Abstract
Backgrounds/Aims
Hepatic resection has only guaranteed long-term survival in patients with colorectal liver metastasis (CRLM) even in the era of effective chemotherapy. The definite role of neoadjuvant chemotherapy (NCT) is to improve outcomes of unresectable CRLMs, but it its role has not been defined for initially resectable CRLMs (IR-CRLMs).
Methods
We reviewed the medical records of 226 patients, who had been diagnosed and treated for IR-CRLM between 2003 and 2008; the patients had the following pathologies: 10% had more than 4 nodules, 11% had tumors larger than 5 cm, and 61% had synchronous CRMLs. Among these patients, 20 patients (Group Y) were treated with NCT, and 206 (Group N) did not receive NCT according to their physician's preference. The median follow-up time was 34.1 months.
Results
The initial surgical plans were changed after NCT to further resection in 20% and to limited resection in 10% of 20 patients. Complication rates of Groups Y (30%) were indifferent from Group N (23%) (p=0.233), but intraoperative transfusions were more frequent in Group N (15%) than in Group Y (5%) (p=0.006). There was one case of hospital mortality (0.44%). Disease-free survival rates in Groups Y and N were 23% and 39%, respectively, and patient survival rates were 42% and 66% (p>0.05). By multivariate analysis, old age (≥60 years), differentiation of primary tumor (poorly/mucinous), resection margin involvement, and no adjuvant chemotherapy were associated with poor patient survival; the number of CRLMs (≥4) was associated with poor disease-free survival.
Conclusions
NCT had neither a positive impact nor a negative impact on survival, even with intraoperative transfusion, as observed on operative outcomes for patients with IR-CRLM. Further study is required to elucidate the role of NCT for treatment of patient with IR-CRLMs.
Keywords: Colorectal liver metastasis, Hepatic resection, Prognostic factor, Neoadjuvant chemotherapy, Adjuvant chemotherapy
INTRODUCTION
Hepatic resection has only guaranteed long-term survival in patients with colorectal liver metastases (CRLM).1,2,3 Recent improvements in perioperative care and in surgical techniques of hepatic resection have produced excellent surgical outcomes and have increased the surgical options available to patients with advanced metastatic tumors.1,3,4,5,6,7,8,9 For this reason, the patients with technically resectable metastases due to CRLM actually had undergone hepatic resection in the large volume centers of hepatic surgery. "Technically resectable" CRLM was defined as the possibility of an oncologically radical operation and the possibility of preserving adequate liver function as introduced. Adequate liver function after hepatic resection included various meanings; in our center, major hepatectomy was decided according to the reference values as follows: a remnant liver volume via a CT volumetry ≥25% of the total liver volume and the parabolic pattern of oral glucose intolerance test or <15% of indocyanine green (ICG) 15-minutes retention test (ICG R15). By contrast, the total number, the size, their unilateral or bilateral presentation, and hilar node metastasis were not considered exclusion criteria. Moreover, as the outcomes of hepatic resection in recent retrospective reviews were excellent,1,3 these selection criteria for hepatic resection have been accepted as the standard in patients with CRLM in our center since 2003.
However, some of the patients, who underwent hepatic resection according to these extended criteria, had shown rapid tumor progression after hepatic resection in spite of effective adjuvant chemotherapy.10,11,12,13 Since 2005, neoadjuvant chemotherapy (NCT) has been prescribed for patients with initially resectable CRLMs (IR-CRLMs) with the expectation that it would prevent early recurrence after hepatic resection, resulting in a survival benefit. For, NCT has been given according to the oncologist's preference, although the role of NCT has typically been mainly down staging or debulking for curative surgery for initially unresectable CRLMs. However, the role of NCT has not been elucidated in patients with IR-CRLMs.
On a retrospective basis, we evaluated the survival outcome of patients with IR-CRLMs treated with NCT and analyzed the factors affecting prognosis after hepatic resection for IR-CRLMs.
METHODS
A total of 226 patients with CRLMs underwent hepatic resection at Seoul National University Hospital from January 2003 to December 2008. A retrospective review of these patients' medical records was conducted and an Institutional Review Board approval was obtained to conduct this study.
Selection criteria of patients with primarily resectable CRLMs
Selection criteria for hepatic resection included the following: controlled primary disease, no extrahepatic disease on preoperative imaging studies revealed (except for selected patients with controlled extrahepatic lesions), hepatic resection could remove all tumors with acceptable surgical margins, and expectation of adequate liver function after hepatic resection. In order for adequate liver functioning, the final liver volume must be more than 25% on preoperative computed tomography (CT) scan. An acceptable liver function test must include a parabolic oral glucose test (OGTT) and/or less than 15% of ICG R15. A case that required additional radiofrequency ablation therapy was excluded. In all patients, preoperative diagnostic workup included 3-phase abdominal and pelvic CT scans. In addition, contrast-enhanced liver magnetic resonance imaging (MRI), contrast chest CT scans, and positron emission tomography (PET) scans were performed according to the surgical plan.
Patients and tumor characteristics
Among 226 patients, 149 (65.9%) were male. The mean age at time of hepatic resection was 63.7 years, and 155 patients (68.6%) were 60 years of age or older. Primary lesions were derived in the colon for 135 patients (59.7%) and in the rectum for 95 patients. According to American Joint Committee on Cancer, 6th edition, the staging of patients' primary colorectal tumors were as follows: T1 in 5 patients (2.2%), T2 in 13 patients (5.8%), T3 in 176 patients (77.9%), and T4 in 23 patients (10.2%). Metastases to a regional lymph node were noted in 152 patients (67.3%). Synchronous liver metastasis was noted in 138 patients (61.0%). Median preoperative carcinoembryogenic antigen (CEA) level was 8.5 ng/ml (range, 1-25, 150 ng/ml), with 57.5% of patients having had CEA of more than 5 ng/ml. A total of 127 patients (56.2%) had a single tumor, and 22 patients (9.7%) had more than 4 tumors. The median size of the largest tumor was 2.6 cm (range, 0.3-18.5 cm); 36 patients (15.9%) had tumors larger than 5 cm. Bilobar distribution of tumors was present in 33 patients (14.6%). Median follow-up was 34.1 months (range, 0-88 months) for all patients (Table 1).
Table 1. Patient characteristics.
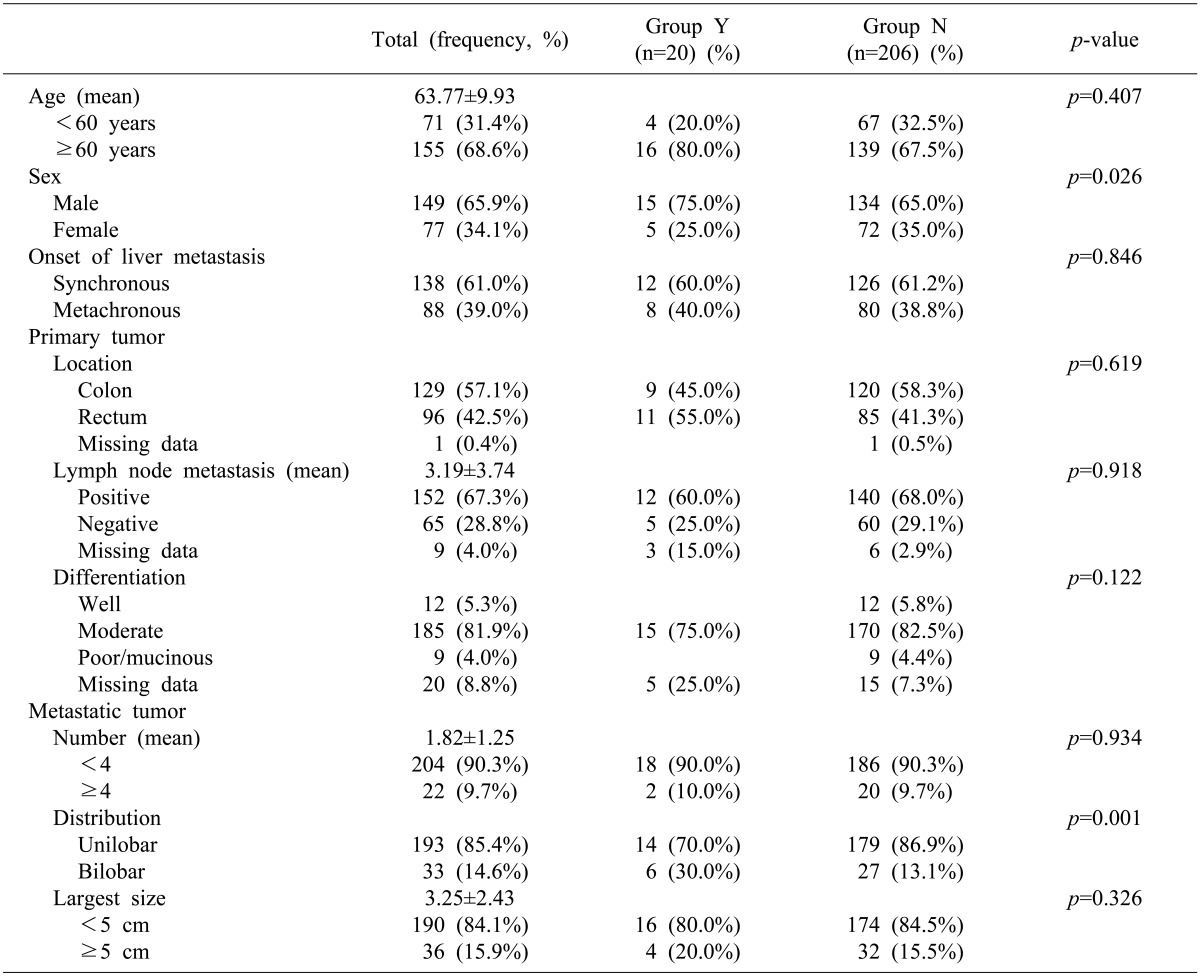
Group Y=primarily resectable CRLM patients with neoadjuvant chemotherapy, Group N=primarily resectable CRLM patients without neoadjuvant chemotherapy.
NCT for patients with IR-CRLMs
Since 2005, NCT has been used to prevent early recurrence in patients with IR-CRLMs that have undergone hepatic resection to prolong disease-free survival. The NCT has been used according to the physician preference. To elucidate the impact of NCT on the outcomes after hepatic resection, we divided the patients into 2 groups: Group Y (n=20) with NCT and Group N (n=206) without. In Group Y, the most commonly used (in 13 [75%] patients) NCT was 5-FU based chemotherapy combined with oxaliplatin. A Xeloda-based chemotherapy was used in 5 patients (20%), with 4 patients receiving treatment combined with oxaliplatin and 1 patient combined with irinotecan. Other target agent chemotherapy was used in 2 patients (5%). There was a median 8 treatment cycles of NCT for patients in the Y group (range, 3-26 times).
Surgical procedures
A standardized hepatic resection was performed under anesthesia in conditions of low central venous blood pressure before parenchymal transection without the Pringle maneuver. Intraoperative ultrasound was carried out to obtain an adequate resection margin and to confirm vascular structures on CT scans and/or MRI.14,15,16 The number, size, and the resection margin of CRLMs were determined by a pathologist.
Resections were defined on the basis of the Brisbane description of hepatic anatomy.17 Resections less than a segmentectomy were defined as wedge resections. Major hepatectomy was defined as resection of more than 2 consecutive segments except left lateral sectionectomy or a remnant liver volume less than 30% of the total liver volume. Minor hepatectomy was defined as a resection other than major hepatectomy.
End point of the study
Survival time was measured from the time of hepatic resection. Living patients were examined at the time of their last follow-up. The variables considered included the biology of the primary tumor (disease stage, differentiation grade, and nodal metastasis), and the factors of the hepatic lesions (number, size, lobar distribution, and preoperative carcinoembryonic antigen (CEA) level of CRLMs, type of hepatic resection, resection margin involvement, estimated blood loss, intraoperative transfusions, postoperative complications, and use of adjuvant chemotherapy after hepatic resection). Complications were graded according to a previously published grading system.18 All complications and deaths within 30 days of surgery were considered postoperative morbidity and mortality.
Statistical analysis
SPSS software, version 17.0 was used for data analysis. Univariate analysis was performed using the chi-squared test or Student's t-test. The Kaplan-Meier method and the log-rank test were used to evaluate patient survival for various prognostic factors. The Cox proportional hazard regression model was used to assess the independent prognostic influence of various factors on patient survival. All variables that were significantly associated with survival on the univariate level were included. Significance levels were set at p<0.05.
RESULTS
Patient profiles
No statistical differences were observed according to age, primary tumor stage, number and size of CRLMs, and adjuvant chemotherapy between Group Y and N (p> 0.05) except gender ratio and lobar distribution of CRLMs (Table 1). There were more males in Group Y (75.0%) than in Group N (65.0%, p=0.026), and the bilobar distribution was more prevalent in Group Y (30.0%) than in Group N (13.1%, p=0.001). NCT has been utilized as a therapy since 2005, thus the follow-up period was significantly shorter in Group Y (27.0±12.6 months) than in Group N (34.1±20.0 months, p=0.008).
Operative outcome
A total of 106 patients (46.9%) underwent major hepatectomies; 120 patients (53.1%) underwent minor resections as the primary procedure (Table 2). The median operative time was 260 minutes (range, 20-750 minutes). The median intraoperative estimated blood loss was 400 mL (range, 25-4,300 ml). Intraoperative transfusion was performed in 32 patients (14.2%). Surgical margins were histologically negative in 257 patients (96.1%) and less than 1 cm in 140 patients (61.9%). The median postoperative hospital stay was 11 days (range, 6-79 days). No differences were observed between Groups Y and N for the followings: major hepatectomy (55.0% vs. 67.2%, p=0.273), operation time (272.8 min vs. 285.0 min, p=0.434), resection margin <1 cm (55.0% vs. 62.6%, p=0.644), resection margin positivity (5.0% vs. 3.9%, p=0.185), estimated blood loss ≥1,000 ml (5.0% vs. 11.7%, p=0.365), and postoperative hospital stay (12.7 days vs. 12.9 days, p=0.598). However, intraoperative transfusion was more frequent in Group N than in Group Y (5% vs. 15%, p=0.006).
Table 2. Summary of hepatic resection.
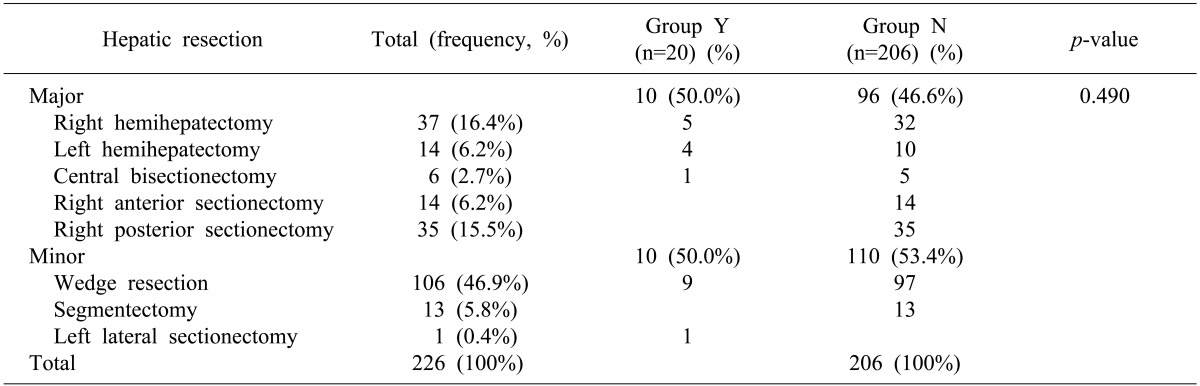
Group Y=primarily resectable CRLM patients with neoadjuvant chemotherapy, Group N=primarily resectable CRLM patients without neoadjuvant chemotherapy.
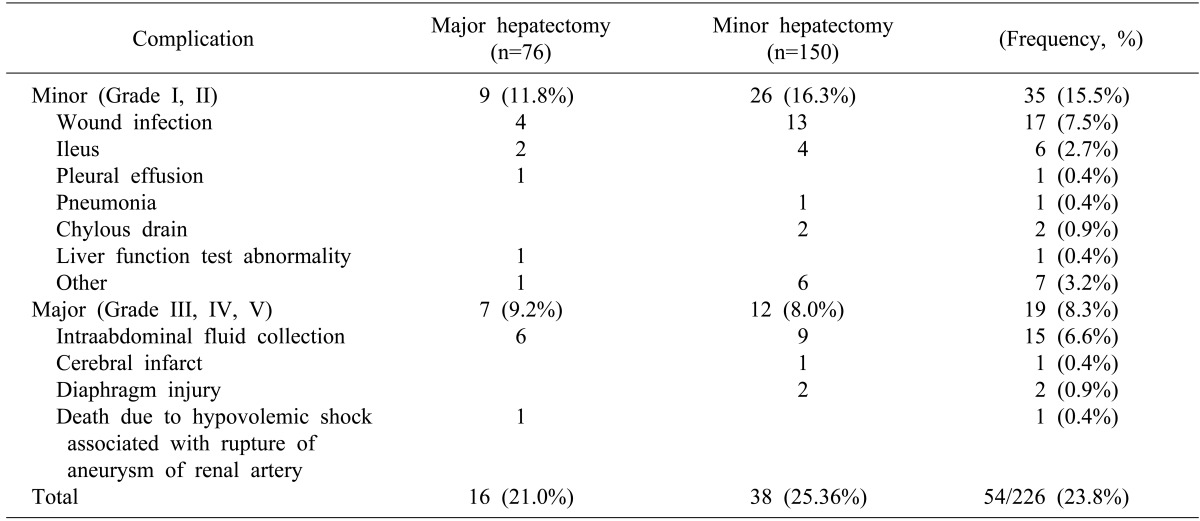
Postoperative complications occurred in 54 patients (23.8%) (Table 3). Major morbidity occurred in 19 patients (8.3%), and minor morbidity occurred in 35 patients (15.5%). The complication rates (21.0% vs. 25.6%, p= 0.117) and the III-IV grade of complications (43.7% vs. 31.5%, p=0.186) were not significantly different between patients undergoing major or minor hepatectomy (p= 0.807). The complication rates (30.0% vs. 23.3%, p= 0.233) and the III-V grade of complications (10.0% vs. 8.3%, p=0.836) were also insignificant between Groups Y and N (p=0.809). There was no operative mortality, but one in-hospital mortality (0.44%). A 78-year-old male patient underwent simultaneous right hemicolectomy and right hemihepatectomy; although he did not experience immediate postoperative complications, he experienced bleeding due to incidental aneurysmal rupture of the left renal artery at postoperative day 7 and died of multi-organ failure at postoperative day 33.
Table 3. Perioperative morbidities according to the type of hepatectomy.
Survival analysis
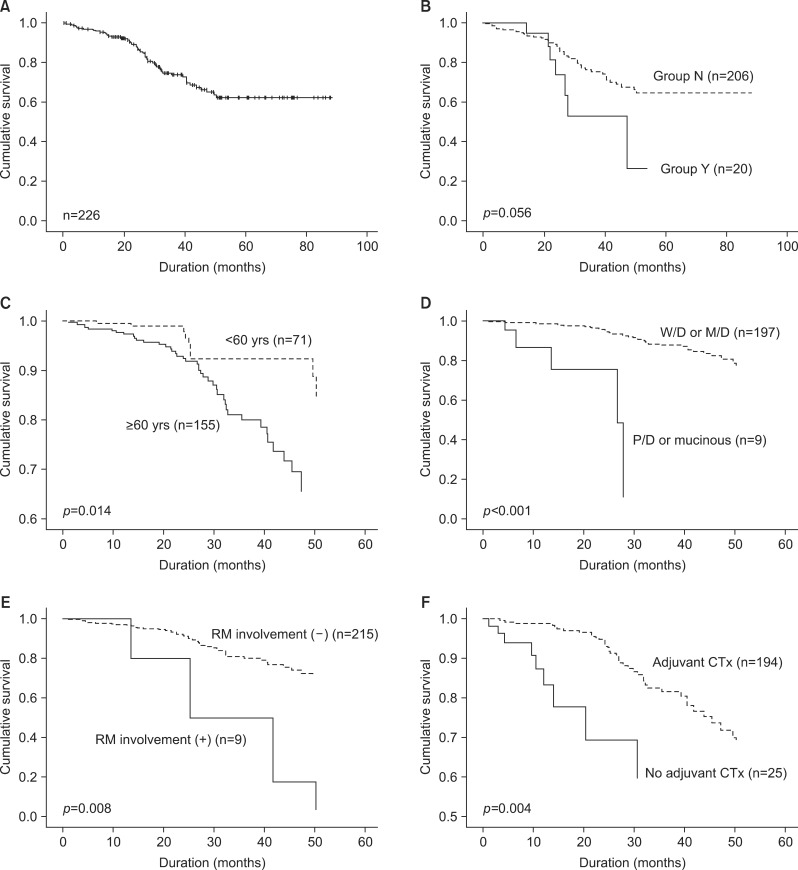
Median follow-up was 34.1 months (range, 0-88 months) for all patients and 31.4 months for survivors. The overall survival rate was 62.2 months (Fig. 1A). The patient survival rate was not different between Groups Y and N (42.0% vs. 65.7%, p=0.056) (Fig. 1B).
Fig. 1. Overall survival after hepatic resection for initially resectable colorectal liver metastasis. (A) Patients' survival rates. (B) Patients survival according neoadjuvant chemotherapy (Group Y: Primarily resectable CRLM patients with neoadjuvant chemotherapy, Group N: Primarily resectable CRLM patients without neoadjuvant chemotherapy). (C) Patient survival related to age, analyzed by the adjusted Cox proportional hazard method. (D) Patient survival related to differentiation of the primary colorectal tumor, analyzed by the adjusted Cox proportional hazard method (W/D, well differentiated; M/D, moderate differentiated; P/D, poorly differentiated). (E) Patient survival related to resection margin (RM) involvement, analyzed by the adjusted Cox proportional hazard method. (F) Patient survival related to adjuvant chemotherapy (CTx), analyzed by the adjusted Cox proportional hazard method.
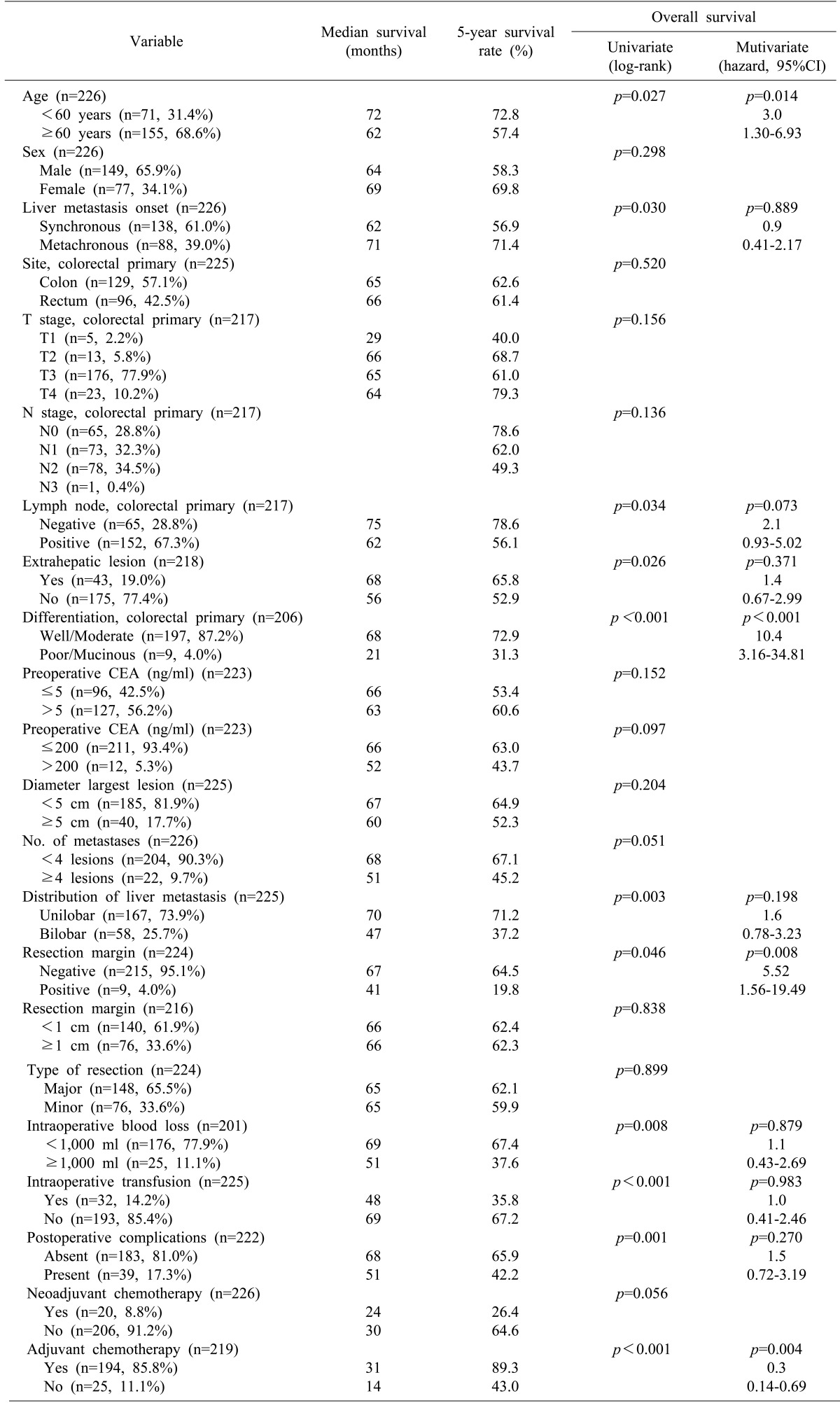
By univariate analysis, age (≥60 years), liver metastasis onset (synchronous), lymph node involvement of primary tumor, differentiation of primary tumor (poor/mucinous), extrahepatic lesion, number of metastases (≥4), distribution of liver metastasis (bilobar distribution), resection margin involvement, intraoperative blood loss (≥ 1,000 ml), intraoperative transfusion, and no adjuvant chemotherapy were associated with poor patient survival (Table 4). Otherwise, NCT and diameter of the largest tumor of ≥5 cm had no marked impact on patient survival. Multivariate analysis revealed that age (≥60 years), differentiation of primary tumor (poor/mucinous), resection margin involvement, and no adjuvant chemotherapy were independent prognostic factors of poor patient survival of IR-CRLMs (Fig. 1C-F).
Table 4. Univariate and multivariate analysis of prognostic factors affecting patient survival.
Recurrence patterns and disease-free survival
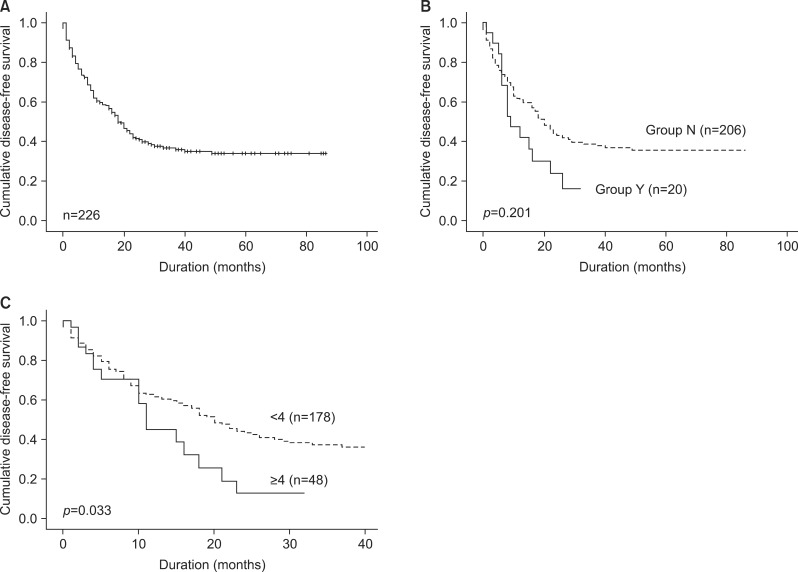
The median disease-free survival, as measured from time of hepatic resection, was 37.1 months (range, 0-86 months, Fig. 2A). The overall disease-free survival rate was 23.0% and 38.8% in Groups Y and N, respectively (p=0.201, Fig. 2B). The disease recurred in 130 patients (57.5%). Of these, 86 patients (66.2%) had their initial recurrence within the first year, 34 (26.2%) in the second year, and 10 (7.6%) after the second year. Thirteen patients (9.2%) underwent repeat hepatic resection of their first recurrence, and of these, 1 patient underwent hepatic resection three times. The most frequent site of initial recurrence was the liver in 54 patients (41.5%), a metastatic lymph node in 32 (24.6%), the lung in 24 (18.5%), peritoneal seeding in 7 (5.4%), and multiple recurrence sites in 13 (10%).
Fig. 2. Disease-free survival after hepatic resection for initially resectable colorectal liver metastasis analyzed by the Kaplan-Meier method. (A) Disease-free survival curve. (B) Disease-free survival according to neoadjuvant chemotherapy (Group Y: Primarily resectable CRLM patients with neoadjuvant chemotherapy, Group N: Primarily resectable CRLM patients without neoadjuvant chemotherapy). (C) Disease-free survival related to number of metastases, analyzed by the adjusted Cox proportional hazard method.
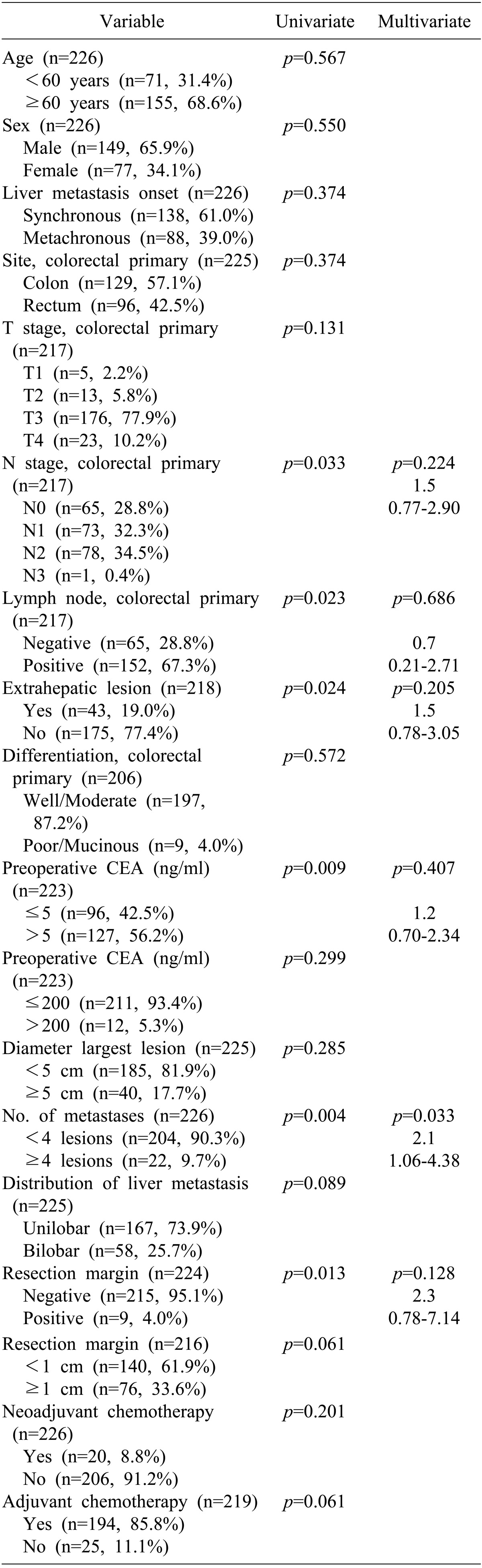
Based on univariate analysis, all of the following were consistent with disease-free survival: lymph node involvement and stage of primary tumor, extrahepatic lesions, preoperative CEA (>5 ng/ml), number of liver metastases(≥4 lesions), resection margin involvement, and resection margin ≤1 cm were associated with shorter disease-free survival (Table 5). On follow-up multivariate analysis, the number of liver metastases (≥4 lesions) was the only factor associated with shorter disease-free survival (Fig. 2C).
Table 5. Univariate and multivariate analysis of prognostic factors affecting tumor recurrence.
Outcome of hepatic resection according to the NCT
Median duration of NCT from diagnosis was 7.5 months (range, 1-16 months). According to the revised RECIST criteria, 4 patients (20%) showed partial response, one patient (5%) showed stable disease and 15 patients (75%) showed progressive disease in Group Y. In 15 patients with progressive disease, CRLMs were increased in both size and number in 3 patients and only in size for 12 patients during NCT.
In 14 patients (70%), NCT had no clinical impact on initial surgical plans. Initial tentative surgical plans of hepatic resections were changed after NCT in 6 patients (30%) to further resection in 4 (20%) and to limited resection in 2 patients (10%).
DISCUSSION
There have been several papers focusing on the role of hepatic resection for CRLMs even in the era of effective chemotherapy. As recent advances in hepatectomy techniques have dramatically improved, operative mortality associated with hepatic resection is less than 1% in experienced surgical centers.14,15,16 Moreover, the recent introduction of effective adjuvant chemotherapies and advanced diagnostic images has been widening the criteria of IR-CRLMs, although the definition has been variable in different centers.19,20,21,22,23,24,25
In spite of advanced criteria of IR-CRLMs, the 5-year survival rate was more than 50% in Group N. Our center is one of the highest volume hepatic resection and living donor liver transplantation centers in Korea. Moreover, early adaption of advanced imaging modalities (ie, supermagnetic iron oxide-enhanced MRI and EOB-Gd-DTPA MRI, has assisted the improvement of surgical and oncological outcomes of IR-CRLMs).26,27,28,29,30 For this reason, one stage hepatic resection in synchronous CRMLs and immediate hepatic resection in metachronous CRLMs without NCT were the main policy when dealing with IR-CRLMs in our institution since 2003.
Recently, some authors insist that the survival benefit from hepatic resection was determined by biological features of liver lesions rather than by early and precise detection of small lesions.31,32,33 For this reason, interval hepatic resection (ie, staged resection for synchronous CRLMs or delayed resection for metachronous CRLMs after NCT), might be recommended to assess the biological behavior of these lesions in patients with IR-CRLMs. In addition, in the era of effective chemotherapy, the tentative surgical plan can change from major to limited resection, in order to save the liver parenchyma.34,35,36,37,38 In this respect, all patients with IR-CRLMs could be candidates for NCT, because the chemotherapeutic agents have been more effective and some reports of initially unresectable CRLMs have supported these attempts.39,40,41 In addition, it must be kept in mind that the potential hazards of chemotherapy to the liver are detrimental in proportion to the number of chemotherapy cycles.
In this study, there was no benefit of NCT for the IR-CRLMs, as well as no harm. NCT was not one of the prognostic factors of DFS and patient survival rates. According to the RECIST criteria, only 10% of patients (n=2) in Group Y could have more than a remnant of their liver saved via a more limited resection than the initial surgical plan. By contrast, 20% of Group Y underwent further hepatic resection than initially planned. It was not surprising because only 20% of initially unresectable patients could be saved by NCT.42 This result might be caused by the disease progression even during NCT. On the other hand, this retrospective study excluded patients who showed complete response to NCT, as well as the patient who dropped out from the resection list due to disease progression; thus the effectiveness of NCT could be not be assessed fully.
The most recent a randomized controlled study, European Organization for Research and Treatment of Cancer (EORTC) phase III study, demonstrated that perioperative FOLFOX improved the 3-year-progression-free survival in the patients with IR-CRLMs. However, the effectiveness of NCT is still debated, because the control group of that study received only hepatic resection without adjuvant chemotherapy.43 Therefore, the answer for the role and the criteria of NCT in patients with IR-CRLMs has been unclear. To elucidate who will respond well to NCT and who will be a candidate for NCT obtaining survival gain may require further studies.
This study is limited by its retrospective design and by the small sample size of the group Y; there were no criteria for NCT in IR-CRLMs, because it had been performed before 2008 when there was no world-wide established NCT guideline for IR-CRLMs. The greater bilobar distribution and male gender in Group Y than in Group N could be explained as a selection bias. However, because the sample size was too small, we couldn't statistically correct this bias.
In order to overcome the limitation of this retrospective study, we reanalyzed the survival outcome according to the survival benefit from the time of diagnosis of IR-CRLMs. However, the survival rate from the initial time of the diagnosis of IR-CRLMs, not from the time of hepatic resection, to the last follow-up or death was also similar between Groups Y and N (p=0.337).
The patient candidates who gained survival time via NCT might be those patients with very early and aggressive recurrence, with an initial presentation of CRLMs was resectable. In this study analysis, age ≥60 years (p=0.014), intraoperative transfusion (p=0.025), and no adjuvant chemotherapy (p=0.004) were significant risk factors for early recurrence after hepatic resection (within 3 months) based on the results of univariate analysis in Group N. However, age ≥60 years was the only significant risk factor of very early recurrence in Group N on multivariate analysis. Therefore, further study is required to determine which IR-CRLMs will be candidates for NCT taking survival benefit or who will respond well to NCT.
In conclusion, NCT had no positive impact on the survival outcome and no negative impact on the operative outcome in the patients with IR-CRLMs although patients who underwent NCT saved intraoperative transfusion. Further study is required to elucidate NCT criteria for patients who have IR-CRLMs, but can get survival benefits according to the tumor biology.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordlinger B, Guiguet M, Vaillant JC, et al. Association Française de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 3.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 4.House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. 752–755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 7.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. doi: 10.1097/00000658-200001000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Cox JV, Blanke C, et al. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 12.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Andres A, Toso C, Moldovan B, et al. Complications of elective liver resections in a Center With Low mortality: a simple score to predict morbidity. Arch Surg. 2011 doi: 10.1001/archsurg.2011.175. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Kamiyama T, Nakanishi K, Yokoo H, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Huang ZQ, Xu LN, Yang T, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 2009;122:2268–2277. [PubMed] [Google Scholar]
- 17.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections . HPB (Oxford) 2000;2:333–339. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiber M, Fingerle AA, Brügel M, Gaa J, Rummeny EJ, Holzapfel K. Detection and classification of focal liver lesions in patients with colorectal cancer: Retrospective comparison of diffusion-weighted MR imaging and multi-slice CT. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.072. (in press) [DOI] [PubMed] [Google Scholar]
- 20.Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The efficacy of diffusion-weighted imaging for the detection of colorectal cancer. Hepatogastroenterology. 2009;56:128–132. [PubMed] [Google Scholar]
- 21.O'Rourke TR, Welsh FK, Tekkis PP, et al. Accuracy of liver-specific magnetic resonance imaging as a predictor of chemotherapy-associated hepatic cellular injury prior to liver resection. Eur J Surg Oncol. 2009;35:1085–1091. doi: 10.1016/j.ejso.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Blyth S, Blakeborough A, Peterson M, Cameron IC, Majeed AW. Sensitivity of magnetic resonance imaging in the detection of colorectal liver metastases. Ann R Coll Surg Engl. 2008;90:25–28. doi: 10.1308/003588408X242303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura K, Kadoya M, Ueda K, Fujinaga Y, Miwa S, Miyagawa S. Detection of hepatic metastases from colorectal carcinoma: comparison of histopathologic features of anatomically resected liver with results of preoperative imaging. J Clin Gastroenterol. 2007;41:789–795. doi: 10.1097/01.mcg.0000225676.22218.08. [DOI] [PubMed] [Google Scholar]
- 24.Kuker RA, Mesoloras G, Gulec SA. Optimization of FDG-PET/CT imaging protocol for evaluation of patients with primary and metastatic liver disease. Int Semin Surg Oncol. 2007;4:17. doi: 10.1186/1477-7800-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titu LV, Breen DJ, Nicholson AA, Hartley J, Monson JR. Is routine magnetic resonance imaging justified for the early detection of resectable liver metastases from colorectal cancer? Dis Colon Rectum. 2006;49:810–815. doi: 10.1007/s10350-006-0537-y. [DOI] [PubMed] [Google Scholar]
- 26.Löwenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–840. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 27.Rappeport ED, Loft A. Liver metastases from colorectal cancer: imaging with superparamagnetic iron oxide (SPIO)-enhanced MR imaging, computed tomography and positron emission tomography. Abdom Imaging. 2007;32:624–634. doi: 10.1007/s00261-007-9297-y. [DOI] [PubMed] [Google Scholar]
- 28.Vidiri A, Carpanese L, Annibale MD, et al. Evaluation of hepatic metastases from colorectal carcinoma with MR-superparamagnetic iron oxide. J Exp Clin Cancer Res. 2004;23:53–60. [PubMed] [Google Scholar]
- 29.Furuhata T, Okita K, Tsuruma T, et al. Efficacy of SPIO-MR imaging in the diagnosis of liver metastases from colorectal carcinomas. Dig Surg. 2003;20:321–325. doi: 10.1159/000071758. [DOI] [PubMed] [Google Scholar]
- 30.Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–478. doi: 10.1148/radiology.196.2.7617863. [DOI] [PubMed] [Google Scholar]
- 31.Wanebo HJ, Berz D. The neoadjuvant therapy of colorectal hepatic metastases and the role of biologic sensitizing and resistance factors. J Surg Oncol. 2010;102:891–897. doi: 10.1002/jso.21691. [DOI] [PubMed] [Google Scholar]
- 32.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Interaction of tumour biology and tumour burden in determining outcome after hepatic resection for colorectal metastases. HPB (Oxford) 2010;12:84–93. doi: 10.1111/j.1477-2574.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasui K, Hirai T, Kato T, et al. A new macroscopic classification predicts prognosis for patient with liver metastases from colorectal cancer. Ann Surg. 1997;226:582–586. doi: 10.1097/00000658-199711000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karanjia ND, Lordan JT, Quiney N, Fawcett WJ, Worthington TR, Remington J. A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur J Surg Oncol. 2009;35:65–70. doi: 10.1016/j.ejso.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Ferrero A, Vigan L, Lo Tesoriere R, Russolillo N, Sgotto E, Capussotti L. Bisegmentectomies as alternative to right hepatectomy in the treatment of colorectal liver metastases. Hepatogastroenterology. 2009;56:1429–1435. [PubMed] [Google Scholar]
- 36.Torzilli G, Donadon M, Marconi M, et al. Systematic extended right posterior sectionectomy: a safe and effective alternative to right hepatectomy. Ann Surg. 2008;247:603–611. doi: 10.1097/SLA.0b013e31816387d7. [DOI] [PubMed] [Google Scholar]
- 37.Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247:109–117. doi: 10.1097/SLA.0b013e3181557e47. [DOI] [PubMed] [Google Scholar]
- 38.Elias D, Baton O, Sideris L, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42. doi: 10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 39.Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 40.Karasaki T, Sano K, Takamoto T, et al. Complete resection of unresectable liver metastases from colorectal cancer without deterioration of liver function after cetuximab and irinotecan: two case reports. Hepatogastroenterology. 2010;57:1526–1528. [PubMed] [Google Scholar]
- 41.Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–1547. doi: 10.1038/sj.bjc.6605940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]