Abstract
To determine bone morphogenetic protein (BMP)-2 protein and Aggrecan in osteoarthritic and healthy cartilage with special regard to localization and degree of cartilage damage 95 samples representing osteoarthritic cartilage and 17 samples out of normal cartilage were graded histological by Mankin Score and were studied by immunohistochemistry for the expression of BMP-2 and Aggrecan. BMP-2 protein was detected intracellular in normal and in osteoarthritic cartilage. Extracellular BMP-2 was detected exclusively in osteoarthritic cartilage and exhibits characteristic extracellular patterns: samples with BMP-2 in the extracellular matrix show BMP-2 negative coronae around BMP-2 positive cells. There is a statistically significant increase in the prevalence of extracellular BMP-2 with increasing ICRS grade/Mankin grade. Aggrecan was detected in the extracellular matrix und exhibited coronas throughout all layers. A decline of extracellular Aggrecan with increasing ICRS grade could be observed. Normal cartilage shows no intracellular Aggrecan whereas an increase in the prevalence of intracellular Aggrecan could be detected in osteoarthritic cartilage. The appearance of intracellular Aggrecan is often associated with extracellular BMP-2. The detection of BMP-2 protein in normal as well as in osteoarthritic cartilage highlights the importance of BMP-2 in tissue homeostasis and point to a putative role for maintaining tissue integrity during the development of osteoarthritis. The co-appearance of extracellular BMP-2 and intracellular Aggrecan indicates a functional relationship. The most interesting result is the characteristic distribution of extracellular BMP-2. These coronas seem to have an impact during progression of osteoarthritis and need to be further investigated.
Keywords: BMP-2, cartilage, coronas, osteoarthritis
Zusammenfassung
Um charakteristische strukturelle Veränderungen des bone morphogenetic proteins (BMP)-2 und des Aggrecans im arthrotischen und gesunden Gelenkknorpel in Abhängigkeit der Lokalisation und des Schädigungsgrades zu identifizieren, wurde in 95 Proben histologisch klassifizierbarer Knorpelschäden (Mankin Score) und 17 Kontrollproben immunhistochemisch die Expression von BMP-2 und Aggrecan untersucht.
Das BMP-2 Protein wurde intrazellulär in normalen und arthrotischen Knorpel exprimiert. Extrazelluläres BMP-2 wurde einzig in arthrotischen Knorpel detektiert und zeigte dabei ein charakteristisches Verteilungsmuster: Extrazellulär exprimiertes BMP-2 bildet BMP-2 negative Höfe um BMP-2 positive Knorpelzellen. Die Prevalenz extrazellulärer BMP-2 Expression nimmt mit steigenden makroskopischen und histologischen Schädigungsgrad (ICRS/Mankin) signifikant zu. Die Expression von Aggrecan in the extrazellulären Matrix nimmt mit steigendem ICRS Schädigungsgrad ab, während die intrazelluläre Expression von Aggrecan im arthrotischen Knorpel zunimmt.
Die intrazelluläre Aggrecan und extrazelluläre BMP-2 Expression ist assoziiert – ein funktioneller Zusammenhang wahrscheinlich. Besonders das charakteristische Verteilungsmuster von BMP-2 in normalen und arthrotischen Knorpel weist auf die Bedeutung des BMP-2 für die Integrität und Homeostase des Knorpelgewebes hin und sollte weiter untersucht werden.
Introduction
Osteoarthritis (OA) causes several changes in cartilage and leads to loss of tissue integrity and function. Most prominent is the breakdown of the extracellular matrix (ECM) indicated through loss of the major matrix molecules including proteoglycans and collagen. This matrix transformation occurs although the chondrocytes are thought to have anabolic activity such as matrix synthesis and proliferation. The cells seem to counteract the matrix destruction during the degenerative events of osteoarthritis [1], [2].
This regenerating action of OA cells may be regulated through a variety of different factors. Transforming growth factor-β (TGF-β) is expressed in OA cartilage and is one of these potent chondrogenic factors. A major subgroup of the transforming growth factor-beta superfamily are the bone morphogenetic proteins (BMPs). Originally BMPs were discovered because of their osteoinductive potential at extraskeletal sites in vivo [3], [4]. Meanwhile it has been shown that BMPs are potent inducers of bone and cartilage formation in vivo and in vitro. BMP signalling has been shown to be essential for chondrogenesis and the postnatal cartilage homeostasis [5]. To date, almost 30 different BMPs could be identified with BMP-2 as a strong inducer of osteochondrogenesis. Moreover it has been shown that BMP-2 is able to induce proteoglycan synthesis in healthy and osteoarthritic cartilage [6]. The effects of BMP-2 in stimulating chondrogenesis and cartilage repair are well known but little is known about the role of endogenously produced BMP-2 in healthy and osteoarthritic cartilage [7]. To date, the expression of BMP-2 in osteoarthritis has been investigated rarely with contradictory results: Nakase et al. detected BMP-2 in OA tissue whereas healthy cartilage appeared to be negative [8]. In contrast, Chen et al. found BMP-2 in normal as well as in OA cartilage [9].
Taken together, we wondered whether BMP-2 could be one of the key regulators of the regenerating action of OA chondrocytes and the up-regulation of Aggrecan synthesis may be a major effect of BMP-2 in OA. The purpose of this study was to detect BMP-2 protein and Aggrecan in OA and normal cartilage using immunohistochemistry with special regard to localization and degree of cartilage damage.
Material and methods
Patients
A total of 35 patients received endoprosthetic knee replacement between January and December 2007 were selected for histological investigations. This study was open-label, prospective and mono centred. We examined 25 patients with primary osteoarthritis (12 women, 13 men) and 10 patients (4 women, 6 men) with osteoarthritis following trauma (secondary osteoarthritis). The cohort’s age ranged between 47 and 80 years (mean age: 65.1 years). Written informed consent was obtained from all patients. Human femoral, tibia and patellar joint surfaces were obtained during total knee replacement surgery with written approval from the local Ethic Committee for Clinical Trials of the Friedrich Schiller University of Jena (1714-01/06). All cases satisfied the classification criteria for osteoarthritis of the knee, i.e. pain, loss of function and mobility combined with radiological signs of osteoarthritis. The total knee replacement surgery was carried out due to clinical relevant knee pain in final stage osteoarthritis assessed by case history.
Normal healthy cartilage was obtained from 4 omissions. Only persons with no indication for any knee related diseases were included and the cartilage samples were harvested within 72 hours after death. Joint surfaces were resected as described for total knee replacement surgery. The age ranged between 17 and 36 years with a mean age of 27.4 years. All persons were male.
Sample preparation
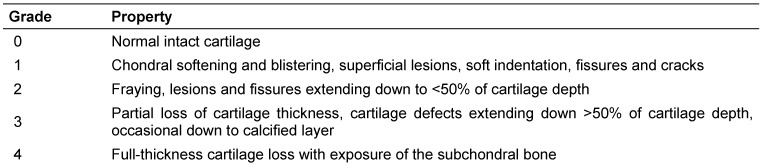
Immediately after resection all joint surfaces were stored in Ringer’s solution and sample preparation was performed. Full thickness osteochondral cylinders including subchondral bone with a diameter of 4 mm were drilled out of various areas using a gouge bit under continuous irrigation with Ringer’s solution for cooling. The degree of cartilage destruction was determined independently by two orthopaedic surgeons using the ICRS classification and the criteria given in Table 1 (Tab. 1) [10]. The drilled out specimen were stored in acetone (Roth, Karlsruhe) at –20°C until further preparation. We could obtain 95 specimens out of the 35 patients. The samples were drilled out of different surface areas and represented various stages of cartilage damage (one ICRS grade 0, 30 grade 1, 34 grade 2 and 30 grade 3). Seventeen samples representing normal cartilage were obtained from the omissions (12 ICRS grade 0, 5 ICRS grade 1).
Table 1. Grading of articular cartilage damage and ulceration based on ICRS classification system.
Sample embedding
The cartilage samples were dehydrated in acetone at –20°C for at least 10 days. After thawing, the specimens were incubated in fresh acetone for one hour. Embedding of the specimen was carried out using Technovit 9100 (Heraeus Kulzer, Wehrheim/Ts.). This polymerisation system is based on methylmethacrylate (MMA) that hardens at low temperatures and has been developed especially for embedding of mineralised tissues.
The acetone-fixed samples were incubated two times in xylene (Roth, Karlsruhe) for one hour each. The first preinfiltration step was performed one hour in a xylene/Technovit Basic Solution (destabilised) mixture. After that the specimen were incubated in Technovit Basic Solution with Hardener 1 for one hour. Infiltration was carried out with Technovit Basic Solution + Hardener 1 + PMMA-Powder in an exsiccator for at least 8 days at room temperature. Finally, samples were put in moulds and polymerisation mixture (according to manufacturers’ protocol) was added. Polymerisation was performed at –7°C for 2–3 days.
The embedded samples were cut in 4 µm sections using a Leica microtome. Ethanol (Roth, Karlsruhe) was used as cutting fluid. Sections were flattened at poly-L-lysine coated slides with 80% ethanol and dried at 56°C over night.
Each sample was stained immunohistochemically for BMP-2 and Aggrecan and histologically for grading according to Mankin [11].
Immunohistochemistry
The polymer of the Technovit-embedded tissue sections was removed by four fold incubation in 2-methoxyethylacetate (Merck, Darmstadt) for 15 min each followed by rehydration through graded alcohol series. Afterwards samples were rinsed in phosphate buffered saline (PBS, Invitrogen, Karlsruhe) and decalcification in 3% acetic acid and ethylenediaminetetraacetic acid (Roth, Karlsruhe) was performed. Slides were rinsed again in PBS and put into Shandon Coverplates (Thermo Fisher, Schwerte). Endogenous peroxidase was blocked using 3% H2O2 for 10 min and subsequently rinsed three times in PBS. Unspecific binding sites were blocked with normal horse serum for one hour at room temperature. Primary antibody (BMP-2: B9553, Sigma Aldrich, München; Aggrecan: MCA4722, AbDSerotec, Düsseldorf) was added and incubated over night at 4°C. Afterwards samples were rinsed three times with PBS and Avidin/Biotin Blocking Kit (Vector Laborities, Inc., Burlinghame/USA) was applied. After another washing step the secondary, biotinylated antibody (Vector Laborities, Inc., Burlinghame/USA) was confessed for 30 min. Removal was performed by rinsing three times with PBS. Then peroxidase-coupled ABC-Reagent (Vector Laborities, Inc., Burlinghame/USA) was added for another 30 min. Finally the antibody binding was visualized by incubation with aminoethylecarbazol-solution (Sigma, München). After staining, the sections were rinsed in PBS and embedded in Kaiser’s glycerine gelatine. The samples were investigated microscopically and documented. Antibody controls were performed by omitting the primary and the secondary antibody. No signal could be detected in these controls.
Histology – microscopic staging
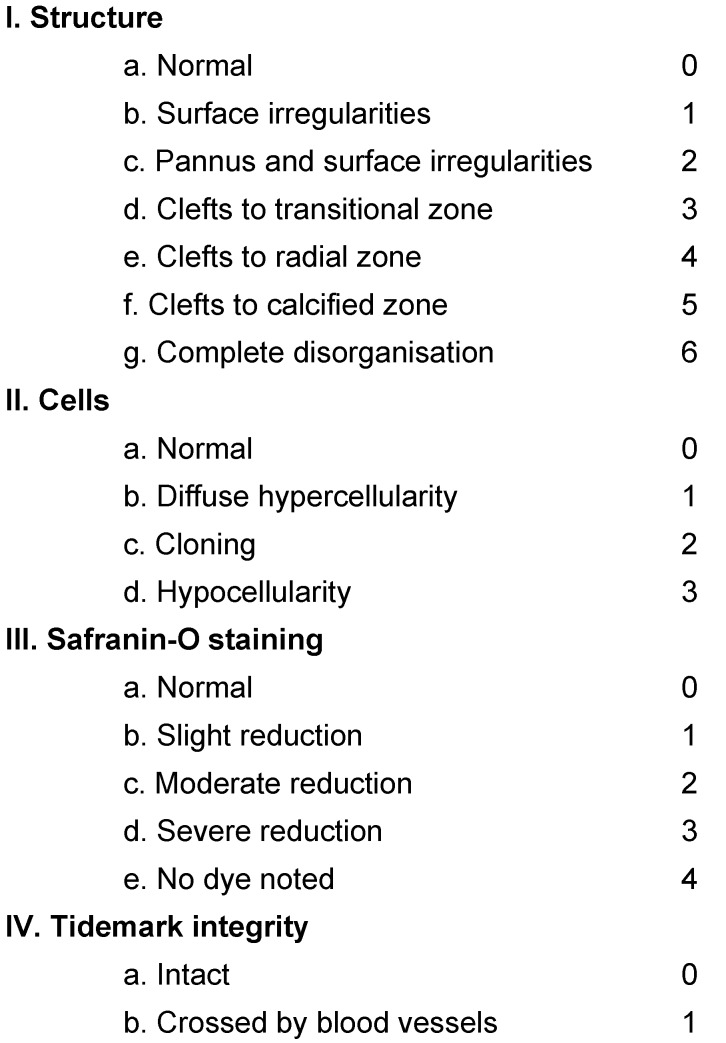
Cartilage samples were staged microscopically according to the modified Mankin Score given in Table 2 (Tab. 2) [11]. The sections were stained with Hematoxylin-Eosin and with Safranin-O and investigated microscopically. The four different aspects of the score were determined and summed up leading to a scale that ranges between 0 and 14. For further analysis the Mankin score was used to discern groups of different stages of cartilage destruction. Mankin score 0–2 represents normal cartilage, 3–5 superficial fibrillation, 6–7 moderately cartilage destruction, 8–10 severely damage of cartilage and over 10 complete loss of cartilage [12].
Table 2. Modified Mankin score is the sum of structure, cells, Safranin-O staining and tidemark integrity.
Statistic analysis
Statistic evaluation of the results was carried out using Microsoft ExcelTM. Significance was determined through Fisher’s exact test. Significance levels were defined as follows: ρ≤0.05 (*), ρ≤0.01 (**) and ρ≤0.001 (***).
Results
BMP-2 can be detected extra- and intracellular
BMP-2 was detected intracellular by immunohistochemistry in normal cartilage and in all stages of cartilage damage occurring during osteoarthritis (Figure 1 (Fig. 1)). BMP-2 was localized in all layers of cartilage tissue, i.e. the upper, middle and deep layer as well as in cell clusters and individual chondrocytes.
Figure 1. BMP-2 immunohistochemistry at different stages of osteoarthritis.
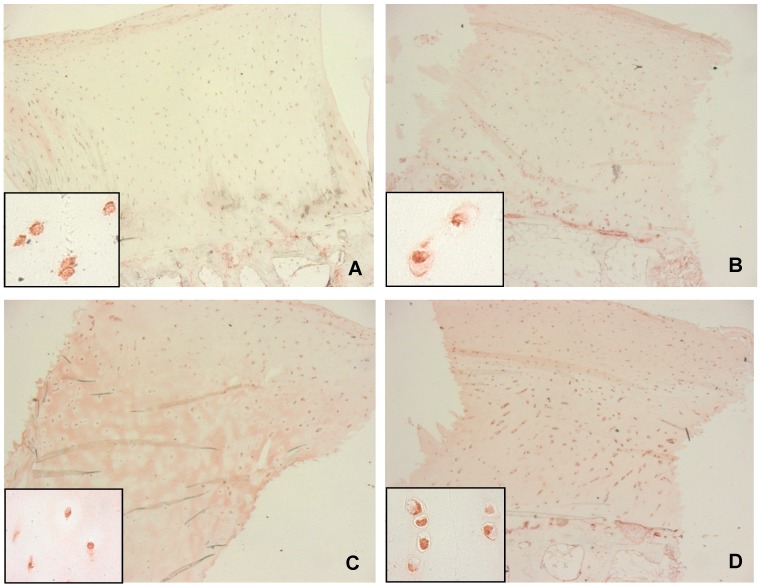
A: normal cartilage (ICRS: 0), B-D: osteoarthritic cartilage with weak (B, ICRS: 1), moderate (C, ICRS: 2) and severely (D, ICRS: 3) damage; all sections show intracellular localization of BMP-2 throughout all cartilage layers, extra cellular BMP-2 could only be observed in osteoarthritic cartilage (C, D), original magnification: 2.5x, inserts: 63x.
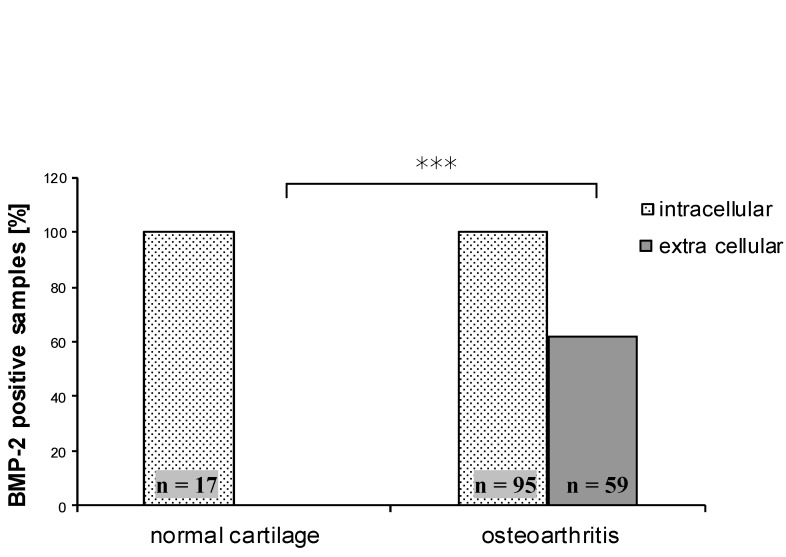
Quantitative analysis showed no extracellular BMP-2 signal in normal cartilage whereas 62.1% of the osteoarthritis tissue specimens were positive for BMP-2 in the ECM. This difference was statistically significant (ρ<0.000). Extracellular localization of BMP-2 seemed to be an exclusive feature of osteoarthritic cartilage whereas normal cartilage showed only intracellular BMP-2 (Figure 2 (Fig. 2)).
Figure 2. Comparison of BMP-2 positive specimen between normal and osteoarthritic cartilage. There is no difference regarding intracellular BMP-2, extra cellular BMP-2 was significantly more prevalent in osteoarthritic cartilage (ρ<0.000).
Furthermore we divided the OA samples according to their aetiology in primary and secondary osteoarthritis and compared them with normal cartilage. No difference was found regarding intracellular BMP-2. The prevalence of extracellular BMP-2 was significantly higher for both primary and secondary OA (ρ<0.000). All control samples were negative for extracellular BMP-2 whereas 60.5% of the primary OA and 66.7% of the secondary OA samples showed extracellular staining for BMP-2. However, our investigations could not detect a difference between primary and secondary OA.
BMP-2 expression increases with ICRS grading
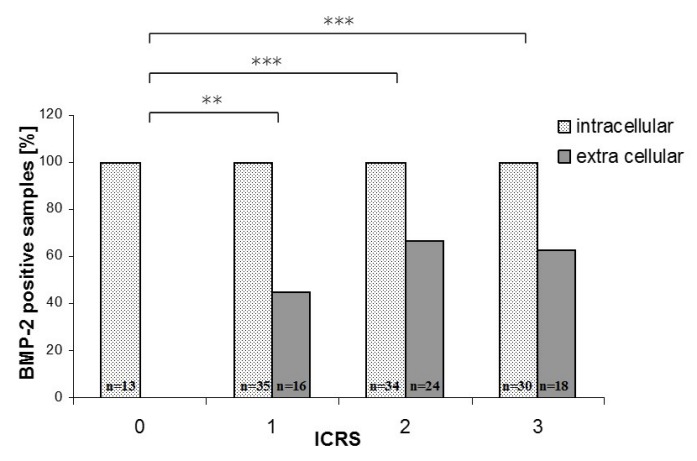
Further analysis showed a relationship between cartilage damage and BMP-2 expression (Figure 3 (Fig. 3)). With increasing ICRS grade the number of samples with extracellular BMP-2 rises. Normal cartilage (ICRS 0) was completely negative for extracellular BMP-2. With increasing cartilage damage the percentage of samples positive for extracellular BMP-2 rises from 45.2% in grade 1 cartilage to 70% for ICRS grade 2 and 60.7% for grade 3 cartilage. All ICRS grades indicating cartilage damage (ICRS 1–3) were statistically different from normal cartilage (ICRS 0). However, no difference was found between the ICRS grades 1, 2 and 3. Moreover, a slight decrease can be observed between ICRS grade 2 and ICRS grade 3.
Figure 3. BMP-2 positive samples in relation to the ICRS grading. All samples exhibited intracellular BMP-2. Samples positive for extra cellular BMP-2 could only be detected in OA samples.
BMP-2 shows characteristic extracellular patterns in osteoarthritis
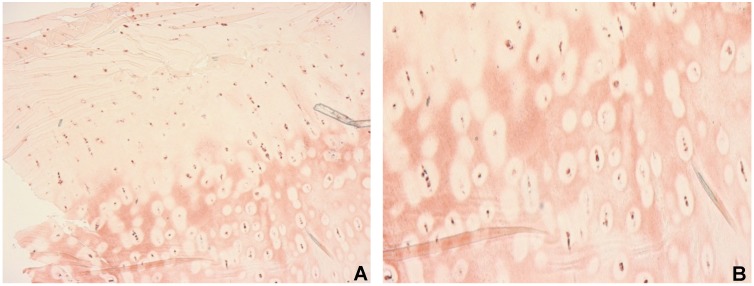
The presence of extracellular BMP-2 is indicative for osteoarthritic cartilage. There is not only a difference in the number of samples positive for extracellular BMP-2 but also qualitative distinction regarding structural features. In samples with extracellular BMP-2 we observed specific structural characteristics. Several samples which stained positive for extracellular BMP-2 showed BMP-2 negative areas around BMP-2 positive cells (Figure 4 (Fig. 4)). These so called coronas were detectable in 32.4% of the primary OA samples and in 12.5% of the secondary OA specimen. The presence of coronas was statistically significant higher in primary OA than in normal cartilage (ρ=0.005). Coronas were mostly present in middle and deep layers.
Figure 4. Immunohistochemical staining for BMP-2. All cells were positive for BMP-2. Extra cellular BMP-2 is present in middle and deep layers and BMP-2 negative areas around positive cells (coronas) are predominant, original magnification: 2.5x (A), 10x (B).
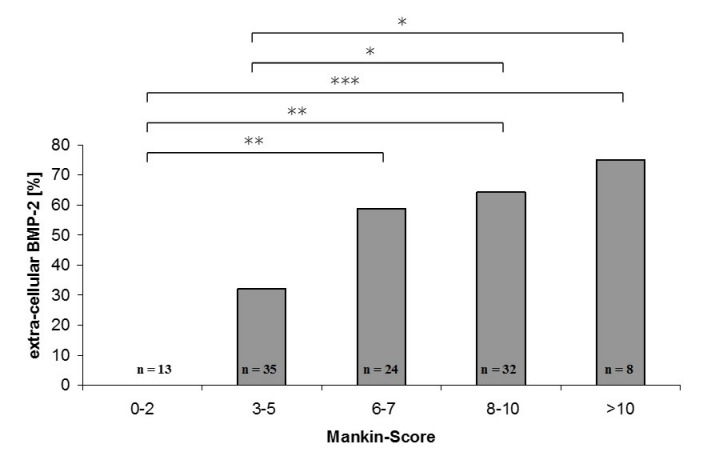
In the next step only samples with extracellular BMP-2 were analyzed. The percentage of samples showing coronas were calculated (Figure 5 (Fig. 5)). Samples derived from primary OA showed coronas in 53.5% of all cases with extra cellular BMP-2 compared to 18.8% for secondary OA. This difference appeared to be statistically significant (ρ=0.02). The formation of BMP-2 negative coronas around BMP-2 positive cells is more prominent in primary than in secondary OA. These results encouraged us to further investigate the samples derived from primary OA. Microscopic score according to Mankin was determined because it gives more detailed information about the degree of cartilage damage. The Mankin Score implicates number and distribution of chondrocytes as well as integrity of the extracellular matrix. The results show that the number of samples with extracellular BMP-2 rises with increasing Mankin Score (Figure 6 (Fig. 6)). Considering the parameters included in the Mankin Score, like Safranin-O-staining, it could be speculated that BMP-2 may be involved in ECM changes occurring during the progression of OA.
Figure 5. Proportion of corona exhibiting samples among specimens with extra cellular BMP-2. 53.5% of primary and only 18.8% of secondary OA samples showed a formation of BMP-2 negative coronas in the ECM.
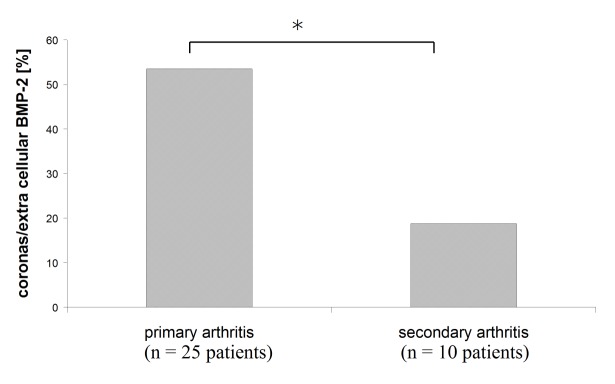
Figure 6. Extra cellular BMP-2 in relation to Mankin Score. The percentage of samples with extra cellular BMP-2 increases with Mankin Score (* ρ<0.05, ** ρ<0.01, *** ρ<0.001).
Intracellular Aggrecan is associated with extracellular BMP-2
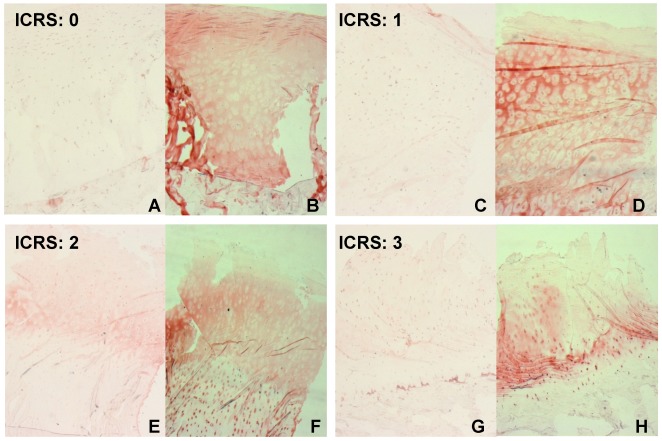
In the next step, a potential effector of BMP-2 was investigated: Aggrecan, the major cartilage proteoglycan, was detected using immunohistochemistry. All samples exhibited extracellular staining for Aggrecan with similar structural characteristics also observed for BMP-2: there were Aggrecan negative areas around the cells. Except these coronas the whole ECM is stained positive for Aggrecan throughout all layers. A decline of Aggrecan with increasing ICRS grade beginning in the upper layers can be observed in OA samples. The same distribution and localisation of BMP-2 and Aggrecan indicates a functional relationship. Moreover we could localize Aggrecan intracellular. This occurs to be more prominent in samples that also stained positive for extracellular BMP-2 (Figure 7 (Fig. 7)). OA specimen with no extracellular BMP-2 exhibited intracellular Aggrecan in 50% of the cases whereas 65% of the samples with extracellular BMP-2 stained positive for intracellular Aggrecan. The Aggrecan positive cells were mostly found in the middle and deep layers and often showed Aggrecan positive ECM around the positive cells instead of the coronas.
Figure 7. BMP-2 (A, C, E, G) and Aggrecan (B, D, F, H) immunohistochemistry. Intracellular BMP-2 is detectable in all ICRS grades whereas extracellular BMP-2 only can be found in osteoarthritic cartilage (E, ICRS: 2); Aggrecan could be detected in the ECM of normal and osteoarthritic cartilage. A decrease of Aggrecan immunolocalisation beginning in the upper layer occurs with increasing ICRS grade (D, F, H) whereas the appearance of intracellular Aggrecan seems to increase (F, H) and often is associated with extracellular BMP-2 (E, F), original magnification: 2.5x.
Discussion
This work describes the expression and distribution of bone morphogenetic protein-2 (BMP-2) in human osteoarthritic tissue. BMP-2 could be detected intracellular in normal and osteoarthritic cartilage. These findings highlight the importance of BMP-2 in the homoeostasis of healthy cartilage and point to a putative role for maintaining tissue integrity during the development of osteoarthritis. BMP-2 was synthesized throughout all layers in individual chondrocytes as wells as in cell clusters. Moreover, we could detect extracellular BMP-2 which is an exclusive feature of osteoarthritic cartilage and shows characteristic patterns (coronas). The current work supports the results of Chen et al. who found BMP-2 mRNA in normal adult and osteoarthritic cartilage [9]. Moreover, we could detect BMP-2 on the protein level which means that not only gene expression of BMP-2 occurs in cartilage but also the functional protein is produced. The presence of BMP-2 protein is a prerequisite for proper BMP signalling which can alter gene expression in target cells [13]. The detection of intracellular BMP-2 can have different reasons. First it could be an indicator for freshly synthesized protein that has not been transported into the extracellular space yet where it could induce BMP signalling. On the other hand it could be internalized BMP-2 as a consequence of recent binding of BMP-2 to the receptors, activation of downstream signalling and finally uptake of the BMP-2 receptor complex into the cytoplasm. With regard to the distribution of extracellular BMP-2 the second explanation may be preferred because the negative coronas could be another indicator for active signalling and uptake of BMP-2 through chondrocytes. The results derived through immunohistochemistry are snapshots and so it is conceivable that synthesis as well as uptake of BMP-2 can be the reason for intracellular staining. Because intracellular BMP-2 can be found in normal as well as in OA cartilage, a switch in BMP-2 activity could be possible. In brief, BMP-2 is expressed in normal cartilage in small amounts to maintain tissue integrity. During progression of osteoarthritis the BMP-2 expression is up-regulated to counteract the disease related cartilage damage. The higher prevalence of extracellular BMP-2 could be a hint to support this hypothesis. These results raise the question what consequences the up-regulation of BMP-2 may have. A possible role of BMP-2 for extracellular matrix production was found in cell culture experiments, where it has been shown that BMP-2 is able to boost matrix turnover [6]. Therefore we investigated Aggrecan in the same cartilage samples and found an increase in the prevalence of intracellular Aggrecan, which is an indicator for freshly synthesized protein, in correlation with elevated prevalence of extracellular BMP-2. Taken together, it could be possible that BMP-2 is able to activate Aggrecan synthesis in OA cartilage to retard breakdown of ECM occurring during OA progression. The correlation between extracellular BMP-2 and increasing cartilage damage (represented through ICRS grade and Mankin Score) leads to the presumption that BMP-2 is activated during progression of osteoarthritis. Therefore we speculated whether the activity of BMP-2 changes during progression of osteoarthritis. It has been shown that BMP activity depends on time, concentration and cellular context and is able to switch during disease progression [14], [15]. A recent review proposed a dual role for BMPs in osteoarthritis: a protective effect through activation of Aggrecan and Collagen synthesis and a destructive through up-regulation of MMP-13 levels [16]. The protective activity seems to be a short term effect of locally produced BMPs and the long term exposure to BMPs drives chondrocytes into terminal differentiation. These different effects may depend on the receptor configuration, appearance of inhibitors, interaction of different BMPs and modulation of downstream signalling. Each BMP binds to a preferential receptor combination and activates signalling [5]. Alterations in receptor configuration may alter the downstream effects. Furthermore, the BMP pathway is modulated through a variety of inhibitors at different stages of signalling: BMP binding to receptors can be blocked via extracellular inhibitors such as noggin and gremlin, Smad stability can be altered through degradation initiated by Smurf or the Smad signalling is modulated by other pathways such as MAPK pathway, just to name a few [17], [18]. Taken together, there is a huge number of possibilities how BMP signalling takes place and therefore BMPs are able to influence a broad spectrum of targets. To further elucidate the role of BMP signalling in OA it is necessary to determine receptor configuration and exact pathways used for activation/inhibition of disease related targets such as aggrecan synthesis.
This study gives some hints regarding a possible role of BMP-2 for protection of cartilage against destruction occurring during OA. However, further studies are needed to define a possible correlation between BMP-2 and structural matrix proteins on a quantitative level.
Notes
Acknowledgements
The authors would like to thank Birgit Lemser for excellent technical assistance.
Funding
This project is supported by the Berufsgenossenschaft der Bauwirtschaft and the Thüringer Aufbaubank (Project: Cartilage destruction and osteoarthritis).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Aurich M, Squires GR, Reiner A, Mollenhauer JA, Kuettner KE, Poole AR, Cole AA. Differential matrix degradation and turnover in early cartilage lesions of human knee and ankle joints. Arthritis Rheum. 2005 Jan;52(1):112–119. doi: 10.1002/art.20740. Available from: http://dx.doi.org/10.1002/art.20740. [DOI] [PubMed] [Google Scholar]
- 2.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003 May;48(5):1261–1270. doi: 10.1002/art.10976. Available from: http://dx.doi.org/10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 3.Urist MR. Bone: formation by autoinduction. Science. 1965 Nov;150(3698):893–899. doi: 10.1126/science.150.3698.893. Available from: http://dx.doi.org/10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 4.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998 Jan;(346):26–37. doi: 10.1097/00003086-199801000-00006. Available from: http://dx.doi.org/10.1097/00003086-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004 Nov;2(11) doi: 10.1371/journal.pbio.0020355. Available from: http://dx.doi.org/10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FA, van den Berg WB, van der Kraan PM. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9(5):R102. doi: 10.1186/ar2305. Available from: http://dx.doi.org/10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddi AH. Cartilage morphogenetic proteins: role in joint development, homoeostasis, and regeneration. Ann Rheum Dis. 2003;62:73–78. doi: 10.1136/ard.62.suppl_2.ii73. Available from: http://dx.doi.org/10.1136/ard.62.suppl_2.ii73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakase T, Miyaji T, Tomita T, Kaneko M, Kuriyama K, Myoui A, Sugamoto K, Ochi T, Yoshikawa H. Localization of bone morphogenetic protein-2 in human osteoarthritic cartilage and osteophyte. Osteoarthr Cartil. 2003 Apr;11(4):278–284. doi: 10.1016/S1063-4584(03)00004-9.. Available from: http://dx.doi.org/10.1016/S1063-4584(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen AL, Fang C, Liu C, Leslie MP, Chang E, Di Cesare PE. Expression of bone morphogenetic proteins, receptors, and tissue inhibitors in human fetal, adult, and osteoarthritic articular cartilage. J Orthop Res. 2004 Nov;22(6):1188–1192. doi: 10.1016/j.orthres.2004.02.013. Available from: http://dx.doi.org/10.1016/j.orthres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003 Apr 01;85(suppl 2):45–57. [PubMed] [Google Scholar]
- 11.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- 12.Pearson RG, Kurien T, Shu KS, Scammell BE. Histopathology grading systems for characterisation of human knee osteoarthritis--reproducibility, variability, reliability, correlation, and validity. Osteoarthr Cartil. 2011 Mar;19(3):324–331. doi: 10.1016/j.joca.2010.12.005. Available from: http://dx.doi.org/10.1016/j.joca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004 Feb;25(1):72–101. doi: 10.1210/er.2003-0007. Available from: http://dx.doi.org/10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 14.Kodach LL, Bleuming SA, Musler AR, Peppelenbosch MP, Hommes DW, van den Brink GR, van Noesel CJ, Offerhaus GJ, Hardwick JC. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer. 2008 Jan;112(2):300–306. doi: 10.1002/cncr.23160. Available from: http://dx.doi.org/10.1002/cncr.23160. [DOI] [PubMed] [Google Scholar]
- 15.Steinert S, Kroll TC, Taubert I, Pusch L, Hortschansky P, Höffken K, Wölfl S, Clement JH. Differential expression of cancer-related genes by single and permanent exposure to bone morphogenetic protein 2. J Cancer Res Clin Oncol. 2008 Nov;134(11):1237–1245. doi: 10.1007/s00432-008-0396-0. Available from: http://dx.doi.org/10.1007/s00432-008-0396-0. [DOI] [PubMed] [Google Scholar]
- 16.van der Kraan PM, Blaney Davidson EN, van den Berg WB. Bone morphogenetic proteins and articular cartilage: To serve and protect or a wolf in sheep clothing's? Osteoarthr Cartil. 2010 Jun;18(6):735–741. doi: 10.1016/j.joca.2010.03.001. Available from: http://dx.doi.org/10.1016/j.joca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 17.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001 Nov;239(1):1–14. doi: 10.1006/dbio.2001.0388. Available from: http://dx.doi.org/10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 18.Tardif G, Pelletier JP, Boileau C, Martel-Pelletier J. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: an immunohistochemical study. Osteoarthr Cartil. 2009 Feb;17(2):263–270. doi: 10.1016/j.joca.2008.06.022. Available from: http://dx.doi.org/10.1016/j.joca.2008.06.022. [DOI] [PubMed] [Google Scholar]