenin in cytoplasm in check through ubiquitination and proteasomal degradation.15-17 The initiation of canonical Wnt signal transduction begins with the interaction of Wnt ligands such as Wnt3a to a receptor complex consisting of Frizzled receptor and low-density lipoprotein receptor-related protein receptor. This is followed by activation of a disheveled protein (Dv1) by phosphorylation. The active Dv1 protein in turn breaks the destruction complex by preventing the binding of GSK-3 with axin. Thus, phosphorylation of β-catenin in the cytoplasm is averted by causing inactivation of GSK-3. The un-phosphorylated β-catenin cannot be subjected to ubiquitination and thus evades its degradation through proteasome.15-17 The available un-phosphorylated stable cytoplasmic β-catenin can then be utilized as a cell junctional adhesion molecule or in nuclear gene transcription42,43 In the nucleus, β-catenin forms a protein complex with lymphoid enhancer factor and T-cell factor (β-catenin /LEF/TCF complex) to facilitate downstream target gene transcription.15-17 At the cell junctions, the un-phosphorylated β-catenin is made available to repair adherens junctional complex and restore endothelial cell barrier integrity.42,43 In this study, we have demonstrated through luciferase reporter assay that in RLMECs, a canonical Wnt protein, Wnt3a, showed an increase in ß-catenin dependent TCF mediated transcriptional activity. However, a non-canonical Wnt protein, Wnt5a failed to show similar response on luciferase reporter assay. This confirms the presence of an active canonical Wnt pathway in RLMECs. Furthermore, Since GSK-3 plays an important role in the canonical Wnt signaling pathway, we have demonstrated through the in-vivo study that inhibition of GSK-3 using a pharmacological specific inhibitor SB216763 can protect against microvascular hyperpermeability following HS in Sprague-Dawley rats. Thus, preventing phosphorylation of β-catenin in the cytoplasm by inhibiting GSK-3 via SB216763 attenuates HS-induced microvascular hyperpermeability.
During HS-induced microvascular hyperpermeability, along with pro-inflammatory mediators, a pro-apoptotic protein BAK gets up-regulated and initiates mitochondria-mediated intrinsic apoptotic cascade. The subsequent events lead to an increase in mitochondrial reactive oxygen species (ROS), a decrease in mitochondrial transmembrane potential, and the release of mitochondrial apoptogenic protein cytochrome c in the cytoplasm leading to activation of downstream executioner caspase-3.28 The active caspase-3 brings about organizational change in the adherens junctional complexes by cleaving β-catenin or altering β-catenin association within the complex.13,14,28,30 This alterations in the adherens junctional complexes affect its barrier integrity resulting in an increase in paracellular endothelial cell permeability.13,28,30 In this study, we have demonstrated that inhibition of GSK-3 by SB216763 prevents BAK-induced caspase-3 enzyme activation in RLMECs. Furthermore, SB216763 also protects against BAK-induced hyperpermeability in RLMECs. However, SB216763 failed to show similar effect against endothelial cell hyperpermeability induced by caspase-3 protein. Also, this is well supported by the earlier studies that GSK-3 can initiate apoptotic signaling by targeting upstream of caspase-3 in the apoptotic pathway.31 Therefore, inhibition of GSK-3 can halt the apoptotic cascade by decreasing caspase-3 activity through its effect on BAK, which lies upstream of the apoptotic pathway. But, inhibiting GSK-3 becomes ineffective in protecting against active caspase-3 protein treatment, since it is a final executionary step downstream of the apoptotic pathway.
Furthermore, a recent study from our laboratory has shown that by inhibiting phosphorylation of β-catenin using GSK-3 specific inhibitor SB216763, there is increase in β-catenin-mediated transcriptional activity in microvascular endothelial cells.13 Therefore, apart from β-catenin mediated protection against HS-induced microvascular hyperpermeability via inhibiting phosphorylation of β-catenin by SB216763. In the current study, we have also demonstrated another possible pathway through which SB216763 can prevent microvascular hyperpermeability following HS and that is via attenuating BAK induced caspase-3 activation. However, we cannot differentiate between dominant/direct or indirect pathway, but we can postulate that both the mechanisms might be occurring simultaneously in protecting against microvascular hyperpermeability following HS. Moreover, through these results we were successful in demonstrating an interaction between Wnt and apoptotic signaling pathways through the effect of GSK-3 inhibition on caspase-3 activity. And also the dynamics between these two signaling pathways has a potential that can be explored further in the context of HS-induced microvascular hyperpermeability.
In conclusion, an active canonical Wnt signaling pathway is present in endothelial cells and inhibition of GSK-3 via SB216763 can protect against microvascular hyperpermeability in Sprague-Dawley rats following HS. This demonstrates that β-catenin phosphorylation by GSK-3 is one of the key mechanisms involved in regulating microvascular hyperpermeability following HS. Furthermore, SB216763 was shown to attenuate BAK-induced caspase-3 enzyme activity and endothelial cell hyperpermeability in-vitro. These observations have opened new dimensions for our own research and to others researchers in future in terms of studying interaction between canonical Wnt signaling and apoptotic signaling pathways in HS-induced microvascular hyperpermeability. It is also important to investigate the role of specific canonical Wnt ligands and Frizzled receptors involved in HS-induced microvascular endothelial barrier dysfunction. Thus, this study highlights the importance of GSK-3 activity and its role in regulating microvascular hyperpermeability following HS. The study is a significant step towards translational research, and its results have therapeutic potential against HS-induced microvascular hyperpermeability targeting GSK-3 and its associated intracellular signaling and molecular network.
Acknowledgements
This work was supported by a grant (1K01HL07815-01A1), from National Heart, Lung and Blood Institute, National Institutes of Health, USA.
Footnotes
Authorship
D.A.S., B.T., and F.A.H. were involved in designing, performing the experiments, analysis, and interpretation of the data and manuscript preparation. E.W.C. was involved in designing, interpretation of the data and manuscript preparation.
References
- 1).Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2).Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).World Health Organization International Injuries [Internet] Available from: http://www.who.int/topics/injuries/about/en/index.html.
- 4).Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912–918. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 5).Childs EW, Udobi KF, Hunter FA. Hypothermia reduces microvascular permeability and reactive oxygen species expression after hemorrhagic shock. J Trauma. 2005;58:271–277. doi: 10.1097/01.ta.0000119203.24601.7e. [DOI] [PubMed] [Google Scholar]
- 6).Childs EW, Udobi KF, Hunter FA, Dhevan V. Evidence of transcellular albumin transport after hemorrhagic shock. Shock. 2005;23:565–570. [PubMed] [Google Scholar]
- 7).Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 8).Gianfranco B, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2008;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 9).Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 10).Vincent PA1, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 11).Sawant DA, Tharakan B, Adekanbi A, Hunter FA, Smythe WR, Childs EW. Inhibition of VE-cadherin proteasomal degradation attenuates microvascular hyperpermeability. Microcirculation. 2011;18:46–55. doi: 10.1111/j.1549-8719.2010.00067.x. [DOI] [PubMed] [Google Scholar]
- 12).Sawant DA, Tharakan B, Hunter FA, Smythe WR, Childs EW. Role of β-catenin in regulating microvascular endothelial cell hyperpermeability. J Trauma. 2011;70:481–487. doi: 10.1097/TA.0b013e31820b3ed7. [DOI] [PubMed] [Google Scholar]
- 13).Tharakan B, Hellman J, Sawant DA, Tinsley JH, Parrish AR, Hunter FA, Smythe WR, Childs EW. β-Catenin dynamics in the regulation of microvascular endothelial cell hyperpermeability. Shock. 2012;37:306–311. doi: 10.1097/SHK.0b013e318240b564. [DOI] [PubMed] [Google Scholar]
- 14).Steinhusen U, Badock V, Baurer A, Behrens J, Wittman-Liebold B, Dörken B, Bommert K. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–16353. doi: 10.1074/jbc.M001458200. [DOI] [PubMed] [Google Scholar]
- 15).Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 16).Komiya Y1, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).MacDonald BT1, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Sugimura R, Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today. 2010;90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 19).Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 20).Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8:4032–4039. doi: 10.4161/cc.8.24.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, Liu J, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN. GSK3beta: role in therapeutic landscape and development of modulators. Br J Pharmacol. 2010;160:1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 24).Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 25).Bazzoni G, Tonetti P, Manzi L, Cera MR, Balconi G, Dejana E. Expression of junctional adhesion molecule-A prevents spontaneous and random motility. J Cell Sci. 2005;118:623–632. doi: 10.1242/jcs.01661. [DOI] [PubMed] [Google Scholar]
- 26).Smith DG, Buffet M, Fenwick AE, Haigh D, Ife RJ, Saunders M, Slingsby BP, Stacey R, Ward RW. 3-Anilino-4-arylmaleimides: potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3) Bioorg Med Chem Lett. 2001;11:635–639. doi: 10.1016/s0960-894x(00)00721-6. [DOI] [PubMed] [Google Scholar]
- 27).Kramer T, Schmidt B, Lo Monte F. Small-Molecule Inhibitors of GSK-3: Structural Insights and Their Application to Alzheimer’s Disease Models. Int J Alzheimers Dis. 2012:381029. doi: 10.1155/2012/381029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2007;292:H3179–H3189. doi: 10.1152/ajpheart.01337.2006. [DOI] [PubMed] [Google Scholar]
- 29).Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 30).Sawant DA, Tharakan B, Tobin RP, Reilly J, Hunter FA, Newell MK, Smythe WR, Childs EW. Microvascular endothelial cell hyperpermeability induced by endogenous caspase 3 activator staurosporine. J Trauma Acute Care Surg. 2013;74:516–523. doi: 10.1097/TA.0b013e31827a0620. [DOI] [PubMed] [Google Scholar]
- 31).Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 33).Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 34).Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, Wipf P, Kagan VE, Fink MP. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245:305–314. doi: 10.1097/01.sla.0000236626.57752.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Uddin MN, Horvat D, Childs EW, Puschett JB. Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1726–R1734. doi: 10.1152/ajpregu.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Uddin MN, McLean LB, Hunter FA, Horvat D, Severson J, Tharakan B, Childs EW, Puschett JB. Vascular leak in a rat model of preeclampsia. Am J Nephrol. 2009;30:26–33. doi: 10.1159/000193220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Childs EW, Tharakan B, Hunter FA, Smythe WR. 17beta-estradiol mediated protection against vascular leak after hemorrhagic shock: role of estrogen receptors and apoptotic signaling. Shock. 2010;34:229–235. doi: 10.1097/SHK.0b013e3181d75b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Tharakan B, Whaley JG, Hunter FA, Smythe WR, Childs EW. (−)-Deprenyl inhibits vascular hyperpermeability after hemorrhagic shock. Shock. 2010;33:56–63. doi: 10.1097/SHK.0b013e3181a7fb7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Sawant DA, Tharakan B, Tobin RP, Stagg HW, Hunter FA, Newell MK, Smythe WR, Childs EW. Inhibition of Fas-Fas ligand interaction attenuates microvascular hyperpermeability following hemorrhagic shock. Shock. 2013;39:161–167. doi: 10.1097/SHK.0b013e31827bba73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Tharakan B, Holder-Haynes JG, Hunter FA, Smythe WR, Childs EW. Cyclosporine A prevents vascular hyperpermeability after hemorrhagic shock by inhibiting apoptotic signaling. J Trauma. 2009;66:1033–1039. doi: 10.1097/TA.0b013e31816c905f. [DOI] [PubMed] [Google Scholar]
- 42).Peifer M, Orsulic S, Pai LM, Loureiro J. A model system for cell adhesion and signal transduction in Drosophila. Dev Suppl. 1993:163–176. [PubMed] [Google Scholar]
- 43).Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol. 2006;169:314–324. doi: 10.2353/ajpath.2006.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]