Abstract
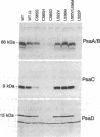
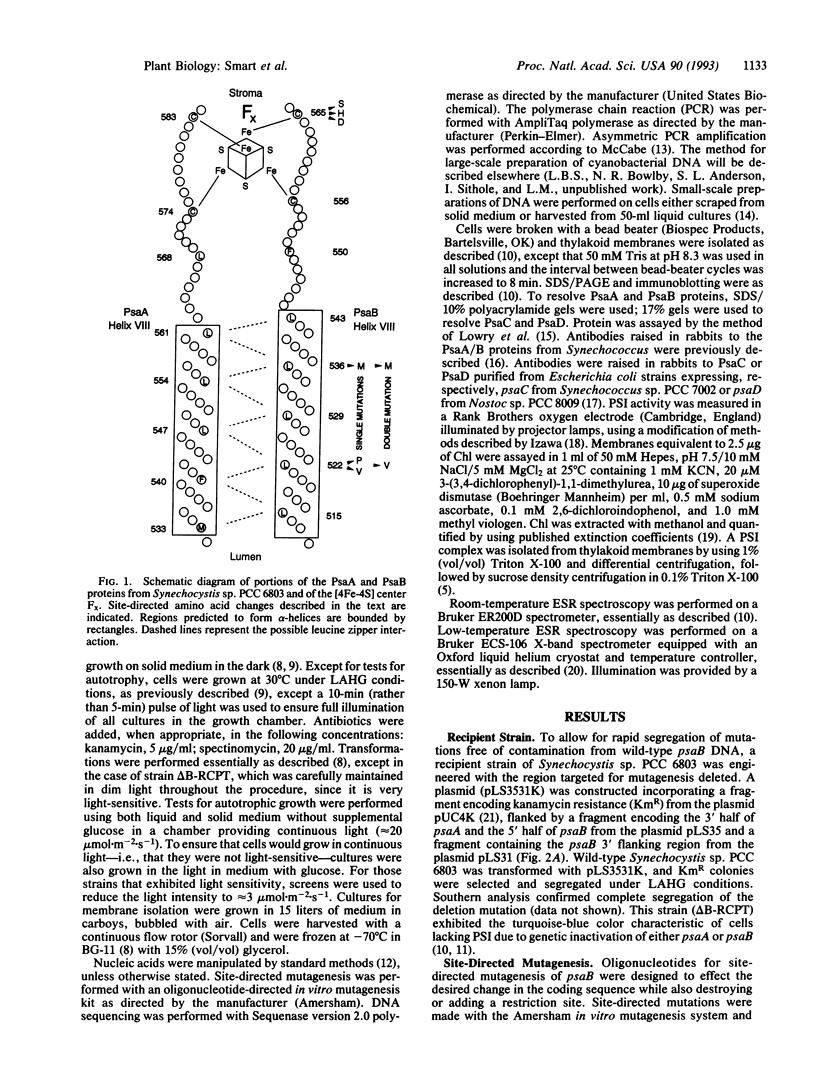
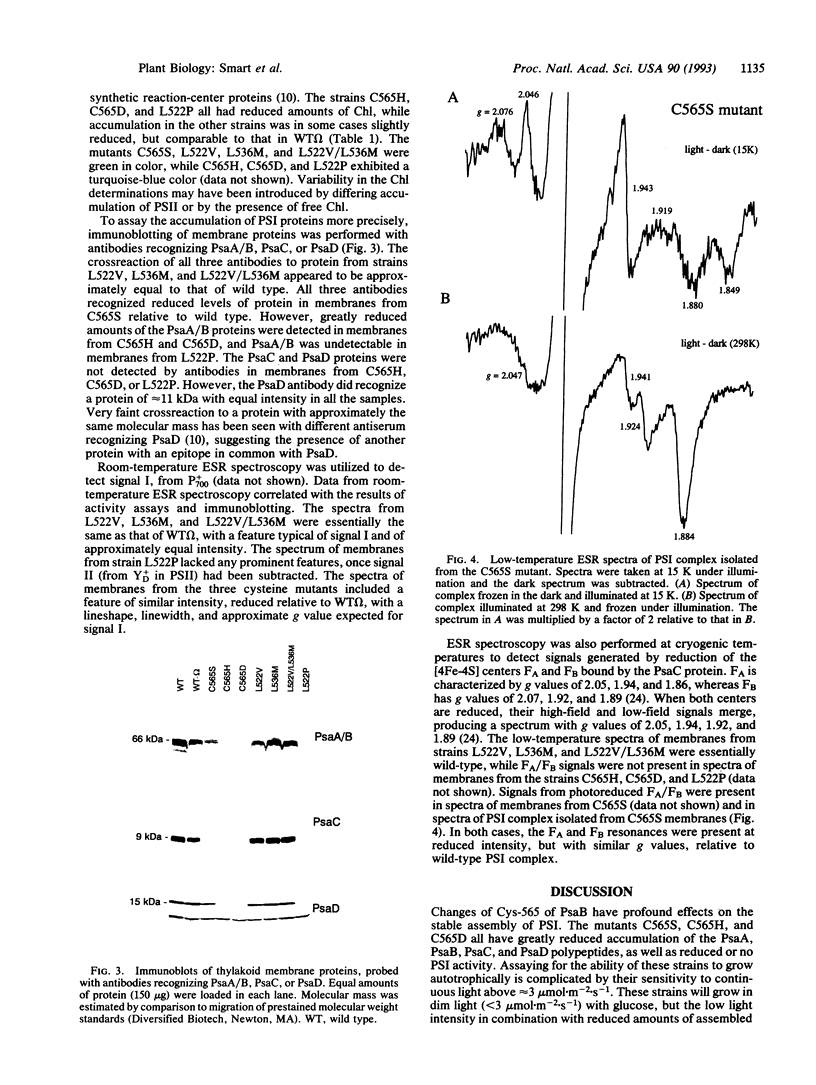
We have utilized the unicellular cyanobacterium Synechocystis sp. PCC 6803 to incorporate site-directed amino acid substitutions into the photosystem I (PSI) reactioncenter protein PsaB. A cysteine residue (position 565 of PsaB) proposed to serve as a ligand to the [4Fe-4S] center Fx was changed to serine, histidine, and aspartate. These three mutants--C565S, C565H, and C565D--all exhibited greatly reduced accumulation of PSI reaction-center proteins and failed to grow autotrophically, indicating that this cysteine most likely does coordinate Fx, which is crucial for PSI biogenesis. Interestingly, the strain C565S accumulated significantly more PSI than the other two cysteine mutants and displayed photoreduction of the [4Fe-4S] terminal electron acceptors FA and FB. Mutations were also introduced into a leucine zipper motif of PsaB, proposed to participate in reaction-center dimerization. The mutants L522V, L536M, and L522V/L536M all exhibited wild-type characteristics and grew autotrophically, whereas the L522P mutation prevented PSI accumulation. These data do not provide support for a major structural role of the leucine zipper in reaction-center dimerization or in assembly of Fx. However, the amino acid substitutions incorporated were conservative and might not have perturbed the leucine zipper.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog O., Shoham G., Michaeli D., Nechushtai R. Monomeric and trimeric forms of photosystem I reaction center of Mastigocladus laminosus: crystallization and preliminary characterization. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5312–5316. doi: 10.1073/pnas.88.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. L., McIntosh L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J Bacteriol. 1991 May;173(9):2761–2767. doi: 10.1128/jb.173.9.2761-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt R. D., Sauer K., Klein M. P., Knaff D. B., Kriauciunas A., Yu C. A., Yu L., Malkin R. Electron spin echo envelope modulation spectroscopy supports the suggested coordination of two histidine ligands to the Rieske Fe-S centers of the cytochrome b6f complex of spinach and the cytochrome bc1 complexes of Rhodospirillum rubrum, Rhodobacter sphaeroides R-26, and bovine heart mitochondria. Biochemistry. 1991 Feb 19;30(7):1892–1901. doi: 10.1021/bi00221a023. [DOI] [PubMed] [Google Scholar]
- Conover R. C., Kowal A. T., Fu W. G., Park J. B., Aono S., Adams M. W., Johnson M. K. Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem. 1990 May 25;265(15):8533–8541. [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Kössel H., Döry I., Igloi G., Maier R. A leucine-zipper motif in photosystem I. Plant Mol Biol. 1990 Sep;15(3):497–499. doi: 10.1007/BF00019166. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Li N., Zhao J. D., Warren P. V., Warden J. T., Bryant D. A., Golbeck J. H. PsaD is required for the stable binding of PsaC to the photosystem I core protein of Synechococcus sp. PCC 6301. Biochemistry. 1991 Aug 6;30(31):7863–7872. doi: 10.1021/bi00245a028. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Membrane-bound iron-sulfur centers in photosynthetic systems. Biochim Biophys Acta. 1978 Oct 23;505(2):147–181. doi: 10.1016/0304-4173(78)90011-3. [DOI] [PubMed] [Google Scholar]
- Manodori A., Cecchini G., Schröder I., Gunsalus R. P., Werth M. T., Johnson M. K. [3Fe-4S] to [4Fe-4S] cluster conversion in Escherichia coli fumarate reductase by site-directed mutagenesis. Biochemistry. 1992 Mar 17;31(10):2703–2712. doi: 10.1021/bi00125a010. [DOI] [PubMed] [Google Scholar]
- McCormack K., Tanouye M. A., Iverson L. E., Lin J. W., Ramaswami M., McCormack T., Campanelli J. T., Mathew M. K., Rudy B. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-oka H., Takahashi Y., Kuriyama K., Saeki K., Matsubara H. The protein responsible for center A/B in spinach photosystem I: isolation with iron-sulfur cluster(s) and complete sequence analysis. J Biochem. 1988 Jun;103(6):962–968. doi: 10.1093/oxfordjournals.jbchem.a122394. [DOI] [PubMed] [Google Scholar]
- Ohad N., Hirschberg J. Mutations in the D1 subunit of photosystem II distinguish between quinone and herbicide binding sites. Plant Cell. 1992 Mar;4(3):273–282. doi: 10.1105/tpc.4.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Smart L. B., Anderson S. L., McIntosh L. Targeted genetic inactivation of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC 6803. EMBO J. 1991 Nov;10(11):3289–3296. doi: 10.1002/j.1460-2075.1991.tb04893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart L. B., McIntosh L. Expression of photosynthesis genes in the cyanobacterium Synechocystis sp. PCC 6803: psaA-psaB and psbA transcripts accumulate in dark-grown cells. Plant Mol Biol. 1991 Nov;17(5):959–971. doi: 10.1007/BF00037136. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Webber A. N., Malkin R. Photosystem I reaction-centre proteins contain leucine zipper motifs. A proposed role in dimer formation. FEBS Lett. 1990 May 7;264(1):1–4. doi: 10.1016/0014-5793(90)80749-9. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li N., Warren P. V., Golbeck J. H., Bryant D. A. Site-directed conversion of a cysteine to aspartate leads to the assembly of a [3Fe-4S] cluster in PsaC of photosystem I. The photoreduction of FA is independent of FB. Biochemistry. 1992 Jun 9;31(22):5093–5099. doi: 10.1021/bi00137a001. [DOI] [PubMed] [Google Scholar]