Abstract
Ophthalmic Plastic and Reconstructive Surgery is a specialized area of ophthalmology that deals with the management of deformities and abnormalities of the eyelids, lacrimal system and the orbit. An ophthalmoplastic surgeon is able to identify and correct abnormalities of the ocular adnexae such as ectropion, lid retraction, conjunctival scarring with severe entropion, that can cause secondary ocular surface disorders; manage patients with watering eye, and when needed intervene with a dacryocystorhinostomy by external or endonasal approach and moreover minimize disfigurement following enucleation or evisceration and prevent further corneal damage, alleviate complains of tearing and grittiness, but also cosmetic complaints in patients with Graves’ orbitopathy.
Aim of this manuscript was to review current established and recently evolving surgical procedures.
1 Surgical anatomy of the eyelid
The eyelids are specialized structures composed of skin, superficial muscle, connective tissue and secretory glands. The knowledge of the location and anatomic relationship of eyelid structures and orbital content is necessary to establish a correct diagnosis and an appropriate management-plan of adnexal disorders.
The eyelid topography is influenced by age, race, and general facial anatomy. The maximum adult horizontal and vertical palpebral aperture is 28–30 mm and 8–12 mm. Upper and lower lid meet medially and laterally respectively at the inner and at the outer canthus; the latter usually sits 2 mm higher than the medial one.
Landmarks are the brow, the lid margin, the lashes, the skin creases, and the orbital rim.
The brow is composed of pilosebaceous units, muscle and fat and lies along the anterior aspect of the superior orbital rim. Brow skin is thick and bears hairs. Contraction of the frontalis muscle elevates the brow, while forced contraction of the orbicularis oculi muscle lowers it.
The lid margin is 2 mm wide and can be divided into an anterior rounded portion holding the lashes, a posterior sharp portion applied to the globe and a lacrimal one which overlies the lacrimal caruncle and the plica semilunaris; the lacrimal punctum opens on the centre of the lacrimal papilla into the lacrimal canaliculus. The lashes protrude from the anterior lid margin and their follicles, with the annexed sebaceous glands of Zeiss, extending deep to the level of the orbicularis and the tarsus. The eyelid creases represent the superficial insertion of the levator aponeurosis and of the lower lid retractors and are located 7–10 mm from the upper lid margin and 4–5 mm from the lower lid margin.
The lid structure in cross section consists of four layers: skin and subcutaneous tissue, orbicularis muscle, septum, tarsal plate and conjunctiva. The lid skin is very thin, allowing unrestricted movement with blinking, lid closure and vertical eye movements. The orbicularis muscle is an elliptical, flat, striated muscle that lies just below the skin of the lid.
The orbital septum consists of a multilamellar fibrous tissue arising from the periosteum at the orbital margin; it thickens towards the lid margin to form the dense tarsal plate and separates the orbital cavity and the eyelid. The tarsal plates provide rigidity to the eyelids and are tethered medially and laterally by the canthal tendons. The medial canthal tendon inserts anterior to the lacrimal sac on the lacrimal crest and maxilla while the lateral canthal tendon inserts on Whitnall’s tubercle just posterior to the orbital rim. The tarsus contains 30–40 holocrine modified sebaceous glands (Meibomiam glands).
The most posterior layer of the lid is the conjunctiva, a thin transparent vascularized mucous membrane that covers the pericorneal surface and the posterior surface of the eyelids.
The eyelid retractors are more developed for the upper lid than for the lower lid. The upper lid retractor provides continued protection of the globe in down gaze as well as unobstructed vision in primary and upward gaze.
The Levator palpebrae superioris arises outside the annulus of Zinn; behind the superior orbital rim the levator becomes more tendinous and a fibrous condensation, the Whitnall´s ligament, attaches to the superior aspect of the levator.
The sympathetic innervated muscle of Müller lies under the levator aponeurosis. It proceeds anteriorly to insert on the superior border of the upper lid tarsus. It adheres firmly to the levator at its origin and to the underlying conjunctiva.
The lower lid retractor, the capsulopalpebral head of the inferior rectus muscle, with the inferior tarsal muscle represent the retractor system of the lower lid.
The lacrimal system and tear film
In order to maintain the refractive power of the cornea and its functional integrity an adequate tear film covering the ocular surface is necessary; this is possible thanks to a lacrimal system composed by a secretory and excretory component.
The secretory portion is composed of the main and of the accessory lacrimal gland. The main lacrimal gland is divided in a larger orbital lobe and a smaller palpebral lobe, the first lies in the so-called lacrimal gland fossa; while the palpebral lobe rests below the levator aponeurosis. The accessory lacrimal glands are scattered around the conjunctival sac in the connective tissue of the conjunctiva, their ducts open directly onto the free surface of the conjunctiva and contribute to the production of the precorneal tear film. The glands secretion is regulated and the relative contribution of main and accessory lacrimal glands appears to vary with the level of stimulation; when the lacrimal fluid reaches the puncta, the capillary attraction is sufficient to draw the fluid from the marginal tear strip into the lacrimal puncta, canaliculi, sac and nasolacrimal duct.
In a simplified model the precorneal tear film consists of three layers: a lipid outer layer, an aqueous middle layer and a mucous inner layer. The lipid layer is produced by the Meibomian glands in the superior and inferior tarsus, by the gland of Zeis in the palpebral margin and by the glands of Moll at the roots of the lashes. The aqueous layer is produced by the accessory glands of Krause, in the upper and lower fornix, and by the accessory glands of Wolfring. They are responsible for the basic secretion while the main lacrimal gland is involved in reflex tearing. The mucin layer is produced by conjunctival goblet cells such as the Manz’ glands and the complex Henle’s crypts.
The blood supply
The ophthalmic artery is the major blood supply of the orbit. It arises from the internal carotid artery and enters the orbital cavity through the optic foramen. The ophthalmic artery reaches the medial wall of the orbit and divides into 2 terminal branches, frontal and dorsal nasal. The branches of the ophthalmic artery are divided into an orbital group (lacrimal artery, supraorbital artery, posterior ethmoidal artery, anterior ethmoidal artery, internal palpebral artery, frontal artery, nasal artery), distributing vessels to the orbit and surrounding parts, and an ocular group, (long ciliary artery, short ciliary artery, anterior ciliary artery, central retinal artery, muscular artery) distributing vessels to the muscles and bulb of the eye:
The venous drainage system is comprised of the following: vortex veins which provide drainage for the uveal tract; superior ophthalmic vein the main venous channel for the superior orbit, which drains to the cavernous sinus; Inferior ophthalmic vein which provides a channel for inferior drainage.
The eyelid innervation
The motor innervation of the lids reach the orbicularis muscle from the temporal and zygomatic branches of the facial nerve (VII), and the levator from oculomotor nerve (III); Müller muscle and the inferior tarsal muscle are supplied by sympathetic fibres from the superior cervical ganglion.
Sensory innervations is provided by the supra- and infratrochlear, as well as the supraorbital and lacrimal branches of the ophthalmic division of the trigeminal nerve (V) and the infraorbital nerve from the maxillary division of the trigeminal nerve (V).
The primary innervations of the lacrimal gland is through the lacrimal nerve, a branch of the ophthalmic division of the trigeminal nerve; few fibres from the zygomatic nerve, a branch of the maxillary division of the trigeminal nerve, may also reach the gland as well as facial nerve and postganglionic cervical sympathetic fibres.
2 Ectropion
The ectropion is an eyelid malposition characterized by outward displacement of the eyelid margin; it can be classified in involutional, paralytic and cicatricial.
2.1 Involutional/senile ectropion
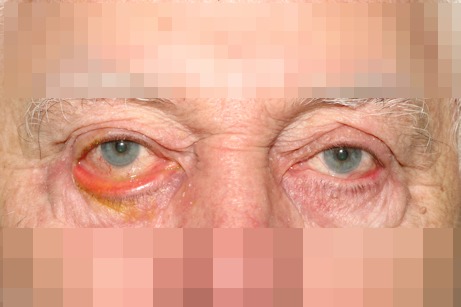
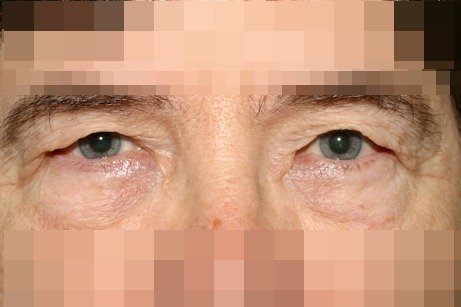
In this condition the lower lid is predominantly involved. While the upper lid can undergo the same changes, the clinical signs are not as dramatic as in the lower lid. The patient may experience dry eye symptoms and reflex epiphora due to a chronic exposure of the ocular surface (Figure 1 (Fig. 1)).
Figure 1. Right eye lower lid senile ectropion.
Procedures to correct involutional ectropion
“Lateral canthal sling”: described for the first time in 1979 by Anderson and Gordon, is suitable for eyelid laxity with poor lateral canthal fixation; re-fixating the eyelid to the lateral orbital tubercle corrects the laxity and restores the contour of the lateral canthal angle [1]. This can also be enforced with periosteal flap raised from just outside the rim to provide lasting lateral canthal fixation [2].
“Wedge resection”: common method that can be used when the canthal tendons are stable and marked horizontal redundancy of lid tissue is present. An inverted pentagonal wedge resection is performed; the lid margin is reconstructed with two interrupted 6.0 long absorbable sutures placed through the lash line and the grey line; tarsus and conjunctiva are repaired and the skin wound closed. This procedure can lead to lateral canthus deformity, in case of overcorrection as well as spill over with tear fluid if dip in the lid margin results.
“Lazy-T” (Tarsoconjunctival diamond excision with horizontal shortening) is suitable in cases of predominantly medial ectropion when the lateral canthal tendon is not particularly lax. A standard pentagon wedge resection is performed in conjunction with a diamond shaped excision of conjunctiva and retractor. This procedure has also been performed with a CO2-laser [3]. Risk of damaging the canalicular system or excessive removal of conjunctiva with consecutive shortening of the fornix are the most serious but rare complications occurring with procedures aiming to correct the medial ectropion.
“Medial canthoplasty” is indicated to promote extra lift to the puncti (e.g. in facial nerve palsy). Lachrymal probes are placed in the canaliculi, and an incision along the lid margin, from the puncti to the inner canthus, is performed leaving the canaliculi in the posterior lamella. Two 6.0 absorbable sutures, passing from the orbicularis muscle above the upper canaliculus to the muscle below the lower canaliculus, are placed and tied to close the inner canthus and invert the puncti [4].
2.2 Paralytic ectropion and lagophthalmos
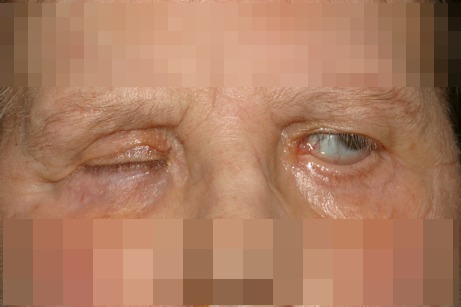
This is often related to VII nerve palsy and results from a loss of orbicularis muscle tone; these patients often present not only lagophthalmos but also secondary upper lid retraction because of unopposed levator action. Decreased blinking and tear production can lead to exposure keratopathy and concomitant fifth cranial nerve lesions can reduce corneal sensation, with consecutive neuroparalytic keratopathy. Early surgical correction is required for severe keratopathy, expected long term persistence of the facial palsy, patients with associated corneal anaesthesia and dry eye as well as in patients with lack of a good Bells phenomenon, and patients expected not to comply with regular medical treatment (Figure 2 (Fig. 2)).
Figure 2. Paralytic ectropion secondary to VII nerve palsy. A loss of orbicularis muscle tone is responsible of lagophthalmos and upper lid retraction because of inadequate levator action.
Procedures to correct paralytic ectropion
“Tarsorrhaphy” has been the classic approach for severe exposure keratopathy because of its simplicity and efficacy. It can be used as a temporary or permanent measure. Temporary tarsorrhaphy provides a solution to difficult but self-limiting short term problems (i.e. lagophthalmos due to Bell’s palsy or exposure keratopathy in a reversibly comatose patient). It is achieved suturing together juxtaposed areas of the upper and the lower eyelids. Tarsorrhaphy with transposition of upper lid tarsus to the lower lid is needed for more permanent results modulating the amount of eyelid closure desired. While a tarsorrhaphy of less than 3 mm can still lead to an acceptable cosmetic result may not be sufficient to treat exposure, a more extensive one, on the other hand, limits cosmesis and visual field.
“Eyelid loading” provides passive lid closure and an increased blink response. The use of gold weight implanted onto the tarsal plate lower the upper lid by means of increased gravity. Postoperative complications include a low grade with the rule corneal astigmatism, ptosis, bulging and extrusion of the implant [5], [6]. In order to prevent the extrusion of the implant Tremolada et al in 2001 suggested covering the gold implant with homologous temporal galea to improve the result of the procedure [7]. Platinum is currently evaluated as an alternative material [8], [9], since due to its higher density (weight/volume) such implants are thinner. Another suggestion is to use a semi flexible chain of implants to allow better adaptation to the tarsal plate.
2.3 Cicatricial ectropion
This is usually due to shortening of the anterior lid lamella. The aim of surgery is to release the tension responsible for the lid eversion.
Procedures to correct cicatricial ectropion
“Z-plasty” is effective when the contracture is present in a single vertical line. Tension is released by tissue transfer.
3 Entropion
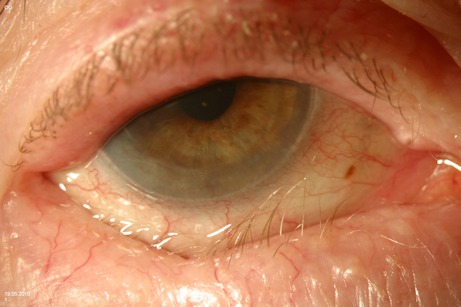
Entropion is the inward rotation of the eyelid margin, resulting in contact of the lid lashes with the ocular surface (Figure 3 (Fig. 3)). A correct eyelid margin position requires an adequate horizontal tone, as well as a balance of forces between the anterior and the posterior lamellae. It is necessary to evaluate the relative contribution of eyelid laxity and mechanical changes in the anterior and posterior lamellae when designing a surgical approach to entropion.
Figure 3. Right eye lower lid senile involutional entropion.
Entropion can be classified into: congenital (rare condition), senile, spastic or cicatricial.
3.1 Senile entropion
While horizontal eyelid laxity is usually best corrected with lateral canthal tendon tightening procedure (paragraph 2.1) different techniques address the other elements [10].
Procedures to correct senile entropion
“Everting sutures” remain the simplest form of surgical correction that is also quick safe and cheap. Double armed 4.0 plain catgut sutures are passed in a horizontal mattress fashion; the first needle engages the lower lid retractors which are seen as subconjunctival infratarsal white band at the lowest point of the inferior conjunctival fornix, while the other arm of the suture is passed 2 mm lateral to the first one in an identical fashion. The sutures are tied tightly to produce 1 mm of postoperative ectropion and are allowed to fall out spontaneously. Without exceptions the procedure has been considered to offer only a temporary remedy for patients with entropion [11], [12] but when combined with a lateral tarsal sling achieve long-lasting correction of involutional entropion [13]. No significant perioperative or postoperative complications have been described.
“Wies procedure”: a full thickness incision of the lower lid parallel to the lid margin, about 5 mm away from the lashes, is performed. Three double-armed sutures are placed through the conjunctiva and the lower lid retractor layer, into the orbicularis muscle anterior to the tarsal plate and then tied over small bits of rubber. The skin edges are closed with 4 interrupted sutures and removed after six days. The procedure offers a more etiologically based approach allowing the tightening of the lower lid retractor under direct visualization [14].
“Quickert procedure” is a combination of a wedge resection and Weis procedure, allows the correction of both horizontal eyelid laxity and inward rotation of the lower eyelid; low recurrence rate is reported after this procedure [15], [16], [17].
“Jones procedure” is used to tighten the lower lid retractors if they have become detached or if spasm is a major factor. If the retractors are obviously detached they can be simply reattached to the inferior tarsal plate border; in case they are still attached, they are tightened by plication [18]. Clinical studies provide evidence that success is greater after Jones procedure than after Wies procedure [19], [20].
3.2 Cicatricial entropion/fornix reconstruction
Progressive scarring of the conjunctiva leads to obliteration of the fornices and formation of symblepharon in a variety of ocular surface disorders. The numerous procedure described over the years to rotate the margin of the eyelid away from the cornea can be subdivided into those characterised by “passive” rotation of the margin with e.g. tarsal fracturing, rotation sutures with or without mucous membrane grafting and those characterized by a “buttress” procedure in which some tarsal substitute is rotated, advanced or grafted into position to reconstruct the eyelid margin. The passive procedures are generally easier and faster to perform but share the tendency for the outward rotation of the eyelid to regress over time due to second intension healing of the surgical wounds, in some cases due to progression of the underlying disease.
Any surgical procedure to correct symblepharon involves excision of the scar tissue, application of a tissue substitute to cover the palpebral and/or the bulbar surface and, in order to prevent readhesion, the use of conformers to keep the graft in place and reduce fornix shrinkage [21]. A conformer should be inserted at the end of the procedure that will result in a lagophthalmos of about 1 mm, indicating that reconstructed fornixes are stretched to the maximum. In the absence of inflammation early fibrin deposition and subsequent contraction will be the determinant of postoperative scar formation.
Procedures to correct cicatricial entropion
“Tarsotomy or tarsal fracture” is indicated in diffuse trichiasis associated with lid margin entropion. In the eyelid affected, a horizontal incision parallel to the lid margin is made through the full thickness of conjunctiva and tarsus. Double-armed vicryl sutures are passed; entering from the proximal cut edge of the tarsus first and brought out through the skin just proximal to the anterior lash line. One or more double-armed suture can be placed depending on the extension of the area involved. The procedure is effective in case of mild cicatricial entropion. Modest overcorrection is initially desired since there is a tendency toward cicatricial contracture and inward rotation of the margin with healing. Reported complications are overcorrection, stitch abscess requiring early suture removal and pyogenic granuloma within the incision site [22].
In “anterior lamellar repositioning” an incision is performed in the skin crease and deepen through the orbicularis muscle to expose the upper part of the tarsal plate throughout its width; after dissection the anterior lamella is pull up to assess the amount of correction of the entropion; if inadequate, an incision along the length of the grey line can allow extra eversion of the lashes. The procedure is indicated in case of upper lid mild or moderate cicatricial entropion with eyelash malposition; it allows advancing the posterior lamella thus everting the lashes. In order to avoid postoperative second retraction of the upper lid the upper lid retractors are recessed at the same time [23].
“Fornix reconstruction”, by established procedures such as labial or buccal mucous membrane grafts, has been widely used to replace the missing conjunctiva. In these procedures bulbar and palpebral surfaces are separated and the transplanted mucous membrane used to prevent recurrent symblepharon formation. In order to avoid early postoperative shrinkage of the reconstructed fornix, conformers or fixation of the transplant to the underlying tissue with a silicone sleeve and full thickness skin sutures are used [21]. Oral mucosa is easily available, but although complications are uncommon, donor site morbidity can limit oral function if large full-thickness grafts are required. If in addition mucin deficiency has to be addressed, e.g. in severe chemical burns or Stevens-Johnson-Syndrome, nasal turbinate mucosa provides an alternative source [24], [25]. Amniotic membrane (AM) has also been suggested for fornix reconstruction; it is the innermost layer of the placenta and consists of three layers: the epithelium, a thick basement membrane and an avascular stroma. It is used not as a conjunctival substitute, but rather as a substrate that supports conjunctival cell migration and regeneration. It supports rapid epithelialization, reduces inflammatory processes as well as vascularization and is capable of suppressing fibrosis [26]. Other advantages include avoidance of donor site morbidity as well as the need for general anaesthesia for harvesting the graft. It can be used fresh, i.e. unpreserved [27] or freeze dried, vacuum packed and gamma-irradiated [28], but cryopreservation in 50% glycerol at –80°C [29] is most widely used since this preserves growth supporting factors in the membrane while enabling convenient long term storage. Since the AM is quite thin and elastic it is well tolerated by the patient and does not become necrotic [30], [31], [32], [33], [34], [35].
The efficacy of fornix reconstruction with AM seems to depend on the underlying aetiology [32]. A successful fornix reconstruction with AM requires 3 prerequisites [34]:
The underlying aetiology should be non-inflammatory
Some healthy conjunctiva should be present in order to repopulate the new surface
Adequate lacrimal function is required to maintain the reconstructed fornix.
Another proposal is to use human amniotic membrane as substrate for culturing oral epithelial cells
4 Distichiasis, trichiasis
Misdirected eyelashes can lead to corneal abrasion, corneal ulceration, vascularization, scarring and even perforation. In trichiasis the eyelid is in a normal position and the eyelashes arise from their normal anatomic site and are typically of normal caliber; usually this condition is related to underlying inflammation, injury or any conjunctival disorder such as cicatricial pemphigoid or Stevens-Johnson syndrome. Distichiasis, on the contrary is a congenital or acquired condition in which an extra row of displastic lashes grows from the posterior aspect of the eyelid margin; the eyelid position is normal, but the offending lashes are often of a finer caliber. Acquired distichiasis is often secondary to chronic inflammation such as blepharitis or meibomiatis. The depth of the abnormal follicles in the tarsal plate is quite variable. The aim of the surgical correction is to redirect the lashes or remove the lash follicle.
Procedures to correct distichiasis and trichiasis
“Epilation” is simple and quick but it is usually ineffective because of eyelash re-growth. “Eyelash electrolysis” is indicated in case of a few abnormal lashes only. Following local anaesthesia a fine electrode wire is inserted into the lash follicle and a low current applied until white discoloration of the lash root arises from the lash follicle. The treatment is usually successful in damaging the eyelash follicle and thus avoiding regrowth of the lashes in about 50% of the follicle treated, but it is relatively tedious to perform and often poorly tolerated by the patient. Excessive electrolysis can also be responsible for scarring of the lid margin [36].
“Argon laser therapy” first presented in 1979 induces less inflammation because of precise tissue destruction but recurrent trichiasis is more common. Laser variables are usually as follow: 1.0-W power, 50-µm spot size, 0.2 seconds duration and blue-green wave length [37], [38]. It may be preferable when inflammation and tissue destruction has to be avoided, e.g. in ocular pemphigoid [39]. Since accurate placement of the burn is required the patients have to be able to remain immobile [40].
“Eyelash cryotherapy” is indicated in case of a large number of abnormal lashes; it is performed by applying a nitrous oxide cryoprobe for 20–30 seconds to the abnormal eyelashes [41]. Postoperative oedema can be quite marked at 48 to 72 hours, but regresses. Potential complications include lid nodge, corneal ulceration, symblepharon formation and activation of conjunctival inflammation and herpes zoster, skin depigmentation and destruction of the meibomian glands with consequent decreased integrity of the tear film [42].
“Lamella division and cryotherapy of the posterior lamella”: In order to apply the cryoprobe immediately to the lashes root, thus improving efficacy while reducing complication the anterior and posterior lamellae can be divided through a grey line incision with subsequent cryotherapy to the posterior lamella. The procedure allows protecting the normal lashes from the effect of freezing, and unnecessary depigmentation of the skin. The technique, as described by Anderson et al, is mainly indicated in case of congenital and acquired distichiasis. Considerable oedema may result and the eyelid margin may remain slightly thickened following this procedure [43].
5 Upper eyelid dermatochalasis and ptosis
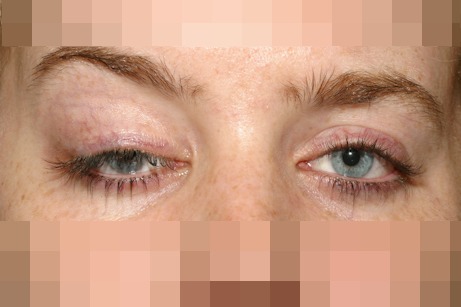
Droppy upper eyelids are frequently a cause of visual symptoms and an indication for surgical intervention. This occurs as result of a number of distinct diagnoses, which are treated by different surgical procedures. Ptosis (or blepharoptosis) is a downward displacement of the upper eyelid margin resulting from myogenic, involutional, neurogenic, mechanical, traumatic, or developmental causes [44] (Figure 4 (Fig. 4)).
Figure 4. Right eye congenital ptosis. In order to achieve sufficient upperlid elevation the patient requires lifting the brow. A brow suspension is planned for correction.
Eyelid ptosis occurs when there is a deficiency in the structure or function of Muller’s muscle or of the levator muscle/aponeurosis complex in the posterior lamella and surgical repair involves tightening or repositioning these structures or replacing their function with a suspension attached to an adjacent structure, in other words all ptosis repair procedures, whether performed through an anterior or posterior approach address posterior lamellar pathology or insufficiency. Dermatochalasis refers to an excess of eyelid skin; the underlying muscle, connective tissue can also prolapse (Figure 5 (Fig. 5)). Although dermatochalasis is most often an involutional problem, the excessive eyelid skin can result from specific disorders such as thyroid eye disease, floppy eyelid syndrom, and trauma. Dermatochalasis (blepharoplasty) surgery is an anterior lamella procedure with cutaneous incision aiming to remove excess of skin and, depending on the anatomic variation of specific patients, orbicularis oculi muscle, orbital septum or orbital fat (repositioned or removed).
Figure 5. Blepharochalasis with excess skin and muscle overcoming the eyelid margin and limiting the lateral visual field.
5.1 Upper lid blepharoplasty
The aim in upper lid blephatoplasty is to remove excess skin and muscle and eventually prolapsed fat: surgical planning includes deciding which techniques (steel blade or CO2 laser) and adjunctive procedure such as brow ptosis, ptosis, etc. will be used.
The procedure is usually performed under local anaesthesia. The excessive skin is preoperatively marked using the eyelid skin crease (apparently 8–10 mm above the lid margin in women and a bit lower in man) as a base, ending medially above the punctum and laterally at the lateral canthus. The mark is then extended laterally and upwards at an angle of 45º as far as the orbital rim. The superior marking allows maximum skin excision (as determined by the “pinch test”) while respecting a 12 mm minimum of required infrabrow supratarsal skin. After excision of the redundant skin and of a strip of orbicularis muscle and opening of the orbital septum, prolapsed fat can be excised from the medial and central compartment. Care must be taken to resect the right amount of skin not to induce lagophthalmos and to perform good hemocauterization to avoid orbital haemorrhage, which can lead to vision loss [45], [46].
5.2. Upperlid ptosis
Ptosis may be classified according to various criteria. Thought several procedures have been established the two main ways to achieve elevation of the upper lid are to shorten the levator palpebrae superioris or the Muller muscle or to carry out a brow/frontalis suspension procedure. For determining the optimal lid margin level during surgery the following guidelines are recommended: in bilateral surgery the lid margin are placed at 1mm or below the superior limbus. In unilateral surgery the margin of the ptotic lid is placed 1–2 mm above that of the contralateral eye [47].
Procedures to correct ptosis
Muller muscle procedure (minimal ptosis 1–2 mm, levator function >10 mm)
“Muller muscle conjunctival resection” consists of a resection of Muller muscle and of conjunctiva via a posterior approach; the procedure is easy, precise, and predictable and gives the possibility to grade the correction [48]. All patient with minimal ptosis should undergo prior to surgery to phenylephrine test, that is carried out by instilling two drops of 2.5% phenilephrine. The test is considered positive if the margin-to-reflex distance increases >1.5 mm, that indicates that the Muller muscle is viable and the procedure indicated [48].
“Fasanella Servat” involves the excision of conjunctiva, “Muller muscle toward the fornix and some accessory lachrymal glands. Resection is carried out according to the following algorithm: 1 mm ptosis: 4 mm resection, 1.5 mm ptosis: 6 mm resection and 3 mm ptosis: 11–12 mm resection. In patients with dry eye syndrome, symptoms may worsen after the surgery, because of the decreased tarsal stability and number of accessory lachrymal glands, thus less basal tear secretion [49].
Levator muscle procedures (moderate ptosis 3–4 mm; levator function 5–10 mm)
“Levator muscle advancement”: With this procedure the levator and its aponeurosis are reattached to the eyelid structures [50].
“Levator muscle resection or tacking”: It is the procedure of choice for patients with reasonable levator function. The amount of resection/tacking can be small (10–13 mm), medium (14–20 mm) or large (21–26 mm) and can be tailored to be smaller or larger depending on the levator function. The procedure can be carried out via anterior or posterior approach [51].
Brow/Frontalis suspension procedure (Severe ptosis >4 mm; levator function < 5 mm; congenital ptosis)
Various brow/frontalis suspension techniques either with autogenous or banked fascia lata or alloplastic material (mersilene, mash, silicone rod) are available [51], [52], [53]. Although many autoplastic and alloplastic material have been proposed it is generally agreed that fascia lata gives the best results. There is little donor site complication but the material is well tolerated. Alloplastic material may be used only if autogenous fascia is not available or when fascia lata is not necessary, like in temporary lid elevation.
“Endoscopic brow lift”: The early 1990s saw the introduction of the endoscope for brow lift surgery. This technique often requires access incisions that are placed inconspicuously just posterior to the hairline to optimize traction on the scalp and forehead. Benefits of this procedure include less scarring, reduced risk of numbness, better patient acceptance and less edema. Nonetheless the endoscopic approach can be challenging particularly in patients with high hairline or sloped forehead [54].
6 Eyelid retraction
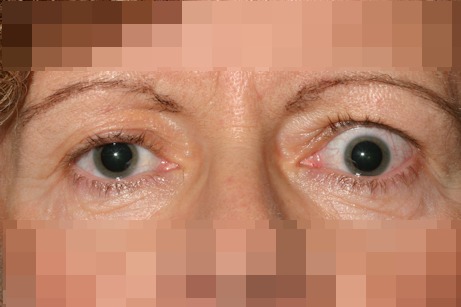
Eyelid retraction is a complex issue resulting from a wide range of pathology and with a large number of management options (Figure 6 (Fig. 6)). The most common cause of eyelid retraction is thyroid related orbitopathy but myopathic, neuropathic, inflammatory, mechanical and traumatic aetiologies also have to be considered.
Figure 6. Left eye upper lid retraction in patient with Graves’ orbitopathy.
6.1 Upper lid retraction
Procedures to correct upper lid retraction
Surgical correction in these patients can be difficult because of the combined inflammatory and cicatricial nature of the condition; it is important to wait that the patient’s thyroid disease and eyelid level have achieved stability. Retraction due to vertical inadequacy of the anterior lamella can be surgically addressed with the techniques described for cicatricial ectropion [55] paragraph 2.3 in other cases other surgical procedures can be uses such as “Lid loading” described in paragraph 2.2.
“Müller-ectomy and levator recession”, performed either through a transcutaneous or transonjunctival approach requires patient co-operation to asses the right eyelid height in the primary, up and down gaze. If after Müller-ectomy the lid is still retracted, overaction of the levator muscle is implicated; overcorrection can be corrected during surgery reattaching the recessed tissue to the tarsus or the skin [56], [57]. Transconjunctival mullerectomy with mechanical disinsertion of the levator muscle of the upper eyelid lowers it on average by 5.0 mm, avoids external scars formation and leaves the external lid structures intact [58]. Potential complications of the procedure include dry eye, over or undercorrection and epithelial implantation cysts [59], [60].
“Full-thickness transcutaneous blepharotomy” consists in a graded full-thickness transcutaneous blepharotomy, which, through a skin crease incision, extends medially and laterally along the superior tarsal border in a graded fashion based on the eyelid height and shape. Of all tissue layers at the end of the procedure only the skin is closed with 6.0 non-absorbable continuous sutures [61]. Henderson in 1965 [62] already described a transconjunctival partial-thickness blepharotomy consisting of an incision of upper lid conjunctiva and retractors combined with a Müller muscle myotomy for mild to moderate retraction and an additional levator aponeurotomy for severe cases.
“Z-myotomy” allows lengthening of the levator aponeurosis using partial width myotomies of the levator aponeurosis. The best candidates for this procedure are patients with severe, long standing lid retraction, persisting after any thyroid abnormalities have been treated [63].
“Interpositional spacers” are usually needed when the eyelid is retracted for more than 3 mm (considering the distance between the eyelid contour and the limbus). Eye bank sclera, either fresh or preserved, fascia lata, tarsus, nasal septal or ear cartilage are all used as spacer material [64], [65], [66], [67], [68], [69]. The main problem concerning the use of non autologous material is the possible presence of pathogenic viruses while autologous material will not be rejected, but needs a second surgical incision for harvesting; it usually does not contract as much as cadaver tissue so it needs to be only 1.5 times the vertically dimension of the intended amount of lid while cadaver tissue, on the contrary, needs to be 2 to 2.5 times the vertical height. In case of upper lid retraction the tissues often preferred as spacers are:
“Sclera” obtained from eye-bank eyes, can be sutured to the tarsal plate and the levator muscle. In the upper lid the required vertical height of the implant is approximately twice the amount of the eyelid retraction [64].
“Tarsus”: The amount of tissue needed should usually be 1–2 mm larger than the defect, and it is sutured into place with running 6.0 nylon suture tied on the anterior surface of the lid [67].
“Nasal cartilage” can’t be harvested without the risk of major complications such as nasal septal perforation and/or nasal bleeding. Such grafts can be used to obtain upper lid repositioning [68], [69].
6.2 Lower lid retraction
It is present when in primary position the sclera is exposed beneath the inferior limbus.
Procedures to correct lower lid retraction
Often it is possible to correct lower lid retraction just by recessing the eyelid retractors or removing scar tissue. Any associated horizontal eyelid laxity should be corrected at the same time [70].
“Medial tarsal suspension” (MTS) allows the suspension of the medial lower eyelid to the superior orbital rim periosteum applying a lifting force to the medial end of the eyelid.
The suspension suture is tied to elevate the medial end of the eyelid to the height desired. It can be combined with a medial canthoplasty as described above. The procedure moves the medial canthal angle medially approximately 3 mm creating some asymmetry when performed unilaterally [71].
“Interpositional spacers”: in the lower lid passive drag due to gravity in addition to active retraction is a factor that needs to be addressed. Graft tissues of choice are:
“Ear cartilage” harvested from the scalpoid fossa between the helix and the antihelix, must be thinned and trimmed so as to fit the defect established when the posterior lamella structures retract from the tarsal plate. Ear cartilage grafts have advantages compared to sclera in that it is not preserved or irradiated and, more important it shows less evidence of shrinkage or resorption; this allows for a one-to-one calculation in estimating the appropriate size of graft material to implant. Complications include infection, dislodgement and excessive bulkiness due to insufficient thinning of the cartilage graft [65], [66], [68].
“Polyethylene” (pPE) already used in the form of implants for orbital volume enhancement, has become available as specifically formed spacer for lower eyelid retraction. Following a subciliary incision with recession of the lower lid retractors a preformed lid implant can at its top end be placed anterior to the tarsus and with its inferior edge rest on the orbital rim. The spacer is well biointegrated thanks to the porosity of the material [72]. It is thinner than hand sculpted cartilage grafts and maintains excellent structural support.
The implant normally shows a good performance in keeping the lower eyelid elevated but overcorrection, evident especially on downgaze, may need to be addressed by reduction of the implant at a later stage [72]. The same implant has also been suggested for the correction of paralytic ectropion [73]. In 2006 Garibaldi et al described a novel drug release pPE eyelid implant, which demonstrated antiangiogenic and ant inflammatory properties which could prove beneficial in the treatment of lower lid retraction [74].
7 Lacrimal/canalicular surgery
7.1 Tearing patient
The tearing patient is a challenge to the ophthalmologist. Hypersecretion, lacrimal pump dysfunction and lacrimal outflow obstruction are all causes of tearing. With the advent of dacryocystorhinostomy (DCR) tearing secondary to nasolacrimal duct obstruction is a treatable condition with a high success rate of resolution [75].
Procedures to correct tearing eye
DCR is a surgical method that allows the direct drainage of tears from the lacrimal sac into the nasal cavity, bypassing the blocked nasolacrimal duct (NLD).
“External DCR”: originally described by Toti in 1904 consisted of resecting the lacrimal sac mucosa, bone, and nasal mucosa through an external skin incision [76]; Dupuy-Dutemp and Bourguet introduced the concept of the nasal and lacrimal mucosal flaps, and modified this technique [77]. This procedure has largely remained unchanged and is still considered the gold standard in the treatment of acquired NLD obstruction with a success rate greater than 90%. Nevertheless cutaneous incision and disruption of the medial canthal ligament with resultant lacrimal pump dysfunction have been cited as significant disadvantages [78], [79].
The “endonasal approach” was first proposed by Caldwell in 1893 [80], who used an electric burr to create a middle meatal osteotomy in the area marked by a metal probe which was passed trough the NLD to identify the area of blockage. West modified the technique in 1914 introducing the idea of a window osteotomy by removal of the lacrimal bone and the superior maxilla to access the nasolacrimal duct [81]. The endonasal technique remained limited in use until the introduction of operating microscopes, rigid and semirigid nasal endoscopes and fiberoptic delivery systems that helped physicians to evaluate the intranasal anatomy. Endonasal DCR can be carried out in various ways, either with or without the help of an endoscope; and with the use of different equipment such as rongeur, drill, chisel and various types of laser. The first clinical study on endoscopic DCR was published 1989 [82] and Massaro et al introduced in 1990 endonasal laser-assisted DCR [78]. Medical lasers have been utilized in clinical practice for decades and have started to play an important role also in ophthalmology. The introduction of fiber-transmissible wavelength, improvements in endoscopic instrumentation and evolving laser technologies have renewed interest in laser assisted endonasal application. Laser in endonasal surgery involves instant vaporisation/ablation of tissue and delayed tissue loss by coagulative effect. As such there is a relative bloodless field that is useful in endoscopic surgery in which the operative space is often limited. Additionally advantages in technology have allowed for slimmer delivery systems that allow for focused application and enhance manoeuvrability. Although laser assisted endonasal DCR techniques initially utilized the laser from the nasal cavity directed towards the lateral nasal wall (Endo-laser DCR) improvements in fiberoptics technologies have allowed for delivery of laser energy via the canaliculus, allowing for laser energy to be directed away from the globe and orbital content and towards the nasal cavity under endoscopic guidance (endocanalicular laser DCR). Different types of lasers have been used in laser assisted endo-nasal DCR including carbon dioxide (CO2); holmium: yttrium aluminium garnet (Ho:YAG); Neodymium: yttrium aluminium garnet (Nd:YAG); Erbium: yttrium aluminium garnet (Erb:YAG); potassium-titanyl phosphate (KTP) and diode [83], [84], [85]. Diode laser being one of the most portable and least expensive of the laser available is the most commonly used for endocanalicular DCR.
Several reports have compared external and endonasal DCR [86]. Many show that external DCR has higher primary success rate, but the preparation of a big rhinostomy also in endo nasal DCR and the preservation of the lacrimal sac and of the nasal mucosa to create mucosal flap leads to similar success rate compared to those obtained with the external approach [87]. Moreover the endonasal approach avoids cutaneous incision, disruption of the medial canthal tendon and of the lacrimal pump function, allows direct access and visualization of the osteotomy site, and shows significant shorter operative times compared with external DCR. The procedure can be performed in local anaesthesia expanding the population eligible for this procedure [75]; on the other hand disadvantages include a steep learning curve and high equipment and instrumentation costs. Reported advantages of external DCR include the unimpaired view of surgical area and the possibility to obtain a lacrimal sac biopsy, as it has been favoured for patients with lacrimal sac neoplasm, evidence of posttraumatic bony deformity of the lacrimal sac or canalicular pathology [88].
The “endocanalicular approach”: Canalicurar obstruction may be congenital or acquired and anatomically divided into proximal obstruction, obstruction of the mid canaliculus and distal obstruction. Successful treatment depends on the site and degree of obstruction. The punctoplasty allows controlled opening of the punctum: retrograde intubation dacryocystorhinostomy is used for proximal and midcanalicular obstructions [89]. In case of distal canalicular obstruction different procedures have been proposed. In 1990 Sisler and Allarakhia [90] first described a transcanalicular trephine, a hollow stainless steel tube that has a cutting edge at one end and a plastic grip at the other. The instrument is inserted via the lacrimal punctum and when the blockage is encountered trephination is performed. More recently Haefliger and Piffaretti described a similar lacrimal trephine with a hollow lumen through which they use a microendoscope during trephination [91]. Antegrade balloon canaliculoplasty is a relatively new technique and can be safely applied for conservative management of partial canalicular and common canalicular stenosis. A guidewire is passed through the lacrimal system via the punctum and the balloon catheter is passed over it until the inflatable section straddles the stenosis [92]. Canaliculodacryocystorhinostomy is indicated in case of distal upper, lower or common canalicular obstruction, that is when 8 mm of the lateral upper or lower canaliculi or both are patent. In this procedure an anastomosis is made between the patent medial ends of the canaliculi or the common canaliculus and the nasal mucosa after excision of the obstructed part. The lacrimal sac mucosa is used to make a posterior flap and the nasal mucosa a very large anterior flap [93].
7.2 Dry eye
Most dry eye patients are aqueous deficient and are treated with topical tear supplements and lubricants, although mucolytics, soft contact lenses, punctal occlusion and retinoic acid may also be used in selected patients. The use of topical tear supplements alone may fail to relieve signs and symptoms in patients not able or willing to instil drops as frequently as needed, or with physical impairment or occupational restriction. Different solutions can be proposed to patients affected by dry eye.
Procedures to correct dry eye
“Punctal occlusion” is an essential part of the management of severe cases of dry eye resulting from an aqueous deficiency. The idea behind punctal occlusion is to increase the aqueous component of the tear film by blocking tear outflow; it helps to retain artificial tears and/or the patient’s own tears on the ocular surface. It can be temporary or permanent. The procedure is not indicated in patients who have normal Schirmer tests or no corneal signs of dry eye [94] and should not be considered if dryness is a temporary condition. Punctal occlusion provides improvement of the tear film stability, ocular surface staining scores, conjunctival squamous metaplasia and globet cell density as well as the impaired functional visual acuity for dry eye patients [95], [96].
Several materials and design have been used for canalicular tamponade: absorbable implants (gelatine, collagen, catgut) versus non-absorbable plugs (polyethylene, N-butyl cyanoacrilate) [97].
“Thermal cautery” is performed with the intention of permanently occluding the punctum, is not uniformly successful, with inadvertent recanalization unpredictably occurring at various intervals after treatment. Two methods have been described, deep and superficial thermal cauterization. The first involves cauterisation with the tip of the needle inserted within the vertical and sometime even into the horizontal portion of the canaliculus, the superficial approach on the contrary involve cauterisation of the edge of the punctum without insertion into the vertical portion of the canaliculus. Comparison of the overall proportions of puncta remaining closed after deep or superficial cauterization show that the first results in at least a twofold greater success rate throughout the follow-up period of over one year after cauterization [98]. Glatt in 1991 and Jeffrey et al in 1992 reported acute bilateral dacryocystitis following punctal thermal cautery occlusion probably due to a pre-existing nasolacrimal obstruction. The authors suggest evaluating the patency of the lacrimal system before performing permanent occlusion of both puncta in any eye [99], [100].
“Canalicular excision”: Putterman described this technique in 1991. The conjunctiva and posterior wall of the canaliculus are incised from the punctum up to the medial canthus, and punctum and canaliculus are then removed up to their entrance into the lacrimal sac. The extirpation of a piece of canaliculus is 100% effective but carries the risk of inversion of the lid margin and eyelashes of the canalicular portion of the eye [101].
“Punctal tarsorrhaphy” was first introduced by Murube del Castillo in 1995; it consists of a medial tarsorrhaphy on the site of the lacrimal puncta. Two rectangular surfaces, 3 mm by 2 mm, which includes the punctum in their lateral half, are excised from the lower and the upper lid margin; the raw surfaces are sutured together and a mattress suture is placed for two weeks in the central third of the lid, in order to prevent blinking. This procedure not only closes the puncta but also reduces the surface of the palpebral fissure diminishing evaporation; the operation is appropriate in severe cases of dry eye, the aesthetic result is acceptable and the operation is easily reversible [102].
“Permanent punctal occlusion” involves removal of the epithelium, from the punctum and the vertical portion of the canaliculus up to a depth of 2 mm, with a corneal rust ring burr. The raw surfaces of the vertical portion of the canaliculus are then brought together and sutured. The procedure provides permanent punctal occlusion; once the punctum is occluded it is difficult to reverse the anatomical and functional blockage thus candidates must be carefully selected [103].
8 Orbita surgery
8.1 Orbital decompression
Graves’ thyroid disease is a relatively common disorder in endocrinology and general internal medicine. Up to 60% of patients with Graves’ hyperthyroidism develop some form of Graves’ orbitopathy that is a sight-threatening chronic autoimmune disorder characterized by an inflammation of retrobulbar tissues leading to accumulation of hydrophilic glycosaminoglycans and/or an increase in orbital adipose tissue. Signs include a wide-open eye appearance, caused by upper eyelid retraction and exophthalmos. Symptoms include photofobia, sandy feeling in the eye, painful eye movement and diplopia; visual acuity may be reduced [104]. Surgery is usually performed either once inflammatory signs are waned to correct residual diplopia, exophthalmos or lid retraction, or in patients with active disease who present with refractory or progressing optic neuropathy or corneal ulcer. Indications for orbital decompression include a stretched optic nerve, prevention of further corneal damage, alleviating complains of tearing and grittiness, but also cosmetic complaints. During orbita decompression a part of the bony walls is removed in order to provide more space for the extraocular muscle and orbital fat. Diplopia correction usually follows orbital decompression of several months and eyelis surgery may be a final step in rehabilitation of a patient with Graves’ orbitopathy.
Procedures to correct exophthalmos
Ideally the planning of decompression surgery should be adequate to the severity of the orbitopathy, patient specific osteology and possible previous surgery. An inferior fornix incision can be used for infero medial bony decompression and/or for removing fat from the medial and lateral inferior orbital quadrants: an upper skin crease incision instead offers a wider access to the lateral orbital wall [105]. The swinning eyelid technique offers an adequate access to the bony orbit and to the lateral fat compartments is a versatile technique that can virtually be used as a standard approach for the greatest majority of patients needing decompression surgery [106]. Orbita decompression by coronal incision is an invasive technique and is for this not to be used as a standard approach to orbita decompression, nevertheless it is not to be abandoned as it can be additional tool in surgeon’s hand when dealing with patents who can better benefit out of a particular tailored rather than standardised approach [107]. With the development of endoscopic surgery the trans nasal removal of the lamina papyracea has resulted in proptosis reduction comparable to the results of other approaches allowing excellent view for safe removal of the medial and inferior orbital wall especially in the region of the ethmoidal ceiling and orbital apex without the need of external incision and with less morbidity, it also reduces the incidence of hypoesthesia secondary to infra-orbitary nerve lesion [108].
8.2. Anophthalmic socket
The aim of socket surgery is to minimize disfigurement either related to a volume deficit following enucleation or evisceration or to a lining defect in contracted sockets in patients with anophthalmia. The volume deficit in the socket should be corrected by positioning a sizeable orbital implant at the time of surgery and subsequently by fitting the patient with an adequate prosthesis [109], [110]. The absence of the implant (or a small implant) can be responsible for post enucleation socket syndrome (PESS), whose features include enophthalmos, deep upper lid sulcus, ptosis or lid retraction, lower lid laxity and shallowing of the lower fornix [110], [111].
Procedures to correct an anophthalmic socket
“Orbital implants”: The ideal implant should adequately replace the orbital volume, support and transmit a satisfactory motility to the prothesis without requiring further surgery or pegging, show a low complication rate, be biointegrable, not expensive and easy to implant during surgery. Many different materials have been used so far including hemispheric implants, spherically alloplastic nonporous implant and more recently porous implants [112].
In the late 80s the introduction of porous orbital implant, such as coralline hydroxyapatite, syntetic hydroxyapatite, porous polyethylene, aluminium oxide, modified the surgical approach towards the rehabilitation of anophthalmic socket [112], [113]. The commonly used material for porous biointegrable implants includes natural coralline hydroxyapatite, syntetic hydroxyapatite, porous polyethylene and aluminium oxide. Hydroxyapatite implants show a good biocompatibility, although their rough surface may contribute to late exposure because of the abrasion of Tenon’s capsule and conjunctiva during the movement of the implant [112]. Wrapping allows precise attachment of the extraocular muscle to the implant and helps positioning of the implant at the time of surgery. Polyglactin mesh is frequently used followed by donor sclera, porcine collagen, facia lata, and bovine pericardium [114]. Porous polyethylene orbital implant do not require wrapping as the muscles can be sutured directly to the implant [115]; Aluminium oxide has good biocompatibility, is bio inert and strong and induces less inflammation when in contact with tissues [116].
Complications associated with orbital implant include discharge, conjunctival dehiscence, infection, pyogenic granuloma formation and exposure. The incidence of exposure may be related to the type of surgery, the material of the implant, the size of the implant, the implant total surface area, the tissue ingrowth and the wrapping material [117], [118], [119].
“Filler” Injectable calcium hydroxylapatite has been suggested as a well-tolerated, simple and cost effective technique to treat volume deficiency in the anophtalmic orbit [120]. Augmentation is achieved injecting in the extraconal space this semi permanent filler (Radiesse) that consists of 30% calcium hydroxylapatite microspheres in a carring vehicle; it has demonstrated a lasting effect in the orbit of approximately 1 year with little volume loss. The filler seems to last longer in areas with less movement, blood supply and lymphatic drainage. Injection can even be performed in an office setting under local anaesthesia and the procedure is repeated until adequate volume is obtained [121].
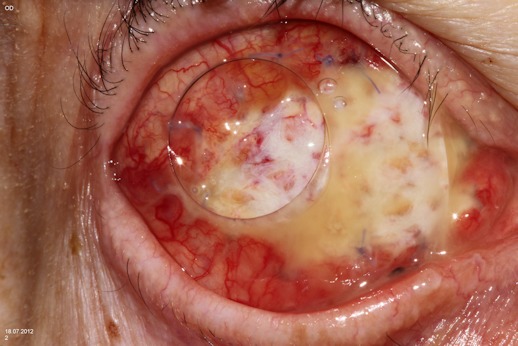
“Autologous dermis fat”: In many cases unfortunately even with an implant placement there is a tendency toward enophthalmos, for this reason many surgeons try to place the largest possible implant. The size of the implant that can be placed is mainly limited by the ability to close Tenon’s capsule and the conjunctiva over the implant. Tension placed on these soft tissues may contribute to reduction of the conjunctival fornix and implant extrusion. These problems may be addressed by using autologous dermis to replace the ocular surface area lost with removal of the globe and excision of the corneoscleral cap. When an eye, in fact, is enucleated or eviscerated, an area of ocular surface equal to or greater then the corneoscleral cap is removed and this reduces the amount of potential surface area to for closure. The loss of ocular surface area is often exacerbated by previous ocular surgery with associated conjunctival shrinkage and scarring, this prevent the positioning of an adequate size implant or forces the surgeon to place the sphere posterior to the normal position of the globe with deleterious effects on the prosthesis motility. A dermis fat implant provides extra surface area preserving the fornixes and avoiding socket contraction [122] (Figure 7 (Fig. 7)).
Figure 7. Primary autologous dermis fat graft after enucleation due to painful glaucomatous eye. The graft is not yet fully epithelialized.
“Eyelid malposition correction in patients with anophthalmia”: Patients with anophthalmia with ptosis or lower lid malposition may pose a real challenge for the plastic surgeon. When the eyelid malposition is related to a volume deficit, it should be corrected with a secondary implant (if no implant is present) or with an orbital floor implant in case of small intraconal implant before correcting the eyelid malposition. Lower lid malposition is usually corrected, prior to ptosis, with a lateral tarsal strip or fascial sling depending on the amount of lid laxity and involvement of the medial and lateral tendon.
“Contracted socket”: A severe contraction may occur following trauma, recurrent inflammation due to inadequate prosthesis or radiotherapy [123]. It makes the patient unable to maintain the prosthesis that becomes a source of irritation, discharge and pain. If contraction is mild it is possible to correct the upper lid entropion with an anterior lamella repositioning and the lower lid entropion with standard surgical procedures. In case of severe contraction it is possible to increase socket lining with mucous membrane graft (if the socket is moisture) or skin graft (if the socket is dry). In all cases the surgery should provide orbital and lid expansion. Auricular cartilage grafting has been proposed to correct lower conjunctival fornix retraction and shortening of the posterior lamella [124].
“Congenital anophthalmia”: Anophthalmia can be a result of primary genetic defect or due to external factors such as infections or drugs that may influence the morphogenic pathway that control eye development [125]. The treatment of congenital anophthalmia is mainly directed towards improving cosmesis through stimulation of both soft tissues and bony orbital growth [126]. Rehabilitation involves progressively enlarging static acrylic conformers to expand the socket, followed by placement of spherical orbital implants, dermis fat graft, or use of either socket or orbital expanders or both. Unfortunately all these methods can still result in poor cosmetic outcome, with retardation of both soft tissue and bony orbital growth adversely affecting mid facial symmetry [127].
9 Conclusion
The ocular adnexae plays an important role in maintaining a healthy ocular surface. Eyelid malposition, fornix shortening and severe aqueous deficiency result in severe, progressive surface disease. It is important to address adnexal problems investigating the cause of the defect and correcting it with the adequate surgical procedure aiming to re-establish the anatomical conditions necessary for a healthy ocular surface.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Anderson RL. Tarsal strip procedure for correction of eyelid laxity and canthal malposition in the anophthalmic socket. Ophthalmology. 1981 Sep;88(9):895–903. doi: 10.1016/s0161-6420(81)80003-6. [DOI] [PubMed] [Google Scholar]
- 2.Lemke BN, Sires BS, Dortzbach RK. A tarsal strip-periosteal flap technique for lateral canthal fixation. Ophthalmic Surg Lasers. 1999 Mar;30(3):232–236. [PubMed] [Google Scholar]
- 3.Korn EL, Glotzbach RK. Carbon dioxide laser repair of medial ectropion. Ophthalmic Surg. 1988 Sep;19(9):653–657. [PubMed] [Google Scholar]
- 4.Anderson RL, Hatt MU, Dixon R. Medial ectropion. A new technique. Arch Ophthalmol. 1979 Mar;97(3):521–524. doi: 10.1001/archopht.1979.01020010265017. Available from: http://dx.doi.org/10.1001/archopht.1979.01020010265017. [DOI] [PubMed] [Google Scholar]
- 5.Schrom T, Goldhahn A, Neumann K, Berghaus A. Risiken der Oberlidgoldimplantation bei peripherer Fazialisparese. [Risks of upper eyelid gold implantation in peripheral facial paralysis]. HNO. 1999 Apr;47(4):262–268. doi: 10.1007/s001060050393. (Ger). Available from: http://dx.doi.org/10.1007/s001060050393. [DOI] [PubMed] [Google Scholar]
- 6.Goldhahn A, Schrom T, Berghaus A, Krause A, Duncker G. Goldimplantation bei Lagophthalmus. Hornhautastigmatismus als besondere Komplikation. [Corneal astigmatism as a special complication after lid-loading in patients with lagophthalmos]. Ophthalmologe. 1999 Aug;96(8):494–497. doi: 10.1007/s003470050443. (Ger). Available from: http://dx.doi.org/10.1007/s003470050443. [DOI] [PubMed] [Google Scholar]
- 7.Tremolada C, Raffaini M, D'Orto O, Gianni AB, Biglioli F, Carota F. Temporal galeal fascia cover of custom-made gold lid weights for correction of paralytic lagophthalmos: long-term evaluation of an improved technique. J Craniomaxillofac Surg. 2001 Dec;29(6):355–359. doi: 10.1054/jcms.2001.0250. Available from: http://dx.doi.org/10.1054/jcms.2001.0250. [DOI] [PubMed] [Google Scholar]
- 8.Schrom T, Goldhahn A, Berghaus A, Duncker GI. Die Oberlidretraktion bei endokriner Orbitopathie. Eine neue Indikation für das „Lidloading“. [Lid retraction in endocrine orbitopathy. A new indication for “lid loading”]. HNO. 2000 Jan;48(1):41–44. doi: 10.1007/s001060050007. (Ger). Available from: http://dx.doi.org/10.1007/s001060050007. [DOI] [PubMed] [Google Scholar]
- 9.Berghaus A, Neumann K, Schrom T. The platinum chain: a new upper-lid implant for facial palsy. Arch Facial Plast Surg. 2003 Mar-Apr;5(2):166–170. doi: 10.1001/archfaci.5.2.166. Available from: http://dx.doi.org/10.1001/archfaci.5.2.166. [DOI] [PubMed] [Google Scholar]
- 10.Shore JW. Changes in lower eyelid resting position, movement, and tone with age. Am J Ophthalmol. 1985 Apr 15;99(4):415–423. doi: 10.1016/0002-9394(85)90008-x. [DOI] [PubMed] [Google Scholar]
- 11.Meadows AE, Reck AC, Gaston H, Tyers AG. Everting sutures in involutional entropion. Orbit. 1999 Sep;18(3):177–181. doi: 10.1076/orbi.18.3.177.2706. Available from: http://dx.doi.org/10.1076/orbi.18.3.177.2706. [DOI] [PubMed] [Google Scholar]
- 12.Wright M, Bell D, Scott C, Leatherbarrow B. Everting suture correction of lower lid involutional entropion. Br J Ophthalmol. 1999 Sep;83(9):1060–1063. doi: 10.1136/bjo.83.9.1060. Available from: http://dx.doi.org/10.1136/bjo.83.9.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rougraff PM, Tse DT, Johnson TE, Feuer W. Involutional entropion repair with fornix sutures and lateral tarsal strip procedure. Ophthal Plast Reconstr Surg. 2001 Jul;17(4):281–287. doi: 10.1097/00002341-200107000-00008. Available from: http://dx.doi.org/10.1097/00002341-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Wies FA. Surgical treatment of entropion. J Int Coll Surg. 1954 Jun;21(6:1):758–760. [PubMed] [Google Scholar]
- 15.Collin JR, Rathbun JE. Involutional entropion. A review with evaluation of a procedure. Arch Ophthalmol. 1978 Jun;96(6):1058–1064. doi: 10.1001/archopht.1978.03910050578018. Available from: http://dx.doi.org/10.1001/archopht.1978.03910050578018. [DOI] [PubMed] [Google Scholar]
- 16.Danks JJ, Rose GE. Involutional lower lid entropion: to shorten or not to shorten? Ophthalmology. 1998 Nov;105(11):2065–2067. doi: 10.1016/S0161-6420(98)91126-5. Available from: http://dx.doi.org/10.1016/S0161-6420(98)91126-5. [DOI] [PubMed] [Google Scholar]
- 17.Hoh HB, Harrad RA. Factors affecting the success rate of the Quickert and Wies procedures for lower lid entropion. Orbit. 1998 Sep;17(3):169–172. doi: 10.1076/orbi.17.3.169.2749. Available from: http://dx.doi.org/10.1076/orbi.17.3.169.2749. [DOI] [PubMed] [Google Scholar]
- 18.Jones LT, Reeh MJ, Tsujimura JK. Senile entropion. Am J Ophthalmol. 1963 Mar;55:463–469. [PubMed] [Google Scholar]
- 19.Boboridis K, Bunce C, Rose GE. A comparative study of two procedures for repair of involutional lower lid entropion. Ophthalmology. 2000 May;107(5):959–961. doi: 10.1016/S0161-6420(00)00027-0. Available from: http://dx.doi.org/10.1016/S0161-6420(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 20.Altieri M, Iester M, Harman F, Bertagno R, Capris P, Venzano D, Baldi F, Altieri G. Comparison of three techniques for repair of involutional lower lid entropion: a three-year follow-up study. Ophthalmologica. 2003 Jul-Aug;217(4):265–272. doi: 10.1159/000070633. Available from: http://dx.doi.org/10.1159/000070633. [DOI] [PubMed] [Google Scholar]
- 21.Karesh JW, Putterman AM. Reconstruction of the partially contracted ocular socket or fornix. Arch Ophthalmol. 1988 Apr;106(4):552–556. doi: 10.1001/archopht.1988.01060130598046. Available from: http://dx.doi.org/10.1001/archopht.1988.01060130598046. [DOI] [PubMed] [Google Scholar]
- 22.Kersten RC, Kleiner FP, Kulwin DR. Tarsotomy for the treatment of cicatricial entropion with trichiasis. Arch Ophthalmol. 1992 May;110(5):714–717. doi: 10.1001/archopht.1992.01080170136042. Available from: http://dx.doi.org/10.1001/archopht.1992.01080170136042. [DOI] [PubMed] [Google Scholar]
- 23.Sodhi PK, Yadava U, Mehta DK. Efficacy of lamellar division for correcting cicatricial lid entropion and its associated features unrectified by the tarsal fracture technique. Orbit. 2002 Mar;21(1):9–17. doi: 10.1076/orbi.21.1.9.2600. Available from: http://dx.doi.org/10.1076/orbi.21.1.9.2600. [DOI] [PubMed] [Google Scholar]
- 24.Wenkel H, Rummelt V, Naumann GO. Long term results after autologous nasal mucosal transplantation in severe mucus deficiency syndromes. Br J Ophthalmol. 2000;84:279–284. doi: 10.1136/bjo.84.3.279. Available from: http://dx.doi.org/10.1136/bjo.84.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naumann GO, Lang GK, Rummelt V, Wigand ME. Autologous nasal mucosa transplantation in severe bilateral conjunctival mucus deficiency syndrome. Ophthalmology. 1990 Aug;97(8):1011–1017. doi: 10.1016/s0161-6420(90)32471-5. [DOI] [PubMed] [Google Scholar]
- 26.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997 Dec;104(12):2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 27.Mejía LF, Acosta C, Santamaría JP. Use of nonpreserved human amniotic membrane for the reconstruction of the ocular surface. Cornea. 2000 May;19(3):288–291. doi: 10.1097/00003226-200005000-00006. Available from: http://dx.doi.org/10.1097/00003226-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Nakamura T, Shimizu Y, Kinoshita S. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004 Jan;45(1):93–99. doi: 10.1167/iovs.03-0752. Available from: http://dx.doi.org/10.1167/iovs.03-0752. [DOI] [PubMed] [Google Scholar]
- 29.Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J, Völcker HE. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol. 2000 Jan;238(1):68–75. doi: 10.1007/s004170050012. Available from: http://dx.doi.org/10.1007/s004170050012. [DOI] [PubMed] [Google Scholar]
- 30.Barabino S, Rolando M. Amniotic membrane transplantation elicits goblet cell repopulation after conjunctival reconstruction in a case of severe ocular cicatricial pemphigoid. Acta Ophthalmol Scand. 2003 Feb;81(1):68–71. doi: 10.1034/j.1600-0420.2003.00019.x. Available from: http://dx.doi.org/10.1034/j.1600-0420.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 31.Honavar SG, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology. 2000 May;107(5):975–979. doi: 10.1016/S0161-6420(00)00026-9. Available from: http://dx.doi.org/10.1016/S0161-6420(00)00026-9. [DOI] [PubMed] [Google Scholar]
- 32.Solomon A, Espana EM, Tseng SC. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology. 2003 Jan;110(1):93–100. doi: 10.1016/S0161-6420(02)01441-0. Available from: http://dx.doi.org/10.1016/S0161-6420(02)01441-0. [DOI] [PubMed] [Google Scholar]
- 33.Barabino S, Rolando M, Bentivoglio G, Mingari C, Zanardi S, Bellomo R, Calabria G. Role of amniotic membrane transplantation for conjunctival reconstruction in ocular-cicatricial pemphigoid. Ophthalmology. 2003 Mar;110(3):474–480. doi: 10.1016/S0161-6420(02)01892-4. Available from: http://dx.doi.org/10.1016/S0161-6420(02)01892-4. [DOI] [PubMed] [Google Scholar]
- 34.Kheirkhah A, Blanco G, Casas V, Hayashida Y, Raju VK, Tseng SC. Surgical strategies for fornix reconstruction based on symblepharon severity. Am J Ophthalmol. 2008 Aug;146(2):266–275. doi: 10.1016/j.ajo.2008.03.028. Available from: http://dx.doi.org/10.1016/j.ajo.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 35.John T, Foulks GN, John ME, Cheng K, Hu D. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology. 2002 Feb;109(2):351–360. doi: 10.1016/s0161-6420(01)00900-9. [DOI] [PubMed] [Google Scholar]
- 36.Bartley GB, Bullock JD, Olsen TG, Lutz PD. An experimental study to compare methods of eyelash ablation. Ophthalmology. 1987 Oct;94(10):1286–1289. doi: 10.1016/s0161-6420(87)80013-1. [DOI] [PubMed] [Google Scholar]
- 37.Bartley GB, Lowry JC. Argon laser treatment of trichiasis. Am J Ophthalmol. 1992 Jan 15;113(1):71–74. doi: 10.1016/s0002-9394(14)75756-3. [DOI] [PubMed] [Google Scholar]
- 38.Wilcsek GA, Francis IC. Argon laser and trichiasis. Br J Ophthalmol. 2003 Mar;87(3):375. doi: 10.1136/bjo.87.3.375. Available from: http://dx.doi.org/10.1136/bjo.87.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahni J, Clark D. Argon laser and trichiasis: a helpful tip. Br J Ophthalmol. 2001 Jun;85(6):762. doi: 10.1136/bjo.85.6.761b. Available from: http://dx.doi.org/10.1136/bjo.85.6.761b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanumanthu S, Webb LA, Lee WR, Williamson J. Histological and morphometric analysis of the effects of argon laser epilation. Br J Ophthalmol. 2003 Aug;87(8):984–987. doi: 10.1136/bjo.87.8.984. Available from: http://dx.doi.org/10.1136/bjo.87.8.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frueh BR. Treatment of distichiasis with cryotherapy. Ophthalmic Surg. 1981 Feb;12(2):100–103. [PubMed] [Google Scholar]
- 42.Wood JR, Anderson RL. Complications of cryosurgery. Arch Ophthalmol. 1981 Mar;99(3):460–463. doi: 10.1001/archopht.1981.03930010462014. Available from: http://dx.doi.org/10.1001/archopht.1981.03930010462014. [DOI] [PubMed] [Google Scholar]
- 43.Anderson RL, Harvey JT. Lid splitting and posterior lamella cryosurgery for congenital and acquired distichiasis. Arch Ophthalmol. 1981 Apr;99(4):631–634. doi: 10.1001/archopht.1981.03930010631008. Available from: http://dx.doi.org/10.1001/archopht.1981.03930010631008. [DOI] [PubMed] [Google Scholar]
- 44.Beard C. A new classification of blepharoptosis. Int Ophthalmol Clin. 1989 Winter;29(4):214–216. doi: 10.1097/00004397-198902940-00002. Available from: http://dx.doi.org/10.1097/00004397-198902940-00002. [DOI] [PubMed] [Google Scholar]
- 45.Horch RE, Arkudas A. Oberlid- und Unterlidblepharoplastik: Entwicklungen der ästhetisch-plastischen periokulären Chirurgie. [Upper and lower eyelid blepharoplasty: development of aesthetic periocular plastic surgery]. Chirurg. 2011 Sep;82(9):775–781. doi: 10.1007/s00104-011-2150-4. (Ger). Available from: http://dx.doi.org/10.1007/s00104-011-2150-4. [DOI] [PubMed] [Google Scholar]
- 46.Oesstreicher J, Mehta S. Complication of blepharoplasty: prevention and management. Plast Surg Int. 2012:252–368. doi: 10.1155/2012/252368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003 May-Jun;27(3):193–204. doi: 10.1007/s00266-003-0127-5. Available from: http://dx.doi.org/10.1007/s00266-003-0127-5. [DOI] [PubMed] [Google Scholar]
- 48.Dresner SC. Ptosis management: A practical approach. In: Chen WP, editor. Oculoplasty Surgery: The essentials. New York: Thieme Medical Publisher; 2001. pp. 1–10. [Google Scholar]
- 49.Iliff JW, Pacheco EM. Ptosis surgery. In: Tasman W, Jaeger EA, editors. Duane's clinical ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 1–18. [Google Scholar]
- 50.Parsa FD, Wolff DR, Parsa NN, Elahi E. Upper eyelid ptosis repair after cataract extraction and the importance of Hering's test. Plast Reconstr Surg. 2001 Nov;108(6):1527–1536. doi: 10.1097/00006534-200111000-00014. Available from: http://dx.doi.org/10.1097/00006534-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Allen RC, Saylor MA, Nerad JA. The current state of ptosis repair: a comparison of internal and external approaches. Curr Opin Ophthalmol. 2011 Sep;22(5):394–399. doi: 10.1097/ICU.0b013e32834994a0. Available from: http://dx.doi.org/10.1097/ICU.0b013e32834994a0. [DOI] [PubMed] [Google Scholar]
- 52.Dailey RA, Wilson DJ, Wobig JL. Transconjunctival frontalis suspension (TCFS) Ophthal Plast Reconstr Surg. 1991;7(4):289–297. doi: 10.1097/00002341-199112000-00010. Available from: http://dx.doi.org/10.1097/00002341-199112000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Spoor TC, Kwitko GM. Blepharoptosis repair by fascia lata suspension with direct tarsal and frontalis fixation. Am J Ophthalmol. 1990 Mar 15;109(3):314–317. doi: 10.1016/s0002-9394(14)74557-x. [DOI] [PubMed] [Google Scholar]
- 54.Connell BF, Lambros VS, Neurohr GH. The forehead lift: techniques to avoid complications and produce optimal results. Aesthetic Plast Surg. 1989 Fall;13(4):217–237. doi: 10.1007/BF01570355. [DOI] [PubMed] [Google Scholar]
- 55.O'Donnell BA. Eyelid retractor surgery as an adjunct to cicatricial ectropion repair. Clin Experiment Ophthalmol. 2000 Aug;28(4):293–297. doi: 10.1046/j.1442-9071.2000.00319.x. [DOI] [PubMed] [Google Scholar]
- 56.Putterman AM, Urist M. Surgical treatment of upper eyelid retraction. Arch Ophthalmol. 1972 Apr;87(4):401–405. doi: 10.1001/archopht.1972.01000020403007. Available from: http://dx.doi.org/10.1001/archopht.1972.01000020403007. [DOI] [PubMed] [Google Scholar]
- 57.Putterman AM, Fett DR. Muller's muscle in the treatment of upper eyelid retraction: a 12-year study. Ophthalmic Surg. 1986 Jun;17(6):361–367. [PubMed] [Google Scholar]
- 58.Lindeman V, Lieb W, Kahaly G, Grehn F. Müller-Ektomie bei endokriner Orbitopathie. [The Muller-ectomy in endocrine orbitopathy]. Ophthalmologe. 1995 Jun;92(3):359–361. (Ger). [PubMed] [Google Scholar]
- 59.George JL, Tercero ME, Angioï-Duprez K, Maalouf T. Risk of dry eye after mullerectomy via the posterior conjunctival approach for thyroid-related upper eyelid retraction. Orbit. 2002 Mar;21(1):19–25. doi: 10.1076/orbi.21.1.19.2602. Available from: http://dx.doi.org/10.1076/orbi.21.1.19.2602. [DOI] [PubMed] [Google Scholar]
- 60.Wuebbolt GE, Zuercher M, O'Donnell B, Collin R. Epithelial implantation cysts of the upper eyelid after lid-lowering procedures. Ophthalmology. 1993 Sep;100(9):1289–1292. doi: 10.1016/s0161-6420(93)31486-7. [DOI] [PubMed] [Google Scholar]
- 61.Elner VM, Hassan AS, Frueh BR. Graded full-thickness anterior blepharotomy for upper eyelid retraction. Trans Am Ophthalmol Soc. 2003;101:67–73. [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson JW. Relief of Eyelid Retraction: A Surgical Procedure. Arch Ophthalmol. 1965 Aug;74:205–216. doi: 10.1001/archopht.1965.00970040207015. Available from: http://dx.doi.org/10.1001/archopht.1965.00970040207015. [DOI] [PubMed] [Google Scholar]
- 63.Grove AS., Jr Eyelid retraction treated by levator marginal myotomy. Ophthalmology. 1980 Oct;87(10):1013–1018. doi: 10.1016/s0161-6420(80)35136-1. [DOI] [PubMed] [Google Scholar]
- 64.Mourits MP, Koornneef L. Lid lengthening by sclera interposition for eyelid retraction in Graves' ophthalmopathy. Br J Ophthalmol. 1991 Jun;75(6):344–347. doi: 10.1136/bjo.75.6.344. Available from: http://dx.doi.org/10.1136/bjo.75.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baylis HI, Rosen N, Neuhaus RW. Obtaining auricular cartilage for reconstructive surgery. Am J Ophthalmol. 1982 Jun;93(6):709–712. doi: 10.1016/0002-9394(82)90464-0. [DOI] [PubMed] [Google Scholar]
- 66.Baylis HI, Perman KI, Fett DR, Sutcliffe RT. Autogenous auricular cartilage grafting for lower eyelid retraction. Ophthal Plast Reconstr Surg. 1985;1(1):23–27. doi: 10.1097/00002341-198501000-00004. Available from: http://dx.doi.org/10.1097/00002341-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Brown BZ. The use of homologous tarsus as a donor graft in lid surgery. Ophthal Plast Reconstr Surg. 1985;1(2):91–95. doi: 10.1097/00002341-198501020-00004. Available from: http://dx.doi.org/10.1097/00002341-198501020-00004. [DOI] [PubMed] [Google Scholar]
- 68.Marks MW, Argenta LC, Friedman RJ, Hall JD. Conchal cartilage and composite grafts for correction of lower lid retraction. Plast Reconstr Surg. 1989 Apr;83(4):629–635. doi: 10.1097/00006534-198904000-00006. Available from: http://dx.doi.org/10.1097/00006534-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Callahan A. Reconstruction of the eyelids with cartilage and mucosa from the nasal septum. Trans Ophthalmol Soc U K. 1976 Apr;96(1):39–44. [PubMed] [Google Scholar]
- 70.Patipa M. The evaluation and management of lower eyelid retraction following cosmetic surgery. Plast Reconstr Surg. 2000 Aug;106(2):438–453. doi: 10.1097/00006534-200008000-00033. Available from: http://dx.doi.org/10.1097/00006534-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 71.Frueh BR, Su CS. Medial tarsal suspension: a method of elevating the medial lower eyelid. Ophthal Plast Reconstr Surg. 2002 Mar;18(2):133–137. doi: 10.1097/00002341-200203000-00007. Available from: http://dx.doi.org/10.1097/00002341-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Wong JF, Soparkar CN, Patrinely JR. Correction of lower eyelid retraction with high density porous polyethylene: The Medpor ((R)) Lower Eyelid Spacer. Orbit. 2001 Sep;20(3):217–225. doi: 10.1076/orbi.20.3.217.2623. Available from: http://dx.doi.org/10.1076/orbi.20.3.217.2623. [DOI] [PubMed] [Google Scholar]
- 73.Toledano Fernández N, García Sáenz S, Arteaga Sánchez A, Díaz Valle D, Sánchez Cruz J. Correccion del ectropion paralitico con implante de espaciador de polietileno poroso por abordaje externo subciliar. [Paralytic ectropion correction with porous polyethylene spacer by subciliar external approach]. Arch Soc Esp Oftalmol. 2002 Oct;77(10):537–542. [PubMed] [Google Scholar]
- 74.Garibaldi DC, Robinson MR, Lee SS, Park DJ, Fine HF, Deitz L, D'Anna S, Yuan P, Ranson N, Grant MP, Iliff NT, Merbs SL. New corticosteroid-eluting porous polyethylene implant for the management of lower eyelid retraction: a pilot study. Ophthal Plast Reconstr Surg. 2006 Nov-Dec;22(6):424–429. doi: 10.1097/01.iop.0000245487.61479.56. Available from: http://dx.doi.org/10.1097/01.iop.0000245487.61479.56. [DOI] [PubMed] [Google Scholar]
- 75.Woog JJ, Kennedy RH, Custer PL, Kaltreider SA, Meyer DR, Camara JG. Endonasal dacryocystorhinostomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2001 Dec;108(12):2369–2377. doi: 10.1016/S0161-6420(01)00945-9. Available from: http://dx.doi.org/10.1016/S0161-6420(01)00945-9. [DOI] [PubMed] [Google Scholar]
- 76.Toti A. Nuovo metodo conservatore di cura radicale delle suppurazioni croniche del sacco lacrimale. (dacriocistorinostomia) Clin Mod Firenze. 1904;10:385. [Google Scholar]
- 77.Dupuy-Dutemps MM, Bourguet ET. Note préliminaire sur un procédé de dacryocystorhinostomie. Ann Ocul (Paris) 1920;157:1445–1447. [Google Scholar]
- 78.Massaro BM, Gonnering RS, Harris GJ. Endonasal laser dacryocystorhinostomy. A new approach to nasolacrimal duct obstruction. Arch Ophthalmol. 1990 Aug;108(8):1172–1176. doi: 10.1001/archopht.1990.01070100128048. Available from: http://dx.doi.org/10.1001/archopht.1990.01070100128048. [DOI] [PubMed] [Google Scholar]
- 79.Woog JJ, Metson R, Puliafito CA. Holmium: YAG endonasal laser dacryocystorhinostomy. Am J Ophthalmol. 1993 Jul 15;116(1):1–10. doi: 10.1016/s0002-9394(14)71736-2. [DOI] [PubMed] [Google Scholar]
- 80.Caldwell GW. Two new operations for obstruction of the nasal duct with preservation of the canaliculi. Am J Ophthalmol. 1983;10:189. [Google Scholar]
- 81.West JM. A Window Resection of the Nasal Duct in Cases of Stenosis. Trans Am Ophthalmol Soc. 1910;12(Pt 2):654–658. [PMC free article] [PubMed] [Google Scholar]
- 82.McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol. 1989 Jun;103(6):585–587. doi: 10.1017/S0022215100109405. Available from: http://dx.doi.org/10.1017/S0022215100109405. [DOI] [PubMed] [Google Scholar]
- 83.Lee S, Yen MT. Laser-assisted dacryocystorhinostomy: a viable treatment option? Curr Opin Ophthalmol. 2011 Sep;22(5):413–418. doi: 10.1097/ICU.0b013e32834994c8. Available from: http://dx.doi.org/10.1097/ICU.0b013e32834994c8. [DOI] [PubMed] [Google Scholar]
- 84.Levin PS, StormoGipson DJ. Endocanalicular laser-assisted dacryocystorhinostomy. An anatomic study. Arch Ophthalmol. 1992 Oct;110(10):1488–1490. doi: 10.1001/archopht.1992.01080220150037. Available from: http://dx.doi.org/10.1001/archopht.1992.01080220150037. [DOI] [PubMed] [Google Scholar]
- 85.Fay AM, Michalos P, Rubin PA. Endocanalicular Nd: YAG laser dacryocystorhinostomy. Int Ophthalmol Clin. 1999 Winter;39(1):177–184. doi: 10.1097/00004397-199903910-00016. Available from: http://dx.doi.org/10.1097/00004397-199903910-00016. [DOI] [PubMed] [Google Scholar]
- 86.Lee DW, Chai CH, Loon SC. Primary external dacryocystorhinostomy versus primary endonasal dacryocystorhinostomy: a review. Clin Experiment Ophthalmol. 2010 May;38(4):418–426. doi: 10.1111/j.1442-9071.2010.02254.x. Available from: http://dx.doi.org/10.1111/j.1442-9071.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- 87.Tsirbas A, Wormald PJ. Mechanical endonasal dacryocystorhinostomy with mucosal flaps. Br J Ophthalmol. 2003 Jan;87(1):43–47. doi: 10.1136/bjo.87.1.43. Available from: http://dx.doi.org/10.1136/bjo.87.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma B. Non endoscopic endonasal dacryocystorhinostomy. J Laryngol Otol. 2008;122:476–479. doi: 10.1017/S0022215107009954. [DOI] [PubMed] [Google Scholar]
- 89.Jonea LT. The cure of epiphora due to canalicular disorders, trauma and surgical failures on the lacrimal passages. Trans Am Acad Ophthalmol Otolaryngol. 1962 Jul-Aug;66:506–524. [PubMed] [Google Scholar]
- 90.Sisler HA, Allarakhia L. New minitrephine makes lacrimal canalicular rehabilitation an office procedure. Ophthal Plast Reconstr Surg. 1990;6(3):203–206. doi: 10.1097/00002341-199009000-00010. Available from: http://dx.doi.org/10.1097/00002341-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Haefliger IO, Piffaretti JM. Lacrimal drainage system endoscopic examination and surgery through the lacrimal punctum. Klin Monbl Augenheilkd. 2001 May;218(5):384–387. doi: 10.1055/s-2001-15907. Available from: http://dx.doi.org/10.1055/s-2001-15907. [DOI] [PubMed] [Google Scholar]
- 92.Athanasiov PA, Prabhakaran VC, Mannor G, Woog JJ, Selva D. Transcanalicular approach to adult lacrimal duct obstruction: a review of instruments and methods. Ophthalmic Surg Lasers Imaging. 2009 Mar-Apr;40(2):149–159. doi: 10.3928/15428877-20090301-04. Available from: http://dx.doi.org/10.3928/15428877-20090301-04. [DOI] [PubMed] [Google Scholar]
- 93.Doucet TW, Hurwitz JJ. Canaliculodacryocystorhinostomy in the management of unsuccessful lacrimal surgery. Arch Ophthalmol. 1982 Apr;100(4):619–621. doi: 10.1001/archopht.1982.01030030621017. Available from: http://dx.doi.org/10.1001/archopht.1982.01030030621017. [DOI] [PubMed] [Google Scholar]
- 94.Cohen EJ. Punctal occlusion. Arch Ophthalmol. 1999 Mar;117(3):389–390. doi: 10.1001/archopht.117.3.389. [DOI] [PubMed] [Google Scholar]
- 95.Dursun D, Ertan A, Bilezikçi B, Akova YA, Pelit A. Ocular surface changes in keratoconjunctivitis sicca with silicone punctum plug occlusion. Curr Eye Res. 2003 May;26(5):263–269. doi: 10.1076/ceyr.26.4.263.15431. Available from: http://dx.doi.org/10.1076/ceyr.26.4.263.15431. [DOI] [PubMed] [Google Scholar]
- 96.Goto E, Yagi Y, Kaido M, Matsumoto Y, Konomi K, Tsubota K. Improved functional visual acuity after punctal occlusion in dry eye patients. Am J Ophthalmol. 2003 May;135(5):704–705. doi: 10.1016/S0002-9394(02)02147-5. Available from: http://dx.doi.org/10.1016/S0002-9394(02)02147-5. [DOI] [PubMed] [Google Scholar]
- 97.Murube J, Murube E. Treatment of dry eye by blocking the lacrimal canaliculi. Surv Ophthalmol. 1996 May-Jun;40(6):463–480. doi: 10.1016/S0039-6257(96)82013-3.. Available from: http://dx.doi.org/10.1016/S0039-6257(96)82013-3. [DOI] [PubMed] [Google Scholar]
- 98.Knapp ME, Frueh BR, Nelson CC, Musch DC. A comparison of two methods of punctal occlusion. Am J Ophthalmol. 1989 Sep 15;108(3):315–318. doi: 10.1016/0002-9394(89)90123-2. [DOI] [PubMed] [Google Scholar]
- 99.Glatt HJ. Acute dacryocystitis after punctal occlusion of keratoconjunctivitis sicca. Am J Ophthalmol. 1991 Jun 15;111(6):769–770. doi: 10.1016/s0002-9394(14)76787-x. [DOI] [PubMed] [Google Scholar]
- 100.Marx JL, Hillman DS, Hinshaw KD, Olson JJ, Putterman AM, Lam S. Bilateral dacryocystitis after punctal occlusion with thermal cautery. Ophthalmic Surg. 1992 Aug;23(8):560–561. [PubMed] [Google Scholar]
- 101.Putterman AM. Canaliculectomy in the treatment of keratitis sicca. Ophthalmic Surg. 1991 Aug;22(8):478–480. [PubMed] [Google Scholar]
- 102.Murube J. Surgical treatment of dry eye. Orbit. 2003 Sep;22(3):203–232. doi: 10.1076/orbi.22.3.203.15614. Available from: http://dx.doi.org/10.1076/orbi.22.3.203.15614. [DOI] [PubMed] [Google Scholar]
- 103.Liu D, Sadhan Y. Surgical punctal occlusion: a prospective study. Br J Ophthalmol. 2002 Sep;86(9):1031–1034. doi: 10.1136/bjo.86.9.1031. Available from: http://dx.doi.org/10.1136/bjo.86.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010 Feb 25;362(8):726–738. doi: 10.1056/NEJMra0905750. Available from: http://dx.doi.org/10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baldeschi L. Small versus coronal incision orbital decompression in Graves' orbitopathy. Orbit. 2010 Aug;29(4):177–182. doi: 10.3109/01676830.2010.508000. Available from: http://dx.doi.org/10.3109/01676830.2010.508000. [DOI] [PubMed] [Google Scholar]
- 106.Sasim IV, de Graaf ME, Berendschot TT, Kalmann R, van Isterdael C, Mourits MP. Coronal or swinging eyelid decompression for patients with disfiguring proptosis in Graves' orbitopathy? Comparison of results in one center. Ophthalmology. 2005 Jul;112(7):1310–1315. doi: 10.1016/j.ophtha.2005.01.049. Available from: http://dx.doi.org/10.1016/j.ophtha.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 107.Rochels R, Beigel A, Mehdorn HM. Der Bügelschnitt als chirurgischer Zugang zur Orbita. [Coronal incision as the surgical approach to the orbits]. Ophthalmologe. 1995 Apr;92(2):212–214. (Ger). [PubMed] [Google Scholar]
- 108.Lima WT, Perches M, Valera FC, Demarco RC. Orbital endoscopic decompression in Graves ophthalmopathy. Braz J Otorhinolaryngol. 2006 Mar-Apr;72(2):283–287. doi: 10.1016/S1808-8694(15)30069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tyers AG, Collin JR. Orbital implants and post enucleation socket syndrome. Trans Ophthalmol Soc U K. 1982 Apr;102(Pt 1):90–92. [PubMed] [Google Scholar]
- 110.Custer PL, Kennedy RH, Woog JJ, Kaltreider SA, Meyer DR. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2003 Oct;110(10):2054–2061. doi: 10.1016/S0161-6420(03)00857-1. Available from: http://dx.doi.org/10.1016/S0161-6420(03)00857-1. [DOI] [PubMed] [Google Scholar]
- 111.Collin JR. A manual of systematic eyelid surgery. 3rd ed. London: Elsevier-Butterworth-Heinemann; 2006. Enucleation, evisceration and socket surgery; pp. 223–228. [Google Scholar]
- 112.Chalasani R, Poole-Warren L, Conway RM, Ben-Nissan B. Porous orbital implants in enucleation: a systematic review. Surv Ophthalmol. 2007 Mar-Apr;52(2):145–155. doi: 10.1016/j.survophthal.2006.12.007. Available from: http://dx.doi.org/10.1016/j.survophthal.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Lyle CE, Wilson MW, Li CS, Kaste SC. Comparison of orbital volumes in enucleated patients with unilateral retinoblastoma: hydroxyapatite implants versus silicone implants. Ophthal Plast Reconstr Surg. 2007 Sep-Oct;23(5):393–396. doi: 10.1097/IOP.0b013e3181462ca8. Available from: http://dx.doi.org/10.1097/IOP.0b013e3181462ca8. [DOI] [PubMed] [Google Scholar]
- 114.Custer PL, Kennedy RH, Woog JJ, Kaltreider SA, Meyer DR. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2003 Oct;110(10):2054–2061. doi: 10.1016/S0161-6420(03)00857-1. Available from: http://dx.doi.org/10.1016/S0161-6420(03)00857-1. [DOI] [PubMed] [Google Scholar]
- 115.Sadiq SA, Mengher LS, Lowry J, Downes R. Integrated orbital implants – a comparison of hydroxyapatite and porous polyethylene implants. Orbit. 2008;27(1):37–40. doi: 10.1080/01676830701512585. Available from: http://dx.doi.org/10.1080/01676830701512585. [DOI] [PubMed] [Google Scholar]
- 116.Jordan DR, Gilberg S, Mawn LA. The bioceramic orbital implant: experience with 107 implants. Ophthal Plast Reconstr Surg. 2003 Mar;19(2):128–135. doi: 10.1097/01.IOP.0000056027.63698.FE. Available from: http://dx.doi.org/10.1097/01.IOP.0000056027.63698.FE. [DOI] [PubMed] [Google Scholar]
- 117.Alwitry A, West S, King J, Foss AJ, Abercrombie LC. Long-term follow-up of porous polyethylene spherical implants after enucleation and evisceration. Ophthal Plast Reconstr Surg. 2007 Jan-Feb;23(1):11–15. doi: 10.1097/01.iop.0000249429.02757.6b. Available from: http://dx.doi.org/10.1097/01.iop.0000249429.02757.6b. [DOI] [PubMed] [Google Scholar]
- 118.Liu D. Evisceration techniques and implant extrusion rates: A retrospective review of two series and a survey of ASOPRS surgeons. Ophthal Plast Reconstr Surg. 2007 Jan-Feb;23(1):16–21. doi: 10.1097/01.iop.0000249430.33159.f3. Available from: http://dx.doi.org/10.1097/01.iop.0000249430.33159.f3. [DOI] [PubMed] [Google Scholar]
- 119.Custer PL, Trinkaus KM. Porous implant exposure: Incidence, management, and morbidity. Ophthal Plast Reconstr Surg. 2007 Jan-Feb;23(1):1–7. doi: 10.1097/01.iop.0000249432.18688.ee. Available from: http://dx.doi.org/10.1097/01.iop.0000249432.18688.ee. [DOI] [PubMed] [Google Scholar]
- 120.Vagefi MR, McMullan TF, Burroughs JR, White GL, Jr, McCann JD, Anderson RL. Injectable calcium hydroxylapatite for orbital volume augmentation. Arch Facial Plast Surg. 2007 Nov-Dec;9(6):439–442. doi: 10.1001/archfaci.9.6.439. Available from: http://dx.doi.org/10.1001/archfaci.9.6.439. [DOI] [PubMed] [Google Scholar]
- 121.Kotlus BS, Dryden RM. Correction of anophthalmic enophthalmos with injectable calcium hydroxylapatite (Radiesse) Ophthal Plast Reconstr Surg. 2007 Jul-Aug;23(4):313–314. doi: 10.1097/IOP.0b013e318068742c. Available from: http://dx.doi.org/10.1097/IOP.0b013e318068742c. [DOI] [PubMed] [Google Scholar]
- 122.Vagefi MR, McMullan TF, Burroughs JR, Isaacs DK, Tsirbas A, White GL, Jr, Anderson RL, McCann JD. Autologous dermis graft at the time of evisceration or enucleation. Br J Ophthalmol. 2007 Nov;91(11):1528–1531. doi: 10.1136/bjo.2007.115543. Available from: http://dx.doi.org/10.1136/bjo.2007.115543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Quaranta-Leoni F. Orbital pathology: the anophthalmic socket.Medico legal ophthalmology. Rome, Venice: Fabiano-SOI; 2007. pp. 158–163. [Google Scholar]
- 124.Smith RJ, Malet T. Auricular cartilage grafting to correct lower conjunctival fornix retraction and eyelid malposition in anophthalmic patients. Ophthal Plast Reconstr Surg. 2008 Jan-Feb;24(1):13–18. doi: 10.1097/IOP.0b013e31815efe35. Available from: http://dx.doi.org/10.1097/IOP.0b013e31815efe35. [DOI] [PubMed] [Google Scholar]
- 125.Ragge NK, Subak-Sharpe ID, Collin JR. A practical guide to the management of anophthalmia and microphthalmia. Eye (Lond) 2007 Oct;21(10):1290–1300. doi: 10.1038/sj.eye.6702858. Available from: http://dx.doi.org/10.1038/sj.eye.6702858. [DOI] [PubMed] [Google Scholar]
- 126.Mazzoli RA, Raymond WR, 4th, Ainbinder DJ, Hansen EA. Use of self-expanding, hydrophilic osmotic expanders (hydrogel) in the reconstruction of congenital clinical anophthalmos. Curr Opin Ophthalmol. 2004 Oct;15(5):426–431. doi: 10.1097/01.icu.0000138618.61059.4c. Available from: http://dx.doi.org/10.1097/01.icu.0000138618.61059.4c. [DOI] [PubMed] [Google Scholar]
- 127.Gundlach KK, Guthoff RF, Hingst VH, Schittkowski MP, Bier UC. Expansion of the socket and orbit for congenital clinical anophthalmia. Plast Reconstr Surg. 2005 Oct;116(5):1214–1222. doi: 10.1097/01.prs.0000181653.38200.eb. Available from: http://dx.doi.org/10.1097/01.prs.0000181653.38200.eb. [DOI] [PubMed] [Google Scholar]


