Abstract
Cannabis has been used throughout the world for centuries. The psychoactive effects of cannabis are largely attributable to Δ9-tetrahydrocannabinol (Δ9-THC), the prototypical cannabinoid that occurs naturally in the plant. More recently, chemically- and pharmacologically-distinct synthetic cannabinoids (SCBs) have emerged as drugs of abuse. As compared to Δ9-THC, the distinct structures of these compounds allow them to avoid legal restrictions (at least initially) and detection in standard drug screens. This has contributed to the popularity of SCBs among drug users who seek to avoid positive drug screens. Importantly, the distinct structures of the SCBs also typically result in increased affinity for and efficacy at cannabinoid CB1 receptors, which are thought to be responsible for the psychoactive effects of Δ9-THC and its analogues. Accordingly, it seems likely that these more powerful cannabimimetic effects could result in increased adverse reactions and toxicities not elicited by Δ9-THC in cannabis. Animal models useful for the study of emerging SCBs include the cannabinoid tetrad, drug discrimination, and assays of tolerance, dependence, and withdrawal. However, these in vivo procedures have not been particularly informative with regards to drug efficacy, where the majority of SCB effects are comparable to those of Δ9-THC. In contrast, essentially all in vitro measures of drug efficacy confirm Δ9-THC as a relatively weak CB1 partial agonist, while the majority of the SCBs detected in commercial preparations are full agonists at the CB1 receptor. As use of these emerging SCBs continues to rise, there is an urgent need to better understand the pharmacology and toxicology of these novel compounds.
Keywords: Cannabis, Synthetic Cannabinoids, SCBs, THC, psychoactive effects, CB1 receptors
Introduction
Cannabis is the most commonly used recreational drug, especially among teens and young adults [1]. Cannabis is primarily abused for its psychoactive effects, (e.g., subjective euphoria, relaxation, and elevated mood), attributed to its main psychoactive constituent Δ9-tetrahydrocannabinol (Δ9-THC). Since the discovery of Δ9-THC, hundreds of novel analogues have been synthesized and used as therapeutic agents, as pharmacological tools to enhance our understanding of the endocannabinoid system (see below), and most recently, as recreational drugs of abuse. Across the United States, commercial preparations of synthetic cannabinoids (SCBs) (e.g., labeled “K2” or “Spice”) have gained much attention among drug users and lawmakers. According to a 2012 survey, SCBs are the 2nd most commonly used illegal drug among young adults, with only cannabis use occurring at a higher rate [2]. In a three-year product surveillance study in our state, 26 individual SCBs were detected in commercial products [3]. The most common SCBs detected in Arkansas were JWH-018, AM2201, JWH-122, JWH-210, and XLR11, and it was not uncommon to find two or three SCBs in combination in commercial products sold in the state [3]. Use of SCBs produces psychoactive effects similar to those of cannabis, they are easily accessible, and difficult to detect in standard urine drug screens [4], which all contribute to their high rate of use. Despite local, state, and federal regulations of the more prevalent synthetic cannabinoids, SCB products can still be purchased with ease from the internet, head shops and convenience stores. Legislators are concerned with finding effective measures to curtail SCB availability, however, this has been particularly challenging given the constantly changing composition of commercial SCB products [3]. This has also frustrated efforts at developing a standardized drug test that screens for SCB use.
Although commercial SCB products are typically sold as a mixture of plant materials (touted as “herbal incense” or “potpourri”), they have also been documented to exist as tablets, capsules, and as powders [3]. According to the Drug Enforcement Administration (DEA), SCBs commonly found in commercial preparations include the aminoalkylindoles JWH-018, JWH-073, and JWH-200, and the cyclohexylphenols CP-47497 and CP-47497 C8, all of which are currently listed as Schedule I controlled substances [5]. Despite strict regulations on these particular SCBs, other analogs are still emerging in commercial products, including AKB48, AM-2201, JWH-081, JWH-122, UR-144 and XLR11 [6, 7]. Commercial preparations of SCBs often contain multiple combinations and varying concentrations of SCBs, even within products marketed with the same name and packaging, leading to marked dose/drug inconsistency from batch to batch [8, 9].
A strong motivation for controlling SCBs, in addition to their cannabis-like intoxicating effects, is the relatively high incidence of adverse effects associated with their use, including acute psychosis, confusion, anxiety, tachycardia, drowsiness, dizziness, agitation, hypertension, seizures, convulsions, vomiting, nausea, high blood pressure and chest pain [10-13], acute central nervous system (CNS) and cardiovascular toxicity [14], and long-term abuse-related effects of dependence and withdrawal [15, 16]. According to the American Association of Poison Control Centers, there were 2,906 reported cases of synthetic cannabinoid exposure in 2010, which more than doubled in 2011 to 6,968 [17]. Amidst a flurry of media attention and strict legal regulations, exposures moderately decreased to 5,228 in 2012 and 2639 in 2013 [17]. The implications of acute SCB use on human health remains poorly understood, and even less is known about the long-term effects of these drugs. Currently, there are no direct treatments for complications arising from SCB use, and only supportive care is provided in these cases.
Much of our understanding of commercial SCB products has primarily been achieved through studying the endocannabinoid system. Below, we briefly introduce the endocannabinoids in order to provide a background against which to compare the newly emerging SCB drugs of abuse. The remainder of this review will focus on current findings on the in vivo pharmacological effects of synthetic cannabinoids, with a particular emphasis on their abuse potential.
Endocannabinoid system
The 1964 identification of the highly lipophilic Δ9-THC set the stage for the eventual discovery of the first cannabinoid (CB1) receptor in 1990, followed three years later by the characterization of the CB2-receptor [reviewed in 18, 19]. Both CB1 and CB2 receptors are members of the seven transmembrane G protein coupled receptor (GPCR) superfamily [20]. CB1 receptors are among the most abundant GPCRs in the mammalian CNS. Outside the brain, CB1 receptors are expressed at lower levels in a variety of peripheral tissues including fat, heart, intestine, liver, endocrine, pancreas and uterus [21]. CB2 receptors are primarily expressed in peripheral tissues, such as liver, lung and kidney, and are closely associated with the immune and hematopoietic system [22, 23]. More recently, increasing evidence supports the existence of CB2 receptors in the brain, strengthening the idea of a functional significance of CB2 receptors in the CNS [24, 25]. In this review, we will primarily focus on the effects of SCBs at CB1 receptors since they are primarily responsible for the abuse-related psychoactive effects of these drugs. The study of endocannabinoids is presently flourishing, and detailed reviews of endocannabinoid synthesis, signaling and metabolism are available [26-28].
Cannabinoid ligands
There are four major chemical classes of exogenous cannabinoid ligands that differ structurally [reviewed in 29] (see Figure 1). Classical cannabinoids include Δ9-THC, AM2389, cannabinol, nabilone, HU-210 and other trycyclic terpenoid derivatives bearing a benzopyran moiety. Non-classical cannabinoids include CP 55,940, HU-308 and other bicyclic and tricyclic analogs of Δ9-THC lacking the pyran ring of classical cannabinoids. Aminoalkylindoles including WIN55,212-2, JWH-018, JWH-073 and AM1241 differ in structure, lipophilicity, and binding activity at the cannabinoid receptors in comparison to the above-mentioned classes. Many of the aminoalkylindoles are currently found in commercial SCB products. Finally, 1,5 biarylpyrazole ligands act as cannabinoid receptor antagonists, and include compounds such as rimonabant and AM251, which are both CB1-receptor selective, and SR144528, which is CB2-receptor selective.
Figure 1. Cannabinoid ligands.
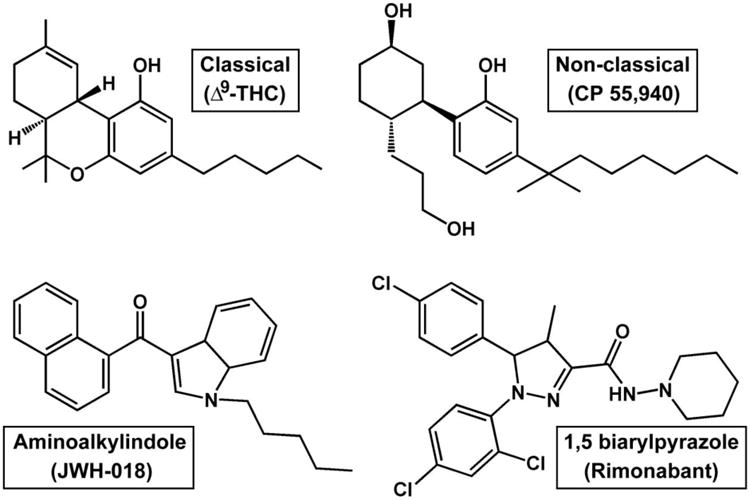
Representative compounds of the four major chemical classes of exogenous cannabinoid ligands. Note that the classical cannabinoid Δ9-THC is a relatively low efficacy partial agonist at cannabinoid receptors, the non-classical cannabinoid CP 55,940 and the aminoalkylindole JWH-018 are both full agonists at CB1 receptors, while the 1,5 biarylpyrazole rimonabant functions as an antagonist/inverse agonist at cannabinoid receptors.
Preclinical in vivo Pharmacology of Synthetic Cannabinoids
Cannabinoid tetrad
Administration of cannabinoid agonists from multiple structural classes elicits a characteristic cluster of effects in laboratory animals. This cluster of the four classical endpoints of hypothermia, analgesia, catalepsy and locomotor suppression has been termed the cannabinoid tetrad [30, 31]. Qualitatively consistent data are reliably observed for cannabinoid agonists across tetrad measures, characterized by dose-dependent decreases in measures of body temperature and motor activity, and dose-dependent increases in measures of analgesia and catalepsy. The advantage of using these tetrad measures to evaluate cannabinoids is that none of these assays require any particular training of the animals. Thus, data can be generated rapidly. Another advantage of the tetrad is that all of these endpoints can be assessed in quick succession in the same subject, decreasing total animal usage.
The cannabinoid tetrad has been extremely useful in the characterization of the biological activity of cannabinoid ligands. Interestingly, Δ9-THC tends to elicit tetrad effects of similar magnitude to higher efficacy cannabinoids such as WIN 55-212 and CP-55,940 [32]. The SCBs JWH-018 and JWH-073 also elicit characteristic tetrad effects in the mouse after intraperitoneal injection [33]. CB1-receptor mediated tetrad effects after exposure to smoke produced from combustion of an herbal incense product containing 5.4% JWH-018 have also been reported [34]. Importantly, a Phase I hydroxylated JWH-018 metabolite retained marked cannabimimetic effects in vivo, eliciting profound hypothermic and locomotor depressant effects in mice which were blocked by pretreatment with AM251, a CB1-receptor selective antagonist [35]. Similarly, the analogous metabolite of JWH-073 also decreased core temperature and locomotor activity in the mouse [33]. However, not all biologically active Phase I hydroxylated metabolites of these compounds are cannabinoid agonists, as pretreatment with a different metabolite of JWH-073 significantly blunted hypothermic effects elicited by JWH-018 in the mouse without altering core temperature on its own [33]. Interestingly, this metabolite of JWH-073 did not alter the analgesic, cataleptic or locomotor effects of JWH-018 in the mouse, perhaps suggesting that it did not penetrate the CNS at the dose tested [33].
Overall, the tetrad assay reveals that commercially available SCBs elicit effects similar to those of the prototypical cannabinoid Δ9-THC. Furthermore, these effects are believed to be CB1-receptor mediated since pretreatment with CB1-receptor antagonists can block these effects. Finally, the activity of Phase I metabolites of some common SCBs raises the intriguing possibility that genetic polymorphisms in cytochrome P450 enzymes may play an important role in determining an individual's response to these drugs. For example, an individual with a liver enzyme profile leading to biased production of antagonist metabolites might experience greatly attenuated drug effects, while an individual with a liver enzyme profile favoring formation of agonist metabolites might experience potentiated or longer-lasting drug effects.
Discriminative stimulus effects
Cannabinoids exert numerous effects on perception and other unobservable variables in humans, thus, drug discrimination is useful as a preclinical and clinical model of these subjective effects. The drug discrimination assay can be thought of as an in vivo procedure to identify drugs with similar subjective effects to a well-characterized training drug. Thus, when studying emerging SCB drugs of abuse, a dose of Δ9-THC is typically used as the standard training stimulus. Centrally-active cannabinoid agonists reliably induce Δ9-THC-like effects in animals trained to discriminate Δ9-THC. For example, full substitution for Δ9-THC was observed with WIN 55212 and 1-butyl-2-methyl-3-(1-naphthoyl)indole in monkeys [36], and with R(-)-methanandamide in rats [37]. Thus, drug discrimination may be particularly useful in the study of cannabinoid in vivo pharmacology, both due to its high degree of pharmacological specificity, and because results from preclinical studies are predictive of the subjective effects of cannabis in humans [38].
To date, only a few studies have examined the discriminative stimulus effects of SCBs present in commercial products. In rats, JWH-018 has been shown to substitute for Δ9-THC and for the endocannabinoid analogue methanandamide [39, 40]. In these studies, consistent with in vitro data, JWH-018 was more potent than Δ9-THC and methanandamide, and the interoceptive effects of JWH-018 were attenuated by pre-treatment of the CB1-receptor selective antagonist rimonabant. Similarly, in rhesus monkeys, JWH-018 and JWH-073 fully substituted for the discriminative stimulus effects of Δ9-THC, and antagonist studies with rimonabant suggest that they did so via interactions with CB1 receptors [41]. Most recently, novel tetramethylcyclopropyl ketone indoles currently emerging as drugs of abuse were tested for Δ9-THC-like discriminative effects in rats. As might be expected, both UR-144 and XLR-11 fully substituted for Δ9-THC, were more potent than Δ9-THC itself, and were antagonized by rimonabant pretreatment [42]. This provides further evidence that the subjective effects of SCBs are mediated through the CB1-receptor.
Reinforcing effects
Assessment of the reinforcing effects of drugs is accomplished using the self-administration technique, in which an operant response results in the immediate administration of the drug. Despite recreational use and abuse of cannabinoids throughout human history, the reinforcing effects of cannabinoids have not been widely investigated in laboratory animals. Intravenous self-administration of low Δ9-THC doses were demonstrated in squirrel monkeys [43, 44]. Furthermore, reports have shown that high efficacy cannabinoids WIN 55,512 and HU-210, both found in some SCB commercial products, maintain intravenous self-administered behavior in mice and rats [45-47]. These data may suggest that other high efficacy cannabinoids, such as those SCBs present in commercial products, might also display reinforcing effects in self-administration procedures, but thus far, no published reports bolster this supposition. Importantly, the reports in squirrel monkeys and rats demonstrate that the reinforcing effects of intravenous cannabinoids are significantly attenuated by pretreatment with CB1-receptor antagonists, strongly suggesting that the abuse-related effects of these substances are indeed mediated by central cannabinoid systems.
Conditioned place preference
Another way to indirectly assess abuse-related effects of cannabinoids in experimental animals is to study their capacity to elicit a conditioned place preference. Studies investigating the capacity of cannabinoids to induce conditioned place preference present a complex and contradictory picture, which is discussed in detail elsewhere [48]. Nevertheless, robust preferences for Δ9-THC-paired contexts have sometimes been reported in rats [49] and mice [50-52] under carefully controlled experimental conditions. Recently, it was demonstrated that the SCB JWH-018 induced a dose-dependent place aversion in previously drug-naïve mice, but elicited place preference in mice with a history of Δ9-THC administration [53]. This finding, coupled with the fact that place preference induced by either Δ9-THC [54] or CP 55,940 [55] is attenuated by pre-treatment of CB1-receptor antagonists, again implies that augmentation of cannabinoid signaling can lead to conditioned rewarding effects in rodents.
Tolerance, dependence and withdrawal
Cannabinoid administration may lead to rapid tolerance to antinociceptive effects, anticonvulsant activity, cataleptic effects, suppression of locomotor activity hypothermia, hypotension, release of corticosteroids, and other effects in multiple species [reviewed in 56]. However, tolerance does not develop to all cannabinoid effects, e.g., Δ9-THC-elicited adrenocorticotropic hormone (ACTH) secretion remained remarkably stable across five daily administrations in the rat [57]. Receptor theory states that tolerance to drug effects produced by treatment with a low efficacy ligand can be at least partially surmounted by administration of a high efficacy agonist, while tolerance to effects induced by repeated treatment with a high efficacy compound will elicit profound cross-tolerance when low efficacy ligands are tested. However, applying this theory of intrinsic efficacy in tolerance and cross-tolerance to cannabinoids is unreliable. In other words, a similar degree of tolerance to the hypothermic effects of Δ9-THC, a low efficacy cannabinoid agonist, CP-55,940 and WIN 55,212, both full efficacy cannabinoid agonists, was reported following chronic Δ9-THC administration, despite the substantial efficacy differences between these drugs [58]. However, a different regimen of Δ9-THC resulted in dramatic tolerance to Δ9-THC effects on locomotor activity, hypothermia and antinociception in mice. Nevertheless, only moderate cross-tolerance was apparent when high efficacy agonists CP-55,940 or WIN 55,212 were tested, and only to some of these effects [32]. Furthermore, Δ9-THC treatment decreased sensitivity to the discriminative stimulus effects of Δ9-THC in rhesus monkeys, but did not alter the Δ9-THC-like interoceptive effects of high efficacy cannabinoids CP-55,940, JWH-073 or JWH-018 [59]. These apparent differences in tolerance to some behavioral and physiological effects are also reflected at the receptor level, as chronic administration of Δ9-THC or WIN 55212-2 resulted in similar levels of tolerance to drug-elicited hypoactivity, hypothermia, and antinociception, but a greater degree of CB1-receptor desensitization was quantified in the Δ9-THC-treated mice in some brain areas (e.g., cerebellum amygdala, nucleus accumbens and hippocampus) despite the substantially lower CB1-receptor efficacy [60]. A better understanding of the relationship between tolerance to in vivo effects and regulation of expression and function of CB1 receptors after chronic administration of cannabinoid agonists with varying efficacies is needed.
Drug dependence is not directly observable in vivo, rather it is assumed to be present when either the discontinuation of drug administration (spontaneous withdrawal) or the administration of an antagonist (precipitated withdrawal) elicits a withdrawal syndrome. The lack of a reliable and readily observed spontaneous withdrawal syndrome after discontinuation of Δ9-THC has caused much debate on the clinical relevance of cannabis withdrawal in humans. However, the terminology “cannabis withdrawal syndrome” has gained acceptance as described by the Diagnostic and Statistical Manual of Mental Disorders [61], and clinical reports of sleep disturbances, strange dreaming, decreased appetite, irritability, anxiety, depressed mood, physical discomfort and drug cravings are said to occur as a result of discontinuation of cannabis use [62, 63]. WIN55,212-2, an aminoaklylindole SCB which acts as a full agonist at CB1 receptors, elicited measurable signs of spontaneous withdrawal in rats [64]. In addition to the higher intrinsic efficacy, WIN55,212-2 is also eliminated more readily from adipose tissue due to its low lipophilicity, in comparison to Δ9-THC, which is stored and eliminated at a slower rate. One might speculate that the structurally related aminoalkylindole SCBs present in commercial preparations (JWH-018, AM2201, etc.) might also elicit a cannabinoid withdrawal syndrome after abrupt discontinuation. To date, there are no preclinical data to directly support this notion, but clinical reports suggest a relatively high incidence of withdrawal in frequent/daily users of K2 and Spice products. The withdrawal syndrome elicited after abrupt discontinuation of SCBs is similar to reports with Δ9-THC, and consists of chills, drug cravings, headaches, insomnia, anorexia, inner unrest, nausea, and nocturnal nightmares, but also includes potentially more severe symptoms such as seizures and hallucinations [6, 15, 16]. Interestingly, Gunderson and colleagues recently reported that the psychoactive effects of commercial SCB preparations substituted for those of cannabis in cannabis-dependent patients, and also blocked or attenuated cannabis withdrawal syndrome in these same patients [14].
Conclusion
Much of our understanding of cannabinoid tolerance, dependence and withdrawal has been based on studies involving Δ9-THC, a relatively weak partial agonist at CB1 and CB2 receptors. However, the SCBs commonly found in quasi-legal commercial products such as K2 and Spice are typically full cannabinoid receptor agonists. Importantly, a drug's efficacy determines how “powerful” its maximal effects may be in biological systems. A low efficacy cannabinoid like Δ9-THC will have a less pronounced maximal effect than a higher efficacy cannabinoid, such as the SCBs present in commercial products, and this difference in maximal effects cannot be overcome simply by increasing the dose of Δ9-THC. In other words, no amount of Δ9-THC can stimulate cannabinoid receptors to the same degree as the SCBs currently emerging as drugs of abuse. This has left researchers working with these high efficacy SCBs in the unusual position of having to determine whether their effects are related to the unprecedented degree of cannabinoid receptor stimulation elicited by these compounds, or whether they are produced by interactions with other, non-cannabinoid receptor systems.
In light of the growing popularity of commercial SCB products, it has become critically important to reevaluate our understanding of cannabinoid abuse. Increasing evidence suggests that there is a strong abuse potential for the high efficacy SCBs, at least comparable to that of cannabis itself. Furthermore, these SCB products are readily accessible and can be purchased easily from the comfort of home through the Internet. As long as there is a market for SCBs, competitive pricings and attractive gimmicks will be used to increase sales. Given the prevalence of SCB consumption there is an urgent need to better understand the pharmacology and toxicology of synthetic cannabinoids. In particular, the role of intrinsic efficacy in abuse-related effects and adverse effects should be targeted in future studies. Finally, commercial SCB products can no longer be viewed as innocuous alternatives to cannabis. Instead, the profound psychoactive effects of these preparations must be recognized, and acknowledged to result from the combined actions of a complex mixture of different SCBs present in commercial preparations, almost all of which are more efficacious than Δ9-THC.
Table 1. Synthetic compounds found in commercial products.
Affinity constants (Ki) and estimates relative to Δ9-THC (top) and efficacy (EC50) at CB1 receptors and estimates relative to the standard CB1 full agonist CP55,940 (bottom) at CB1 for SCBs JWH-018, AM-2201, UR-144 and XLR-11. Lower Ki values indicate higher affinity for the CB1 receptor. An efficacy equivalent (100%) to that of CP indicates full agonist effects at the CB1 receptor. Brents et al. (2011) and Chimalakonda et al. (2012) data were obtained in mouse whole-brain homogenates, while data from Wiley et al. (2013) were obtained from human cloned CB1 membranes. Assay conditions among laboratories will lead to variability in measures when the same compounds are tested, but the pattern of results is always the same: the SCBs in commercial preparations have higher affinity for CB1 receptors than Δ9-THC, and exhibit full agonist efficacy.
| Compound | CB1 affinity Ki (nM) | CB1 affinity (x THC) | Reference |
|---|---|---|---|
| THC | 15.29 | - | Brents et al., 2011; Chimalakonda et al., 2012 |
| JWH-018 | 1.22 | 12.53 | |
| AM-2201 | 0.40 | 38.71 | |
| THC | 67.00 | - | Wiley et al., 2013 |
| UR-144 | 24.00 | 2.79 | |
| XLR-11 | 29.00 | 2.31 | |
| Compound | CB1 efficacy EC50 (nM) | CB1 efficacy (% CP) | Reference |
| CP55,940 | 3.36 | - | Brents et al., 2011; Chimalakonda et al., 2012 |
| JWH-018 | 6.82 | 100 | |
| AM-2201 | ND | 100 | |
| CP55,940 | 25.00 | - | Wiley et al., 2013 |
| UR-144 | 159.00 | 100 | |
| XLR-11 | 98.00 | 100 |
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Sherrica Tai and William E. Fantegrossi declare they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Articles of particular interest are annotated below:
* Of importance
** Of particular importance
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2013. (NSDUH Series H-46). HHS Publication No. (SMA) 13-4795. [Google Scholar]
- 2.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. University of Michigan News Service; Ann Arbor, MI: 2012. The rise in teen marijuana use stalls, synthetic marijuana use levels, and use of ‘bath salts’ is very low. Retrieved 09/24/2013 from http://www.monitoringthefuture.org. [Google Scholar]
- **3.Seely KA, et al. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: A three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Science International. 2013;233(1-3):416–22. doi: 10.1016/j.forsciint.2013.10.002. This study details efforts to track the compositions of commercial SCB products in the state of Arkansas over a three-year period during which several major state and federal legislative changes aimed at controling the proliferation of these substances were enacted. The major psychoactive constituents on commercial SCB products are described, as are the effects of legislation on drug availability. [DOI] [PubMed] [Google Scholar]
- 4.Auwarter V, et al. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? Journal of Mass Spectrometry. 2009;44(5):832–7. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 5.Drug Enforcement Administration. Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I. Microgram Bulletin. 2011;44(3):21–30. [Google Scholar]
- 6.Musshoff F, et al. Driving under the influence of synthetic cannabinoids (“Spice”): a case series. International Journal of Legal Medicine. 2013 doi: 10.1007/s00414-013-0864-1. [DOI] [PubMed] [Google Scholar]
- 7.Drug Enforcement Administration. Schedules of controlled substances: temporary placement of three synthetic cannabinoids into Schedule I. Final order. Federal Register. 2013;78(95):28735–9. [PubMed] [Google Scholar]
- 8.Lindigkeit R, et al. Spice: a never ending story? Forensic Science International. 2009;191(1-3):58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Piggee C. Investigating a not-so-natural high. Analytical Chemistry. 2009;81(9):3205–7. doi: 10.1021/ac900564u. [DOI] [PubMed] [Google Scholar]
- 10.Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug and Alcohol Dependence. 2011;117(2-3):152–7. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Forrester MB. Adolescent synthetic cannabinoid exposures reported to Texas poison centers. Pediatric Emergency Care. 2012;28(10):985–9. doi: 10.1097/PEC.0b013e31826c9a97. [DOI] [PubMed] [Google Scholar]
- 12.Pant S, et al. Spicy seizure. The American Journal of the Medical Sciences. 2012;344(1):67–8. doi: 10.1097/MAJ.0b013e31824cf5c2. [DOI] [PubMed] [Google Scholar]
- 13.Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. Journal of Medical Toxicology. 2012;8(1):62–4. doi: 10.1007/s13181-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Gunderson EW, et al. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. The American Journal on Addictions. 2012;21(4):320–6. doi: 10.1111/j.1521-0391.2012.00240.x. Several cases of SCB use among THC-dependent subjects are described. The fact that SCBs prevent or alleviate THC withdrawal implies that these drugs are likely to share a common site and mechanism of action. [DOI] [PubMed] [Google Scholar]
- 15.Nacca N, et al. The synthetic cannabinoid withdrawal syndrome. Journal of Addiction Medicine. 2013;7(4):296–8. doi: 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann US, et al. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Deutsches Ärzteblatt International. 2009;106(27):464–7. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Association of Poison Control Centers. Synthetic Marijuana Data. 2013 Retrieved 02/10/2013 from http://www.aapcc.org/alerts/synthetic-marijuana/
- 18.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 19.Montero C, et al. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. European Journal of Medicinal Chemistry. 2005;40(1):75–83. doi: 10.1016/j.ejmech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Begg M, et al. Evidence for novel cannabinoid receptors. Pharmacology & Therapeutics. 2005;106(2):133–45. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Tucci SA, et al. Therapeutic potential of targeting the endocannabinoids: implications for the treatment of obesity, metabolic syndrome, drug abuse and smoking cessation. Current Medicinal Chemistry. 2006;13(22):2669–80. doi: 10.2174/092986706778201512. [DOI] [PubMed] [Google Scholar]
- 22.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira-Clerc F, et al. The endocannabinoid system as a novel target for the treatment of liver fibrosis. Pathologie-Biologie. 2008;56(1):36–8. doi: 10.1016/j.patbio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Gutierrez MS, et al. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. British Journal of Pharmacology. 2012;165(4):951–64. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi ZX, et al. Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nature Neuroscience. 2011;14(9):1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grotenhermen F. Pharmacology of cannabinoids. Neuro Endocrinology Letters. 2004;25(1-2):14–23. [PubMed] [Google Scholar]
- 27.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2012;367(1607):3216–28. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2012;367(1607):3353–63. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chemistry and Physics of Lipids. 2002;121(1-2):3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 30.Compton DR, et al. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. The Journal of Pharmacology and Experimental Therapeutics. 1992;260(1):201–9. [PubMed] [Google Scholar]
- 31.Little PJ, et al. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. The Journal of Pharmacology and Experimental Therapeutics. 1988;247(3):1046–51. [PubMed] [Google Scholar]
- 32.Fan F, et al. Development of cross-tolerance between delta-9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. The Journal of Pharmacology and Experimental Therapeutics. 1994;271(3):1383–90. [PubMed] [Google Scholar]
- 33.Brents LK, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochemical Pharmacology. 2012;83(7):952–61. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Wiebelhaus JM, et al. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug and Alcohol Dependence. 2012;126(3):316–23. doi: 10.1016/j.drugalcdep.2012.05.034. This is the first demonstration that cannabimimetic effects of a commercial SCB product are induced in mice after “smoking.” All of the in vivo endpoints of the cannabinoid tetrad are utilized, and these effects are blunted in mice previously administered a CB1 antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **35.Brents LK, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. This paper assesses CB1 receptor affinity and efficacy for a number of hydroxylated Phase I metabolistes of the SCB JWH-018. In vitro, it is shown that many of these metabolites retain high affinity for the CB1 receptor, and exhibit a range of efficacies from full agonism to antagonism. In vivo, one of these metabolites elicits hypothermic effects via activation of CB1 receptors. The formation of these active metabolites will likely differ among individuals as a function of CPY450 polymorphisms, and may be a mechanism for toxicity in some individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiley JL, et al. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug and Alcohol Dependence. 1995;40(1):81–6. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- 37.Jarbe TU, et al. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156(4):369–80. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- 38.Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neuroscience and Biobehavioral Reviews. 1992;16(1):55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- *39.Jarbe TU, et al. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta(9)-tetrahydrocannabinol) discrimination for rats. Behavioural Pharmacology. 2011;22(5-6):498–507. doi: 10.1097/FBP.0b013e328349fbd5. The experiments described in this report highlight the utility of the drug discrimination procedure in the study of emerging SCBs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarbe TU, et al. Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol. Psychopharmacology (Berl) 2010;208(1):87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginsburg BC, et al. JWH-018 and JWH-073: Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2012;340(1):37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiley JL, et al. Cannabinoids in disguise: Delta(9)-Tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–54. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justinova Z, et al. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169(2):135–40. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 44.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature Neuroscience. 2000;3(11):1073–4. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 45.Fattore L, et al. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156(4):410–6. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- 46.Martellotta MC, et al. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85(2):327–30. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 47.Navarro M, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. The Journal of Neuroscience. 2001;21(14):5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Justinova Z, et al. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacology, Biochemistry, and Behavior. 2005;81(2):285–99. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepore M, et al. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sciences. 1995;56(23-24):2073–80. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 50.Castane A, et al. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. The European Journal of Neuroscience. 2003;17(1):155–9. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 51.Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147(4):436–8. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- 52.Valjent E, et al. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. British Journal of Pharmacology. 2002;135(2):564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyatt WS, Fantegrossi WE. Delta-9-THC exposure attenuates aversive effects and reveals appetitive effects of K2/“Spice” constituent JWH-018 in mice. Behavioural Pharmacology. 2014 doi: 10.1097/FBP.0000000000000034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braida D, et al. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. The European Journal of Neuroscience. 2004;506(1):63–9. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 55.Braida D, et al. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001;104(4):923–6. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 56.Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91(11):1585–614. [PubMed] [Google Scholar]
- 57.Dewey WL, Peng TC, Harris LS. The effect of 1-trans-delta 9-tetrahydrocannabinol on the hypothalamo-hypophyseal-adrenal axis of rats. European Journal of Pharmacology. 1970;12(3):382–4. doi: 10.1016/0014-2999(70)90094-4. [DOI] [PubMed] [Google Scholar]
- 58.Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. British Journal of Pharmacology. 1993;110(4):1483–90. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2012;342(3):843–9. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxaz inyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. The Journal of Pharmacology and Experimental Therapeutics. 2002;303(1):36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 61.American Psychiatric Association. DSM-IV-TR. (4th text rev) 2000 [Google Scholar]
- 62.Allsop DJ, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allsop DJ, et al. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug and Alcohol Dependence. 2011;119(1-2):123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. European Journal of Pharmacology. 2001;416(1-2):75–81. doi: 10.1016/s0014-2999(01)00873-1. [DOI] [PubMed] [Google Scholar]


