Abstract
Purpose
In spite of the recent improved results of hepatectomy for huge hepatocellular carcinomas (HCC), the prognosis of patients with huge HCCs is still poor compared to that of patients with small HCCs. This study was performed to compare the results of hepatectomy between patients with huge HCCs and those with small HCCs, to identify the prognostic factors in patients with huge HCCs, and to determine the preoperative selection criteria.
Methods
We retrospectively analyzed 51 patients who underwent hepatectomy, between July 1994 and February 2009 at Dankook University Hospital. Patients with HCC≥10 cm were classified in large (L) group and others were classified in small (S) group. The clinicopathological features, operative procedures, and postoperative outcome were compared between both groups and various prognostic factors were investigated in group L.
Results
Eleven patients were classified in group L. Tumor size, vascular invasion, and tumor stage were higher in group L. Postoperative morbidity was higher in group L, but mortality was not different between the groups. Disease-free survivals were significantly lower in group L than in group S (36.4%, and 24.2% vs. 72.0%, and 44.0% for 1- and 3-year), but overall survival rates were similar in both groups (45.5%, and 15.2% in group L vs. 60.3%, and 41.3% in group S for 3- and 5-year). Presence of satellite nodules was the only prognostic factor in multivariate analysis after surgery for huge HCC.
Conclusion
Regardless of tumor size, huge HCCs deserve consideration for surgery in patients with preserved liver function. Furthermore, the effect of surgery could be maximized with appropriate selection criteria, such as huge HCC without satellite nodules.
Keywords: Carcinoma, Hepatocellular, Hepatectomy, Prognosis
Introduction
According to recent publications on cancer incidence in Korea by the Korea Central Cancer Registry,1 the incidence of hepatocellular carcinoma (HCC) and its proportion among total carcinomas has decreased slightly over the past several years. However, HCC is still the fifth most common carcinoma in the Korean population as of 2008. While it is true that early detection of HCC has become easier due to increasing interest in regular checkup in high risk patients and upgraded imaging techniques, considerably enlarged tumors are not infrequently encountered in clinical practice.
Huge HCC, which is generally defined when its greatest diameter is 10 cm or more, although there is a variation according to literatures, has a poorer prognosis than that of smaller HCC due to higher incidence of vascular invasion and more aggressive tumor biology. Unfortunately, patients with huge HCC are not eligible for other treatment modalities such as liver transplantation, percutaneous ethanol injection therapy (PEIT), radiofrequency ablation (RFA), and while some centers tries transarterial embolization (TAE), the 5-year survival rate is less than 10%. Consequently, surgical resection remains the only treatment option that offers opportunities for long-term survival or complete cure. However, surgical resection of huge HCCs is a great challenge to the liver surgeons, because surgery for these tumors entails prolonged operative time and has an increased risk for massive bleeding or liver failure after major hepatectomies in patients with chronic hepatitis or early stage cirrhosis; furthermore, rapid recurrence after surgery is not infrequent.
An increasing number of studies have reported favorable outcomes after hepatectomy for huge HCC,2,3,4,5,6,7 but the results of surgical intervention in huge HCCs still remain poor compared to that of small HCCs. To maximize the effect of surgical resection through prolongation of survival duration and improving the quality of life in patients with huge HCC, adequate selection of candidates for surgery is of great importance.
This study was performed to investigate the effect of hepatectomy for huge HCC by comparing the surgical outcomes of patients with huge HCC to those of patients with small HCC, and to establish surgical strategy for preoperative patient selection by identification of independent prognostic factors in patients with huge HCC.
Methods
1. Patients and surgical treatment
Between July 1994 and February 2009, 51 patients with HCC underwent partial hepatectomy without gross residual tumor with intent of radical surgery at Dankook University Hospital. Retrospective analysis was made by reviewing the medical records and if required, by telephonic communication. Patients with HCC≥10 cm were classified in large (L) group, and others were classified in small (S) group.
Both groups were compared with respect to the following preoperative demographic and clinicopathologic characteristics, such as age, gender, presenting symptoms, etiology of HCC, Child-Pugh classification, platelet count, prothrombin time, serum total bilirubin, serum alpha-fetoprotein (AFP), aspartate transaminase (AST), alanine transaminase (ALT), serum albumin, and preoperative TAE; histopathological factors, such as gross classification of tumor, tumor size, tumor cell differentiation, presence of satellite nodules, presence of macroscopic and microscopic vascular invasion, presence of capsular invasion, ruptured tumor, radical resection, resection margin, tumor TNM stage, and presence of liver cirrhosis; factors related to surgery, such as type of hepatectomy, operative time, intraoperative blood loss, perioperative transfusion, postoperative hospital stay, morbidity rate, and mortality rate; surgical outcomes, such as postoperative disease-free survival and overall survival. Then these factors were also analyzed to identify significant prognostic factors for patients with huge HCC.
Gross classification of HCC was determined according to the classification suggested by Baer et al.8 in 1989, which is the most widely employed classification at present, well predicts the resectability of HCC, and divides HCC into 3 types; hanging, pushing, and invasive type. Modified UICC stage (4th ed) of the Liver Cancer Study Group of Japan, which was adopted in the practice guidelines for management of hepatocellular carcinoma 2009 of the Korean Liver Cancer Study Group, was used for tumor staging.9
Surgery was determined by taking into consideration the degree of liver cirrhosis and disease progression simultaneously. Candidates with HCC selected for surgery were those in whom all the tumors, regardless of tumor size and number and vascular invasion, would be included in the extent of resection estimated by liver function, and there was no extrahepatic metastasis nor tumor thrombosis in the main portal vein and inferior vena cava on preoperative imaging studies. Liver function was assessed by using the Child-Pugh classification, serum total bilirubin, the 15-minute retention rate for indocyanine green (ICG R15), presence of portal hypertension, and liver volumetry. In principle, surgery was not performed in patients with huge HCC who were in portal hypertension or Child-Pugh class B.
Type of hepatectomy was divided into major resection; which was the resection of more than 3 segments according to the Couinaud classification of liver anatomy, and minor resection; in which less than 2 segments were removed. Laparoscopic surgery was not separately classified and was analyzed together with open surgery in this study. Intraoperative ultrasonography was willingly used to assess the resection plane related to tumor location, the relationship between the tumor and vessels, and the additional tumors not preoperatively detected. The method for parenchymal division was different according to the period of surgery, and consisted of the fracture technique with either Kelly clamps or fingers and resection with waterjet or CUSA (Cavitron ultrasonic surgical aspirator). Whether to use and how to perform the intraoperative temporal block of the hepatic inflow differed according to the period of surgery and the surgeon's preference. Curative resection was defined as grossly and microscopically complete removal of the tumor.
According to the classification suggested by Dindo et al.10 in 2004, postoperative complications which required surgical, endoscopic, or radiologic intervention, or were life-threatening were categorized into major complications, and the rest were into minor complications. Operative mortality was defined as death before discharge from the hospital or death within 30 days after surgery. Postoperative recurrent disease was basically diagnosed by radiologic imaging modalities.
2. Analysis of prognostic factors for overall survival in patients with huge HCC
Factors that showed significant statistical difference between the two groups and those factors that have frequently been described as significant in the previous literature were selected as prognostic factors for overall survival in patients with huge HCC. The factors subsequently selected were gender (male vs. female), age (≥60 years vs. <60 years), AFP levels (≥1,000 ng/ml vs. <1,000 ng/ml), preoperative TAE (yes vs. no), operative time (≥500 min vs. <500 min), intraoperative blood loss (≥2,500 ml vs. <2,500 ml), perioperative transfusion (yes vs. no), gross classification of tumor (noninvasive vs. invasive), tumor cell differentiation (well or moderately differentiated vs. poorly or undifferentiated), satellite nodules (yes vs. no), macroscopic vascular invasion (yes vs. no), microscopic vascular invasion (yes vs. no), capsular invasion (yes vs. no), ruptured tumor (yes vs. no), resection margin (≥10 mm vs. <10 mm), tumor TNM stage (II or III vs. IV), and liver cirrhosis (yes vs. no).
Each of the selected factors was first subjected to univariate analysis in order to determine the statistical significance, and only the significant factors then went through multivariate analysis to identify the final significant independent prognostic factors for overall survival in patients with huge HCC.
3. Statistical analysis
Statistical analyses were performed by PASW for Windows 18.0 (SPSS Inc, Chicago, IL, USA). In univariate analysis, the Fisher's exact test and Mann-Whitney test were used to compare the demographic, clinicopathological, and histopathological factors between the two groups. Survival duration, of which starting point was defined as the operation date, was calculated by the Kaplan-Meier method, and the Log-rank test was employed to compare the survival rates between the two groups. As for analysis of significant prognostic factors in patients with huge HCC, the Log-rank test was used for univariate analysis, and the Cox-proportional hazards model was used for multivariate analysis. p-values less than 0.05 were considered as statistically significant.
Results
1. Clinicopathological and histopathological characteristics
During this study period, the number of patients who received partial hepatectomy for HCC was 51, of which 11 (21.6%) patients had tumor size larger than 10 cm (group L). Comparison of the clinicopathological and histopathological characteristics between group L and group S is shown in Table 1 and Table 2. Among the total 51 patients, the male patients (42, 82.4%) outnumbered the female patients; the male : female ratio in group L was almost equal (6 : 5), while in group S this ratio was significantly shifted towards males (9 : 1, p=0.015). The mean age was similar in the two groups (52.4 years in group L and 54.6 years in group S respectively). The most common cause of chronic liver disease was viral hepatitis B in both groups. All patients in group L were in Child-Pugh class A, while a small proportion (12.5%) of patients in group S were in Child-Pugh class B. The pushing type HCC was found to be the most common gross type (63.6% in group L, 62.5% in group S), and there was no difference in the distribution of gross classification between both groups. Curative resection was achieved in all group L patients, which was similar in group S patients (90%). While tumor size (145.0 mm vs. 38.1 mm), macroscopic (81.8% vs. 12.5%) and microscopic (63.6% vs. 22.5%) vascular invasion , and tumor stage (T2: 18.2% vs. 67.5%, T3: 72.7% vs. 27.5%) were significantly higher in group L, no significant differrence between the two groups was found in terms of tumor cell differentiation, satellite nodules, capsular invasion, ruptured tumor, and resection margin. The incidence of liver cirrhosis showed significant difference between group L (36.4%) and group S (92.5%), which explains the higher proportion of Child-Pugh A patients in group L.
Table 1. Comparison of clinicopathological features between patients with hepatocellularcarcinoma larger than 10 cm (group L) and patients with smaller tumors (group S).
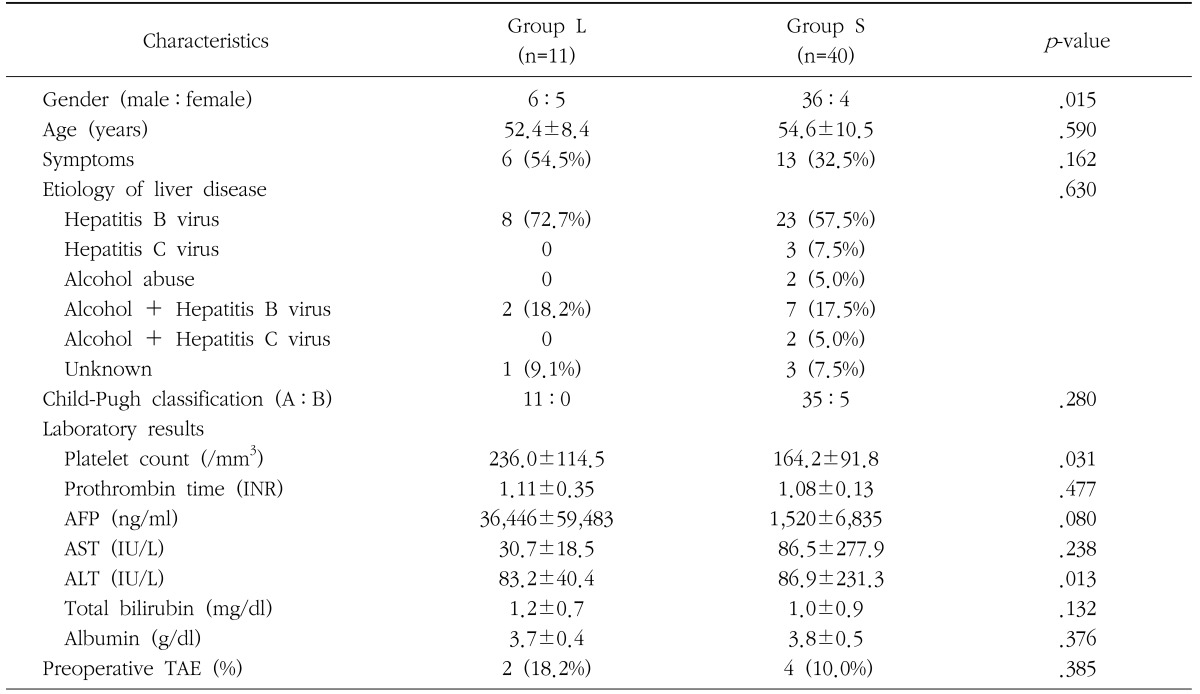
AFP=alpha fetoprotein; AST=aspartate transferase; ALT=alanine transferase; TAE=transarterial embolization
Table 2. Comparison of histopathological results between patients with hepatocellularcarcinoma larger than 10 cm (group L) and patients with smaller tumors (group S).
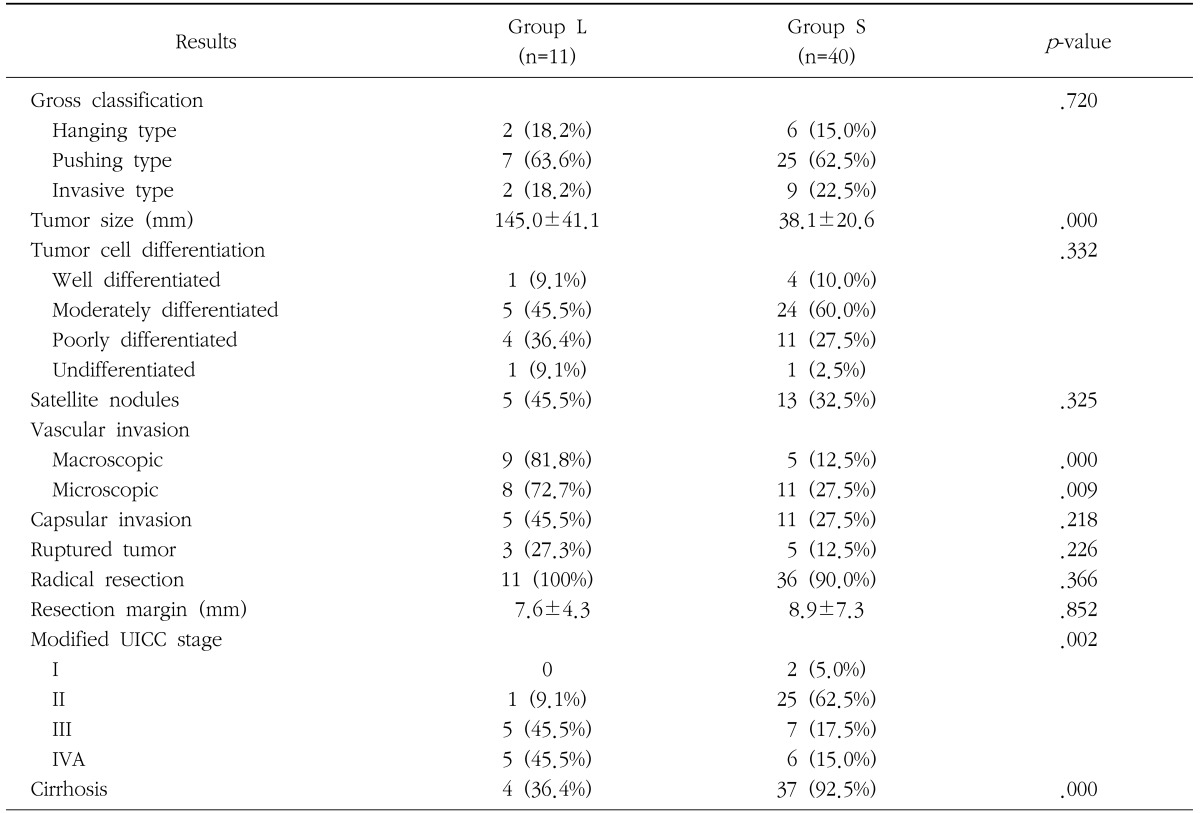
UICC=the international union against cancer
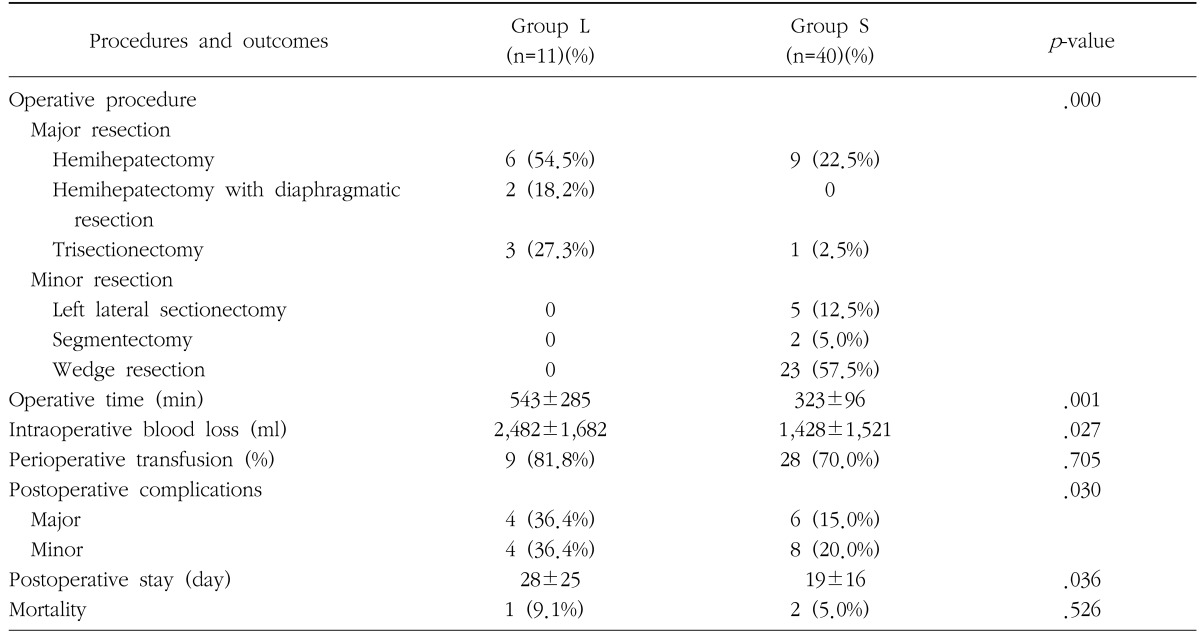
2. Outcomes related to surgery and postoperative complications
Proportion of major hepatectomy, operative time, intraoperative blood loss, postoperative hospital stay, and morbidity rate were significantly higher in group L, however perioperative transfusion and mortality rate were similar in both groups (Table 3). All patients in group L underwent major hepatectomy, among which 2 cases of right hemihepatectomy with partial resection of diaphragm were included. In contrast, major hepatectomy was performed in only 25% of patients in group S, thereby showing a marked difference between the two groups. Perioperative transfusion rates were relatively high in both groups. Morbidity rate was two-fold higher in group L compared to group S. Major complications comprised bile leakage, intraabdominal bleeding and abscess, pleural effusion, liver abscess, wound dehiscence, and minor complications were wound seroma, ascites, minor bile leakage, and mild pleural effusion. There was one death due to liver failure in group L, and two deaths due to intraabdominal bleeding in group S.
Table 3. Comparison of operative procedures and outcomes between patients with hepatocellularcarcinoma larger than 10 cm in diameter (group L) and patients with smaller tumors (group S).
3. Postoperative recurrence and survival analysis
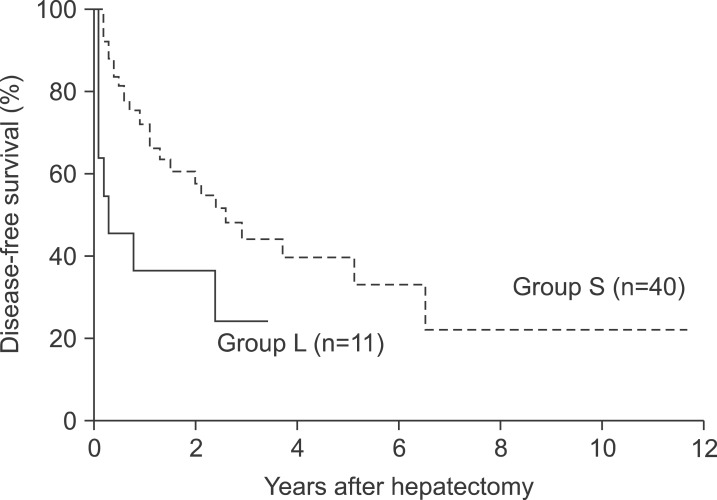
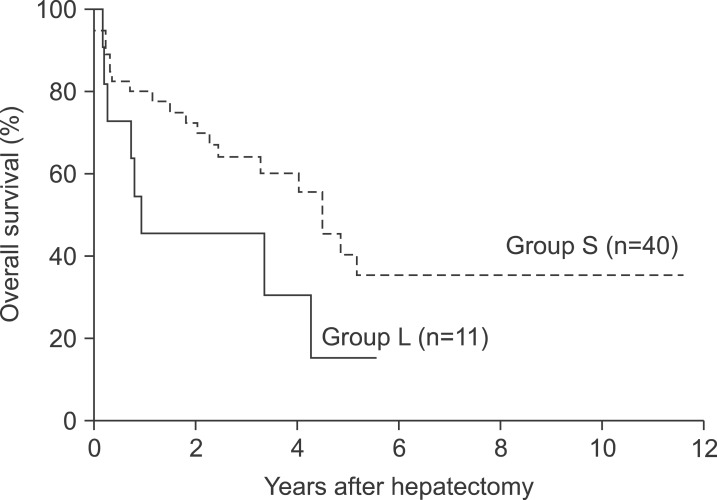
The median follow-up duration for survival analysis was 30 months. During this period, postoperative recurrence was detected in 8 patients (72.7%) in group L and 17 patients (42.5%) in group S. The 1- and 3-year disease-free survival rates in group L were 36.4% and 24.2%s, respectively, which were significantly lower than those in group S (72.0% and 44.0%, respectively, p=0.030, Fig. 1). In addition, the median disease-free survival was significantly lower in group L (4.0 months) compared to 31.0 months in group S (p=0.030). In contrast, the 1-year, 3-year, and 5-year survival rates in group L (45.5%, 45.5%, and 15.2%, respectively) were lower than those in group S (80.0%, 60.3%, and 41.3%, respectively), but this difference was not statistically significant (Fig. 2).
Fig. 1. Cumulative disease-free survival curves of the two groups.
Fig. 2. Cumulative overall survival curves of the two groups.
4. Prognostic factors for overall survival in patients with huge HCC
Univariate analysis revealed presence of satellite nodules was the only independent factor affecting overall survival of group L (Table 4). In multivariate analysis, where the potential prognostic factors of p-value less than 0.1 in univariate analysis, such as age, AFP level, microscopic vascular invasion, and tumor TNM stage were analyzed together with satellite nodules, the only significant prognostic factor was again satellite nodules. The 1-year survival rate in patients with huge HCC accompanied with satellite nodules was 0%, while patients without satellite nodules showed the survival rate was 83.3% at 1 and 3 years.
Table 4. Significant prognostic factors for overall survival in patients with HCC larger than 10 cm in diameter by univariate analysis.
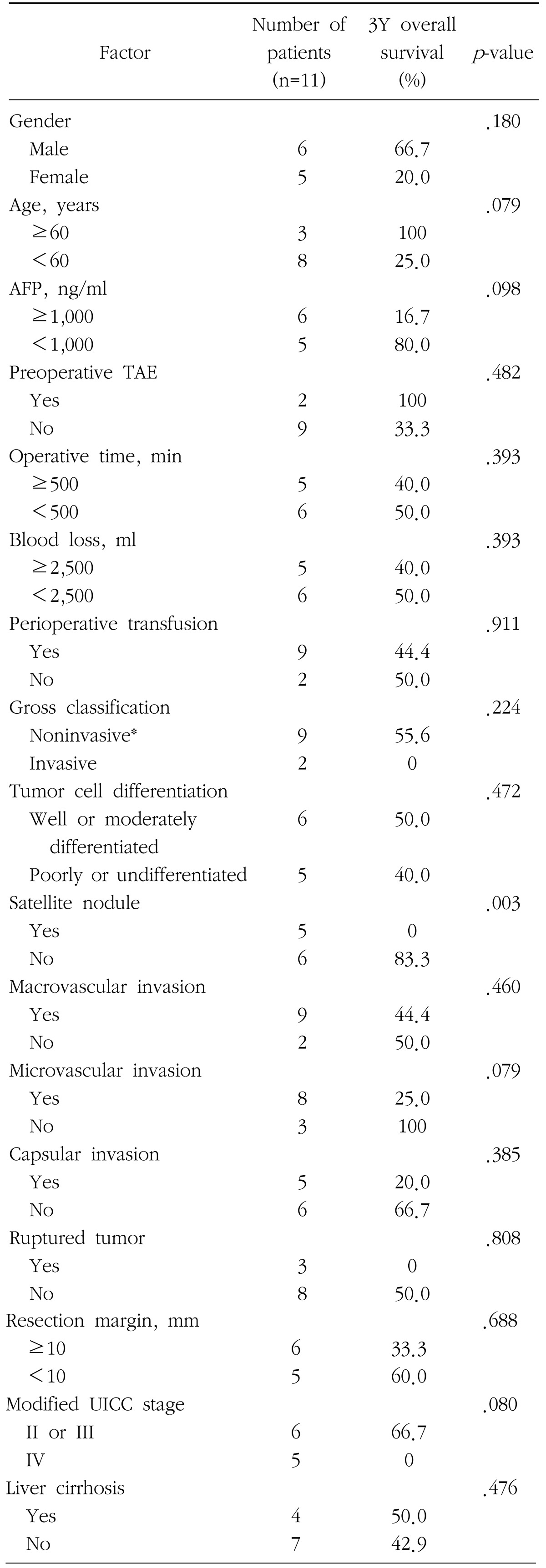
AFP=alpha fetoprotein; TAE=transarterial embolization; UICC=the International Union Against Cancer; *Noninvasive includes hanging type and pushing type
Discussion
In general, the prognosis of HCC has been known to become poorer as the tumor size increases due to progression of vascular invasion and aggravation of tumor biology.11 In the present study, macroscopic and microscopic vascular invasion were more frequent in group L, but tumor cell differentiation showed no significant difference between the two groups. In addition, although numerical values of survival rates of both group were different, these difference had no statistical significance. Furthermore, it is not uncommon to find literature that reports no difference in survival between patients with huge HCC and small HCC.6,7,12
In a series of Shah et al.,7 the 5-year overall survival rate for patients with huge HCC has been reported to be as high as 54%, while the majority of researchers, who conducted the study under similar conditions, have reported the survival rate to be 20~40%.4,5,6,11,13,14,15,16,17,18,19,20 The reported 5-year survival rates continue to remain high, especially in the recent studies. In contrast, the 5-year survival for patients with huge HCC in this study was a slightly lower (15.2%), and this is thought to be attributable to the inclusion of patients who underwent surgery a long time ago, and the small number of patients enrolled in the study. Pandey et al.16 reported a 5-year survival rate of 28.6% after surgery for huge HCC, which increased to 57.7% in the absence of significant independent factors for survival such as vascular invasion, liver cirrhosis, and satellite nodules, and decreased to 22.5% when at least 1 independent factor was present. Similarly, Shimada et al.21 reported that in patients with a single nodular huge HCC without gross tumor thrombus, the postoperative 5-year survival rate was 69.8%, thereby demonstrating high survival rates in selected patients with huge HCC. As in a similar study by Taniai et al.,17 those patients who had liver cirrhosis with gross vascular invasion or with multiple nodular huge HCC were excluded from the selected group for surgery. These suggest that in order to improve postoperative survival, a meticulous selection of patients for surgery is requisite.
As seen to date from the results of numerous studies,2,7,13,17,18 the present study showed that early recurrence rate within the first year after surgery and the overall recurrence rate were significantly high in patients with huge HCC. Early recurrence within the first year after surgery occurred in 7 (63.6%) patients in group L, and this rate was significantly higher than that of patients (10, 25.0%) in group S. This high recurrence rate of huge HCCs is probably not only due to the biologic characteristics of easy recurrence, but also to a certain extent, due to tumor cell dissemination that results from manipulation of tumor or mobilization of the liver containing tumor during operation. Accordingly, in order to minimize such tumor cell dissemination during operation for huge HCCs, the employment of the anterior approach or the liver hanging maneuver has increased in recent years.22,23,24,25,26,27
Among the 10 patients in group L, 3 were alive at the time of this study. Two of these surviving patients had shown no evidence of recurrence for 12 and 32 months after surgery, respectively. The other one patient was suspected to have recurrent disease at the postoperative 29th month, but no evidence of recurrence had been demonstrated for 37 months after second operation; a total 66-month survival. The last patient was the only survivor for more than 5 years in group L, and was also the longest survivor in this group. The patient who survived for 66 months was a male aged 68 years at the time of surgery and had a 13 cm-sized HCC diagnosed incidentally in medical checkup. No evident etiology for HCC was found, and the AFP level was normal at 1 ng/ml. The tumor was hanging type located in the left lateral section and macroscopic vascular invasion was suspected. Histopathological examination revealed that it was the only well differentiated HCC among group L, without evidence of microscopic vascular invasion or satellite nodules, but early stage of liver cirrhosis was present. Another patient who had been free of disease for 32 months was a 58-year-old male and was a hepatitis B carrier. He had a HCC of 18 cm in diameter incidentally detected without symptoms. Serum AFP level was normal at 3 ng/ml and the tumor was pushing type located in the right hemiliver and macroscopic vascular invasion was present. Histopathological examination proved the tumor without satellite nodules or liver cirrhosis, but poorly differentiated and with microscopic vascular invasion. With the exception of a patient with a short disease-free survival of 12 months, both patients with long-term survivals of 32 and 66 months had several common characteristics such as: incidentally detected tumors without symptoms, normal AFP levels, noninvasive type of gross tumor classification, and absence of satellite nodules. However, further studies based on more patients are required for the above mentioned characteristics to have any clinical impact.
Independent factors that influence survival after surgery for HCCs vary between studies, but common factors suggested are: tumor size, tumor stage, resection margin, vascular invasion, tumor cell differentiation, and satellite nodules.11 Confined to analyses of surgery for huge HCCs, reported independent factors comprise gross classification of tumor, liver cirrhosis, tumor stage, portal vein invasion, radicality of surgery, and multiplicity of tumor.2,6,16,17,18,20,21
The present study revealed that satellite nodules were the only independent factors for survival, and the presence or absence of satellite nodules significantly affected postoperative survival in patients with huge HCC (1-year survival rate: 0% vs. 83.3%). Studies that have demonstrated satellite nodules as an independent factor for survival in huge HCC are rare. Pandey et al.16 observed that vascular invasion and liver cirrhosis along with satellite nodules were the independent factors for survival in patients with huge HCC. Shimada et al.21 also showed a high 5-year survival rate of 69.8% in patients with a single large HCC ≥10 cm but without gross tumor thrombus, indirectly suggesting that satellite nodules or multiple tumors were related with a poor prognosis. Similar conclusions were made by Yang et al.,12 who stated that clinical characteristics and surgical outcomes of single huge (>5 cm) HCCs were similar to those of small HCCs.
Different gross classifications of tumor have been employed between researchers; Eggel et al.28 classified HCCs into three categories, nodular type, massive type, and the diffuse type according to tumor size and morphology in 1901, while in 1984 Okuda et al.29 classified HCCs according to pattern of tumor growth - expanding type, spreading type, and the multifocal type. Kanai et al.30 suggested another classification in 1987, which is presently adopted by the Liver Cancer Study Group of Japan, in which four types have been described according to tumor morphology; single nodular type, single nodular type with extranodular growth, confluent multinodular type, and the infiltrative type. Thus, it can be seen, that there had been no uniform consensus on the gross classification of HCCs, which had led to inconsistent research and assessment. In 1989, Baer et al.8 presented a classification - hanging type, pushing type, and the invasive type, that predicts resectability of liver tumors, including HCCs, and which is widely being adopted at present. As the present study is an investigation of hepatectomy for huge HCCs, the classification suggested by Baer et al.8 was incorporated. While several studies have reported the clinical characteristics and surgical outcomes of the whole HCCs with respect to gross morphology, studies limited to huge HCCs have only recently emerged.12,18 Regardless of kind of gross classification, non-invasive huge HCCs demonstrate a more favorable prognosis, and this study was also able to confirm that patients with non-invasive gross type showed longer survival, despite the limitation of small number of study cases.
Taniai et al.17 and Pawlik et al.11 concluded that the presence of liver cirrhosis in patients with huge HCCs was a significant prognostic factor, and Taniai et al.17 further postulated that huge HCCs with gross vascular invasion or multiple tumors in cirrhotic background were not appropriate for surgery. Since liver cirrhosis itself is a risk factor for recurrence of HCC in the remnant liver, it is an independent prognostic factor for survival, irrespective of tumor size. In this study, despite the fact that liver cirrhosis was present in 4 (36.4%) patients with huge HCCs, there was no significant difference in survival according to liver cirrhosis. This may be due to mild cirrhosis with relatively preserved liver function of Child-Pugh classification A. Another possible reason is that survival was determined by other more significant factors related to tumor, rather than cirrhosis.
It is the common expectation of all liver surgeons to maximize the surgical outcomes by appropriate selection of patients with huge HCCs for surgery, based upon studies of independent prognostic factors for survival. In this context, Poon et al.2 and Shimada et al.21 emphasized that surgical outcomes could be maximized in patients of a single nodular huge HCC without gross vascular invasion or tumor thrombosis. To date, recent trends have concentrated on the presence of vascular invasion and multiplicity of tumors as the guideline for establishing the selection criteria for surgery in HCC patients, rather than tumor size.12
To improve the prognosis of patients with huge HCC, major hepatectomies, which resect 3 or more segments according to the Couinaud's classification of liver anatomy, are mostly performed. These hepatectomies may sometimes be combined with other resections of neighboring organs or tissues for radical surgery. While reports on such combined resections are rare, Chen et al.19 performed combined resections in 17.2% of HCC patients, and partial resection of diaphragm was conducted in 2 (18.2%) patients of this study.
Conclusion
Although the number of cases was small and further studies are required in future, considering the results of this study that there was no significant statistical difference in overall survival after surgery in both groups, as the similar results of the previous studies on surgical treatment for huge HCC, surgical resection for huge HCCs should be actively attempted, irrespective of tumor size, as long as the patient's condition is acceptable. Furthermore, to enhance surgical outcome of huge HCC and to improve quality of life in these patients, careful and appropriate selection of candidates for surgery should be based on the independent prognostic factors, such as satellite nodules.
Footnotes
The author would like to express his gratitude to Dr. Wonae Lee of The Department of Pathology, Dankook University College of Medicine, for the assistance in reviewing the HCC slides and collection of patient information.
The present research was conducted by the research fund of Dankook University in 2009.
References
- 1.Korea Central Cancer Registry. 2008 Cancer Statistics in Korea. Ministry for Health, Welfare, and Family Affairs; 2010. [Google Scholar]
- 2.Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- 3.Hanazaki K, Kajikawa S, Shimozawa N, et al. Hepatic resection for hepatocellular carcinoma in diameter of >or=10 cm. Hepatogastroenterology. 2002;49:518–523. [PubMed] [Google Scholar]
- 4.Zhou XD, Tang ZY, Ma ZC, et al. Surgery for large primary liver cancer more than 10 cm in diameter. J Cancer Res Clin Oncol. 2003;129:543–548. doi: 10.1007/s00432-003-0446-6. [DOI] [PubMed] [Google Scholar]
- 5.Nagano Y, Tanaka K, Togo S, et al. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg. 2005;29:66–71. doi: 10.1007/s00268-004-7509-y. [DOI] [PubMed] [Google Scholar]
- 6.Liau KH, Ruo L, Shia J, et al. Outcome of partial hepatectomy for large (>10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 7.Shah SA, Wei AC, Cleary SP, et al. Prognosis and results after resection of very large (>or=10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589–595. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 8.Baer HU, Gertsch P, Matthews JB, et al. Resectability of large focal liver lesions. Br J Surg. 1989;76:1042–1044. doi: 10.1002/bjs.1800761019. [DOI] [PubMed] [Google Scholar]
- 9.Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 12.Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118–123. doi: 10.1097/SLA.0b013e3181904988. [DOI] [PubMed] [Google Scholar]
- 13.Mok KT, Wang BW, Lo GH, et al. Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surg. 2003;197:730–738. doi: 10.1016/j.jamcollsurg.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CN, Lee WC, Chen MF. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. 2003;10:1070–1076. doi: 10.1245/aso.2003.03.072. [DOI] [PubMed] [Google Scholar]
- 15.Chen XP, Qiu FZ, Wu ZD, Zhang BX. Chinese experience with hepatectomy for huge hepatocellular carcinoma. Br J Surg. 2004;91:322–326. doi: 10.1002/bjs.4413. [DOI] [PubMed] [Google Scholar]
- 16.Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 17.Taniai N, Yoshida H, Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:410–416. doi: 10.1007/s00534-007-1317-3. [DOI] [PubMed] [Google Scholar]
- 18.Choi GH, Han DH, Kim DH, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009;198:693–701. doi: 10.1016/j.amjsurg.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen XP, Qiu FZ, Wu ZD, Zhang BX. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol. 2006;12:4652–4655. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 21.Shimada K, Sakamoto Y, Esaki M, Kosuge T. Role of a hepatectomy for the treatment of large hepatocellular carcinomas measuring 10 cm or larger in diameter. Langenbecks Arch Surg. 2008;393:521–526. doi: 10.1007/s00423-007-0264-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244:194–203. doi: 10.1097/01.sla.0000225095.18754.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizawa T, Kokudo N, Makuuchi M. Right hepatectomy for hepatocellular carcinoma: is the anterior approach superior to the conventional approach? Ann Surg. 2008;247:390–391. doi: 10.1097/SLA.0b013e3181640207. [DOI] [PubMed] [Google Scholar]
- 24.Belghiti J. Editorial perspective: resection of large hepatocellular carcinoma using combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1879–1880. doi: 10.1007/s00268-010-0593-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1874–1878. doi: 10.1007/s00268-010-0546-9. [DOI] [PubMed] [Google Scholar]
- 26.Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. doi: 10.1097/00000658-200007000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TH, Lee SG, Song GW, et al. Right hemihepatectomy using an anterior approach technique for a hepatocellular carcinoma >10 cm in size. Korean J Hepatobiliary Pancreat Surg. 2008;12:232–237. [Google Scholar]
- 28.Eggel H. Uber des primare Carcinom des Leber. Beitrage zur Pathologischen Anatomie und zur Allgemeinen Pathologie. 1901;30:506–604. [Google Scholar]
- 29.Okuda K, Peters RL, Simson IW. Gross anatomic features of hepatocellular carcinoma from three disparate geographic areas. Proposal of new classification. Cancer. 1984;54:2165–2173. doi: 10.1002/1097-0142(19841115)54:10<2165::aid-cncr2820541017>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987;60:810–819. doi: 10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]