Abstract
Background: Plastic and reconstructive uterus operations are performed in congenital uterine anomalies or benign uterine conditions. Congenital uterine anomalies are relatively rare diseases with various approaches for surgical treatment. Therefore, to address the question of the usefulness of a minimally invasive approach in plastic uterus operations, the most common uterine condition which requires reconstructive surgery, namely myomectomy, is discussed.
Method: Searches were conducted in PubMed and The Cochrane Library to identify relevant literature.
Findings: Compared with myomectomy by laparotomy and minilaparotomy, laparoscopic myomectomy is associated with improved short-term outcomes. Laparoscopy is further associated with less adhesion formation. Pregnancy rates after myomectomy in symptomatic patients might be higher after laparoscopy than after laparotomy. Although uterine ruptures following laparoscopic myomectomy are described in the literature, it seems to be a rare event. Concerning the recurrence, there is evidence that rates are similar after laparoscopy and laparotomy.
Conclusion: Myomectomy by laparoscopy has several advantages over abdominal myomectomy (by conventional laparotomy and minilaparotomy) and should be the standard procedure. Despite the advantages of laparoscopy, abdominal myomectomy is still a frequently performed procedure. Lack of training in advanced laparoscopic procedures hampers the wide-spread use of laparoscopic myomectomy. Due to the advantages of laparoscopic surgery, efforts should be made to implement this procedure into daily practice. To provide the best care, physicians should offer patients the opportunity of a laparoscopic treatment of myomas.
Keywords: minimally invasive surgery, myomectomy, plastic and reconstructive surgery, infertility, uterine rupture
Zusammenfassung
Hintergrund: Plastische und rekonstruktive Uterusoperationen werden aufgrund von angeborenen genitalen Fehlbildungen oder gutartigen Uteruserkrankungen durchgeführt. Bei angeborenen genitalen Fehlbildungen handelt es sich um relativ seltene Erkrankungen mit unterschiedlichsten Behandlungsansätzen. Zur Beantwortung der Frage, ob es sinnvoll ist plastische Uterusoperationen minimalinvasiv durchzuführen, wird daher die häufigste gutartige Uteruserkrankung, welche eine Rekonstruktion des Uterus erfordert, nämlich die Myomentfernung, besprochen.
Methodik: Es wurde eine Literaturrecherche in PubMed und der Cochrane Library durchgeführt.
Ergebnisse: Die laparoskopische Myomentfernung ist, verglichen mit der Myomentfernung per Laparotomie und Minilaparotomie, mit einem besseren kurzfristigen Patientenoutcome verbunden. Zudem kann die Entstehung von Adhäsionen durch den Einsatz der Laparoskopie vermindert werden. Die Schwangerschaftsrate nach laparoskopischer Myomentfernung ist bei symptomatischen Patientinnen höher als nach Myomentfernung per Laparotomie. Obwohl Uterusrupturen nach laparoskopischer Myomentfernung in der Literatur beschrieben sind, scheint dies dennoch ein seltenes Ereignis zu sein. Bezüglich des Auftretens von Rezidiven gibt es Hinweise, dass es keinen Unterschied nach Laparoskopie und Laparotomie gibt.
Schlussfolgerung: Die laparoskopische Myomentfernung hat gegenüber der Myomentfernung per Laparotomie (herkömmliche Laparotomie und Minilaparotomie) verschiedene Vorteile und sollte die Standardoperation darstellen. Trotz der Vorteile der Laparoskopie ist die Myomentfernung per Laparotomie immer noch ein häufig durchgeführter Eingriff. Ein Mangel an Training in technisch anspruchsvollen laparoskopischen Operationen erschwert den weitverbreiteten Einsatz der laparoskopischen Myomentfernung. Aufgrund der Vorteile der Laparoskopie, sollte man sich jedoch um die Implementierung dieser Operation in die tägliche Praxis bemühen. Um die bestmögliche Versorgung zu gewährleisten, sollten Ärzte ihren Patienten die Möglichkeit bieten Myome laparoskopisch entfernen zu lassen.
Background
Reconstructive surgery of the uterus is required in congenital uterine anomalies or benign uterine conditions, like myomas, adenomyosis or uterine wall abnormalities, in women wishing to preserve their uterus for reproductive or personal reasons [1]. The restoration of the regular uterine anatomy is important to diminish the negative impact on fertility and pregnancy outcomes that some of these disorders may have [2], [3].
Congenital uterine anomalies are a group of various uterine malformations caused by either unilateral development or incomplete midline fusion due to disturbances in early development of the Müllerian system [4]. The incidence of congenital uterine anomalies ranges from 0.1–2% among all women up to 4% among infertility patients [4]. Treatment of these diseases varies from hysteroscopic resection to complex reconstructive procedures depending on the type of anomaly [2], [5].
However, the most common benign tumor of the uterus in women of reproductive age is the uterine leiomyoma (uterine fibroid, fibroid, myoma) [6] (Figure 1 (Fig. 1)). In a large ultrasonographic study, the cumulative incidence of uterine myomas by age 50 was over 80% for black women and nearly 70% for white women [7]. Although not all women with myomas develop symptoms, myomas have a great clinical impact [8], [9]. The majority of hysterectomies are performed due to symptomatic uterine myomas [10], [11]. Symptoms include abnormal uterine bleeding, pelvic pressure and pain, and reproductive dysfunction [8]. If future pregnancy is desired or if women want to preserve their uterus for personal reasons, an appropriate alternative to hysterectomy has to be found for their treatment.
Figure 1. Location of uterine myomas.
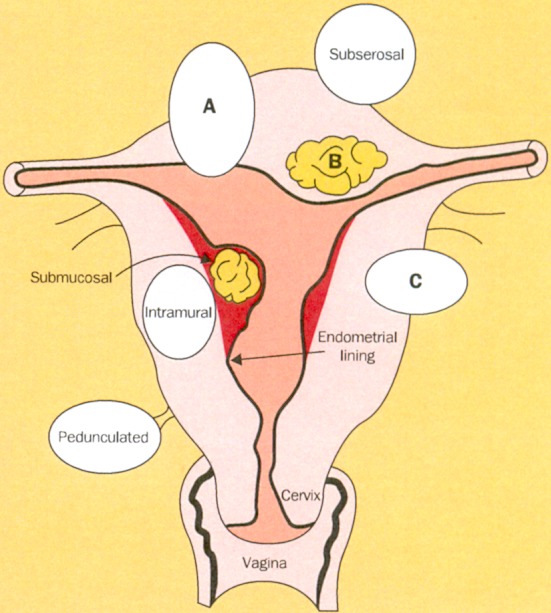
Submucosal fibroids intrude into or are contained in the uterine cavity; intramural fibroids are contained within the wall of the uterus, and subserosal ones create the characteristic irregular feel of the myomatous uterus. Most myomas are of mixed type, however, as illustrated by A, B, and C (reprinted from [8] with permission from Elsevier).
Whereas abdominal myomectomy is performed routinely for many decades, in the recent years, various minimally invasive alternatives to laparotomy have been developed [12]. At present, a vast number of minimally invasive approaches for the treatment of myomas exist including abdominal myomectomy (by minilaparotomy [13] or ultraminilaparotomy [14]), vaginal myomectomy [15], laparoscopic myomectomy (also gasless laparoscopy [16], single access laparoscopy [17] or robotic assisted laparoscopy [18]), uterine artery embolization (UAE) [19], uterine artery occlusion [20], myolysis [21], magnetic resonance imaging-guided focused ultrasound [22] and medical treatment [23]. Only a few of the treatment options are investigated in randomised, controlled trials and some of them still need to be investigated for safety and efficacy. To answer the question about the usefulness of a minimally invasive approach for reconstructive uterine surgery, this article focus on myomectomy as the most common plastic and reconstructive uterine procedure. Myomectomy by laparoscopy is compared to myomectomy by laparotomy, minilaparotomy and robotic assisted laparoscopic myomectomy. Moreover, frequent concerns associated with laparoscopic myomectomy are discussed.
Methods
The PubMed database was searched using the search terms “myomectomy” alone and in combination with “adhesions”, “infertility OR fertility outcome”, “uterus rupture”, “recurrence”, “costs” and “surveys” with the limitation on articles published in English and German. Additionally, the PubMed database was searched using the search term “laparoscopy and learning curve”. The Cochrane Library was also searched for the search term “myomectomy”. Articles were included in the review if the title indicated any relevance to the topic. Statements in the articles were scrutinised by searching the corresponding articles listed in the references sections. The reference lists were also searched for relevant literature.
Findings
Myomectomy
Depending on the preference of the surgeon, different modifications of the technique are possible, concerning trocar placement, instruments used, methods to reduce bleeding or suture material used. The following section provides a brief overview of the basic steps of myomectomy [24], [25].
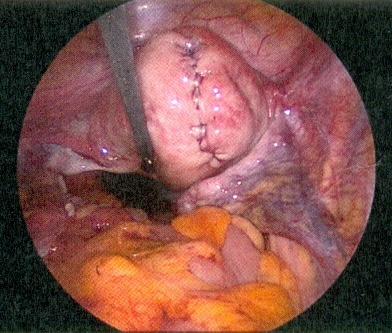
During the procedure, the use of a uterus manipulator facilitates myomectomy and suturing as it enables the positioning of the uterus depending on the location of the myoma (Figure 2 (Fig. 2)). At the beginning of the procedure, diluted vasopressin is injected between the myoma capsule and the normal muscle layer which is an effective technique to reduce haemorrhage [26]. Although rare, some severe complications, associated with the use of vasopressin, were reported including pulmonary oedema, severe hypotension and bradycardia with eventual cardiac arrest [27]. Therefore, the possible occurrence of these complications should be kept in mind when diluted vasopressin is used. After injection, the myometrium overlying the myoma become pale and the myometrium can be incised in horizontal or vertical direction. A horizontal incision may facilitate the subsequent suturing of the myometrial defect [28]. To further reduce the risk of bleeding, the incision is made with a monopolar instrument (hook or scissor) or a harmonic scalpel [6], [28]. Once the myoma pseudocapsule is reached, the myoma can be grasped with a forceps or a myoma screw, enabling traction and countertraction on the myoma which is necessary for the enucleation (Figure 3 (Fig. 3)). If the myoma is enucleated along the avascular cleavage plane, the enucleation should be easily possible. Attachments to the myometrium can be lysed with a bipolar forceps or a monopolar scissor [24]. The enucleated myoma is temporary placed in the cul-de-sac and is removed at the end of the procedure by mechanical or electric morcellation. Suturing of the myometrial defect is of great importance for the strength of the uterine scar. Depending on the depth of the defect, a single or multilayer closure is necessary to minimise the risk of haematoma, post-operative bleeding or uterine rupture in subsequent pregnancies (Figure 4 (Fig. 4)) [27], [28].
Figure 2. Fundal myoma.
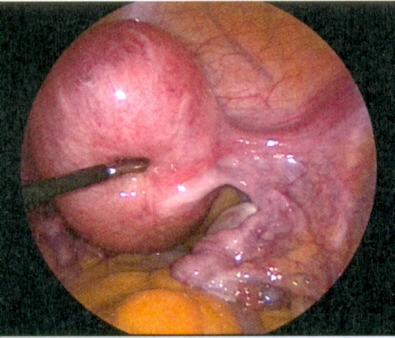
Figure 3. The myometrium overlying the myoma is opened and the myoma is visible. A myoma screw was inserted into the myoma.
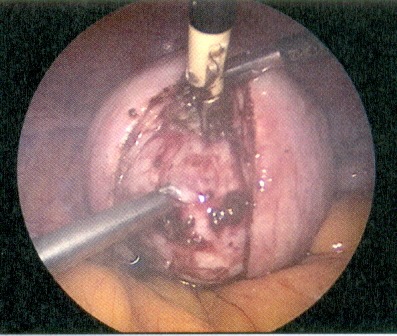
Figure 4. Uterus after myomectomy with hysterotomy suture.
Laparoscopic myomectomy versus abdominal myomectomy by conventional laparotomy
First reports of abdominal myomectomy as an alternative to hysterectomy are published over 100 years ago [29], [30]. Back then, reasons for uterus preservation already included the women’s desire for future childbearing as well as the women’s wish for organ preservation in order to avoid emotional distress caused by the experience of an organ loss [31], [32]. Despite these early advocacies for myomectomy, it took decades before abdominal myomectomy was generally accepted as a treatment option for uterine fibroids [33], [34]. Additionally, in 1979, Semm introduced the laparoscopic myomectomy as a new promising surgical approach for the treatment of uterine myomas [35]. Since then, numerous articles were published concerning the feasibility and safety of laparoscopic myomectomy [36], [37], [38]. However, only a few studies compared laparoscopic myomectomy with abdominal myomectomy whereas only some of them are prospective, randomised trials [39], [40], [41], [42], [43]. The retrospective trials revealed that laparoscopic myomectomy is associated with lower haemoglobin drop or less blood loss, respectively, lower morbidity and a shorter hospital stay [39], [40]. These findings are in line with the prospective, randomised studies [41], [42], [43] (Table 1 (Tab. 1)). Moreover, Holzer et al. demonstrated in a double-blind study that laparoscopic myomectomy is associated with lower postoperative pain [43]. In the recent years, however, publications about myomectomy by minilaparotomy as a minimally invasive alternative to conventional laparotomy are increasing. Prospective, randomised studies exist, comparing myomectomy by laparoscopy and minilaparotomy. Therefore, the next section provides a more detailed comparison of these two minimally invasive fibroid treatments.
Table 1. Laparoscopic myomectomy vs. abdominal myomectomy by conventional laparotomy.
Laparoscopic myomectomy versus abdominal myomectomy by minilaparotomy
Minilaparotomy is a modification of laparotomy where the skin incision does not exceed 5–6 centimetres [13], [44]. Although minilaparotomy was already described in the 1990s [45], only in the last decade, an increasing number of articles have been published concerning minilaparotomy as a minimally invasive treatment option for myomectomy [13], [46], [47]. Authors, who encourage myomectomy by minilaparotomy, state that this procedure has several advantages over laparoscopic myomectomy including the ability to palpate the uterus, the possibility to operate large myomas, no need for extra equipment and no need for advanced technical skills, especially in suturing the uterine incision [13], [48]. In comparison with conventional laparotomy, minilaparotomy showed indeed advantages of minimally invasive surgery like a shorter hospital stay [49], [50]. However, prospective, randomised trials comparing minilaparotomy and laparoscopy, confirmed that laparoscopy is associated with better short-term outcomes like a significantly lower decline in haemoglobin concentrations, lower postoperative pain, lower analgesic requirements and a shorter hospital stay [44], [51], [52] (Table 2 (Tab. 2)).
Table 2. Laparoscopic myomectomy vs. abdominal myomectomy by minilaparotomy.
Concerning complications associated with laparoscopic and abdominal myomectomy, Alessandri et al. reported in their study one laparoconversion due to difficulties of hemostasis and one case of diffuse peritonitis caused by ileal perforation in the laparoscopic group [51]. Interestingly, in the study of Palomba et al. six laparoconversions occurred in the minilaparotomy group. These laparoconversions were due to posterior isthmic and infraligamentary location of the leiomyomas and the authors mentioned that in these cases the degree of surgical difficulty was similar to that of laparoscopy. In this study, location of the main myoma rather than the size of the myoma was the main factor that influences the results. The authors stated that myomectomy of anterior, fundal and lateral myomas was simpler and faster when minilaparotomy was conducted. However, there were five (7.4%) postoperative complications in the minilaparotomy group including one case of fever >38°C, two wound infections and one case of wound dehiscence. In the laparoscopic group, two (2.9%) postoperative complications occurred including one case of fever >38°C and one case of urinary tract infection [52]. Cicinelli et al. reported two intraoperative complications in the laparoscopic group. In one patient, moderate subcutaneous emphysema developed at pneumoperitoneum creation and in the other patient the procedure was converted to minilaparotomy due to difficulty in reconstructing the uterine wall. Postoperatively, five patients in the laparoscopy group (12.5%) and ten patients in the minilaparotomy group (25%) developed fever [44]. Compared with myomectomy by minilaparotomy, laparoscopic myomectomy is associated with better short-term outcomes. Furthermore, laparoscopic myomectomy carries a low risk of minor and major complications.
Laparoscopic myomectomy versus robotic-assisted laparoscopic myomectomy
In the last decade, robotic surgery has been introduced in gynecology and is described as “an enhancement along the continuum of laparoscopic technocological advances” [18]. Robotic surgery provides a 3-dimensional image, absence of tremor, superior instrument articulation, comfort for the surgeon and a faster learning curve [53]. At present, only retrospective studies are available comparing robotic-assisted laparoscopic myomectomy (RALM) with laparotomy or laparoscopy. Compared with laparotomy, RALM is associated with a decrease in blood loss, fewer complications and a shorter hospital stay [54], [55], [56]. Compared with laparoscopic myomectomy, RALM seems to have similar short-term outcomes [56], [57], [58]. Furthermore, Nezhat et al. stated that RALM does not offer any major advantages over laparoscopy when laparoscopy is performed by a skilled surgeon [58]. However, removal of large, unfavourable localised myomas as well as suturing the uterine incision is challenging for many surgeons and hampers the widespread adoption of laparoscopy [54], [59]. Although robotic surgery can overcome these difficulties [56], [60], the higher costs currently lead to an obvious drawback of this possible approach [54], [61]. In case of persisting higher costs, robotic surgery is unlikely to be adopted by all hospitals in the near future. At present, therefore, laparoscopy remains the preferred approach if myomectomy should be conducted by a minimally invasive approach.
Postoperative adhesions
Adhesions are fibrin strands between two anatomical sites which are normally not attached to each other. After a previous laparotomy, adhesions were found in 93% of patients during a second procedure [62]. Complications associated with adhesions are small bowel obstruction (SBO) [63], chronic pelvic pain [64], infertility [65] and the risk for inadvertent bowel injuries in subsequent procedures [66]. A recent review of 2,000 laparoscopies conducted for the treatment of acute SBO, declared that adhesions were accountable for 84.9% of the small bowel obstructions [67]. Although adhesions are described as an important cause of chronic pelvic pain, its real impact remains controversial [64]. One further major concern about adhesions is the unfavourable influence that they could have on future fertility. Adhesions can lead to an impaired interaction between the Fallopian tube and the ovary and it is assumed that adhesions cause 20–40% of female infertility [68], [69]. It is known that some gynaecological procedures carry a higher risk of adhesion development than others [70] whereas myomectomy is associated with a high risk for adhesion formation [1]. Bearing this in mind, it is important to find ways to reduce adhesion formation after myomectomy, as this procedure is often performed to restore childbearing potential.
Comparing laparoscopy and laparotomy in their adhesiogenic potential, conflicting data exist. Whereas laparoscopy was long regarded to be less adhesiogen, it was demonstrated that the laparoscopic environment itself functions as a cofactor in adhesion formation. The pressure used to maintain the pneumoperitoneum leads to tissue hypoxia and thereby to alterations in the fibrinolytic system which is a key factor in adhesion formation. Furthermore, the use of cold and dry insufflation gas could lead to peritoneal damage through tissue desiccation, although tissue desiccation is also a problem during open surgery [71]. Nevertheless, studies investigating adhesion formation after myomectomy by laparoscopy or laparotomy revealed that adhesions occur less often after laparoscopy (RefProf). The published incidence of adhesions after myomectomy varies as shown by the following studies. Tinelli et al. investigated in a prospective blinded observational study the effect of an anti-adhesion agent after both laparoscopic myomectomy and abdominal myomectomy. A large number of patients (n=546) with comparable baseline characteristics and no difference in the dimension of the fibroid were assessed during a second procedure conducted for several reasons. The incidence of adhesions in the different groups was as follows: abdominal myomectomy (AM) without adhesion barrier (AB) (28.1%), laparoscopic myomectomy (LM) without AB (22.6%), AM with AB (22%) and LM with AB (15.9%) [72]. Kubinova et al. assessed adhesions during a second-look laparoscopy for adhesiolysis after abdominal or laparoscopic myomectomy. In this study, 96.65% of patients had adhesions after laparotomy (n=28) compared with 71.43% of patients after laparoscopy (n=68). If adhesions were present, patients after abdominal myomectomy had more dense adhesions than patients after laparoscopy. Furthermore, after abdominal myomectomy 89.29% of patients had de novo adnexal adhesions which might compromise fertility. In the laparoscopic group de novo adnexal adhesions were observed in 10.6% of patients [73]. Another study also assessed the occurrence of adhesions after laparoscopic myomectomy during a second procedure and found adhesions in only 1.6% of patients (2/121) [74]. Although the use of laparoscopy is not able to prevent adhesion formation completely, it can be shown that the occurrence of adhesions is reduced after laparoscopy.
Several factors associated with myomectomy influence the formation of adhesions. Some studies revealed that myomas of the posterior uterine site lead to more adhesions than fundal or anterior myomas [75], [76]. Further influencing factors are the size and the number of removed myomas [77]. Suturing of the uterine surface can increase the risk of adhesion formation [78], [79], [80]. Furthermore, the skill of the surgeon may also have an impact on the development of adhesions [77]. Thus, following the principles of gentle tissue handling is important to avoid extensive trauma to the peritoneum which could result in adhesions. These principles include constant tissue moistening and reduced use of electrocautery [81]. In addition, in high risk procedures like myomectomy, the use of an anti-adhesion agent should be considered [82].
Myomectomy and fertility
The role of fibroids as a cause for infertility, is still discussed controversial. There is agreement that large submucosal fibroids are associated with increased miscarriage rate and reduced fertility and that removal of submucosal fibroids improve fertility outcomes. As submucosal myomas are mainly removed hysteroscopically, they are not included in this article [83]. Whereas subserosal fibroids seem to have no impact on fertility, evidence on the impact of intramural fibroids on fertility is conflicting [84]. In a recent systematic review, the implantation rate and the on-going pregnancy rate were found to be significantly lower in the presence of intramural fibroids, whereas the spontaneous abortion rate was significantly higher [85]. These data were obtained including only prospective trials. A further restriction to studies, which used a high-quality method to assess the uterine cavity, revealed that the implantation rate was still significantly impaired, but the other parameters do not longer reach significance. Moreover, advising infertile patients with intramural fibroids on surgery is controversial due to limited data on the impact of myomectomy on improving fertility [85]. Somigliana et al. proposed to make the decision for surgery based on “(i) the age of the woman; (ii) the location, dimension and number of the fibroids; (iii) the concomitant presence of fibroid-related symptoms such as menorrhagia or hypermenorrhea and (iv) the presence of other causes of infertility and whether or not there is an indication to IVF” [86].
If surgery is recommended, the best approach has to be chosen for the patient not to further compromise fertility. Additionally, not all myomectomies conducted in women of childbearing age are performed in infertile patients. Since more and more women decide to postpone their childbearing to a later age, myomectomies are frequently performed in symptomatic patients with a desire for subsequent pregnancies [87]. Hence, it is important to decide which the best approach is for both infertility and symptomatic patients to improve fertility outcomes. At present, only two randomised controlled trials are available comparing fertility outcomes after laparoscopic and abdominal myomectomy [42], [52]. Seracchioli et al. investigated 131 patients with otherwise unexplained infertility and found no significant differences in the pregnancy and abortion rate between the two groups. However, patients in the laparoscopic group showed better short term outcomes ([42], Table 1). A more recent study by Palomba et al. investigated the reproductive outcomes in both infertility and symptomatic patients (n=136). In case of infertility, no difference in the cumulative pregnancy rate, abortion rate and live-birth rate between laparoscopy and minilaparotomy was found. The authors stated that the study was probably underpowered to demonstrate a significant difference. Comparing only patients with myomectomy for symptomatic myomas, however, cumulative pregnancy rate, pregnancy rate per cycle and live-birth rate per cycle were significantly higher in the laparoscopic group. Furthermore, the time to first pregnancy and live-birth was significantly lower after laparoscopic myomectomy [52]. Thus, laparoscopy performed for the removal of symptomatic myomas may not only have advantages in short-term outcomes, but also in fertility outcome. In the future, large-scaled, prospective, randomised studies are needed to confirm these findings.
Uterine rupture
The main concern after laparoscopic myomectomy in women of childbearing age is about the strength of the myomectomy scar during subsequent pregnancies. Although it seems to be a rare event, reports of uterine rupture after abdominal myomectomy also exist in the literature [88], [89], [90]. However, especially pregnancies after laparoscopic myomectomy have been a matter of concern since laparoscopic suturing is regarded as a demanding task. Several factors may contribute to the development of a weak scar with the subsequent risk for uterine rupture. The extensive use of electrocoagulation instead of sutures to achieve hemostasis can lead to tissue necrosis followed by an impaired wound healing [91]. Further, the presence of infection or hematoma formation within the myometrium, the extent of local tissue destruction and individual healing characteristics are also factors which could influence wound healing in the myometrium [92]. Another important contributing factor to the development of a weak scar may be an inadequate suturing of the myometrial defect. A recent review of 19 case reports of uterine rupture after laparoscopic myomectomy revealed that in 7 cases the uterine defect was not repaired (3 subserosal myomas and 4 subserosal pedunculated myomas), in 3 cases it was repaired with a single suture (1 subserosal myoma and 2 intramural myomas), in 4 cases it was repaired in only 1 layer (intramural myomas) and in 1 case only the serosa was closed (subserosal myoma) [92]. Depending on the depth of the myometrial defect, a multilayer closure may be necessary to eliminate dead space and to achieve an adequate wound closure [91], [93].
Considering several studies on fertility outcome after laparoscopic myomectomy, uterine rupture seems to be also a rare event after laparoscopy [28]. A large review including 626 pregnancies after laparoscopic myomectomy found only 1 case of uterine rupture [28]. In the above-mentioned review of case reports, time of uterine rupture range from 17 to 40 weeks of gestation [92]. Thus, the possibility of uterine rupture should already be taken into consideration before start of labour and patients should be appropriately counseled. Additionally, the mode of delivery, vaginally or by cesarean section, must be discussed with the patients. Kumakiri et al. prospectively investigated the safety of vaginal birth after laparoscopic myomectomy by using the criteria for a vaginal birth after cesarean section. The authors concluded that in selected patients vaginal delivery could be successfully accomplished if the myomectomy wound is appropriately sutured [93]. Therefore, pregnancies after laparoscopic myomectomy carry a low risk of uterine rupture if laparoscopy is conducted by a surgeon who has sufficient expertise.
Myoma recurrence
The risk for myoma recurrence after laparoscopic myomectomy compared with abdominal myomectomy is still a matter of debate. It is assumed that the inability to palpate the uterus during laparoscopy leads to a higher recurrence rate due to small intramural myomas which are left behind in the uterus. These myomas could grow and could be responsible for the recurrence of symptoms [94]. The 5 year cumulative recurrence rate after laparotomy varies from 5.7% to 11.1% if the recurrence rate is not assessed through systematic ultrasound investigations [94]. If transvaginal ultrasonography is used, the recurrence rate after abdominal myomectomy is much higher and varies from 15.4% to 62% [94], [95], [96]. In their study, Nezhat et al. revealed a 5 year cumulative recurrence rate after laparoscopic myomectomy of 51.4% evaluated through chart reviews, returned questionnaires and telephone interviews. The authors concluded that the recurrence rate after laparoscopy may be higher than reported after laparotomy [97], [98]. In a prospective, randomised study, the recurrence rate between abdominal and laparoscopic myomectomy was compared in 81 patients. Transvaginal ultrasonography was used for assessment and after a study period of 40 month, the recurrence rates were similar in both groups (27% laparoscopy and 23% laparotomy, respectively). Furthermore, in this study, none of the women with myoma recurrence required additional surgery during the study period [99]. In another large study, investigating 512 patients who underwent laparoscopic myomectomy, the cumulative recurrence rate at 5 years (at 8 years, respectively) was 52.9% (84.4%, respectively), whereas the cumulative probability of reoperation for recurrent myoma was 6.7% at 5 years (16% at 8 years, respectively) [100]. Factors influencing myoma recurrence may be age, number of myomas, uterine size and childbirth after myomectomy [94], [95], [100], although other authors did not find a relationship between these factors and myoma recurrence [99]. However, further long-term, prospective, randomised studies are needed comparing the recurrence rate after laparoscopic and abdominal myomectomy including skill factors. Moreover, it is important to evaluate the clinical impact of myoma recurrence, measured through the need for subsequent treatment, as well as the influencing factors. Thus, patients should be appropriately counselled about probability and risk factors for myoma recurrence.
Conclusion
Laparoscopic myomectomy has several advantages over abdominal myomectomy and even over myomectomy by minilaparotomy, given that minilaparotomy is suggested as a minimally invasive alternative to laparotomy. These advantages include a lower decline in haemoglobin concentrations, lower postoperative pain, lower analgesic requirements, a shorter hospital stay and a faster postoperative recovery (Table 1 (Tab. 1) and Table 2 (Tab. 2)). Moreover, myomectomy by laparoscopy decreases the risk of adhesion formation which could potentially lead to serious complications. Compared with abdominal myomectomy, fertility outcomes in infertile patients seem to be similar after laparoscopy, whereas in symptomatic patients, laparoscopy may lead to higher pregnancy rates. Furthermore, if the procedures are performed by a surgeon who is skilled in laparoscopic surgery, uterine ruptures after laparoscopic myomectomies are rare events. Therefore, laparoscopy should be the standard approach for myomectomy. It is recommended that laparoscopic myomectomy should include patients with not more than 4–7 myomas and a myoma diameter of <8–10 cm [99], [101].
One frequently mentioned concern about laparoscopic myomectomy is the expected higher costs associated with laparoscopic procedures. A review of studies comparing abdominal and laparoscopic hysterectomies demonstrated that although laparoscopy was associated with higher direct costs, the indirect costs were lower and might compensate [102]. At present, studies comparing the costs of abdominal and laparoscopic myomectomy are sparse. In a recent study, abdominal myomectomy was the least expensive approach compared with robotic-assisted laparoscopic myomectomy [61]. No significant difference in the average costs of abdominal and laparoscopic myomectomy was found [39]. Thus, further studies are needed to compare costs of the procedures, including indirect costs as well as long-term costs if additional treatment is required.
Despite the above-mentioned advantages of laparoscopic myomectomy, abdominal myomectomy is still a frequently performed procedure. In France, 37,787 patients required an intervention for uterine myomas in 2005. The study data were obtained through analysis of a national hospital activity database. Treatment of myomas included 22,540 (59.7%) hysterectomies, 6,291 hysteroscopic resections and 571 UAEs. A total of 8,385 myomectomies were conducted including 2,277 laparoscopic and 6,108 abdominal myomectomies [103]. In Germany, hospital admissions due to interventions for uterine myomas were identified through DRG (diagnosis-related group) codes. In 2005, 64,299 patients were admitted for uterine myomas. 54,577 (84.9%) patients were treated with hysterectomy and in 1,527 patients the myoma were removed through hysteroscopic resection. A total of 8,195 myomectomies were conducted including 315 vaginal myomectomies, 4,692 laparoscopic myomectomies and 3,188 abdominal myomectomies (including 504 laparoconversions). In Germany, more laparoscopic than abdominal myomectomies were performed, although the number of conducted laparotomies was still high [103]. Since acquiring laparoscopic skills is more challenging than acquiring skills needed for conduction of open surgery, not all surgeons are able to perform advanced laparoscopic procedures like myomectomy [104]. A UK survey, published in 2006, revealed that only 11% of the respondents perform laparoscopic myomectomy (response rate 59%) [105]. In a recent Canadian survey, 24.5% of the respondents perform laparoscopic myomectomy and 3.1% stated that more than 50% of their myomectomies are conducted laparoscopically. These rates might be overestimated as the response rate was only 41.4% and it is likely that the questionnaires were answered rather by gynaecologist who were interested in the topic or perform laparoscopic myomectomy. According to this survey, the main obstacle to perform laparoscopic myomectomy was the lack of training in the procedure (70.7% of respondents) [106]. During residency, only a few residents have the opportunity to gain practical experience in advanced laparoscopic procedures like myomectomy [107]. However, for the implementation of laparoscopy, training of basic laparoscopic skills during residency is also important, as laparoscopies are rather performed by surgeons who received explicit training during residency [108]. It was shown that simulator training can be an effective tool to enhance basic laparoscopic skills leading to a better performance during following procedures [109]. After finishing residency, acquirement of advanced laparoscopic skills can be difficult if there is no opportunity for an appropriate teaching and training. Hiring an experienced laparoscopic surgeon who is interested in teaching other surgeons, in combination with surgeons who are interested in learning advanced laparoscopic procedures, has proven to be an effective method to implement advanced laparoscopic procedures into daily practice [108], [110]. Although not all surgeons are similarly skilled [111], personal efforts should be made by every surgeon who performs laparoscopy to continuously enhance personal laparoscopic skills, and thereby enhancing the safety of patients. As Walid recently mentioned: “Gynecologists need to improve their laparoscopic skills, as minimally invasive surgery is becoming the sine qua non of a modern surgeon” [112]. In the future, it is likely that there will be a steadily increasing demand for minimally invasive procedures by patients [106]. Thus, if the patient is a candidate for laparoscopic myomectomy, the procedure should be offered to the patient, either performed personally or through referral to an experienced colleague, for providing the best care.
At present, research findings suggest that minimally invasive surgery is a useful and feasible approach for myomectomy as the most common plastic and reconstructive uterus operation. Nevertheless, further prospective, randomised studies are needed to compare long-term outcomes between different invasive and noninvasive treatment options in uterine myomas including skill evaluation.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pistofides G. Postoperative adhesion after laparoscopic uterine reconstructive surgery. In: De Wilde RL, Schmidt EH, editors. State-of-the-art prevention of adhesions in gynecology. Bremen: UNI-MED; 2010. pp. 62–77. [Google Scholar]
- 2.Brucker SY, Rall K, Campo R, Oppelt P, Isaacson K. Treatment of congenital malformations. Semin Reprod Med. 2011;29(2):101–112. doi: 10.1055/s-0031-1272472. Available from: http://dx.doi.org/10.1055/s-0031-1272472. [DOI] [PubMed] [Google Scholar]
- 3.Somigliana E, Vercellini P, Benaglia L, Abbiati A, Barbara G, Fedele L. The role of myomectomy in fertility enhancement. Curr Opin Obstet Gynecol. 2008;20(4):379–385. doi: 10.1097/GCO.0b013e3283073ac9. Available from: http://dx.doi.org/10.1097/GCO.0b013e3283073ac9. [DOI] [PubMed] [Google Scholar]
- 4.Letterie G. Management of congenital uterine abnormalities. Reprod Biomed Online. 2011;23(1):40–52. doi: 10.1016/j.rbmo.2011.02.008. Available from: http://dx.doi.org/10.1016/j.rbmo.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e.V. Weibliche genitale Fehlbildungen. Berlin: DGGG; 2010. [2011 Dec 19]. Available from: http://www.dggg.de/fileadmin/public_docs/Leitlinien/1-1-4-Weibliche-genitale-Fehlbildungen-2010.pdf. [Google Scholar]
- 6.Agdi M, Tulandi T. Minimally invasive approach for myomectomy. Semin Reprod Med. 2010;28(3):228–234. doi: 10.1055/s-0030-1251479. Available from: http://dx.doi.org/10.1055/s-0030-1251479. [DOI] [PubMed] [Google Scholar]
- 7.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. Available from: http://dx.doi.org/10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 8.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. Available from: http://dx.doi.org/10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 9.Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the united states: a summary of published estimates. J Womens Health. 2005;14(8):692–703. doi: 10.1089/jwh.2005.14.692. Available from: http://dx.doi.org/10.1089/jwh.2005.14.692. [DOI] [PubMed] [Google Scholar]
- 10.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, Marchbanks PA. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34. doi: 10.1016/j.ajog.2007.05.039. Available from: http://dx.doi.org/10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Stang A, Merrill R, Kuss O. Hysterectomy in Germany. Dtsch Arztebl Int. 2011;108(30):508–514. doi: 10.3238/arztebl.2011.0508. Available from: http://dx.doi.org/10.3238/arztebl.2011.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WH. Uterine myomas: management. Fertil Steril. 2007;88(2):255–271. doi: 10.1016/j.fertnstert.2007.06.044. Available from: http://dx.doi.org/10.1016/j.fertnstert.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Minim Invasive Gynecol. 2005;12(3):275–283. doi: 10.1016/j.jmig.2005.03.009. Available from: http://dx.doi.org/10.1016/j.jmig.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Ciavattini A, Tsiroglou D, Litta P, Frizzo H, Tranquilli AL. Ultra-minilaparotomy myomectomy: a minimally invasive surgical approach for the treatment of large uterine myomas. Gynecol Obstet Invest. 2009;68(2):127–133. doi: 10.1159/000227764. Available from: http://dx.doi.org/10.1159/000227764. [DOI] [PubMed] [Google Scholar]
- 15.Faivre E, Surroca MM, Deffieux X, Pages F, Gervaise A, Fernandez H. Vaginal myomectomy: literature review. J Minim Invasive Gynecol. 2010;17(2):154–160. doi: 10.1016/j.jmig.2009.12.007. Available from: http://dx.doi.org/10.1016/j.jmig.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Damiani A, Melgrati L, Marziali M, Sesti F. Gasless laparoscopic myomectomy. Indications, surgical technique and advantages of a new procedure for removing uterine leiomyomas. J Reprod Med. 2003;48(10):792–798. [PubMed] [Google Scholar]
- 17.Lee JH, Choi JS, Jeon SW, Son CE, Lee SJ, Lee Y. Single-port laparoscopic myomectomy using transumbilical GelPort access. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):81–84. doi: 10.1016/j.ejogrb.2010.07.020. Available from: http://dx.doi.org/10.1016/j.ejogrb.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28(2):77–82. [PubMed] [Google Scholar]
- 19.Freed MM, Spies JB. Uterine artery embolization for fibroids: a review of current outcomes. Semin Reprod Med. 2010;28(3):235–241. doi: 10.1055/s-0030-1251480. Available from: http://dx.doi.org/10.1055/s-0030-1251480. [DOI] [PubMed] [Google Scholar]
- 20.Helal A, Mashaly AE, Amer T. Uterine Artery Occlusion for Treatment of Symptomatic Uterine Myomas. JSLS. 2010;14(3):386–390. doi: 10.4293/108680810X12924466007403. Available from: http://dx.doi.org/10.4293/108680810X12924466007403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Hum Reprod Update. 2000;6(6):609–613. doi: 10.1093/humupd/6.6.609. Available from: http://dx.doi.org/10.1093/humupd/6.6.609. [DOI] [PubMed] [Google Scholar]
- 22.Fennessy FM, Tempany CM. A review of magnetic resonance imaging-guided focused ultrasound surgery of uterine fibroids. Top Magn Reson Imaging. 2006;17(3):173–179. doi: 10.1097/RMR.0b013e3180337e1f. Available from: http://dx.doi.org/10.1097/RMR.0b013e3180337e1f. [DOI] [PubMed] [Google Scholar]
- 23.Sankaran S, Manyonda I. Medical management of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):655–676. doi: 10.1016/j.bpobgyn.2008.03.001. Available from: http://dx.doi.org/10.1016/j.bpobgyn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt EH, De Wilde RL. Operationen bei Myomen. In: Schmidt EH, De Wilde R, editors. Standardverfahren der minimal-invasiven Chirurgie in der Frauenheilkunde. Stuttgart: Thieme; 1998. pp. 125–136. [Google Scholar]
- 25.Tchartchian G, Dietzel J, Hackethal A, De Wilde RL, Bojahr B. Die ambulante Myomenukleation. Chir prax. 2009;71:97–107. [Google Scholar]
- 26.Shimanuki H, Takeuchi H, Kitade M, Kikuchi I, Kumakiri J, Kinoshita K. The effect of vasopressin on local and general circulation during laparoscopic surgery. J Minim Invasive Gynecol. 2006;13(3):190–194. doi: 10.1016/j.jmig.2006.01.015. Available from: http://dx.doi.org/10.1016/j.jmig.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Mattei A, Cioni R, Bargelli G, Scarselli G. Techniques of laparoscopic myomectomy. Reprod Biomed Online. 2011;23(1):34–39. doi: 10.1016/j.rbmo.2010.09.011. Available from: http://dx.doi.org/10.1016/j.rbmo.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Hurst BS, Matthews ML, Marshburn P. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83(1):1–23. doi: 10.1016/j.fertnstert.2004.09.011. Available from: http://dx.doi.org/10.1016/j.fertnstert.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Braithwaite J. Removal of a submucous fibroid by section of the uterus (myomectomy) Br Med J. 1900;1(2040):251. doi: 10.1136/bmj.1.2040.251. Available from: http://dx.doi.org/10.1136/bmj.1.2040.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamberlain G. The master of myomectomy. J R Soc Med. 2003;96(6):302–304. doi: 10.1258/jrsm.96.6.302. Available from: http://dx.doi.org/10.1258/jrsm.96.6.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bullard RT. When to operate upon uterine fibromyomata: myomectomy. Cal State J Med. 1905;3(11):361–363. [PMC free article] [PubMed] [Google Scholar]
- 32.Bonney V. Myomectomy or hysterectomy. Br Med J. 1918;1(2984):278–280. doi: 10.1136/bmj.1.2984.278. Available from: http://dx.doi.org/10.1136/bmj.1.2984.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross JW. Surgery in the uterine fibroid, a plea for myomectomy. Am J Obstet Gynecol. 1947;53(2):266–270. doi: 10.1016/0002-9378(47)90343-8. [DOI] [PubMed] [Google Scholar]
- 34.Guarnaccia MM, Rein M. Traditional surgical approaches to uterine fibroids: abdominal myomectomy and hysterectomy. Clin Obstet Gynecol. 2001;44(2):385–400. doi: 10.1097/00003081-200106000-00024. Available from: http://dx.doi.org/10.1097/00003081-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Semm K. New methods of pelviscopy (gynecologic laparoscopy) for myomectomy, ovariectomy, tubectomy and adnectomy. [Neue Methoden der Pelviskopie (gynäkologische Laparoskopie) zur Myom-, Ovar-, Tub- und Adnektomie]. Endoscopy. 1979;11(2):85–93. doi: 10.1055/s-0028-1098329. Available from: http://dx.doi.org/10.1055/s-0028-1098329. [DOI] [PubMed] [Google Scholar]
- 36.Dubuisson J, Fauconnier A, Babaki-Fard K, Chapron C. Laparoscopic myomectomy: a current view. Hum Reprod Update. 2000;6(6):588–594. doi: 10.1093/humupd/6.6.588. Available from: http://dx.doi.org/10.1093/humupd/6.6.588. [DOI] [PubMed] [Google Scholar]
- 37.Koh C, Janik G. Laparoscopic myomectomy: the current status. Curr Opin Obstet Gynecol. 2003;15(4):295–301. doi: 10.1097/01.gco.0000084243.09900.5a. Available from: http://dx.doi.org/10.1097/01.gco.0000084243.09900.5a. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi H, Kuwatsuru R. The indications, surgical techniques, and limitations of laparoscopic myomectomy. JSLS. 2003;7(2):89–95. [PMC free article] [PubMed] [Google Scholar]
- 39.Stringer N, Walker JC, Meyer P. Comparison of 49 laparoscopic myomectomies with 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457–464. doi: 10.1016/S1074-3804(05)80039-8. Available from: http://dx.doi.org/10.1016/S1074-3804(05)80039-8. [DOI] [PubMed] [Google Scholar]
- 40.Marret H, Chevillot M, Giraudeau B The Study Group of the French Society of Gynaecology and Obstetrics (Ouest Division) A retrospective multicentre study comparing myomectomy by laparoscopy and laparotomy in current surgical practice: What are the best patient selection criteria? Eur J Obstet Gynecol Reprod Biol. 2004;117(1):82–86. doi: 10.1016/j.ejogrb.2004.04.015. Available from: http://dx.doi.org/10.1016/j.ejogrb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis G. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174(2):654–658. doi: 10.1016/S0002-9378(96)70445-3. Available from: http://dx.doi.org/10.1016/S0002-9378(96)70445-3. [DOI] [PubMed] [Google Scholar]
- 42.Seracchioli R, Rossi S, Govoni F, Rossi E, Venturoli S, Bulletti C, Flamigni C. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15(12):2663–2668. doi: 10.1093/humrep/15.12.2663. [DOI] [PubMed] [Google Scholar]
- 43.Holzer A, Jirecek ST, Illievich UM, Huber J, Wenzl R. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102(5):1480–1484. doi: 10.1213/01.ane.0000204321.85599.0d. Available from: http://dx.doi.org/10.1213/01.ane.0000204321.85599.0d. [DOI] [PubMed] [Google Scholar]
- 44.Cicinelli E, Tinelli R, Colafiglio G, Saliani N. Laparoscopy vs minilaparotomy in women with symptomatic uterine myomas; a prospective randomized study. J Minim Invasive Gynecol. 2009;16(4):422–426. doi: 10.1016/j.jmig.2009.03.011. Available from: http://dx.doi.org/10.1016/j.jmig.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Benedetti-Panici P, Maneschi F, Cutillo G, Scambia G, Congiu M, Mancuso S. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996;87(3):456–459. doi: 10.1016/0029-7844(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 46.Thomas RL, Winkler N, Carr BR, Doody KM, Doody KJ. Abdominal myomectomy – a safe procedure in an ambulatory setting. Fertil Steril. 2010;94(6):2277–2280. doi: 10.1016/j.fertnstert.2010.02.019. Available from: http://dx.doi.org/10.1016/j.fertnstert.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Malzoni M, Tinelli R, Cosentino F, Iuzzolino D, Surico D, Reich H. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short-term and fertility results. Fertil Steril. 2010;93(7):2368–2373. doi: 10.1016/j.fertnstert.2008.12.127. Available from: http://dx.doi.org/10.1016/j.fertnstert.2008.12.127. [DOI] [PubMed] [Google Scholar]
- 48.Fanfani F, Fagotti A, Bifulco G, Ercoli A, Malzoni M, Scambia G. A prospective study of laparoscopy versus minilaparotomy in the treatment of uterine myomas. J Minim Invasive Gynecol. 2005;12(6):470–474. doi: 10.1016/j.jmig.2005.07.002. Available from: http://dx.doi.org/10.1016/j.jmig.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Benassi L, Marconi L, Benassi G, Accorsi F, Angeloni M, Besagni F. Minilaparotomy vs laparotomy for uterine myomectomies: a randomized controlled trial. Minerva Ginecol. 2005;57(2):159–163. [PubMed] [Google Scholar]
- 50.Cagnacci A, Pirillo D, Malmusi S, Arangino S, Alessandrini C, Volpe A. Early outcome of myomectomy by laparotomy, minilaparotomy and laparoscopically assisted minilaparotomy. A randomized prospective study. Hum Reprod. 2003;18(12):2590–2594. doi: 10.1093/humrep/deg478. Available from: http://dx.doi.org/10.1093/humrep/deg478. [DOI] [PubMed] [Google Scholar]
- 51.Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. J Minim Invasive Gynecol. 2006;13(2):92–97. doi: 10.1016/j.jmig.2005.11.008. Available from: http://dx.doi.org/10.1016/j.jmig.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Palomba S, Zupi E, Russo T, Falbo A, Marconi D, Tolino A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: short-term outcomes. Fertil Steril. 2007;88(4):942–951. doi: 10.1016/j.fertnstert.2006.12.048. Available from: http://dx.doi.org/10.1016/j.fertnstert.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 53.Quaas AM, Einarsson JI, Srouji S, Gargiulo A. Robotic myomectomy: a review of indications and techniques. Rev Obstet Gynecol. 2010;3(4):185–191. [PMC free article] [PubMed] [Google Scholar]
- 54.Advincula AP, Xu X, Goudeau S, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14(6):698–705. doi: 10.1016/j.jmig.2007.06.008. Available from: http://dx.doi.org/10.1016/j.jmig.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Ascher-Walsh CJ, Capes TL. Robot-assisted laparoscopic myomectomy is an improvement over laparotomy in women with a limited number of myomas. J Minim Invasive Gynecol. 2010;17(3):306–310. doi: 10.1016/j.jmig.2010.01.011. Available from: http://dx.doi.org/10.1016/j.jmig.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-Assisted, Laparoscopic, and Abdominal Myomectomy: A Comparison of Surgical Outcomes. Obstet Gynecol. 2011;117(2 Part 1):256–266. doi: 10.1097/AOG.0b013e318207854f. Available from: http://dx.doi.org/10.1097/AOG.0b013e318207854f. [DOI] [PubMed] [Google Scholar]
- 57.Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201(6):566. doi: 10.1016/j.ajog.2009.05.049. Available from: http://dx.doi.org/10.1016/j.ajog.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 58.Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy – a retrospective matched control study. Fertil Steril. 2009;91(2):556–559. doi: 10.1016/j.fertnstert.2007.11.092. Available from: http://dx.doi.org/10.1016/j.fertnstert.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 59.Payne TN, Pitter MC. Robotic-assisted surgery for the community gynecologist: Can it be adopted? Clin Obstet Gynecol. 2011;54(3):391–411. doi: 10.1097/GRF.0b013e31822b4998. Available from: http://dx.doi.org/10.1097/GRF.0b013e31822b4998. [DOI] [PubMed] [Google Scholar]
- 60.Lönnerfors C, Persson J. Robot-assisted laparoscopic myomectomy; a feasible technique for removal of unfavorably localized myomas. Acta Obstet Gynecol Scand. 2009;88(9):994–999. doi: 10.1080/00016340903118026. Available from: http://dx.doi.org/10.1080/00016340903118026. [DOI] [PubMed] [Google Scholar]
- 61.Behera M, Likes CE3, Judd JP, Barnett JC, Havrilesky LJ, Wu JM. Cost analysis of abdominal, laparoscopic, and robotic-assisted myomectomies. J Minim Invasive Gynecol. 2011 doi: 10.1016/j.jmig.2011.09.007. Available from: http://dx.doi.org/10.1016/j.jmig.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Menzies D, Ellis H. Intestinal obstruction from adhesions – how big is the problem? Ann R Coll Surg Engl. 1990;72(1):60–63. [PMC free article] [PubMed] [Google Scholar]
- 63.Barmparas G, Branco BC, Schnüriger B, Lam L, Inaba K, Demetriades D. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14(10):1619–1628. doi: 10.1007/s11605-010-1189-8. Available from: http://dx.doi.org/10.1007/s11605-010-1189-8. [DOI] [PubMed] [Google Scholar]
- 64.Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Fedele L. Chronic pelvic pain in women: etiology, pathogenesis and diagnostic approach. Gynecol Endocrinol. 2009;25(3):149–158. doi: 10.1080/09513590802549858. Available from: http://dx.doi.org/10.1080/09513590802549858. [DOI] [PubMed] [Google Scholar]
- 65.Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7(6):567–576. doi: 10.1093/humupd/7.6.567. Available from: http://dx.doi.org/10.1093/humupd/7.6.567. [DOI] [PubMed] [Google Scholar]
- 66.van der Krabben AA, Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, Schaapveld M, van Goor H. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87:467–471. doi: 10.1046/j.1365-2168.2000.01394.x. Available from: http://dx.doi.org/10.1046/j.1365-2168.2000.01394.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Connor DB, Winter DC. The role of laparoscopy in the management of acute small-bowel obstruction: a review of over 2,000 cases. Surg Endosc. 2011;26(1):12–17. doi: 10.1007/s00464-011-1885-9. Available from: http://dx.doi.org/10.1007/s00464-011-1885-9. [DOI] [PubMed] [Google Scholar]
- 68.Trew G. Postoperative adhesions and their prevention. Rev Gynaecol Perinatal Pract. 2006;6(1-2):47–56. doi: 10.1016/j.rigapp.2006.02.001. Available from: http://dx.doi.org/10.1016/j.rigapp.2006.02.001. [DOI] [Google Scholar]
- 69.De Wilde RL, Trew G. Postoperative abdominal adhesions and their prevention in gynaecological surgery. Expert consensus position. Gynecol Surg. 2007;4(3):161–168. doi: 10.1007/s10397-007-0338-x. Available from: http://dx.doi.org/10.1007/s10397-007-0338-x. [DOI] [Google Scholar]
- 70.Lower AM, Hawthorn RJ, Clark D, Boyed JH, Finlayson AR, Knight AD, Crowe AM Surgical and Clinical Research (SCAR) Group. Adhesion-related readmissions following gynaecological laparoscopy or laparotomy in Scotland: an epidemiological study of 24 046 patients. Hum Reprod. 2004;19(8):1877–1885. doi: 10.1093/humrep/deh321. Available from: http://dx.doi.org/10.1093/humrep/deh321. [DOI] [PubMed] [Google Scholar]
- 71.Ott DE. Laparoscopy and adhesion formation, adhesions and laparoscopy. Semin Reprod Med. 2008;26(4):322–330. doi: 10.1055/s-0028-1082390. Available from: http://dx.doi.org/10.1055/s-0028-1082390. [DOI] [PubMed] [Google Scholar]
- 72.Tinelli A, Malvasi A, Guido M, Tsin DA, Hudelist G, Hurst B, Stark M, Mettler L. Adhesion formation after intracapsular myomectomy with or without adhesion barrier. Fertil Steril. 2011;95(5):1780–1785. doi: 10.1016/j.fertnstert.2010.12.049. Available from: http://dx.doi.org/10.1016/j.fertnstert.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 73.Kubinova K, Mara M, Horak P, Kuzel D, Dohnalova A. Reproduction after myomectomy: comparison of patients with and without second-look laparoscopy. Minim Invasive Ther Allied Technol. 2011 doi: 10.3109/13645706.2011.573797. Available from: http://dx.doi.org/10.3109/13645706.2011.573797. [DOI] [PubMed] [Google Scholar]
- 74.Di Gregorio A, Maccario S, Raspollini M. The role of laparoscopic myomectomy in women of reproductive age. Reprod Biomed Online. 2002;4 Suppl 3:55–58. doi: 10.1016/s1472-6483(12)60118-7. [DOI] [PubMed] [Google Scholar]
- 75.Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82(2):213–215. [PubMed] [Google Scholar]
- 76.Dubuisson JB, Fauconnier A, Chapron C, Kreiker G, Nörgaard C. Second look after laparoscopic myomectomy. Hum Reprod. 1998;13(8):2102–2106. doi: 10.1093/humrep/13.8.2102. Available from: http://dx.doi.org/10.1093/humrep/13.8.2102. [DOI] [PubMed] [Google Scholar]
- 77.Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakiri J, Takeda S. Influencing factors of adhesion development and the efficacy of adhesion-preventing agents in patients undergoing laparoscopic myomectomy as evaluated by a second-look laparoscopy. Fertil Steril. 2008;89(5):1247–1253. doi: 10.1016/j.fertnstert.2007.05.021. Available from: http://dx.doi.org/10.1016/j.fertnstert.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 78.Trew G, Pistofidis G, Pados G, Lower A, Mettler L, Wallwiener D, et al. Gynaecological endoscopic evaluation of 4% icodextrin solution: a European, multicentre, double-blind, randomized study of the efficacy and safety in the reduction of de novo adhesions after laparoscopic gynaecological surgery. Hum Reprod. 2011;26(8):2015–2027. doi: 10.1093/humrep/der135. Available from: http://dx.doi.org/10.1093/humrep/der135. [DOI] [PubMed] [Google Scholar]
- 79.Wallwiener CW, Kraemer B, Wallwiener M, Brochhausen C, Isaacson KB, Rajab T. The extent of adhesion induction through electrocoagulation and suturing in an experimental rat study. Fertil Steril. 2010;93(4):1040–1044. doi: 10.1016/j.fertnstert.2008.12.002. Available from: http://dx.doi.org/10.1016/j.fertnstert.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 80.O'Leary DP. Role of sutures and suturing in the formation of postoperative adhesions. In: diZerega G, editor. Peritoneal surgery. Stuttgart: Thieme; 2000. pp. 201–214. [Google Scholar]
- 81.De Wilde RL, Trew G. Postoperative abdominal adhesions and their prevention in gynaecological surgery. Expert consensus position. Part 2-steps to reduce adhesions. Gynecol Surg. 2007;4(4):243–253. doi: 10.1007/s10397-007-0333-2. Available from: http://dx.doi.org/10.1007/s10397-007-0333-2. [DOI] [Google Scholar]
- 82.Metwally M, Watson A, Lilford R, Vanderkerchove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006;(2):CD001298. doi: 10.1002/14651858.CD001298.pub3. Available from: http://dx.doi.org/10.1002/14651858.CD001298.pub3. [DOI] [PubMed] [Google Scholar]
- 83.Agdi M, Tulandi T. Endoscopic management of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):707–716. doi: 10.1016/j.bpobgyn.2008.01.011. Available from: http://dx.doi.org/10.1016/j.bpobgyn.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198(4):357–366. doi: 10.1016/j.ajog.2007.12.039. Available from: http://dx.doi.org/10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 85.Pritts EA, Parker WH, Olive D. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. Available from: http://dx.doi.org/10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 86.Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007;13(5):465–476. doi: 10.1093/humupd/dmm013. Available from: http://dx.doi.org/10.1093/humupd/dmm013. [DOI] [PubMed] [Google Scholar]
- 87.Nezhat C. The “cons” of laparoscopic myomectomy in women who may reproduce in the future. Int J Fertil Menopausal Stud. 1996;41(3):280–283. [PubMed] [Google Scholar]
- 88.Levi AA. Rupture of the pregnant uterus. Relationship to previous myomectomy. Obstet Gynecol. 1961;18:223–229. [PubMed] [Google Scholar]
- 89.Garnet J. Uterine rupture during pregnancy. An analysis of 133 patients. Obstet Gynecol. 1964;23:898–905. [PubMed] [Google Scholar]
- 90.Golan D, Aharoni A, Gonen R, Boss Y, Sharf M. Early spontaneous rupture of the post myomectomy gravid uterus. Int J Gynecol Obstet. 1990;31(2):167–170. doi: 10.1016/0020-7292(90)90716-X. Available from: http://dx.doi.org/10.1016/0020-7292(90)90716-X. [DOI] [PubMed] [Google Scholar]
- 91.Dubuisson JB, Fauconnier A, Deffarges J, Norgaard C, Kreiker G, Chapron C. Pregnancy Outcome And Deliveries Following Laparoscopic Myomectomy. Hum Reprod. 2000;15(4):869–873. doi: 10.1093/humrep/15.4.869. Available from: http://dx.doi.org/10.1093/humrep/15.4.869. [DOI] [PubMed] [Google Scholar]
- 92.Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554. doi: 10.1016/j.jmig.2010.04.015. Available from: http://dx.doi.org/10.1016/j.jmig.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 93.Kumakiri J, Takeuchi H, Itoh S, Kitade M, Kikuchi I, Shimanuki H, Kumakiri Y, Kuroda K, Takeda S. Prospective Evaluation for the feasibility and safety of vaginal birth after laparoscopic myomectomy. J Minim Invasive Gynecol. 2008;15(4):420–424. doi: 10.1016/j.jmig.2008.04.008. Available from: http://dx.doi.org/10.1016/j.jmig.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602. doi: 10.1093/humupd/6.6.595. Available from: http://dx.doi.org/10.1093/humupd/6.6.595. [DOI] [PubMed] [Google Scholar]
- 95.Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881. doi: 10.1097/01.AOG.0000156298.74317.62. Available from: http://dx.doi.org/10.1097/01.AOG.0000156298.74317.62. [DOI] [PubMed] [Google Scholar]
- 96.Nishiyama S, Saito M, Sato K, Kurishita M, Itasaka T, Shioda K. High recurrence rate of uterine fibroids on transvaginal ultrasound after abdominal Myomectomy in Japanese Women. Gynecol Obstet Invest. 2006;61(3):155–159. doi: 10.1159/000090628. Available from: http://dx.doi.org/10.1159/000090628. [DOI] [PubMed] [Google Scholar]
- 97.Nezhat FR, Roemisch M, Nezhat C. Long-term follow-up of laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3(4 Suppl 1):35. doi: 10.1016/S1074-3804(96)80253-2. Available from: http://dx.doi.org/10.1016/S1074-3804(96)80253-2. [DOI] [PubMed] [Google Scholar]
- 98.Nezhat FR, Roemisch M, Nezhat CH, Seidman DS, Nezhat C. Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1998;5(3):237–240. doi: 10.1016/S1074-3804(98)80025-X. Available from: http://dx.doi.org/10.1016/S1074-3804(98)80025-X. [DOI] [PubMed] [Google Scholar]
- 99.Rosetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001;16(4):770–774. doi: 10.1093/humrep/16.4.770. Available from: http://dx.doi.org/10.1093/humrep/16.4.770. [DOI] [PubMed] [Google Scholar]
- 100.Yoo EH, Lee PI, Huh CY, Kim DH, Lee BS, Lee JK, Kim D. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697. doi: 10.1016/j.jmig.2007.06.003. Available from: http://dx.doi.org/10.1016/j.jmig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 101.Donnez J, Mathieu PE, Bassil S, Smets M, Nisolle M, Berliere M. Laparoscopic myomectomy today. Fibroids: management and treatment: the state of the art. Hum Reprod. 1996;11(9):1837–1840. doi: 10.1093/oxfordjournals.humrep.a019502. [DOI] [PubMed] [Google Scholar]
- 102.Bijen CB, Vermeulen KM, Mourits MJ, de Bock GH, Abdel-Aleem H. Costs and effects of abdominal versus laparoscopic hysterectomy: systematic review of controlled trials. PLoS One. 2009;4(10):e7340. doi: 10.1371/journal.pone.0007340. Available from: http://dx.doi.org/10.1371/journal.pone.0007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez H, Farrugia M, Jones SE, Mauskopf JA, Oppelt P, Subramanian D. Rate, type, and cost of invasive interventions for uterine myomas in Germany, France, and England. J Minim Invasive Gynecol. 2009;16(1):40–46. doi: 10.1016/j.jmig.2008.09.581. Available from: http://dx.doi.org/10.1016/j.jmig.2008.09.581. [DOI] [PubMed] [Google Scholar]
- 104.Hiemstra E, Kolkman W, Jansen FW. Skills training in minimally invasive surgery in Dutch obstetrics and gynecology residency curriculum. Gynecol Surg. 2008;5(4):321–325. doi: 10.1007/s10397-008-0402-1. Available from: http://dx.doi.org/10.1007/s10397-008-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chapman L, Magos A. Surgical and radiological management of uterine fibroids in the UK. Curr Opin Obstet Gynecol. 2006;18(4):394–401. doi: 10.1097/01.gco.0000233933.13684.05. Available from: http://dx.doi.org/10.1097/01.gco.0000233933.13684.05. [DOI] [PubMed] [Google Scholar]
- 106.Liu G, Zolis L, Kung R, Melchior M, Singh S, Cook F. The laparoscopic myomectomy: a survey of canadian gynaecologists. J Obstet Gynaecol Can. 2010;32(2):139–148. doi: 10.1016/S1701-2163(16)34428-0. [DOI] [PubMed] [Google Scholar]
- 107.Kolkman W, Wolterbeek R, Jansen F. Implementation of advanced laparoscopy into daily gynecologic practice: difficulties and solutions. J Minim Invasive Gynecol. 2006;13(1):4–9. doi: 10.1016/j.jmig.2005.11.002. Available from: http://dx.doi.org/10.1016/j.jmig.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Kolkman W, Engels LE, Smeets MJ, Jansen FW. Teach the teachers: an observational study on mentor traineeship in gynecological laparoscopic surgery. Gynecol Obstet Invest. 2007;64(1):1–7. doi: 10.1159/000098315. Available from: http://dx.doi.org/10.1159/000098315. [DOI] [PubMed] [Google Scholar]
- 109.Kolkman W, Wolterbeek R, Jansen FW. Gynecological laparoscopy in residency training program. Surg Endosc. 2005;19(11):1498–1502. doi: 10.1007/s00464-005-0291-6. Available from: http://dx.doi.org/10.1007/s00464-005-0291-6. [DOI] [PubMed] [Google Scholar]
- 110.Fowler DL, Hogle N. The impact of a full-time director of minimally invasive surgery: Clinical practice, education, and research. Surg Endosc. 2000;14(5):444–447. doi: 10.1007/s004640000158. Available from: http://dx.doi.org/10.1007/s004640000158. [DOI] [PubMed] [Google Scholar]
- 111.Mayooran Z, Rombauts L, Brown T, Tsaltas J, Fraser K, Healy D. Reliability and validity of an objective assessment instrument of laparoscopic skill. Fertil Steril. 2004;82(4):976–978. doi: 10.1016/j.fertnstert.2004.05.067. Available from: http://dx.doi.org/10.1016/j.fertnstert.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 112.Walid MS, Heaton R. The role of laparoscopic myomectomy in the management of uterine fibroids. Curr Opin Obstet Gynecol. 2011;23(4):273–277. doi: 10.1097/GCO.0b013e328348a245. Available from: http://dx.doi.org/10.1097/GCO.0b013e328348a245. [DOI] [PubMed] [Google Scholar]