Abstract
Backgrounds/Aims
For patients with acute cholecystitis, conversion from laparoscopic cholecystectomy to open surgery is not uncommon due to possibilities of serious hemorrhage at the liver bed and bile duct injury. Recent studies reported successful laparoscopic subtotal cholecystectomy for acute cholecystitis. The purpose of this study was to determine the efficacy and feasibility of such an operation based on the experience of surgeons at our facility.
Methods
In this study, we enrolled 144 patients who had received either laparoscopic subtotal cholecystectomy (LSC), laparoscopic cholecystectomy (LC), or open cholecystectomy (OC) for acute cholecystitis from January 2004 to December 2009 at the Department of Surgery of our hospital. Their symptoms, signs, operative findings, pathologic results and postoperative results were compared and analyzed.
Results
There were 26 patients in the LSC group 80 in the LC group and 38 in the OC group. There were no differences in mean age, sex, and symptoms of acute cholecystitis. The LSC group showed higher CRP levels (p<0.001) and a higher grade according to the Tokyo criteria (p=0.001). The mean operative time was 115.6 minutes and mean blood loss was 158.9 ml without intra-operative or postoperative transfusion. There weren't any bile duct injuries during the operation. No group suffered bile leakage. Drains were removed 3.3 days after the operation in the LC group, the shortest time compared to the other groups (p<0.001). LC and LSC groups demonstrated shorter postoperative hospital days and time to diet resumption than the OC group (p<0.001).
Conclusions
LSC appears to be a safe and effective treatment in cases of severe acute cholecystitis that require consideration of conversion to open surgery.
Keywords: Acute cholecystitis, Laparoscopic subtotal cholecystectomy
INTRODUCTION
Laparoscopic cholecystectomy (LC) was introduced in 1989 as a standard treatment for benign gallbladder diseases, but was not well enforced initially for acute cholecystitis. However, ever since the first treatment of acute cholecystitis with LC,1 experience with LC has accumulated and techniques have improved, and LC has become the treatment of choice for acute cholecystitis. Nevertheless, in cases with severe acute cholecystitis demonstrating severe swelling and edema of the gallbladder, fibrosis of surrounding organ, fibrosis of Calot's triangle, and anatomic deformity, it is difficult to perform LC successfully. Furthermore, when massive bleeding from the liver bed and bile duct injury are suspected, surgeons are forced to switching to open conversion.2,3 As an alternative treatment, laparoscopic subtotal cholecystectomy (LSC) was introduced, but in the beginning there was controversy regarding its safety. Still, LSC has continuously been applied to acute cholecystitis, and studies have shown this treatment to be feasible and safe.4,5,6 Hence, the purpose of this study was to analyze experiences with LSC and determine its safety and efficacy for acute cholecystitis.
METHODS
Patients
Among 1,069 patients who received LC at one medical center from January, 2004 to December, 2009 by a single surgeon, 144 patients were diagnosed with acute cholecystitis and their clinical data were selected for this retrospective study. Body temperature, blood tests, and more than one imaging study among abdomen ultrasonography, abdomen computed tomography (CT), and hepatobiliary scintigraphy were done for every patient suspected of having acute cholecystitis. Patients suspected, from imaging studies, of having a common bile duct stone were further investigated with preoperative endoscopic retrograde cholangiopancreatography (ERCP) or endoscopic ultrasonography (EUS). When a common bile duct stone was identified, endoscopic stone extraction and endoscopic sphincterotomy were performed. Intraoperative cholangiography was not performed. When the patients demonstrated bile duct obstruction signs such as increased total bilirubin and alkaline phosphatase (ALP), postoperatively, examinations for remnant bile duct stones were carried out.
The diagnosis of acute cholecystitis was made according to the Tokyo guidelines7,8 based on local symptoms of inflammation, systemic signs, and imaging studies, and the severity of acute cholecystitis was divided into Grade I (mild), II (moderate), and III (severe). According to the methods of surgery, the 144 patients were divided into an LSC group, an LC group, and an open group, and their symptoms, signs, operative findings, pathologic results, and postoperative results were compared and analyzed. The thickness of the gallbladder wall was assessed inside the operating theatre with a ruler, measuring the thickest part of the resected gallbladder's wall. For statistical analysis, the chi-square test and a one-way ANOVA test were used. To distinguish the differences between the groups, Tukey's honestly significant difference (HSD) test was carried out, and a p-value of less than 0.05 was considered statistically significant.
Operative procedure
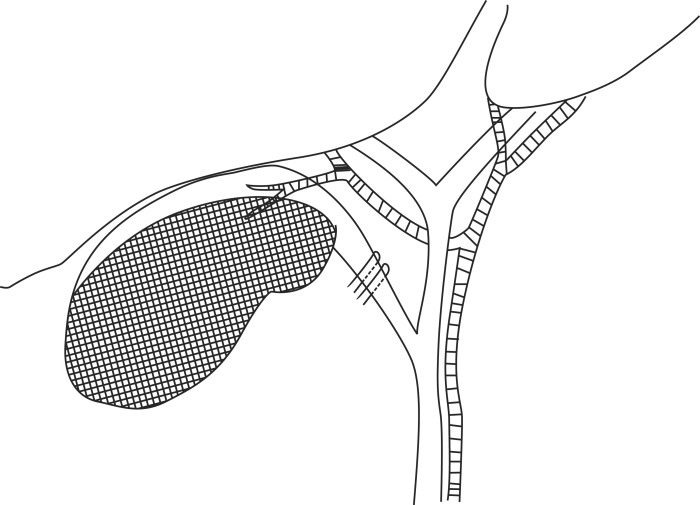
The operation was initially laparoscopic for all patients: 26 patients received LSC because LC was thought to be difficult to dissect the Calot's triangle due to severe inflammation and to identify the anatomy of the cystic artery and the cystic duct. Bile juice was aspirated from the gallbladder with a needle. When pus was evaluated, it was sent for microbial culture. Upon exploring Calot's triangle, either the cystic duct or the structure assumed to be the cystic duct after opening its serosa was ligated with an endo-clip but not divided. The cystic artery was not separately dissected. Then, the infundibulum of the gallbladder was transsected near Hartmann's pouch, and the anterior wall of the gallbladder was removed with an electrocautery device. As a result, there remained 25 to 35 percent of the gallbladder. Small gallbladder stones were suctioned out while big stones were removed using an endobag. The posterior wall attached to the liver bed was not removed. The mucosa of the posterior wall, however, was destroyed by electrocautery (Fig. 1). Finally, the operative field was washed with saline and after placing a drain, the operation was completed.
Fig. 1. Illustration of the surgical technique. The gallbladder infundibular serosa dissected and the cystic duct was clipped twice. The anterior wall of the gallbladder was dissected and mucosa of the remnant posterior wall was destroyed.
RESULTS
There were 26 patients who received LSC, 80 who received LC, and 38 who received open cholecystectomy. Their mean ages were 55.9 years, 54.2 years, and 60.1 year, respectively, and were not different (p=0.196). There were no differences in gender (p=0.544). As for symptoms of acute cholecystitis, all 144 patients suffered from right upper quadrant pain and tenderness, while Murphy's sign was demonstrated by 30.8% of the LSC group, 42.5% of the LC group, and 47.4% of the open group, again without a significant difference (p=0.406). Fever higher than 37.8℃ was observed in 53.8% of the LSC group, 57.5% of the LC group, and 71.1% of the open group, but these differences were not being significant (p=0.279). Patients with leukocytosis (a white cell count >10,200/mm3) was seen in 57.7%, 70.0%, and 71.1%, respectively (p=0.454). Mean CRP levels were 18.4 mg/dl in the LSC group, 8.2 mg/dl in the LC group, and 11.0 mg/dl in the open group. The LSC group showed the highest level and it was significantly different (p<0.001). Imaging studies included abdominal computed tomography, ultrasonography, and hepatobiliary scintigraphy, and showed no differences between groups. There were also no significant differences in American Society of Anesthesiology (ASA) scores (p=0.361). Severity according to the Tokyo guidelines was highest in the LSC group (p=0.001) (Table 1), but there was not a linear correlation of severity among the groups (p=0.310).
Table 1. Preoperative characteristics of 144 patients who underwent cholecystectomy.

ASA, american society of anesthesiology.
Among 26 patients of the LSC group, there was an adhesion between the gallbladder and its surrounding tissues in 69.2%. All patients had gallbladder wall thickening, 8.1 mm being average. Necrosis of the gallbladder was evident in 34.6%, and pus drainage in 53.8% (Table 2).
Table 2. Operative findings of 26 patients who underwent laparoscopic subtotal cholecystectomy.

The mean operative time for the LSC group was 115.6 minutes, and the mean blood loss was 158.9 ml. There was no need for intra-operative or postoperative transfusion, and no intraoperative bile duct injury. The surgical diagnosis made from operative findings included 9 cases of acute cholecystitis, 14 cases of gallbladder congestion, 1 case of gallbladder perforation, and 2 cases of acalculous cholecystitis (Table 3).
Table 3. Operative results of 26 patients who underwent laparoscopic subtotal cholecystectomy.
No group suffered from postoperative bile leakage. There was 1 case of wound infection in the LC group who recovered with conservative management without reoperation. One patient who underwent open cholecystectomy received a transfusion due to bleeding from liver cirrhosis, and died of acute lung injury. The drain was removed 3.3 days on average after LC, which was the shortest time among the groups. The mean overall hospital stay, the postoperative hospital stay, and the mean time to restart a food diet were lessed in the LC and LSC groups than in the open group (p<0.001) (Table 4).
Table 4. Postoperative outcomes of 144 patients who underwent cholecystectomy.

DISCUSSION
LC has become the treatment of choice for acute cholecystitis. Yet, open conversion is common in many severe cases, where severe inflammation and edema of the gallbladder and adhesion between adjacent structures don't indicate clear anatomical identification of cystic artery and duct under laparoscopic vision, thus resulting in increased risk of bile duct injury and bleeding. Open conversion for these severe cases has been regarded as adequate to date.
In 1985, Bornman and Terblanche introduced open partial cholecystectomy as an alternative treatment for acute cholecystitis,9 but there were concerns about accumulation of secretion from the remnant gallbladder's posterior wall mucosa and abscess formation. Also, LSC was noted for the possibility of missed gallstones during retraction and unconfirmed cystic duct resulting in bile leakage, bile abscess, and fistula.15 Soon thereafter, safety and efficacy of open partial cholecystectomy were verified by Johansson et al.,16 and recent papers have reported the usefulness of LC as a treatment for severe cholecystitis.5,10,11,12,13,14 Bile leakage from LSCs range from 3.0%12 to 17.9%;14 intra-abdominal abscess and remnant gallstones range from 3.8%13 to 16.2%.11 These complications did not occur in our study. Remnant gallstones can be minimized by removing small stones with suction devices and big stones with an endobag. Also, even if the cystic duct cannot be clearly isolated, it can be ligated to minimize bile leakage. We initially had concerns with abscess formation and accumulation of secretions from remnant mucosa, which we electrocauterized to prevent such complications. These complications, if they occurred, were scheduled to be treated with conservative treatment or ERCP treatment such as bile duct stent insertion.
We ligated the infundibulum with an endoclip. Recent studies reported usage of endo-loops and a GIA stapler. 2,4,6,11,12,18 Sinha et al. performed LSC without ligating the infundibulum, resulting in bile leakage in 17.9% of case, and was treated non-operatively with conservative treatment or stent insertion by ERCP.14 This suggests that LSC is feasible even without complete identification of anatomic structures.
Our study did not implicate that severer cholecystitis according to the Tokyo guideline requires certain types of surgery. However, the LSC group's grade was higher than that of the LC group (p=0.001), while it was similar with that of the open group (p=0.260), which implies that consideration of LSC or open surgery is required for severer cholecystitis according to Tokyo guidelines. As open conversion is considered during LC due to severe cholecystitis, LSC may be tried first owing to the better postoperative results of laparoscopic surgery than open surgery. Furthermore, LSC is safe when performed by surgeons experienced with laparoscopy surgery. LSC should be limited to severe cholecystitis in which identification of the cystic duct and artery is impossible due to severe edema and swelling of gallbladder, fibrosis and adhesion of Calot's triangle, and necrosis of gallbladder wall, and thus increased risk of injury of bile duct and bleeding is anticipated.
Patients who received open surgery, compared to laparoscopic surgery, tended to have a higher incidence of wound complications, more pain, and longer hospital stays, postoperative hospital stays, and time to diet resumption. There was one case of wound infection in the LC group, who was treated with conservative treatment. There was no severe wound complication requiring reoperation in any of the groups. Drains were removed earlier from the LC group than from other groups (p<0.001). There was no difference between LSC and OC groups (p=0.488). It may have been due to concerns that the LSC group suffers from bile duct leakage and was observed for a longer period without removing the drain. There was no mortality in the laparoscopic group, but one patient in the open group died of a transfusion-related acute lung injury. The transfusion was prescribed because of bleeding from liver cirrhosis. The operation for this patient was converted to an open technique due to massive bleeding from the liver bed. Since LSC does not involve dissection of the liver bed, bleeding is less. Thus, in the case of a patient such as the one mentioned above who is predicted to develop massive bleeding due to liver cirrhosis or thrombocytopenia, LSC rather than open conversion is advised.4,5,6,12 Furthermore, since open conversion done on patients of old age or poor general condition may induce a prolonged operative time, aggravation of cardiopulmonary function from increased pain, and delayed recovery of bowel movement, LSC should be considered. Frazee et al. also reported fewer postoperative cardiopulmonary complications with laparoscopic surgery compared to open surgery for patients with poor pulmonary function.19
When performing LSC for severe acute cholecystitis, suspected malignancy prior to operation with imaging studies such as CT and MRI (magnetic resonance imaging) should never be made light of. Open conversion is mandatory when malignancy is demonstrated during LSC.17 Furthermore, serial ultrasonography, CT, MRI, ERCP, and serum tumor markers should be carried out to follow-up on signs of malignancy. The LSC group in this study had a mean follow-up period of 2.3 months after operation, during which no remnant gallbladder progressed to carcinoma. There was one case in the LC group who suffered from postoperative cholangitis due to bile duct obstruction, for which examination was carried out to reveal a distal common bile duct cancer. The patient underwent pylorus preserving pancreatoduodenectomy. So far, there have not been any reports of remnant GB after LSC progressing to carcinoma. Yet, due to the short follow-up period, this cannot be confirmed and thus, further studies with longer follow-up periods are necessary.
The standard treatment of benign gallbladder disease is LC. Nonetheless, LC may not be so successful for severe acute cholecystitis. In most cases, open conversion has been inevitable. When LSC is applied, however, reduced pain, shorter hospital stays, and earlier diet resumption can be anticipated. Hence, when considering open conversion for severe acute cholecystitis, LSC is a safe and effective alternative.
ACKNOWLEDGEMENTS
This work was supported by INHA University Research Grant.
References
- 1.Jacobs M, Verdeja JC, Goldstein HS. Laparoscopic cholecystectomy in acute cholecystitis. J Laparoendosc Surg. 1991;1:175–177. doi: 10.1089/lps.1991.1.175. [DOI] [PubMed] [Google Scholar]
- 2.Cox MR, Wilson TG, Luck AJ, Jeans PL, Padbury RT, Toouli J. Laparoscopic cholecystectomy for acute inflammation of the gallbladder. Ann Surg. 1993;218:630–634. doi: 10.1097/00000658-199321850-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palanivelu C, Rajan PS, Jani K, et al. Laparoscopic cholecystectomy in cirrhotic patients: the role of subtotal cholecystectomy and its variants. J Am Coll Surg. 2006;203:145–151. doi: 10.1016/j.jamcollsurg.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Chowbey PK, Sharma A, Khullar R, Mann V, Baijal M, Vashistha A. Laparoscopic subtotal cholecystectomy: a review of 56 procedures. J Laparoendosc Adv Surg Tech A. 2000;10:31–34. doi: 10.1089/lap.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 5.Michalowski K, Bornman PC, Krige JE, Gallagher PJ, Terblanche J. Laparoscopic subtotal cholecystectomy in patients with complicated acute cholecystitis or fibrosis. Br J Surg. 1998;85:904–906. doi: 10.1046/j.1365-2168.1998.00749.x. [DOI] [PubMed] [Google Scholar]
- 6.Ransom KJ. Laparoscopic management of acute cholecystitis with subtotal cholecystectomy. Am Surg. 1998;64:955–957. [PubMed] [Google Scholar]
- 7.Sekimoto M, Takada T, Kawarada Y, et al. Need for criteria for the diagnosis and severity assessment of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:11–14. doi: 10.1007/s00534-006-1151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornman PC, Terblanche J. Subtotal cholecystectomy: for the difficult gallbladder in portal hypertension and cholecystitis. Surgery. 1985;98:1–6. [PubMed] [Google Scholar]
- 10.Bickel A, Shtamler B. Laparoscopic subtotal cholecystectomy. J Laparoendosc Surg. 1993;3:365–367. doi: 10.1089/lps.1993.3.365. [DOI] [PubMed] [Google Scholar]
- 11.Beldi G, Glättli A. Laparoscopic subtotal cholecystectomy for severe cholecystitis. Surg Endosc. 2003;17:1437–1439. doi: 10.1007/s00464-002-9128-8. [DOI] [PubMed] [Google Scholar]
- 12.Ji W, Li LT, Li JS. Role of laparoscopic subtotal cholecystectomy in the treatment of complicated cholecystitis. Hepatobiliary Pancreat Dis Int. 2006;5:584–589. [PubMed] [Google Scholar]
- 13.Philips JA, Lawes DA, Cook AJ, et al. The use of laparoscopic subtotal cholecystectomy for complicated cholelithiasis. Surg Endosc. 2008;22:1697–1700. doi: 10.1007/s00464-007-9699-5. [DOI] [PubMed] [Google Scholar]
- 14.Sinha I, Smith ML, Safranek P, Dehn T, Booth M. Laparoscopic subtotal cholecystectomy without cystic duct ligation. Br J Surg. 2007;94:1527–1529. doi: 10.1002/bjs.5889. [DOI] [PubMed] [Google Scholar]
- 15.Hashizume M, Sugimachi K, MacFadyen BV. The clinical management and results of surgery for acute cholecystitis. Semin Laparosc Surg. 1998;5:69–80. doi: 10.1177/155335069800500202. [DOI] [PubMed] [Google Scholar]
- 16.Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005;92:44–49. doi: 10.1002/bjs.4836. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima J, Sasaki A, Obuchi T, Baba S, Nitta H, Wakabayashi G. Laparoscopic subtotal cholecystectomy for severe cholecystitis. Surg Today. 2009;39:870–875. doi: 10.1007/s00595-008-3975-4. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg J, Bisgaard T. The difficult gallbladder: technical tips for laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2000;10:249–252. [PubMed] [Google Scholar]
- 19.Frazee RC, Roberts JW, Okeson GC, et al. Open versus laparoscopic cholecystectomy. A comparison of postoperative pulmonary function. Ann Surg. 1991;213:651–653. doi: 10.1097/00000658-199106000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]