Abstract
The ability of human deciduous tooth dental pulp cells (HDDPCs) to differentiate into odontoblasts that generate mineralized tissue holds immense potential for therapeutic use in the field of tooth regenerative medicine. Realization of this potential depends on efficient and optimized protocols for the genetic manipulation of HDDPCs. In this study, we demonstrate the use of a PiggyBac (PB)-based gene transfer system as a method for introducing nonviral transposon DNA into HDDPCs and HDDPC-derived inducible pluripotent stem cells. The transfection efficiency of the PB-based system was significantly greater than previously reported for electroporation-based transfection of plasmid DNA. Using the neomycin resistance gene as a selection marker, HDDPCs were stably transfected at a rate nearly 40-fold higher than that achieved using conventional methods. Using this system, it was also possible to introduce two constructs simultaneously into a single cell. The resulting stable transfectants, expressing tdTomato and enhanced green fluorescent protein, exhibited both red and green fluorescence. The established cell line did not lose the acquired phenotype over three months of culture. Based on our results, we concluded that PB is superior to currently available methods for introducing plasmid DNA into HDDPCs. There may be significant challenges in the direct clinical application of this method for human dental tissue engineering due to safety risks and ethical concerns. However, the high level of transfection achieved with PB may have significant advantages in basic scientific research for dental tissue engineering applications, such as functional studies of genes and proteins. Furthermore, it is a useful tool for the isolation of genetically engineered HDDPC-derived stem cells for studies in tooth regenerative medicine.
Keywords: drug selection, electroporation, genetically modified, human deciduous tooth dental pulp cells, PiggyBac
Introduction
Primary dental pulp cells (DPCs) consist of several cell types, including fibroblasts and undifferentiated mesenchymal cells.1 Characterization of these cells has contributed to our understanding of dental pulp (DP) tissue.2,3,4 DPCs also have clinical applications in tooth regenerative medicine. However, little is known about efficient gene transfer systems for these cells, thus hampering our understanding of DPCs at the molecular level.
Several methods have been used to introduce foreign DNA into human DPCs for the ectopic expression of a gene of interest. Electroporation, one of the most commonly used methods for the generation of transiently and stably transfected cells, has been used to transfect human DPCs (ref. 5) and bovine DPCs.6,7 Several commercial liposome-based methods have also been used in immortalized human DPCs.8 Rat DP stem cells have been successfully transfected using calcium phosphate nanoparticles.9 Retroviral vectors can also be used to over express cDNA in human DP stem cells.10 Each of these transfection approaches has advantages and disadvantages. The gene transfer efficiency achieved with the use of nonviral DNA such as plasmids is lower than that achieved with viral vectors, such as retro-, adeno-, and lentiviruses.11,12 However, the preparation of viral DNA is time-consuming and laborious. Therefore, simple, efficient, and convenient methods for the transfer of nonviral DNA to mammalian cells are needed.
The PiggyBac (PB) system, derived from the cabbage looper moth Trichoplusia ni,13 was developed in mutant baculovirus strains, hence the name “PiggyBac”.14,15 In the PB system, a transgene inserted between inverted repeat elements in the PB transposon is excised and integrated into the host genome via the transposition activity of the PB transposase enzyme encoded on a separate vector. This system allows for “cut and paste” transposition of a transgene into the genome at TTAA nucleotide elements.15,16 The PB system can integrate large DNA fragments (9–14 kb) in mice,17 mediate gene delivery into the mouse germline (104 integration sites), and act as a mutagen. PB has also been used for in vitro transfection in various mammalian cells,18 in vivo gene transfer in mice,19 the production of inducible pluripotent stem (iPS) cells,20,21 the analysis of whole genome function22 and cancer gene discovery.23 Recently, the PB system has been considered as a delivery method for gene therapy in humans.24
The aim of this study was to demonstrate the ability of the PB-based gene delivery system to enable highly efficient transfection of human deciduous tooth dental pulp cells (HDDPCs) when used with the Neon Transfection System (Invitrogen, Cologne, Germany), an electroporation-based method in which optimized electroporation parameters promote the delivery of plasmid DNA into the cell nucleus, thereby enhancing gene expression.
Materials and Methods
Culture of primary HDDPCs
HDDPCs were obtained with informed patient consent, and the protocols used in this study were approved by the Ethics Committee of Kagoshima University Graduate School of Medical and Dental Sciences. HDDPCs were isolated as previously described25,26 with slight modifications. Pulp tissue was removed from the deciduous teeth of three young patients (aged 8–10 years) and digested in a solution of 3 mg·mL−1 collagenase type I (#17100-017; Invitrogen, Carlsbad, CA, USA) and 4 mg·mL−1 dispase (#410810077; Roche Applied Science, Upper Bavaria, Germany) for 30–60 min at 37 °C. Next, 4 mL Dulbecco's modified Eagle's Medium (DMEM; #11995-081; Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; #SFMB30-2239; Equitech Bio, Kerrville, TX, USA), 50 U·mL−1 penicillin, and 50 mg·mL−1 streptomycin (#15140-122; Invitrogen, Carlsbad, CA, USA) (DMEM/10% FBS) was added to stop the digestion reaction. The resulting single cell suspension was seeded onto 60-mm gelatin-coated dishes (Iwaki Glass, Tokyo, Japan) containing α-modified minimum essential medium (MEMα #135-15175; Wako Pure Chemical Industries, Osaka, Japan) containing 20% FBS, 100 μmol·L−1 L-ascorbic acid-2-phosphate, 50 U·mL−1 penicillin, and 50 mg·mL−1 streptomycin (MEMα/20% FBS) and was cultured at 37°C in an atmosphere of 5% CO2 in air. After 4–6 passages, HDDPCs were used for transfection experiments.
PB-related plasmids
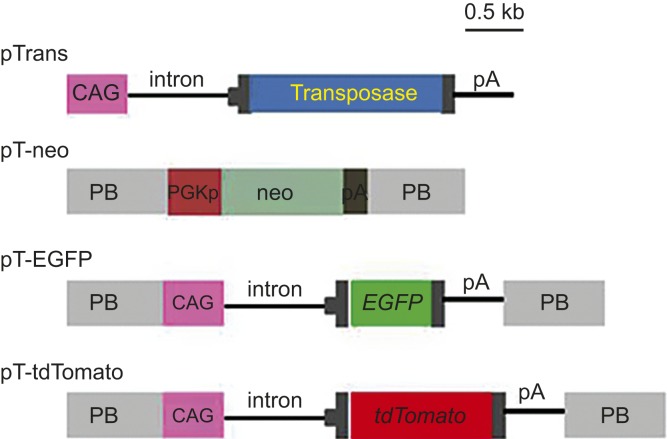
PB expression vectors (Figure 1) were generated using standard cloning procedures. Briefly, the pPB vector contains two PB acceptors with inverted repeats.24 pT-neo (formerly referred to as pTpB) is a pPB-based vector that carries a neomycin resistance gene (neo) expression unit (CMV promoter + neo + poly(A) sites). pT-EGFP is a pPB-based vector that carries an enhanced green fluorescent protein (EGFP) expression unit (CAG promoter27 + EGFP cDNA + poly(A) sites). pT-tdTomato is a pPB-based vector that carries a tandem dimer Tomato (tdTomato) cDNA expression unit (kindly provided by Dr. Roger Tsien) under the control of the CAG promoter. The pTrans vector expresses transposase under the CAG promoter.
Figure 1.
Schematic representation of the expression vectors used in this study. The plasmid backbone is not shown in the figure. CAG, cytomegalovirus enhancer + chicken β-actin promoter; pA, poly(A) sites; EGFP, enhanced green fluorescent protein cDNA; neo, neomycin resistance gene; PB, acceptor site in the PiggyBac system; PGKp, mouse phosphoglycerate kinase promoter; tdTomato, tandem dimer Tomato cDNA; transposase, PB transposase gene.
Generation of HDDPC-derived iPS cells and induction of differentiation
iPS cells (∼105 cells) were transfected with plasmids carrying Yamanaka factors, as described previously.28 Plasmids carrying Yamanaka factor cDNAs were purchased from Addgene (Cambridge, MA, USA).28 pCXLE-hOCT3/4-shp53 carries human OCT-3/4 cDNA and shRNA for human p53. pCXLE-hUL carries human L-MYC and LIN28 cDNAs. pCXLE-hSK carries human SOX2 and KLF4 cDNAs. The plasmids were propagated in Escherichia coli, and the DNA was purified using a Qiagen Plasmid Midi kit (Qiagen, Hilden, Germany).
iPS cell lines were derived and cultured in accordance with the guidelines of the Ethics Committee of Kagoshima University Graduate School of Medical and Dental Sciences. For transfection, HDDPCs (5 × 104) were electroporated using a Neon Transfection System (Invitrogen, Cologne, Germany) in 100 µL of R-buffer (Invitrogen, Carlsbad, CA, USA) containing 1 µg of pCXLE-hOCT3/4-shp53, 1 µg of pCXLE-hUL, 1 µg of pCXLE-hSK, and 0.5 µg of pmaxGFP (an indicator plasmid for monitoring transfection efficiency; Lonza GmbH, Cologne, Germany) under electroporation condition #4 (1 electrical pulse, 1 600 V, 20 ms pulse length). Electroporated cells were then seeded in three wells of a gelatin-coated 24-well plates (Iwaki Glass, Tokyo, Japan) containing DMEM/20% FBS. One day after transfection, the cells were inspected for green fluorescence under ultraviolet (UV) illumination to confirm that the cells had been successfully transfected. The cells were cultivated further in the same medium. The medium was changed every day or every other day. Seven days after transfection, the cells were trypsinized and subsequently reseeded onto mouse embryonic fibroblasts (MEFs) or STO mouse stromal cell line treated with mitomycin C (MMC; #M4287; Sigma-Aldrich, St. Louis, MO, USA) in a 60-mm gelatin-coated dish in human embryonic stem (ES) cell culture medium (iPSellon, #007001; Cardio, Kobe, Japan) supplemented with 5 ng·mL−1 recombinant human basic fibroblast growth factor (bFGF; Wako Pure Chemical Industries, Osaka, Japan). This passage (P) was designated P1. Fifteen days after seeding the electroporated HDDPCs onto the feeder cells, the dish, which contained emerging small ES-like colonies, was washed once with phosphate-buffered saline (PBS) without Ca2+ and Mg2+. The cells were then incubated with PBS containing 10 mg·mL−1 collagenase IV (#17104-019; Invitrogen, Carlsbad, CA, USA), 1 mol·L−1 CaCl2/PBS, 20% Knockout Serum Replacement (KSR; #10828-028; Invitrogen, Carlsbad, CA, USA), and 0.25% trypsin (#15090-046; Invitrogen, Carlsbad, CA, USA) at 37 °C for approximately 5 min and then reseeded onto new feeder cells in a 60-mm gelatin-coated dish. This passage was designated P2. Six to eight days after reseeding, the growing colonies were again dissociated using the same method, split 1:5, and reseeded onto new feeder cells in a 60-mm gelatin-coated dish. This passage was designated P3. The cells were passaged in a similar manner until P26. The medium was changed every day.
Transfection
Experiment 1
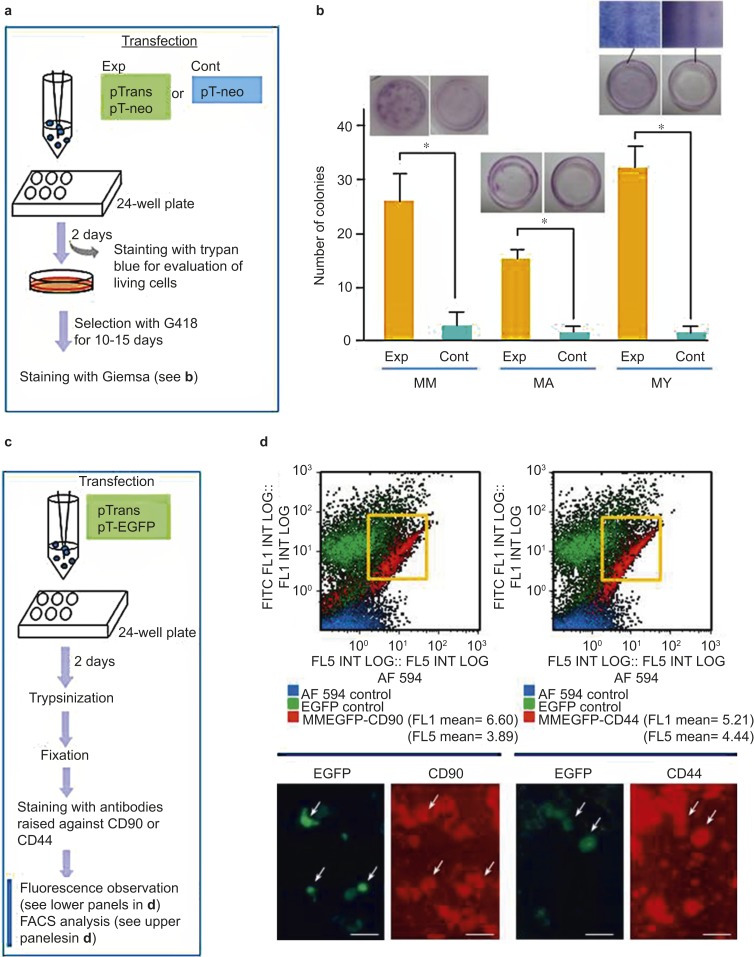
First, the superiority of the PB-based gene delivery system over traditional methods based on simple transfection of plasmid DNA was assessed, as depicted in Figure 2a. For transfection, HDDPCs (5 × 104) were electroporated in a solution of R-buffer (100 µL; Invitrogen, Carlsbad, CA, USA) containing pT-neo + pTrans (0.5 μg each) for the experimental group or pT-neo alone (0.5 μg) for the control group using a Neon Transfection System under the recommended electroporation condition #4 (1 600 V, 20 ms, 1 pulse). Electroporated cells were then seeded in three wells of a gelatin-coated 24-well plates (Iwaki Glass, Tokyo, Japan) containing drug-free DMEM/20% FBS. Two days after transfection, cells were harvested. A portion of these cells was subjected to a hemocytometer-based trypan blue dye exclusion assay29 to test cell viability before and after transfection. At least 100 cells per group were counted. The remaining cells were seeded onto 30-mm gelatin-coated dishes (Iwaki Glass, Tokyo, Japan). Six days after transfection, cells transfected with pT-neo + pTrans or pT-neo alone were cultured in the presence of G418 (400 µg·mL−1). After 10 days of selection, emerging colonies were fixed with 4% paraformaldehyde (PFA) in PBS for 5 min at room temperature. Colonies were then stained with a Giemsa staining kit (#079-04391; Wako Pure Chemical Industries, Osaka, Japan), according to the manufacturer's recommended methods, and then colonies were counted.
Figure 2.
Efficiency of the PB-based gene delivery system for acquisition of stable transfectants. (a) A flowchart of the experiments used to test the activity of PB transposase in HDDPCs established from three donors (MM, MA, and YY). pTrans was used as a positive control for PB transposase expression in the experimental group (Exp). In the control group (Cont), only pT-neo was used for transfection. After transfection, cells were plated onto a 24-well plates and then selected in the presence of G418 for 10–15 days until colonies emerged. The cells were fixed with 4% PFA and stained with Giemsa stain for colony counting. (b) Graphical representation of the number of colonies that survived after drug selection. The experiments were repeated three times, and the mean ± standard deviations of the number of colonies is shown above each bar. The photomicrographs above the bars show representative images of Giemsa-stained cells. A magnified view of the Giemsa-stained colonies is shown for YY. (c) A flowchart of the experiments used to test whether PB-based gene delivery permits transfection of certain specific populations of cells included in the HDDPCs. (d) Fluorescence-activated cell sorting (FACS) (upper panels) and EGFP fluorescence (lower panels) analyses of MM HDDPCs one day after transfection with pTrans and pT-EGFP and subsequent staining with antibodies for CD90 or CD44. The majority of HDDPCs positively stained for these antibodies, and of these, the transfected green fluorescent cells (arrows) are visible. Note that there is no appreciable deviation in the distribution of between EGFP- and CD44- or CD90-positive cells (shown in quadrants). CD, cluster of differentiation; EGFP, enhanced green fluorescent protein; HDDPC, human deciduous tooth dental pulp cell; PFA, paraformaldehyde.
To examine the possibility that certain types of HDDPCs may be preferentially transfected with exogenous DNA, HDDPCs (5 × 105) were electroporated in a solution of R-buffer containing pT-EGFP (0.5 μg), using the same electroporation conditions described above. Two days after transfection, cells were trypsinized, fixed with 4% PFA in PBS(−) for 5 min at room temperature and inspected for fluorescence expression under a fluorescence microscope, as described below. Then, fixed cells were incubated overnight at 4 °C with the following antibodies: anti-CD44 (1/100; phycoerythrin (PE)-labeled monoclonal antibody against hyaluronate/lymphocyte homing associated cell adhesion molecule-HCAM; #BBA10, R&D Systems, Minneapolis, MN, USA) and anti-CD90 (1/100; PE-labeled monoclonal antibody against Thy-1; #MAB2067, R&D Systems, Minneapolis, MN, USA). Both cell-surface molecules are widely recognized as mesenchymal stem cell markers.30,31 Labeled cells were then subjected to flow cytometric analysis, as described below.
Experiment 2_1
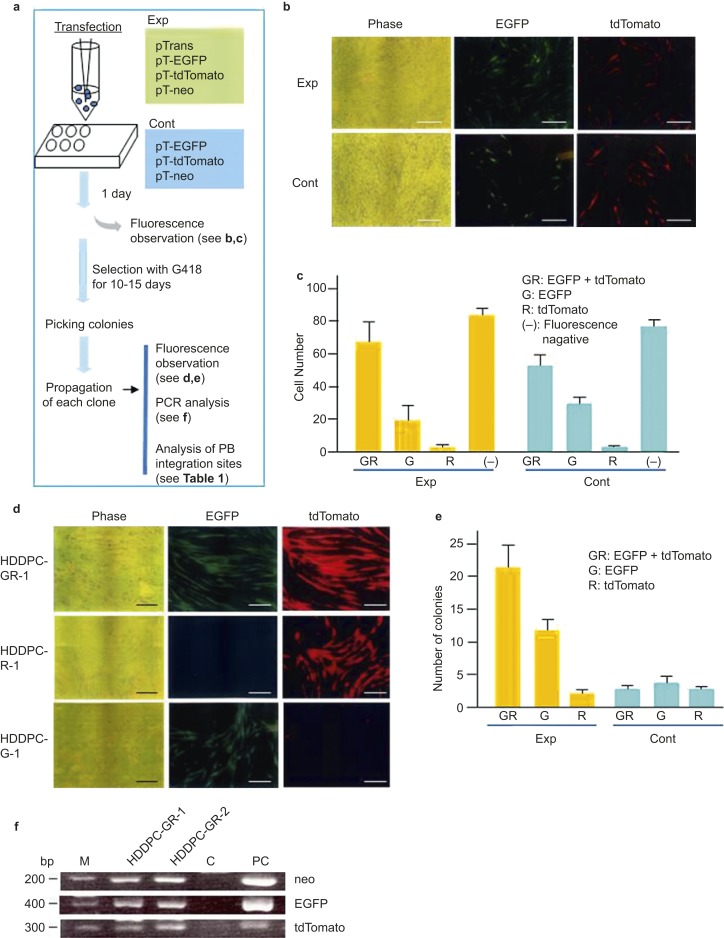
Next, the usefulness of the PB-based gene delivery system for acquisition of stable transfectants carrying multiple transgenes was assessed, as depicted in Figure 3a. To acquire stable transfectants carrying three transposon plasmids (pT-EGFP, pT-tdTomato, and pT-neo; listed in Figure 1), HDDPCs (5 × 104) in the experimental group were electroporated in a solution (100 µL) containing pT-neo + pT-EGFP + pT-tdTomato + pTrans (0.5 μg each), using the same electroporation conditions described in Experiment 1. HDDPCs in the control group were transfected with the same plasmids as the experimental group; however, pTrans was omitted. After transfection, the cells were plated in three wells of a gelatin-coated 24-well plates (Iwaki Glass, Tokyo, Japan) containing drug-free DMEM/20% FBS. The next day, fluorescence was inspected and recorded using a fluorescence microscope, as described below. Six days after transfection, cells were cultured in the presence of G418 (400 µg·mL−1). After 10 days of selection, fluorescence in the emerging colonies was assessed under a fluorescence microscope, as described below. Colonies were then picked using a small 3 mm Whatman filter paper disc, which had been dipped in 0.125% trypsin/0.01% elhylene diamine tetraacetic acid (EDTA), as described previously.32 The discs were then transferred to the wells of a gelatin-coated 48-well plates (Iwaki Glass, Tokyo, Japan) with G418-containing DMEM/20% FBS. The cells were cultured for 20–30 days until they reached confluency. They were then propagated further in a stepwise manner. Fluorescence and the presence of the exogenous constructs were assessed as described below.
Figure 3.
Efficiency of the PB-based gene delivery system for the acquisition of stable transfectants carrying multiple transgenes. (a) A flowchart of the experiments used to test for the simultaneous transfection of two fluorescent markers, EGFP and tdTomato, into a single cell derived from MM HDDPCs. pTrans was used as a positive control in the experimental group (Exp). In the control group (Cont), pTrans was omitted. (b) Fluorescence in HDDPCs one day after transfection. Note that cells in both groups exhibited similar fluorescence patterns, suggesting the transfection efficiency was similar in the experimental and control groups. Phase images were taken under a light microscope; EGFP, images were taken under light + UV illumination to detect EGFP-derived green fluorescence; tdTomato, images were taken under light + UV illumination to detect tdTomato-derived red fluorescence. Scale bar = 50 μm. (c) Graphical representation of the number of surviving colonies after drug selection. After transfection, cells were plated onto a 24-well plates. Four days later, transfected cells were selected for in the presence of G414 for 10–15 days. The colonies were stained with Giemsa stain and then inspected under a fluorescence microscope. GR, colonies with both green (G) and red (R) fluorescence; G, colonies with only green fluorescence; R, colonies with only red fluorescence; (−), non-fluorescent colonies. The experiments were repeated three times, and the mean ± SD of the number of colonies is shown above each bar. (d) Classification of emerging colonies in experimental (Exp) and control (Cont) groups with regard to fluorescence expression. Colonies are inspected for fluorescence and classified as having dual fluorescence (GR), EGFP alone (G), or tdTomato (R) alone. The experiments were repeated three times, and the mean ± SD of the number of colonies is shown above each bar. (e) Fluorescence in cells propagated from colonies generated after transfection and subsequent selection with G418. HDDPC-GR-1 exhibited both green and red fluorescence. HDDPC-R-1 exhibited red fluorescence only, whereas HDDPC-G-1 exhibited green fluorescence only. Bar = 50 μm. (f) PCR analysis of genomic DNA isolated from cells exhibiting both red and green fluorescence (HDDPC-GR-1 and HDDPC-GR-2). As expected, both cells had both EGFP- and tdTomato-derived sequences in their genome. C, genomic DNA from un-transfected MM cells subjected to PCR as a negative control; PC, plasmid DNA subjected to PCR as a positive control; EGFP, enhanced green fluorescent protein; HDDPC, human deciduous tooth dental pulp cell; PCR, polymerase chain reaction; UV, ultraviolet.
Experiment 2_2
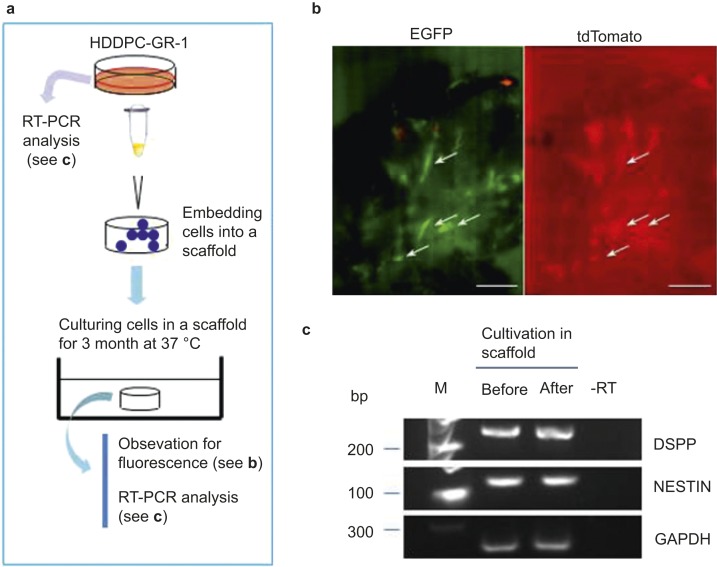
As depicted in Figure 4a, we examined whether expression of exogenous DNA in cells stably transfected via the PB-based gene delivery system could persist for a long period of time in the absence of drug selection. For prolonged cultivation of the transfectants, approximately 200 μL of medium containing fluorescent HDDPCs (5 × 105) was transferred to a scaffold (Calcium Phosphate Scaffold, #354617; BD Bioscience, Tokyo, Japan). The cells were then cultured for approximately three months in a 60-mm tissue culture dish (Iwaki Glass, Tokyo, Japan) containing 4 mL normal medium (DMEM/20% FBS). During this period, the medium was changed twice a week. After cultivation, the scaffold was cut in half, and the inner surface was inspected under a dissecting fluorescence microscope (Olympus, Tokyo, Japan), as described below. The other half was processed for a molecular biological analysis to assess whether expression of endogenous genes (dentin sialophosphoprotein (DSPP) and Nestin) had changed after prolonged cultivation.
Figure 4.
Continuous expression of exogenous genes introduced via the PB system in the absence of the selective drug. (a) A flowchart of depicting the characterization of transfectants cultured over a long period. (b) Fluorescence in HDDPC-GR-1 cells cultured in a scaffold for three months. The scaffold was cut in half, and the inner surface was inspected under a fluorescence microscope. Several clusters of cells with both red and green fluorescence (arrows) were apparent. Bar = 50 μm. (c) RT-PCR analysis of HDDPC-GR-1 cells before and after the long cultivation. After isolation of mRNA, cDNA was synthesized using oligo(dT) and then used for PCR. -RT, RT-PCR using water as template; M, 100-bp ladder markers; DSPP, dentin sialophosphoprotein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; EGFP, enhanced green fluorescent protein; HDDPC, human deciduous tooth dental pulp cell; RT-PCR, reverse transcriptase-polymerase chain reaction.
Experiment 3
We next tested the possibility that iPS cells can be efficiently transfected with the PB-based gene delivery system. For this experiment, iPS cells (5 × 104) in the experimental group were electroporated in a solution (100 µL) containing pT-neo + pT-EGFP + pT-tdTomato + pTrans (0.5 μg each) using the same electroporation conditions used in Experiment 1. iPS cells in the control group were transfected with the same plasmids; however, pTrans was omitted. After transfection, the cells were plated in three wells of a gelatin-coated 24-well plates (Iwaki Glass, Tokyo, Japan) containing drug-free iPSellon and MMC-treated STO cells. Six days after transfection, cells were cultured in the presence of G418 (400 µg·mL−1). After 10 days of selection, fluorescence in the emerging colonies was assessed under a fluorescence microscope, as described below.
To induce embryoid body (EB) formation, HDDPC-iPS colonies were dissected mechanically using a pipette tip under a stereomicroscope and then seeded onto an ultra-low attachment 30-mm dish (#MS-9035X; Sumitomo Bakelite, Tokyo, Japan) containing DMEM/10% FBS. Ten days after cultivation, emerging EBs were transferred into a gelatin-coated 30-mm dish (Iwaki Glass, Tokyo, Japan) and cultured for an additional 10 days in DMEM/10% FBS, allowing for enhanced differentiation into various cell types.
Flow cytometry
Flow cytometric analysis was performed on a Coulter Epics XL Flow Cytometer (Beckman Coulter, Miami, FL, USA). A 15 mW argon ion laser operating at 488 nm was used to excite the fluorophores. Flow cytometric data were analyzed using the program, Expo32ADC (Beckman Coulter, Miami, FL, USA). Fluorescence was measured with an FL-1 sensor using a 525 nm band-pass filter to detect EGFP and an FL-5 sensor using a 670 nm band-pass filter to detect Alexa Fluor (AF) 594. Two-dimensional plots of EGFP versus AF 594 fluorescence were drawn.
Immunostaining of iPS cells and EBs
For immunocytochemical staining of ES markers, cells fixed with 4% PFA in PBS were permeabilized with 0.05% Triton X-100 (#T8787; Sigma-Aldrich, St. Louis, MO, USA) if necessary and blocked with Aqua Block (#PP82; East Coast Biologics, North Berwick, ME, USA). iPS cells were stained with primary antibodies against stage-specific embryonic antigen-4 (SSEA-4; #MABA4304-20; 1:200; Millipore-Chemicon, Darmstadt, Germany) or TRA-1-60 (#MABA4360-20; 1:200; Millipore-Chemicon, Darmstadt, Germany). iPS cells were then incubated with the following secondary antibodies: AF 594-conjugated goat anti-mouse IgM (A21044; 1:200; Invitrogen, Carlsbad, CA, USA) or fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (#AP503F; 1:200; Millipore-Chemicon, Darmstadt, Germany). Nuclear staining was performed using 6-diamidino-2-phenylindole (DAPI) (#H-1200; Funakoshi, Tokyo, Japan).
Similarly, cells differentiated from EBs were stained with primary antibodies specific for endodermal (α-fetoprotein (AFP); #HPA010607; 1:200; Atlas Antibodies, Stockholm, Sweden), ectodermal (β III-tubulin; #NB110-57611; 1:200; Novus Biologicals, Littleton, CO, USA), and mesodermal (α-smooth muscle actin (α-SMA); #NB600-531; 1:200; Novus Biologicals, Littleton, CO, USA) tissues. The cells were then labeled with the following secondary antibody: AF 647-conjugated goat anti-rabbit IgG Fab2 (#4414s; 1:200; Cell Signaling Technology, Tokyo, Japan).
Cytochemical detection of alkaline phosphatase activity
After passage 3, cells were subjected to cytochemical staining for alkaline phosphatase (ALP) activity using the Leukocyte Alkaline Phosphatase kit (Sigma-Aldrich, St. Louis, MO, USA), which is based on ALP-mediated conversion of α-naphtholum coupled with diazonium salt to a reddish-brown product that can be visualized.
Fluorescence detection
The fluorescence of transfected cells was examined using a BX60 fluorescence microscope (Olympus, Tokyo, Japan) with DM505 filters (BP460-490 and BA510IF; Olympus, Tokyo, Japan) and DM600 filters (BP545-580 and BA6101F; Olympus, Tokyo, Japan), which were used for EGFP-derived green fluorescence and AF 594-, AF 647- or tdTomato-derived red fluorescence, respectively. Photomicrographs were taken using a digital camera (FUJIX HC-300/OL; Fuji Film, Tokyo, Japan) attached to the fluorescence microscope.
Polymerase chain reaction analysis
Genomic DNA from HDDPCs was isolated as previously described (Blin and Stafford, 1976), with several modifications.33 Polymerase chain reaction (PCR) was performed in a total reaction volume of 10 μL containing 10 mmol·L−1 Tris-HCl (pH 8.3), 50 mmol·L−1 KCl, 1.5 mmol·L−1 MgCl2, 0.25 mmol·L−1 of each dNTP, 1 mmol·L−1 of each primer (forward and reverse), 2 μL of genomic DNA (∼5 ng), and 0.5 units of rTaq polymerase (#R001; TaKaRa Shuzo, Tokyo, Japan). The primer sets used for the detection of neo in pT-neo, EGFP in pT-EGFP, and tdTomato in pT-tdTomato were described previously.34 pT-neo, pT-EGFP, and pT-tdTomato were detected as 297-, 400-, and 206-bp bands, respectively. As a negative control, 0.5 μg of genomic DNA from un-transfected HDDPCs was used. For positive controls, 5 ng of each plasmid listed in Figure 1 was used. PCR was performed under the following thermocycler conditions: 40 cycles of 96 °C for 10 s, 56 °C for 1 min, and 72 °C for 2 min. The PCR products (5 µL) were separated on a 2% agarose gel and visualized with ethidium bromide.
Reverse transcriptase-polymerase chain reaction analysis
To measure the expression of endogenous DSPP and Nestin mRNA by reverse transcriptase (RT)-PCR, total RNA (∼4 μg) was isolated from HDDPCs before or after cultivation in a scaffold by RNeasy Mini kit (#74104; Qiagen, Hilden, Germany) and reverse-transcribed using a First-Strand cDNA Synthesis kit (#18080-051; Invitrogen, Carlsbad, CA, USA).
The undiluted cDNA samples (1 µL) were then amplified by PCR in a total volume of 20 μL using AmpliTaq Gold 360 Master Mix (#4398881; Applied Biosystems, Foster City, CA, USA). PCR was performed as follows for 38 cycles: 95 °C for 30 s (denaturation), 58 °C for 30 s (annealing), and 72 °C for 60 s (extension), using a PC708 thermal cycler (Astec, Fukuoka, Japan). A negative, no-template control (designated -RT) was included for each reaction. Information regarding each PCR primer set is listed in Supplementary Table 1 (refs. 53,54,55,56). The products (5 µL) were then analyzed by 2% agarose gel electrophoresis and visualized after staining with ethidium bromide.
Mapping insertion sites by splinkerette PCR
Genomic DNA was extracted from the stably transfected clones cultured in Experiment 2_1. Six clones carrying pT-EGFP were mixed and lysed, and the genomic DNA was isolated, as described above.
Splinkerette PCR was performed to map the PB integration sites in the transfectants, as described previously.35,36 Sau3AI-digested genomic DNA was ligated using a splinkerette adapter generated by annealing HMSpAa and HMSpBb. Junction fragments were PCR amplified using the primers HMSp1 and PB-L-Sp1 or PB-R-Sp1. Nested PCR was performed using primers HMSp2 and PB-L-Sp2 or PB-R-Sp2. PCR products were cloned into the TA-cloning vector pCR2.1 (Invitrogen, Carlsbad, CA, USA) and sequenced with standard primers.
Statistical analysis
Statistical analysis of the data obtained from Experiment 1 was performed using PRISM 5 for Windows software (GraphPad, La Jolla, CA, USA). Data were analyzed by one-way repeated analysis of variance and expressed as the mean ± standard deviations for at least three independent experiments. The statistical significance was determined by Student's t-test. P-values <0.05 were considered statistically significant.
Results
Experiment 1: the PB-based gene delivery system allows efficient acquisition of stable transfectants
To evaluate the efficiency of the PB system in generating stable transfectants, three primary cultured HDDPC lines (MM, MA, and YY; 5 × 104 cells each) were transfected using the Neon Transfection System in the presence of pT-neo + pTrans (experimental group) or pT-neo alone (control group), as shown in Figure 2a. The trypan blue dye exclusion assay was used to determine the viability of MM, MA, and YY HDDPCs after transfection at ∼80%, ∼70%, and ∼70%, respectively (data not shown). Six days after transfection, cells were treated with G418 for 10–15 days. Colonies were then Giemsa stained and counted. For MM, MA, and YY cells, the number of G418-resistant colonies was 13-fold, 16-fold, and 32-fold higher, respectively, in the experimental (PB-mediated gene delivery) groups than in the control group (Exp vs. Cont in Figure 2b). These results indicated that PB-mediated gene delivery is an efficient method for obtaining stable transfectants from a small number of HDDPCs.
HDDPCs are derived from primary cultured human DPCs, and they are a mixture of several cell types. Therefore, in the next experiment, we examined whether the PB-based gene delivery system could be used to transfect all of the cell types or only specific sub-populations of HDDPCs. HDDPCs (MM) were first transfected with pT-EGFP + pTrans (Figure 2c). Two days later, cells were trypsinized and briefly fixed with 4% PFA. The fixed cells were then stained with two antibodies raised against the cell-surface molecules CD90 and CD44, both of which are specifically expressed by mesenchymal stem cells.30,31 Almost all HDDPCs (MM) were positive for CD90 and CD44 upon analysis of antibody labeled cells under a fluorescence microscope (lower panels in Figure 2d). Therefore, transfection of HDDPCs with pT-EGFP + pTrans also resulted in transfection of CD90- or CD44-positive cell populations (Figure 2d, arrows). These results indicated that gene delivery via electroporation was not confined to a specific HDDPC population. This notion was further supported by FACS analysis (Figure 2d). The majority of CD90- or CD44-positive cells were also positive for EGFP expression.
Experiment 2_1: the PB-mediated gene delivery system is efficient for generating stable transfectants carrying multiple transgene constructs
To examine whether the PB-mediated gene delivery system could generate stable transfectants carrying multiple transgene constructs, HDDPCs (MM; 5 × 104) were transfected with pTrans + pT-EGFP + pT-tdTomato + pT-neo (experimental, Exp) or pT-EGFP + pT-tdTomato + pT-neo (control, Cont), as depicted schematically in Figure 3a.
One day after transfection, 50%–60% of the cells exhibited both red and green fluorescence in both the experimental and control groups. There was no significant difference in the transfection efficiency between each group (Figure 3b and 3c). This suggested that the transfection efficiency was almost equal between the two groups and that multiple constructs could be simultaneously incorporated into a single cell at a relatively high rate using this system.
At 10–15 days after G418 treatment, the emerging colonies were counted and their fluorescence was assessed. In the experimental group, 60.0% of the colonies exhibited dual fluorescence (Figure 3d and 3e; HDDPC-GR-1), whereas 5.7% (∼3 in average) or 34.3% (∼12 in average) exhibited either red or green fluorescence, respectively (Figure 3d and 3e). The PCR using genomic DNA showed that cells with both red and green fluorescence contained all of the transfected transgenes (Figure 3f). Notably, there was no appreciable change in the proliferation rate or cellular morphology before and after transfection and subsequent drug selection when using MM cells (data not shown). In contrast, few colonies (∼4 in average) exhibited dual fluorescence in the control group (Figure 3e), suggesting that the PB-mediated gene delivery system is superior for generating transfectants with multiple constructs.
Experiment 2_2: gene expression was maintained after prolonged cultivation of stable transfectants generated by the PB system
Next, we examined whether gene expression was maintained after prolonged cultivation of stable transfectants. For this experiment, we used a 3-D scaffold culture system, which supports the growth of a large number of cells without requiring cell passage and enables the cells to form a 3-D structure. The scaffold was inoculated with HDDPC-GR-1 cells (shown in Figure 3d and 3f) and maintained in normal medium for three months (Figure 4a). The scaffold was then cut in half, and the inner surface of the scaffold was inspected under a fluorescence microscope. Several cell clusters within the scaffold exhibited both red and green fluorescence (Figure 4b, arrows). RNA was isolated from cells within the remaining half of the scaffold to determine whether gene expression changed over the course of the long culture period. RT-PCR analysis detected mRNA expression of two HDDPC-related endogenous proteins (DSPP and Nestin) before and after culture in the scaffold (Figure 4c), and the expression levels of these genes were not significantly different after prolonged culture.
With PB-mediated gene delivery, transgenes integrate into AT-rich regions of the host's genome at TTAA target sites.24 To verify this mechanism, we examined the sequences flanking the transgenes. Genomic DNA isolated from HDDPC-GR-1 cells was analyzed using the splinkerette PCR method,36 where junctional sequences between the host genome and the exogenous foreign transgene are identified. As shown in Table 1, there were at least two PB insertion sites in HDDPC-GR-1 cells. Sequencing analysis demonstrated that the PB transposon inserted exclusively into TTAA target sites that were duplicated upon insertion. Furthermore, the insertion sites had adjacent sequences that were unrelated to the PB vector. We were able to map each insertion site using a BLAST search of the NCBI database (Table 1), suggesting the PB vectors integrated successfully into known genes.
Table 1. Splinkerette PCR analysis results indicating PB-mediated integration sites in the human genome.
| No. | Sequence corresponding to the endogenous porcine genome (5′–3′) | Known sequences showing similarity (>80%) to the endogenous porcine genome |
|---|---|---|
| 1 | TTAATAGTCATTCTCTTAGTCCTTTAAGCAACATGG | Mus musculus chromosome 1, clone RP23-23I4, complete sequence |
| Sequence ID: gb|AC131577.16| | ||
| 2 | TTAATAGTCATTCTCTTAGTCCTTTAAGCAACATGG | Sus scrofa 5 BAC CH230-4L11, clone RP23-23I4, complete sequence |
| Sequence ID: gb|AC094527.7| |
Experiment 3: PB-based gene delivery confers efficient generation of stable transfectants derived from iPS cells
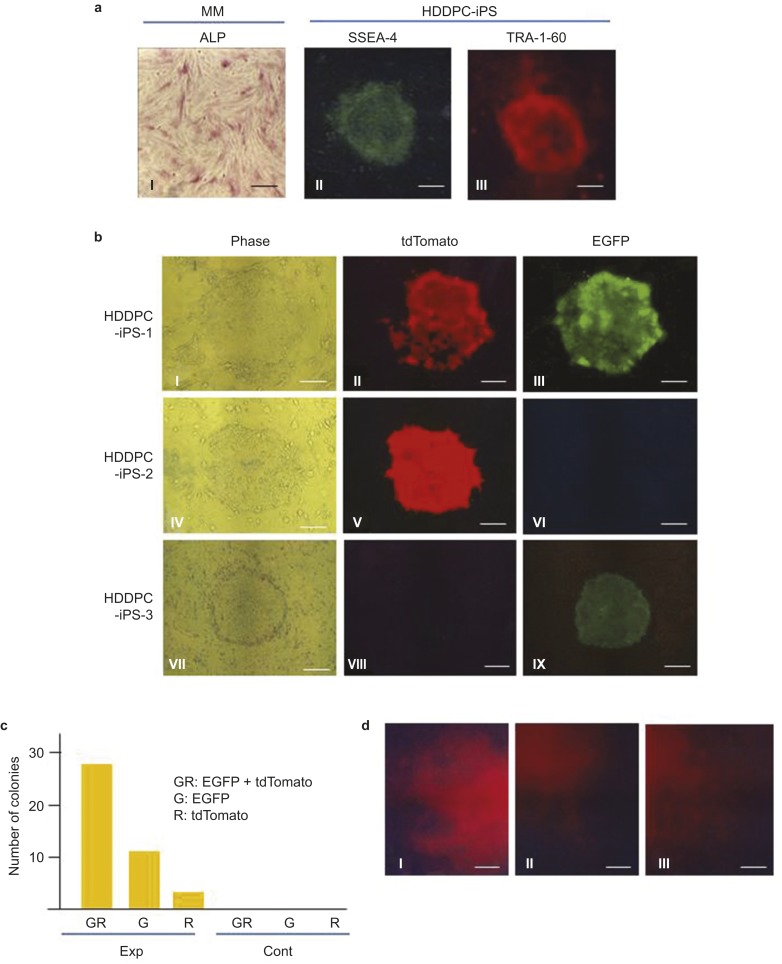
We next examined whether PB-based gene delivery could generate stable transfectants derived from iPS cells. To establish iPS cells from HDDPCs, we used the MM line because it exhibited higher ALP activity (Figure 5aI) than the other two cell lines (MA and YY). ALP is highly expressed in immature cells such as stem cells and ES cells.37,38 Transfection of HDDPCs with plasmids carrying the Yamanaka factor genes led to the successful generation of iPS colonies (submitted for publication). These colonies were positive for the ES/iPS cell-specific antigens SSEA-4 (Figure 5aII) and TRA-1-60 (Figure 5aIII). When these established colonies were transfected with pTrans + pT-EGFP + pT-tdTomato + pT-neo (experimental group) or pT-EGFP + pT-tdTomato + pT-neo (control group), only cells in the experimental group generated viable colonies (Figure 5b and 5c): no colonies emerged in the control group. Upon fluorescence inspection, 66.6% of the colonies in the experimental group exhibited dual fluorescence (Figure 5bI–III and 5c) and 33.4% exhibited green or red fluorescence (Figure 5bIV–IX and 5c). To confirm that our HDDP-derived iPS cells have the potential to differentiate into three germ layers, we first induced EB formation and then differentiation in vitro by cultivating EBs in the presence of differentiation medium on a plastic dish. Ten days after induction, cells on the dish were fixed and then stained with antibodies against markers specific for endodermal (AFP), mesodermal (α-SMA), and ectodermal (βIII-tubulin) cells. Immunostaining revealed the presence of differentiated cells from all three germ layers in the HDDPC-iPS cell culture (Figure 5d), indicating the multipotency of the HDDPC-iPS cells tested.
Figure 5.
PB-based gene delivery generates stable transfectants derived from iPS cells. (a) Characterization of HDDPC cells (MM) and HDDPC-derived iPS cells (HDDPC-iPS). MM exhibited ALPase activity. HDDP-iPS cells expressed SSEA-4 and TRA-1-60, which are markers for ES/iPS cells. The HDDP-iPS cells were subjected to Neon-based transfection in the presence of pTrans + pT-neo + pT-tdTomato + pT-EGFP (Exp) or pT-neo + pT-tdTomato + pT-EGFP (Cont). The transfected cells were then cultivated on feeder cells in the presence of G418 for 10 days. Scale bars = 50 µm. (b) Fluorescence in colonies after selection with G418. The HDDPC-iPS-1 colony exhibited both green and red fluorescence. The HDDPC-iPS-2 colony exhibited red fluorescence only, whereas the HDDPC-iPS-3 colony exhibited green fluorescence only. Scale bars = 50 µm. (c) Graphical representation of colony number shown in b. GR, both green and red fluorescence; G, green fluorescence only; R, red fluorescence only. (d) Immunocytochemical staining of cells 10 days after in vitro differentiation of EBs from HDDPC-iPS cells by typical endodermal (AFP, a), ectodermal (βIII-tubulin, b) and mesodermal (α-SMA, c) markers. Scale bar = 50 µm. EGFP, enhanced green fluorescent protein; HDDPC, human deciduous tooth dental pulp cell; iPs, inducible pluripotent stem.
Discussion
The PB-based gene delivery system is a powerful tool for generating stable transfectants from a variety of cells, including those from humans,39 pigs,40 cattle,41 mice,42 and goats.18 A key property of the PB system is its ability to transfect cells more efficiently than electroporation-based transfection methods using plasmid DNA. For example, Wilson et al. (2011) demonstrated that the number of colonies obtained after transfection of human HEK-293 cells with a transposase expression construct was two- to four-fold higher than the number obtained after transfection with a native sleeping beauty system.24 The high transfection efficiency does not appear to be due to the high gene transfer rate of the PB system itself because similar transfection efficiencies were obtained in the presence and absence of the transposase expression construct in the present study (see Exp vs. Cont in Figure 3b and 3c). The PB system may facilitate the integration of transposons into the host genome at TTAA nucleotide elements (see Table 1), as previously described.14 As expected, in the three HDDPC lines tested, the efficiency of stable transfection was 10- to 20-fold higher with PB vectors + pTrans than with transposons alone (see Figure 2b).
There are several ways to transfect mammalian cells with nonviral DNA, such as plasmid DNA. The method used most frequently is liposomal transfection, which uses liposomal reagents that allow the effective incorporation of DNA into a cell.43 Electroporation is also a promising transfection method; it relies on the transient generation of micropores in a cell membrane through which a cell can take up DNA.44 Electroporation requires expensive apparatus, namely, an electroporator. However, electroporation is generally considered a more effective transfection method than liposomal transfection. We found that electroporation using the Neon Transfection System was more effective than liposome-mediated transfection of human HDDPCs: the former yielded transfection efficiencies ranging from 50% to 80%, whereas the latter ranged from 20% to 30% (data not shown). The combination of electroporation and the PB system can efficiently transfect a small number of cells, including cell types that are difficult to transfect, such as lymphoblastic cells. In fact, Miura et al. successfully transfected non-adherent HepG2 cells using this system.45
Another unique property of PB-based gene delivery is the ability to deliver multiple constructs to a single cell simultaneously. For example, Kahling et al. successfully transfected four transposons into a single cell.46 Similarly, we simultaneously introduced seven transgenes into porcine cells (manuscript in preparation). Our success may be due in part to the PB-based system's higher rate of transfection, when compared to that of other plasmid-based transfection systems, as noted previously by Ivics et al.47 The ability to introduce multiple constructs will be beneficial for researchers who intend to investigate the functions of multiple gene products.
To date, gene delivery to human ES cells or iPS cells has been achieved using viral vectors,48 liposomal transfection,49 and electroporation-mediated gene transfer.50 In this study, we demonstrated that the combined use of PB-based gene transfer and Neon-based electroporation was effective for generating stable ES/iPS cells. In contrast, Neon-based electroporation using transposons without PB transposase failed to generate visible iPS-like colonies.51 Notably, we successfully transferred at least three constructs (EGFP, tdTomato, and neo) into a single iPS cell; the emerging colonies exhibited dual fluorescence and resistance against G418 (see Figure 5b). Reintroduction of the PB transposase gene into transposon-carrying cells removes the transposons without leaving any trace.52 In this context, the PB-based gene transfer system is ideal for gene-based manipulation of mammalian cells including HDDPCs.
Conclusion
The PB transposon system was highly efficient for producing stable transfectants in HDDPCs and iPS cells. Because of this, simultaneous delivery of two marker genes, EGFP and tdTomato, was possible. This PB-based gene transfer system has significant advantages for use in basic scientific research in the field of dental tissue engineering, and it has the potential to be a valuable tool for investigating gene and protein function related to tooth regeneration.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) (grant no. 25463192) from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
Footnotes
Supplementary Information for this article can be found on International Journal of Oral Science's website (http://www.nature.com/ijos/)
Supplementary Information
References
- 1Kenneth MH, Harold E G, Samuel S. Seltzer and Bender's dental pulp. Balrin: Quintessence Publishing, 2002. [Google Scholar]
- 2Huang GT, Sonoyama W, Chen J et al. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 2006; 324(2): 225–236. [DOI] [PubMed] [Google Scholar]
- 3Thibodeau B, Teixeira F, Yamauchi M et al. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod 2007; 33(6): 680–689. [DOI] [PubMed] [Google Scholar]
- 4Wei X, Ling J, Wu L et al. Expression of mineralization markers in dental pulp cells. J Endod 2007; 33(6): 703–708. [DOI] [PubMed] [Google Scholar]
- 5Galler KM, Schweikl H, Thonemann B et al. Human pulp-derived cells immortalized with simian virus 40 T-antigen. Eur J Oral Sci 2006; 114(2): 138–146. [DOI] [PubMed] [Google Scholar]
- 6Thonemann B, Schmalz G. Immortalization of bovine dental papilla cells with simian virus 40 large t antigen. Arch Oral Biol 2000; 45(10): 857–869. [DOI] [PubMed] [Google Scholar]
- 7Thonemann B, Schmalz G. Bovine dental papilla-derived cells immortalized with HPV 18 E6/E7. Eur J Oral Sci 2000; 108(5): 432–441. [DOI] [PubMed] [Google Scholar]
- 8Suguro H, Asano M, Kaneko Y et al. Characterization of human dental pulp-derived cell lines. Int Endod J 2008; 41(7): 609–616. [DOI] [PubMed] [Google Scholar]
- 9Yang X, Walboomers XF, van den Dolder J et al. Non-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriers. Tissue Eng Part A 2008; 14(1): 71–81. [DOI] [PubMed] [Google Scholar]
- 10Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res 2008; 87(2): 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Wang GZ, Liu JH, Lu SZ et al. Ultrasonic destruction of albumin microbubbles enhances gene transfection and expression in cardiac myocytes. Chin Med J 2011; 124(9): 1395–1400. [PubMed] [Google Scholar]
- 12Ohlfest JR, Freese AB, Largaespada DA. Nonviral vectors for cancer gene therapy: prospects for integrating vectors and combination therapies. Curr Gene Ther 2005; 5(6): 629–641. [DOI] [PubMed] [Google Scholar]
- 13Cary LC, Goebel M, Corsaro BG et al. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 1989; 172(1): 156–169. [DOI] [PubMed] [Google Scholar]
- 14Fraser MJ, Cary L, Boonvisudhi K et al. Assay for movement of Lepidopteran transposon IFP2 in insect cells using a baculovirus genome as a target DNA. Virology 1995; 211(2): 397–407. [DOI] [PubMed] [Google Scholar]
- 15Fraser MJ, Ciszczon T, Elick T et al. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol 1996; 5(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 16Bauser CA, Elick TA, Fraser MJ. Proteins from nuclear extracts of two lepidopteran cell lines recognize the ends of TTAA-specific transposons piggyBac and tagalong. Insect Mol Biol 1999; 8(2): 223–230. [DOI] [PubMed] [Google Scholar]
- 17Ding S, Wu X, Li G et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 2005; 122(3): 473–483. [DOI] [PubMed] [Google Scholar]
- 18Bai DP, Yang MM, Chen YL. PiggyBac transposon-mediated gene transfer in Cashmere goat fetal fibroblast cells. Biosci Biotechnol Biochem 2012; 76(5): 933–937. [DOI] [PubMed] [Google Scholar]
- 19Nakanishi H, Higuchi Y, Kawakami S et al. piggyBac transposon-mediated long-term gene expression in mice. Mol Ther 2010; 18(4): 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Yusa K, Rad R, Takeda J et al. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods 2009; 6(5): 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Nagy K, Sung HK, Zhang P et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev 2011; 7(3): 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Balu B, Chauhan C, Maher SP et al. piggyBac is an effective tool for functional analysis of the Plasmodium falciparum genome. BMC Microbiol 2009; 9: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Rad R, Rad L, Wang W et al. PiggyBac transposon mutagenesis: a tool for cancer gene discovery in mice. Science 2010; 330(6007): 1104–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Wilson MH, Coates CJ, George AL Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol Ther 2007; 15(1): 139–145. [DOI] [PubMed] [Google Scholar]
- 25Gronthos S, Brahim J, Li W et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81(8): 531–535. [DOI] [PubMed] [Google Scholar]
- 26Gronthos S, Mankani M, Brahim J et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000; 97(25): 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108(2): 193–199. [DOI] [PubMed] [Google Scholar]
- 28Okita K, Matsumura Y, Sato Y et al. A more efficient method to generate integration-free human iPS cells. Nat Methods 2011; 8(5): 409–412. [DOI] [PubMed] [Google Scholar]
- 29Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 2001; Appendix 3: Appendix 3B. [DOI] [PubMed]
- 30Suchanek J, Visek B, Soukup T et al. Stem cells from human exfoliated deciduous teeth – isolation, long term cultivation and phenotypical analysis. Acta Medica 2010; 53(2): 93–99. [DOI] [PubMed] [Google Scholar]
- 31Karaoz E, Dogan BN, Aksoy A et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 2010; 133(1): 95–112. [DOI] [PubMed] [Google Scholar]
- 32Sato M, Akasaka E, Saitoh I et al. Targeted toxin-based selectable drug-free enrichment of mammalian cells with high transgene expression. Biology 2013; 2: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Sato M, Iwase R, Kasai K et al. Direct injection of foreign DNA into mouse testis as a possible alternative of sperm-mediated gene transfer. Anim Biotechnol 1994; 5(1): 19–31. [Google Scholar]
- 34Sato M, Ohtsuka M, Miura H et al. Determination of the optimal concentration of several selective drugs useful for generating multi-transgenic porcine embryonic fibroblasts. Reprod Domest Anim 2012; 47(5): 759–765. [DOI] [PubMed] [Google Scholar]
- 35Chew SK, Rad R, Futreal PA et al. Genetic screens using the piggyBac transposon. Methods 2011; 53(4): 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Potter CJ, Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS One 2010; 5(4): e10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Navabazam AR, Sadeghian Nodoshan F, Sheikhha MH et al. Characterization of mesenchymal stem cells from human dental pulp, preapical follicle and periodontal ligament. Iran J Reprod Med 2013; 11(3): 235–242. [PMC free article] [PubMed] [Google Scholar]
- 38Berrill A, Tan HL, Wuang SC et al. Assessment of stem cell markers during long-term culture of mouse embryonic stem cells. Cytotechnology 2004; 44(1/2): 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Kettlun C, Galvan DL, George AL et al. Manipulating piggyBac transposon chromosomal integration site selection in human cells. Mol Ther 2011; 19(9): 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Clark KJ, Carlson DF, Foster LK et al. Enzymatic engineering of the porcine genome with transposons and recombinases. BMC Biotechnol 2007; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Kim S, Saadeldin IM, Choi WJ et al. Production of transgenic bovine cloned embryos using piggybac transposition. J Vet Med Sci 2011; 73(11): 1453–1457. [DOI] [PubMed] [Google Scholar]
- 42Li R, Zhuang Y, Han M et al. piggyBac as a high-capacity transgenesis and gene-therapy vector in human cells and mice. Dis Model Mech 2013; 6(3): 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Gubin AN, Koduru S, Njoroge JM et al. Stable expression of green fluorescent protein after liposomal transfection of K562 cells without selective growth conditions. Biotechniques 1999; 27(6): 1162–1164, 1166–1170. [DOI] [PubMed] [Google Scholar]
- 44Mir LM. Electroporation-based gene therapy: recent evolution in the mechanism description and technology developments. Methods Mol Biol 2014; 1121: 3–23. [DOI] [PubMed] [Google Scholar]
- 45Miura H, Inoko H, Inoue I et al. piggyBac-mediated generation of stable transfectants with surface human leukocyte antigen expression from a small number of cells. Anal Biochem 2013; 437(1): 29–31. [DOI] [PubMed] [Google Scholar]
- 46Kahlig KM, Saridey SK, Kaja A et al. Multiplexed transposon-mediated stable gene transfer in human cells. Proc Natl Acad Sci U S A 2010; 107(4): 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Ivics Z, Li MA, Mates L et al. Transposon-mediated genome manipulation in vertebrates. Nat Methods 2009; 6(6): 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Hasegawa K, Cowan AB, Nakatsuji N et al. Efficient multicistronic expression of a transgene in human embryonic stem cells. Stem Cells 2007; 25(7): 1707–1712. [DOI] [PubMed] [Google Scholar]
- 49Cadinanos J, Bradley A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res 2007; 35(12): e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Wang W, Lin C, Lu D et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci U S A 2008; 105(27): 9290–9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Woltjen K, Michael IP, Mohseni P et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009; 458(7239): 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Nakayama A, Sato M, Shinohara M et al. Efficient transfection of primarily cultured porcine embryonic fibroblasts using the Amaxa Nucleofection system. Cloning Stem Cells 2007; 9(4): 523–534. [DOI] [PubMed] [Google Scholar]
- 53Sato M, Ohtsuka M, Miura H et al. Determination of the optimal concentration of several selective drugs useful for generating multi-transgenic porcine embryonic fibroblasts. Reprod Dom Anim 2012; 47(5): 759–765. [DOI] [PubMed] [Google Scholar]
- 54Maciejewska I, Chomik E. Hereditary dentine diseases resulting from mutations in DSPP gene. J Dent 2012; 40(7): 542–548. [DOI] [PubMed] [Google Scholar]
- 55Tabata K, Matsumoto K, Minami S et al. Nestin is an independent predictor of cancer-specific survival after radical cystectomy in patients with urothelial carcinoma of the bladder. PLoS One 2014; 9(5); e91548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Kondo S, Kubota S, Mukudai Y et al. Binding of glyceraldehyde-3-phosphate dehydrogenase to the cis-acting element of structure-anchored repression in ccn2 mRNA. Biochem Biophys Res Commun 2011; 405(3); 382–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.