Abstract
Primary immunodeficiencies (PIDs) are a group of genetically heterogeneous disorders that present with very similar symptoms, complicating definitive diagnosis. More than 240 genes have hitherto been associated with PIDs, of which more than 30 have been identified in the last 3 years. Next generation sequencing (NGS) of genomes or exomes of informative families has played a central role in the discovery of novel PID genes. Furthermore, NGS has the potential to transform clinical molecular testing for established PIDs, allowing all PID differential diagnoses to be tested at once, leading to increased diagnostic yield, while decreasing both the time and cost of obtaining a molecular diagnosis. Given that treatment of PID varies by disease gene, early achievement of a molecular diagnosis is likely to enhance treatment decisions and improve patient outcomes.
Keywords: Whole genome sequencing, Whole exome sequencing, Primary immunodeficiency, Next generation sequencing, Single nucleotide variation, Single nucleotide polymorphism
Introduction
Clinical immunologists, rheumatologists, infectious disease physicians, endocrinologists, oncologists, as well as general physicians, continue to face diagnostic dilemmas in the evaluation of immunodeficiency diseases, both primary (PIDs) and secondary immunodeficiencies, more so due to the explosive increase in the discovery of novel PIDs [1]. The incidence of each individual PID has a wide range: from 1 in 300 live births for selective IgA deficiency to 1 in 200,000 live births for chronic granulomatous disease [1] and extremely rare incidence for some of the newer PIDs [2]. Hence, the evaluation of immunodeficiency depends on the index of suspicion based on patient's clinical manifestations. Recurrent infections, severe or life-threatening infections, infections in unusual site, or infections caused by unusual organism are the most common manifestations of PIDs [1, 3]. Based on the type of infections and the immune cell/protein involved, PIDs were traditionally classified as defects in cellular and humoral immunity [4]. As additional defects were discovered, PIDs were classified as follows: (1) B cell and antibody defects, (2) T cell defects, (3) combined defects, (4) phagocyte defects, (5) complement defects, and (6) well-defined immunodeficiency syndromes [5]. However, it is now understood that many of the known genetic changes that cause PIDs also give rise to noninfectious immune features [6] such as malignancies [7], lymphoproliferation [8], granulomatosis [9, 10], atopy [11], autoimmunity [12, 13], autoinflammatory disorders [14, 15], and hemophagocytosis [1, 16, 17]. Moreover, nonimmune features are prominent manifestations of several PIDs including skeletal anomalies, dental anomalies, dysmorphic facies, albinism, and ectodermal dysplasia [1, 2, 11, 18–22]. Hence, a classification system based on the type of immune cell/protein and resulting infections is no longer sufficient for the complexity of known immunodeficiency phenotypes. As new PIDs are being discovered, it is important to use a diagnostic approach that does not rely solely on traditional classifications, but is suitable for patients with ambiguous or atypical phenotypes and is capable of detecting newly discovered genes. The most recent classification by the International Union of Immunological Societies Expert Committee demonstrates the complexity of PIDs and classifies PIDs into nine categories, namely (1) combined immunodeficiencies; (2) combined immunodeficiencies with associated or syndromic features; (3) predominantly antibody deficiencies; (4) diseases of immune dysregulation; (5) congenital defects of phagocyte number, function, or both; (6) defects in innate immunity; (7) autoinflammatory disorders; (8) complement deficiencies; and (9) phenocopies of PIDs [23•]. While some of the PIDs fit into more than one of these categories, signs and symptoms of various PIDs from different categories overlap considerably. Therefore, diagnostic workup can be time consuming and challenging.
Standard immunodeficiency evaluation of patients is guided by clinical presentation followed by initial screening tests that include phenotypic and functional studies like complete blood count with differential, peripheral blood smear, immunoglobulin levels, specific antibody titers, complement levels, and enumeration of lymphocyte subsets by flow cytometry. Based on the results of the screening tests, advanced second tier laboratory tests can be performed including antibody response to booster immunization, in vitro immunoglobulin production in response to mitogens or cytokines, in vitro lymphocyte proliferative response to mitogens and antigens, cytotoxicity assay, chemotaxis assay, cytokine production in response to toll-like receptor agonists, and bacterial/fungal killing assays [1, 24, 25, 26•]. While the basic evaluation is a useful guide for disease management, these test results categorize PID into groups of heterogeneous disorders in spite of variable phenotype and outcome (low specificity), as exemplified by common variable immunodeficiency (CVID) [27, 28]. Similarly, Epstein-Barr virus (EBV)-associated lymphoproliferative immunodeficiencies are lumped into a single category based on standard evaluation unless molecular testing is pursued [29–32]. Furthermore, standard tests can be normal in PIDs (low sensitivity) and the diagnosis may be missed if a specific molecular diagnosis is not achieved [33]. Molecular diagnosis has been increasingly sought in light of high area under the curve (AUC) of the receiver operating characteristic (ROC). Early molecular diagnosis and accurate identification of causative variants and immunological pathways is crucial as it provides the opportunity for timely treatment to prevent life-threatening infections and irreversible organ damage, prognostic determination, and guidance with respect to familial recurrence. Indeed, delayed diagnosis of primary immunodeficiencies is associated with increased morbidity and mortality [3]. Definitive genetic diagnosis provides insight into the mechanistic nature of the immunodeficiency and can indicate the desired course of treatment such as immunoglobulin replacement, stem cell transplantation, or the use of new investigative drugs that may target the specific pathway involved. Furthermore, molecular diagnosis provides important information about phenotype-genotype relationships, particularly in PIDs with broad clinical heterogeneity.
The approach to molecular diagnosis of PIDs, over the past two decades, has included Sanger sequencing of exons or mutation harboring regions of specific candidate genes, single nucleotide polymorphism (SNP) arrays, linkage analysis, homozygosity mapping, and clinicopathologic correlation with results of functional testing [34••, 35••, 36••]. Yet, a significant portion of such evaluations does not result in a definitive molecular diagnosis. This is due to numerous factors. Currently, less than 50 % of genes known to be associated with PID have clinical testing available in the USA (http://www.genetests.org/). Furthermore, for genes that can be tested, the traditional approach has been to order serial univariate or small panel genetic tests for genes on the differential diagnosis. This process is complicated by the clinical and genetic heterogeneity and overlap between many of the PIDs. In addition, the cost of ordering multiple clinical genetic tests, which can be more than $3,000 per gene tested, is frequently prohibitive. Furthermore, one can extrapolate from the rate of recent discoveries that not all genes associated with PIDs are currently known. Taken together, these factors have resulted in a suboptimal molecular testing strategy that can take months to years and may never identify a patient's molecular diagnosis.
An exponential increase in newly discovered genetic etiologies for immunodeficiency disorders has been reported in recent years. The Immunological Societies Expert Committee for Primary Immunodeficiency recognized 180 PIDs in November of 2011 [37]. In the following year, an additional 19 new PIDs were described [38], and by April of 2014 the Expert Committee listed 210 [23•]. At present, there are more than 240 single gene and structural variations that exhibit compromised immune function (Table 1). In the last 5 years, next generation sequencing (NGS) has revolutionized the field of immunogenetics and, more recently, has begun to shift the paradigm of clinical genetic testing [39–42, 43••]. Interestingly, the majority of the newly described PID genes were identified using NGS methodologies (Table 2). This review focuses on the capabilities and challenges associated with use of NGS for PID diagnosis and gene discovery. We include reports of cases of PIDs discovered and diagnosed using NGS at our and other centers as examples of how NGS may transform clinical practice in PID diagnosis and management.
Table 1.
Known genes causing primary immune deficiencies
| ACP5 | CD79A | G6PD | MBL2 | RHOH | TNFRSF13C |
| ACTB | CD79B | GATA2 | MCM4 | RMRP | TNFRSF1A |
| ADA | CD81 | GFI1 | MEFV | RNASEH2A | TNFRSF4 |
| ADAM17 | CD8A | HAX1 | MPO | RNASEH2B | TNFSF12 |
| ADAR | CEBPE | ICOS | MRE11A | RNASEH2C | TRAC |
| AICDA | CFB | IFNGR1 | MS4A1 | RNF168 | TRAF3 |
| AIRE | CFD | IFNGR2 | MTHFD1 | RNF31 | TRAF3IP2 |
| AK2 | CFH | IGHG1 | MVK | RPSA | TREX1 |
| AP3B1 | CFHR1 | IGHM | MYD88 | RTEL1 | TTC7A |
| APOL1 | CFHR2 | IGKC | NBN | SAMHD1 | TYK2 |
| ATM | CFHR3 | IGLL1 | NCF1 | SBDS | UNC119 |
| BLM | CFHR4 | IKBKB | NCF2 | SEMA3E | UNC13D |
| BLNK | CFHR5 | IKBKG | NCF4 | SERPING1 | UNC93B1 |
| BLOC1S6 | CFI | IKZF1 | NFKB2 | SH2D1A | UNG |
| BTK | CFP | IL10 | NFKBIA | SH3BP2 | USB1 |
| C1QAC1QA | CHD7 | IL10RAIL10RA | MBL2MBL2 | SLC29A3 | VPS13BVPS13B |
| C1QBC1QB | CIITA | IL10RBIL10RB | NHEJ1NHEJ1 | SLC35C1 | VPS45VPS45 |
| C1QCC1QC | CLEC7A | IL12BIL12B | NHP2NHP2 | SLC37A4 | WASWAS |
| C1RC1R | COLEC11 | IL12RB1IL12RB1 | NKX2NKX2-5 | SLC46A1 | WIPF1WIPF1 |
| C1SC1S | CORO1A | IL17FIL17F | NLRP12NLRP12 | SMARCAL1 | XIAPXIAP |
| C2C2 | CR2 | IL17RAIL17RA | NLRP3NLRP3 | SP110 | ZAP70ZAP70 |
| C3C3 | CSF2RA | IL1RNIL1RN | NOD2NOD2 | SPINK5 | ZBTB24ZBTB24 |
| C4AC4A | CTSC | IL21IL21 | NOP10NOP10 | STAT1 | |
| C4BC4B | CXCR4 | IL2RAIL2RA | NRASNRAS | STAT3 | |
| C5C5 | CYBA | IL2RGIL2RG | ORAI1ORAI1 | STAT5B | |
| C6C6 | CYBB | IL36RNIL36RN | PGM3PGM3 | STIM1 | |
| C7C7 | DCLRE1C | IL7RIL7R | PIGAPIGA | STK4 | |
| C8AC8A | DKC1 | IRAK4IRAK4 | PIK3CDPIK3CD | STX11 | |
| C8BC8B | DNMT3B | IRF8IRF8 | PIK3R1PIK3R1 | STXBP2 | |
| C8GC8G | DOCK8 | ISG15ISG15 | PLCG2PLCG2 | TAP1 | |
| C9C9 | ELANE | ITCHITCH | PMS2PMS2 | TAP2 | |
| CARD11CARD11 | FADD | ITGB2ITGB2 | PNPPNP | TAPBP | |
| CARD14CARD14 | FAS | ITKITK | POLE | TAZ | |
| CARD9CARD9 | FASLG | JAK3JAK3 | PRF1 | TBK1 | |
| CASP10CASP10 | FCGR1A | KRASKRAS | PRKCD | TBX1 | |
| CASP8CASP8 | FCGR2A | LAMTOR2LAMTOR2 | PRKDCPRKDC | TCF3 | |
| CD19CD19 | FCGR2B | LCKLCK | PSMB8PSMB8 | TCN2 | |
| CD247CD247 | FCGR3A | LIG4LIG4 | PSTPIP1 | TERC | |
| CD27CD27 | FCGR3B | LPIN2LPIN2 | PTPRCPTPRC | TERT | |
| CD3DCD3D | FCGRT | LRBALRBA | RAB27A | THBD | |
| CD3ECD3E | FCN3 | LRRC8ALRRC8A | RAC2RAC2 | TICAM1 | |
| CD3GCD3G | FERMT3 | LYSTLYST | RAG1RAG1 | TINF2 | |
| CD40CD40 | FOXN1 | MAGT1MAGT1 | RAG2RAG2 | TLR3 | |
| CD40LGCD40LG | FOXP3 | MALT1MALT1 | RFX5RFX5 | TMC6 | |
| CD46CD46 | FPR1 | MASP1MASP1 | RFXANKRFXANK | TMC8 | |
| CD59CD59 | G6PC3 | MASP2MASP2 | RFXAPRFXAP | TNFRSF13B |
Table 2.
Genes discovered as causing primary immunodeficiencies since 2011 and the method of discovery
| Gene name (HUGO) | Alt gene name | Method | PMID |
Year | ||
|---|---|---|---|---|---|---|
| Reference 1 | Reference 2 | Reference 3 | ||||
| ADAM17 | Homozygosity mapping+targeted NGS | 22010916 | 2011 | |||
| BLOC1S6 | PLDN | Candidate gene; exome | 22461475 | 21665000 | 2011 | |
| GATA2 | Candidate gene; exome | 21670465 | 21765025 | 2011 | ||
| IL36RN | Exome | 21848462 | 2011 | |||
| MTHFD1 | Exome | 21813566 | 23296427 | 2011 | ||
| TICAM1 | TRIF | Candidate gene | 22105173 | 2011 | ||
| TRAC | Genome-wide linkage and candidate gene | 21206088 | 2011 | |||
| CD27 | TNFRSF7 | Candidate gene; exome | 22197273 | 22801960 | 2012 | |
| CR2 | CD21 | Candidate gene | 22035880 | 2012 | ||
| ISG15 | Exome | 22859821 | 2012 | |||
| LCK | Candidate gene | 22985903 | 2012 | |||
| LRBA | Candidate gene; exome | 22608502 | 22981790 | 2012 | ||
| MCM4 | Homozygosity mapping+targeted NGS; genome-wide linkage and candidate gene | 22354170 | 22354167 | 2012 | ||
| NKX2-5 | Exome and mouse model | 22560297 | 2012 | |||
| PIK3R1 | Exome | 22351933 | 2012 | |||
| PLCG2 | Exome | 23000145 | 2012 | |||
| PRKCD | Exome | 23319571 | 23430113 | 2012 | ||
| RHOH | Genome-wide linkage and exome | 22850876 | 2012 | |||
| STK4 | MST1 | Genome-wide linkage and candidate gene; exome | 22294732 | 22174160 | 22952854 | 2012 |
| TBK1 | Candidate gene | 22851595 | 2012 | |||
| UNC119 | Candidate gene | 22184408 | 2012 | |||
| WIPF1 | Candidate gene | 22231303 | 2012 | |||
| CARD11 | Exome | 23561803 | 2013 | |||
| CORO1A | Genome-wide homozygosity mapping and exome | 23522842 | 2013 | |||
| IKBKB | Homozygosity mapping | 24369075 | 2013 | |||
| IL21 | Exome and candidate gene | 23440042 | 2013 | |||
| MALT1 | Homozygosity mapping+genome; exome | 23727036 | 24332264 | 2013 | ||
| PIK3CD | Exome | 24136356 | 24165795 | 2013 | ||
| RPSA | Exome | 23579497 | 2013 | |||
| TTC7A | Exome | 23830146 | 2013 | |||
| PGM3 | Exome | 24589341 | 2014 | |||
Next Generation Sequencing Approaches
Next generation sequencing is a high throughput, massively parallel technology involving simultaneous sequencing of a large number of template DNA or cDNA fragments in parallel. DNA sequencing can be performed on the entire genome or targeted to specific regions. Examples of the latter include a subset of genes (i.e., an NGS multiplex panel) and sequencing of all genetic coding regions, termed exome sequencing. For the purposes of this review, we will highlight three NGS sequencing approaches and describe how they have been used in the clinical evaluation of PIDs and how they may be utilized in the future. In-depth reviews of NGS methods and technologies are available elsewhere [44–46].
Targeted Panel
Sequencing the mutation harboring regions of a subset of genes, often referred to as targeted panels, offers specific advantages over exome or genome sequencing. Three methods are commonly employed for targeted sequencing: multiplex PCR, hybridization, and molecular inversion probes [47]. As compared to exome and genome sequencing, we and others have shown that targeted panels typically allow for increased depth of sequencing coverage, which results in higher accuracy, improved sensitivity, and fewer regions not analyzed [42, 48–51]. In part, this reflects the greater bait optimization that is possible with an oligogenic panel and, in part, is a product of the deeper sequencing associated with a smaller total target size. An additional benefit of targeted panel sequencing is that it reduces the risk of incidental findings, such as detection of carrier status, future disease risk, and presymptomatic disease state [52–63]. The impact of the incidentalome is especially important to consider for sequencing tests that are predominantly used for children, who have little or no autonomy [58], as is often the case with PIDs. Furthermore, targeted panel sequencing can be less expensive than exome or genome sequencing due to the smaller total target size [51]. However, a limitation of targeted panel sequencing is that it is confined to the set of genes and/or regions on the panel, meaning it is unable to identify novel causes of genetic disorders; this is of particular concern as over the last 3 years, on average, 10 new genes per year have been associated with PID.
At least two targeted panels have been developed that contain a significant number of PID causing genes. A targeted panel of 170 genes was recently reported by researchers in the Netherlands as a diagnostic tool for PIDs [35••]. The authors of this study reported that 4 (15 %) of 26 patients that had previously been refractory to genetic diagnosis received a definitive molecular diagnosis. Those patients had disease-causing variants in DOCK8, PSTPIP1, XIAP, and CXCR4 [35••]. Interestingly, three of the four patients that received a genetic diagnosis were described to have atypical presentations of known PIDs, a feature known as unanticipated clinical heterogeneity. We have developed an NGS panel, Targeted Gene Sequencing and Custom Analysis (TaGSCAN), as a tool for diagnosis of childhood genetic disorders. Of the 515 genes featured on TaGSCAN, 55 are known to cause PIDs. TaGSCAN is a validated CLIA-compliant test available for clinical use by physicians. We describe a case of a child with a rare PID who was diagnosed by TaGSCAN: The patient was a previously healthy 13-year-old Caucasian male who presented with 4–6 weeks of progressive nontender swelling along both sides of his neck. The history was notable for pneumonia requiring hospitalization at age 2, a paternal uncle who had tuberculosis as a teenager, and an unspecified immune problem in the maternal grandmother. On physical examination, numerous firm, nontender, nonmobile, and nonerythematous bilateral anterior and posterior cervical lymph nodes as well as inguinal and femoral lymph nodes (largest 2.5 cm) were palpable. Oral thrush was noted. Laboratory data revealed normal complete blood count with a white blood cell count of 10,720/μL, elevated erythrocyte sedimentation rate (ESR) of 50 mm/h, and C-reactive protein (CRP) of 1.9 mg/dL. HIV antibodies were negative; EBV antibody panel was consistent with prior infection. An excisional biopsy of a cervical lymph node was obtained, and the histopathology revealed chronic lymphadenitis with reactive lymphoid hyperplasia, negative for malignancy. Aerobic bacterial culture of the lymph node grew Salmonella Enteritidis (group D). Anaerobic, acid fast bacilli and fungal cultures were negative. Due to the very unusual presentation of his Salmonella infection, an underlying immunodeficiency was suspected. Testing for chronic granulomatous disease by flow cytometry showed normal oxidative burst. Quantitative immunoglobulins revealed an elevated IgG of 4,930 mg/dL (normal 613–1,295 mg/dL), mildly elevated IgM of 346 mg/dL (normal 53–334 mg/dL), normal IgA of 178.0 mg/dL, and normal IgE of 62.4 kU/L. Flow cytometry for T and B cell subsets demonstrated an elevated number of CD25 and HLA-DR-positive T cells, a nonspecific indicator of T cell activation that occurs in a variety of infectious states. After treatment of his infection, repeat testing was unremarkable. Since interferon 12 receptor beta 1 gene (IL-12Rβ1) deficiency is associated with unusual Salmonella infections, mycobacterial infections, and thrush [33, 64], a TaGSCAN panel was ordered. Within 6 weeks, the test revealed two heterozygous, deleterious variants in the IL12RB1 gene. The first, p.Trp118X (c.354G>A), was predicted to result in a premature stop codon. The second, p.Ala573Leufs*22 (c.1791+2T>G), was a known disease-causing variant [65]. The presence of two different variants, as in this case, was consistent with a form of autosomal recessive disease inheritance known as compound heterozygosity. The variants were confirmed by Sanger sequencing.
Upon completion of 3 weeks of ciprofloxacin and fluconazole therapy, there was resolution of the thrush, marked improvement of the lymphadenopathy, and normalization of the ESR and CRP. About 2–3 weeks after completing ciprofloxacin, the lymphadenopathy returned. Laboratory testing revealed an ESR of 34 mm/h and CRP of 1.5 mg/dL. A contrasted CT of the neck, chest, abdomen, and pelvis revealed multifocal nonspecific adenopathy involving the cervical chains, submandibular, periparotid, mediastinal, mesenteric, and bilateral inguinal regions. An excision of left inguinal lymph node was performed, and histopathology revealed reactive lymphoid hyperplasia with chronic inflammation. An acid fast bacilli stain was negative. Aerobic bacterial culture of the lymph node again grew Salmonella, group D. Anaerobic, acid fast bacilli, and fungal cultures were negative. A mammalian susceptibility to mycobacterial disease (MSMD) screen by flow cytometry showed normal interferon-γ receptor 1 (IFNGR1) protein expression on the surface of peripheral blood mononuclear cells, normal signal transducers and activators of transcription family 1 (STAT1) phosphorylation in response to IFN-γ, decreased phosphorylation of STAT4 in response to IL-12, but normal in response to interferon-α. He received 6 weeks of oral ciprofloxacin and has remained free of infection for more than 10 months. He continues to take azithromycin for prophylaxis against mycobacterial infections.
IL12RB1 encodes a chain of the receptor for IL-12 and IL-23. IL-12 promotes cell-mediated immunity to intracellular pathogens by inducing TH1 responses and IFN-γ production and binding to IL-12 β1/β2 receptors on T cells and natural killer cells. IL-23 is thought to play a role in pathogens like extracellular bacteria and Candida albicans. The first three cases of PID associated with IL12RB1 gene defects were reported in 1998 and had susceptibility to mycobacterial and Salmonella infections [33]. Since then, 70 unique pathogenic IL12RB1 mutations with autosomal recessive inheritance have been reported in 198 individuals [64].
Exome
Exome sequencing, in theory, covers all known coding regions of all genes. Thus, exome sequencing, in theory, enables detection of the majority of important pathogenic variants. However, in practice, mutation-harboring introns (the noncoding regions between exons) are not fully included, and variants in some exons (particularly exon one) may be missed. Baits cannot be optimized for all targets and the effects of allele-specific enrichment are poorly understood. In addition, novel exons in known genes and novel genes are not targeted. Recently, nevertheless, clinical exome sequencing has been implemented at multiple institutions and early reports have demonstrated its clinical utility in diagnosing rare genetic conditions [66–69]. Although no studies have specifically looked at exome sequencing as a first line diagnostic tool specifically in PID patient populations, multiple case reports describe the use of exome sequencing as a diagnostic and a discovery tool for novel PID genes (Table 2). We summarize some of the recent novel genes whose association with homogeneous PID presentations were elucidated with exome sequencing in this review and present a comprehensive list in Table 2.
Examples of Recent PID Gene Defects Discovered or Diagnosed by NGS
LPS-Responsive Vesicle Trafficking, Beach and Anchor Containing (LRBA) (OMIM ID: 606453)
Two separate studies used NGS to link variants in LRBA to immunodeficiency [70, 71]. In the first study, five affected individuals from four consanguineous families of Arab, Sicilian, and Iranian origin were diagnosed with childhood-onset CVID, presenting with hypogammaglobulinemia, autoimmunity, bronchiectasis/chronic lung disease, and inflammatory bowel disease [70]. These patients had decreased switched memory B cells, elevated B cell apoptosis, and diminished B cell autophagy. Linkage analysis and candidate gene sequencing identified homozygous mutations in LRBA in all affected cases (missense: c.7970T>G, p.Ile2657Ser, exon 54; stop codon: c.5047C>T, p.Arg1683, exon 30; stop codon: c.175G>T, p.Glu59, exon 3; deletion: g.152211739_152222852del111114, chr4, NCBI build 36). LRBA was suspected to be the candidate gene as it is inducible by lipopolysaccharide (LPS), a bacterial cell wall component that is an immunogen. In the second study, five affected patients from two sets of consanguineous parents who shared a common set of grandparents underwent exome sequencing. They all had chronic diarrhea, and some had autoimmune cytopenias, EBV-associated lymphoproliferative disease, and recurrent arthritis. Among them, two patients had normal lymphocyte subsets, immunoglobulins, and T cell proliferation to mitogen stimulation, while three patients had normal lymphocyte subsets but low immunoglobulin levels and reduced T cell proliferation to mitogen stimulation. Exome sequencing showed a homozygous variant c.6657_6658del, pGlu2219Aspfs*3 in exon 44 of LRBA [71]. The variant was predicted to cause premature truncation of the protein that was confirmed by Western blot of lymphoblast lysates of patients.
Biogenesis of Lysosomal Organelles Complex-1, Subunit 6, Pallidin (BLOC1S6 (PLDN)) (OMIM ID: 604310)
We used exome sequencing to identify a novel Hermansky-Pudlak-like PID in a 17-year-old Italian female with oculocutaneous albinism, nystagmus, and recurrent cutaneous infections [72]. Exome sequencing revealed a homozygous nonsense mutation in exon 3 of pallidin (PLDN), official HUGO gene name BLOC1S6 (c.232C>T; p.Q78X, chr15:45895305C>T, human genome build 37), which resulted in deficient protein expression. Functional studies demonstrated impaired NK cell cytolytic activity. The same homozygous nonsense mutation in PLDN was reported in a 9-month-old Indian male with albinism and nystagmus but without other symptoms or signs of Hermansky-Pudlak syndrome. However, this patient did not have signs and symptoms of immunodeficiency at the time of presentation likely due to his diagnosis at an early age [73].
CD27 (OMIM ID: 186711)
Homozygosity mapping and exome sequencing were used to study three patients of Turkish origin and three of Lebanese origin, who presented with primary infectious mononucleosis or EBV and/or suspected hemophagocytic lymphohistiocytosis [74]. The patients displayed no CD27+ cells and one of them had near absent invariant natural killer T cells (iNKT). Some had hypogammaglobulinemia. Candidate gene sequencing was used to identify a homozygous potential disease-causing variant in CD27. Follow-up homozygosity mapping and exome sequencing were then performed in the family and three affected siblings to rule out other potential disease-causing mutations in genes. Homozygosity mapping revealed four chromosomal regions present only in affected siblings. Exome sequencing did not show any additional genetic mutations within these regions. The affected siblings had two candidate intervals containing CD27. The variant found was a homozygous missense mutation (c.G158A, p.Cys53Tyr). Further exome sequencing in two additional unrelated patients also revealed the same homozygous missense mutation in CD27, providing added evidence to support disease causation [74]. Another study included two siblings of consanguineous Moroccan family [75]. One brother presented with infectious mononucleosis and aplastic anemia and died from fulminant gram-negative sepsis. His brother had EBV viremia and hypogammaglobulinemia and impaired T cell-dependent B cell response. Genetic testing revealed a homozygous mutation in CD27 (c.G24A, p.Trp8X) in both patients. CD27 is a member of the TNF receptor family. It acts as a transmembrane receptor and participates in co-stimulation of lymphocytes. The authors suggest that CD27 may affect terminal differentiation of B cells into memory and plasma cells [75].
Phosphoglucomutase 3 (PGM3) (OMIM ID: 172100)
Eight patients from two families (one with consanguineous parents of Egyptian origin) presented with severe atopic dermatitis, other atopic diseases, and elevated IgE. They had recurrent infections despite increased Ig levels and protective antibody titers. Recurrent skin infections included staphylococcal infections, molluscum contagiosum, and flat warts. Recurrent sinopulmonary infections showed moderate response to antibiotic prophylaxis but did not improve with immunoglobulin replacement therapy. Other infections included bronchiectasis among older patients causing death in one patient and lung transplantation in another. Autoimmune cytopenias, glomerulonephritis, cutaneous leukocytoclastic vasculitis, facial dysmorphism, developmental delay, ataxia, dysarthria, sensorineural hearing loss, myoclonus, and seizures were other features. They had low CD8+ T cells and CD27+ memory B cells, elevated IgG, IgA, IgE, protective vaccine titers, and presence of rheumatoid factor. Exome sequencing was performed. PGM3 was the only candidate gene with a rare, compound heterozygous variant with maternal missense variant (c.1585G>C, p.E529Q) and paternal 5-bp deletion (c.1438_1442del, p.L480Sfs*10) causing a frame shift and premature stop. These variations were confirmed by Sanger sequencing [76].
Phosphatidylinositol-4,5-bisphosphate 3-kinase, Catalytic Subunit Delta (PIK3CD: p110delta) (OMIM ID: 602839)
Nine patients from seven unrelated families presented with recurrent sinopulmonary infections, lymphoproliferation, chronic EBV infection, and/or cytomegalovirus (CMV) viremia. The patients lacked CD27+ memory B cells, showed increased transitional CD10+ B cells, CD4+ lymphopenia with increased CD8+ T cells, impaired T cell proliferation in vitro, reduced CD45RA+CCR7+ naive T cells, and increased CD45RA−CCR7− effector memory T cells and CD45RA+ effector memory T cells. Elevated IgM was noted in a few patients. Exome sequencing was used to generate a candidate gene list with nonsynonymous novel rare variants. Patients and family members were found to be heterozygous for one of the three mutations (c.C1002A, p.N334K in C2 domain; c.G1573A, g.E525K in helical domain; c.G3061A, p.E1021K in C-lobe of the kinase domain). Functional prediction software indicated these mutations to be a potential gain-of-function mutation in PIK3CD encoding p110delta subunit of PI3K, an enzyme that was confirmed by Sanger sequencing. Functional studies showed that this enzyme converts phosphatidylinositol-2-phosphate to phosphatidylinositol-3-phosphate which activates Akt and in turn activates mammalian target of rapamycin 1 (mTOR1), leading to increased glycolysis. Increased glycolysis causes CD8+ T cells to differentiate into effector cells and turn into senescent cells and reduces the number of long-lived memory CD8+ Tcells, leading to the PID noted in the patients.
Phosphoinositide-3-kinase, Regulatory Subunit 1 Alpha (PIK3R1) (OMIM ID: 171833)
A 19-year-old patient from a consanguineous family of Chinese/Peruvian origin presented with colitis and severe defects in B cell development with absent B cells, markedly reduced or absent pro-B cells, and agammaglobulinemia [77]. Exome sequencing revealed a homozygous premature stop codon in PIK3R1 (c.G298A, Trp to premature stop codon in exon 6) and both parents were heterozygous. Fifty-five patients with defects in B cell development of unclear etiology had exome sequencing done but did not reveal mutations in PIK3R1. Two older brothers and two maternal uncles had died of acute infections before 2 years of life. She was treated with immunoglobulin replacement. Filters used to identify the mutation included discarding synonymous variants, public and in-house SNP databases to exclude common variants, homozygosity mapping followed by use of functional predication software, which left PIK3R1 as the likely candidate gene. The findings in this individual case suggest pathogenicity of PIK3R1; however, supporting functional studies and/or detection of deleterious variants in this gene in similarly affected individuals are necessary for confirmation of PID gene causality.
Caspase Recruitment Domain 11 (CARD11) (OMIM ID: 607210)
A female born to consanguineous parents of Central European origin presented at 6 months of age with Pneumocystis jirovecii pneumonia. She had agammaglobulinemia with normal T and B cell counts, impaired lymphocyte proliferation, absent expression of proinflammatory cytokines, and absent monocytic cells. Immunophenotyping showed naive CD4+ and CD8+ T lymphocytes (CD45RA+ CCR7+) and naive B cells (CD27−), mostly transitional B cells, with the absence of regulatory T cells (CD4+, CD25+, CD127-, CD45RO+, and CCR4+). She was diagnosed with severe combined immunodeficiency (SCID). Exome sequencing revealed a variant in CARD11 (c.2833C>T, p.Q945*) that induced a premature stop codon. Sanger sequencing of the parents and patient confirmed heterozygous and homozygous mutations, respectively, consistent with autosomal recessive inheritance. The control subject was included in the study. The patient was treated successfully with a peripheral blood stem cell transplant [78]. CARD11 is a scaffold protein that participates in TCR and BCR induced NF-kappa B activation and gene transcription of cytokines. CARD11 double knockout mice (card11−/−) demonstrate antibody deficiencies and impaired T and B cell activation. Upon analyzing the signaling pathways in T and B cells, JNK activation was impaired in both cells and after TLR4 stimulation via lipopolysaccharide. Similarly, IκBα degradation was impaired downstream of protein kinase C (PKCθ and PKCβ). Thus, CARD11 plays a role in both innate and adaptive immune responses [79].
Tetratricopeptide Repeat Domain-7A (TTC7A) (OMIM ID: 609332)
Exome sequencing and homozygosity mapping revealed mutations in the gene tetratricopeptide repeat domain-7A (TTC7A) as the cause of congenital multiple intestinal atresia (MIA). Seven French-Canadian families with MIA were evaluated. Five patients from four different families were found to have homozygous four base intronic deletions (c.53344_53347delAAGT in 5′ splice donor site of exon 7, encoding TPR protein) in TTC7A adjacent to the consensus GT splice donor site. Heterozygous mutations were found in the parents of these four families and two other families in which the patient's DNA was not available. In a seventh family, the two affected siblings had compound heterozygous mutations for the same 4 bp deletion (maternal) and second missense variant c.A133074G, p.L823P in C-terminal of exon 20 (paternal) [80]. In a separate study, exome sequencing and Sanger confirmatory testing in eight unrelated patients with combined immunodeficiency-MIA showed seven different mutations in TTC7A. These families were of Arabian, Serbian, Bosniak, French-Canadian/European, Slavian, and Italian origin [81]. TTC7A, a protein expressed in cells like thymic epithelial cells and colonic epithelial cells, appears to be responsible for causing CID-MIA.
Protein Kinase C Delta (PRKCD) (OMIM ID: 176977)
The index proband, born to consanguineous parents of Turkish origin, presented with recurrent infections since infancy [82]. At 15 months of age, the patient developed nephrotic syndrome, hepatosplenomegaly, lymphadenopathy, relapsing polychondritis, latent hypothyroidism, aseptic endocarditis, pulmonary embolism, and antiphospholipid syndrome. The patient had hypogammaglobulinemia but elevated IgA and M, with decreased CD19+, class switched and nonswitched B memory cells, and increased CD21low B cells, mildly decreased T cell lymphoproliferative response, and absence of isohemagglutinins. The patient was treated with immunoglobulin replacement followed by anti-CD20 therapy, mycophenolate-mofetil, and corticosteroids. Sanger sequencing showed a single CTLA4 variant in the father and proband, which did not explain the phenotype. Exome sequencing and homozygosity mapping were performed. Homozygous variants in UBXN1 (c.G686A, p.Thr229Met) and PKCD (c.1352+ 1G>A) were identified. PKCD was considered as the likely cause of the PID since no pathogenic role of UBXN1 was recognized. Assessment of the functional significance of the PKCD variant showed reduced expression of the PKC target MARCKS in immunoblots of EBV cell line from the proband and increased IL-6 production [82]. PKCD is required for proapoptotic signaling and B cell tolerance.
Another group studied a Hispanic male who presented with recurrent infections, hepatosplenomegaly, lymphadenopathy, and lupus-like rash [83]. His laboratory evaluation showed autoantibodies, B cell lymphocytosis, persistent EBV, decreased NK cells, and low NK cell cytolytic activity. IL-6 was not increased, while IL-10 level was noted to be increased. Gene testing for autoimmune lymphoproliferative syndrome (ALPS) and ALPS-like syndrome and X-linked lymphoproliferative disease genes did not reveal any mutations. He was treated with corticosteroids and rapamycin. Exome sequencing identified a homozygous variant (c.1840C>T, p.R614W) in PKCD that was confirmed by Sanger sequencing [83].
Stromal Interaction Molecule 1 (STIM1) (OMIM ID: 605921)
A 2-year-old patient of Turkish origin, born to consanguineous parents, presented with a history of lymphadenopathy, hepatosplenomegaly, autoimmune hemolytic anemia, and Kaposi sarcoma (KS) of lip, which progressed to disseminated KS. Serum immunoglobulins, lymphocyte subsets, and T cell proliferation to mitogen/antigen stimulation were normal. HIV testing was negative [84]. Exome sequencing revealed a homozygous missense variant (c.1538-1G>A of 5′-exon 8) in STIM1. STIM1 is an endoplasmic reticulum (ER) transmembrane protein that participates in store-operated calcium entry resulting into influx of extracellular calcium and increase in intracellular calcium concentration. The calcium release from ER was normal but the extracellular calcium influx was noted to be absent in the patient's EBV-B cells. Combined with the biochemical and cellular evidence of functional effects of the variant, STIM1 was suggested to be the cause of the PID.
Genome
Genome sequencing offers potential benefits compared to targeted panel and exome sequencing. Specifically, genome sequencing can detect intronic mutations, has more consistent coverage across genes, and enables detection of very large deletions (Noll et al., in preparation) [85]. Conversely, only very small (1-25 nucleotide) insertions and deletions can reliably be detected with exome sequencing and targeted panels. In addition, rapid genome sequencing platforms, as employed for infants at our institution [43••] (Soden et al., submitted), enable diagnosis within days to weeks of symptom onset, thereby accelerating the rate of definitive treatments. For severe PIDs such as SCID, this means the potential to intervene with hematopoietic stem cell transplant more quickly, potentially reducing morbidity, mortality, and cost. To date, only one report of genome sequencing assisting in the diagnosis of PIDs has been published [86]. However, we have reported a proof of principle study demonstrating the utility of rapid whole genome sequencing for infants in the NICU [43••] and predict that this method will have efficacy for suspected severe cases of PID. It is likely that as the cost of genome sequencing and analysis falls that the number of instances in which genome sequencing is used to assist in the diagnosis or discovery of PIDs will increase.
Mucosa-Associated Lymphoid Tissue Lymphoma Translocation Gene 1 (MALT1 (OMIM ID: 604860)
Two siblings with consanguineous parents of Lebanese origin developed recurrent pneumonia, mastoiditis, cheilitis, and gingivitis since 4 months age [86]. They also had bronchiectasis, CMV in urine, and duodenal candidiasis and were subsequently given immunoglobulin replacement, but died due to respiratory failure. They both had absent isohemagglutinins and vaccine titers, impaired T cell proliferation, reduced NK cells, but normal T and B cell counts. Genome sequencing performed revealed three possible candidates. Of these, only MALT1 was in a region of homozygosity. Sanger sequencing confirmed a homozygous missense variant (c.266G>T, p.Ser89Ile on chromosome 18). In order to study the functional significance of the mutation, mRNA and protein expression of MALT1 were performed. MALT1 protein was absent in T cells in one of the patients, suggesting rapid degradation of MALT1 mutant transcripts. MALT1 is a protease that activates NF-kappa B that helps to initiate IL-2 gene transcription. IL-2 expression was noted to be reduced in the patient, suggesting disrupted NF-kappa B activation, leading to reduced IL-2 gene transcription.
Limitations/Challenges of NGS
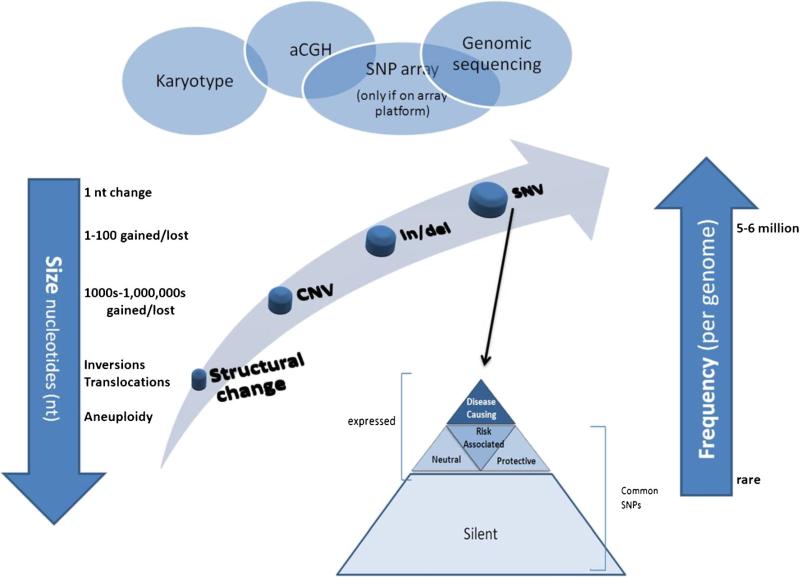
Although the above examples highlight the clinical potential of NGS for discovery and diagnosis of PIDs, there are multiple limitations and challenges that need to be considered. First, most NGS technologies and bioinformatic tools still are unable to routinely and accurately identify certain genetic variations, including most copy number variants, inversions, translocations, and nucleotide repeat expansions. Array comparative genomic hybridization (aCGH) is better suited for detection of large copy number variants, but insensitive to most small events (Fig. 1). For example, 27 subjects from three families had various combinations of recurrent infections, antibody deficiency, autoimmune disease or presence of autoantibodies, granulomatous disease, and cold-induced urticaria. Linkage analysis from one family revealed 24 candidate genes, but genome sequencing did not reveal any novel variants. Since gene encoding phospholipase C, gamma-2 (PLCG2, OMIM ID: 600220) was the primary candidate gene due to its role in B cell and NK cell signaling, Sanger sequencing was performed and revealed heterozygous deletions in PLCG2 involving C-terminal Src homology 2 (cSH2) in exon 19 as well as exons 20 through 22. These deletions were confirmed by post hoc analysis of genome [14]. In addition, bioinformatic analysis of genes with highly homologous sequence, such as HLA regions; genes that have pseudogenes (nonfunctional regions that share sequence similarity with coding genes and that arise by gene duplication); and genes with large repetitive elements can be difficult. Consequently, it is critical to be aware of PID genes on the differential diagnosis that have disease-causing mutations that are not well detected by NGS. In such cases, consultation with a clinical and/or molecular geneticist is recommended to determine which test is most appropriate.
Fig. 1.
Human genetic variation and common detection methods
A unique challenge in the field of genomics is that the molecular diagnosis precedes a clear understanding of the pathophysiology leading to the clinical presentation in some patients. When potential novel genes or phenotypes are detected, functional studies and model systems are needed to understand the biological and pathogenic effect(s) of the variant(s). Lastly, if a disease is not monogenetic, targeted panel, exome or genome sequencing results will be nondiagnostic. Methods to measure the impact of epigenetic factors such as DNA methylation and histone modification, or epistasis, are not yet available for use in diagnostic evaluations. Although defects in epigenetic regulation have not yet been associated with PIDs, they may be in the future, and as such, detection methods will become pertinent to clinicians.
Conclusions
NGS clinical and research applications have been transformative via novel PID gene discovery and refined phenotype-genotype correlations. Furthermore, NGS methods have proven extremely useful in identifying the causes of atypical cases. For example, we have previously described the use of exome sequencing to identify two deleterious, homozygous mutations in DOCK8 (Dedicator of cytokinesis, OMIM ID: 611432) and CLEC7A (C-type lectin domain family 7, member A, OMIM ID: 606264) in three siblings, who presented with IPEX-like disease [87]. NGS has the potential to drastically improve the diagnosis rate of PIDs while simultaneously reducing both the cost and time of testing as compared to standard clinical genetic testing. Accelerating the implementation of treatment, particularly via rapid genomes for critically ill children, has the potential for a significant reduction in morbidity and mortality for such patients. For instance, we have reported the use of exome sequencing to diagnosis siblings with infantile onset inflammatory bowel disease due to mutations in IL10RA (interleukin 10 receptor, alpha, OMIM ID: 146933) [88]. This diagnosis subsequently led to a change in clinical management and hematopoietic stem transplantation [88]. We conclude that the discovery of PIDs is rapidly evolving. As NGS and technologies in informatics continue to improve, the utility of diagnostic genome sequencing will continue to increase.
Acknowledgments
The project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number KL2 TR000089 to Darrell L. Dinwiddie, PhD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
This article is part of the Topical Collection on Pediatric Allergy and Immunology
Compliance with Ethics Guidelines
Conflict of Interest Nikita Raje, Sarah Soden, Christina E. Ciaccio, and Stephen F. Kingsmore declare no conflict of interest. Douglas Swanson declares grants from Pfizer, Inc., outside the submitted work.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any authors.
Contributor Information
Nikita Raje, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA; University of Missouri—Kansas City, Kansas City, MO, USA.
Sarah Soden, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA; University of Missouri—Kansas City, Kansas City, MO, USA.
Douglas Swanson, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA; University of Missouri—Kansas City, Kansas City, MO, USA.
Christina E. Ciaccio, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA University of Missouri—Kansas City, Kansas City, MO, USA.
Stephen F. Kingsmore, Children's Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108, USA University of Missouri—Kansas City, Kansas City, MO, USA.
Darrell L. Dinwiddie, Department of Pediatrics and Clinical Translational Science Center, University of New Mexico Health Sciences Center, 1 University of New Mexico, MSC08 4635, Albuquerque, NM 87131, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–S63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 2.Dotta L, Parolini S, Prandini A, et al. Clinical, laboratory and molecular signs of immunodeficiency in patients with partial oculo-cutaneous albinism. Orphanet J Rare Dis. 2013;8:168. doi: 10.1186/1750-1172-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbarayan A, Colarusso G, Hughes SM, et al. Clinical features that identify children with primary immunodeficiency diseases. Pediatrics. 2011;127(5):810–6. doi: 10.1542/peds.2010-3680. [DOI] [PubMed] [Google Scholar]
- 4.Fudenberg H, Good RA, Goodman HC, et al. Primary immunodeficiencies. Rep of a World Health Organ Comm Pediatr. 1971;47(5):927–46. [PubMed] [Google Scholar]
- 5.Chapel H, Geha R, Rosen F, et al. Primary immunodeficiency diseases: an update. Clin Exp Immunol. 2003;132(1):9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317(5838):617–9. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- 7.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 8.Elenitoba-Johnson KS, Jaffe ES. Lymphoproliferative disorders associated with congenital immunodeficiencies. Semin Diagn Pathol. 1997;14(1):35–47. [PubMed] [Google Scholar]
- 9.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133(2):198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold DF, Wiggins J, Cunningham-Rundles C, et al. Granulomatous disease: distinguishing primary antibody disease from sarcoidosis. Clin Immunol. 2008;128(1):18–22. doi: 10.1016/j.clim.2008.03.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong PF, Freeman AF, Engelhardt KR, et al. An update on the hyper-IgE syndromes. Arthritis Res & Ther. 2012;14(6):228. doi: 10.1186/ar4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gathmann B, Mahlaoui N, for C, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 13.Xiao X, Miao Q, Chang C, et al. Common variable immunodeficiency and autoimmunity—an inconvenient truth. Autoimmun Rev. 2014;13(8):858–64. doi: 10.1016/j.autrev.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Ombrello MJ, Remmers EF, Sun G, et al. Cold urticaria, immuno-deficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366(4):330–8. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Lee GS, Brady J, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91(4):713–20. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak CC, Sandford A, Fong A, et al. Maternal T-cell engraftment associated with severe hemophagocytosis of the bone marrow in untreated X-linked severe combined immunodeficiency. J Pediatr Hematol Oncol. 2008;30(5):396–400. doi: 10.1097/MPH.0b013e318168e7a0. [DOI] [PubMed] [Google Scholar]
- 17.Parekh C, Hofstra T, Church JA, et al. Hemophagocytic lymphohistiocytosis in children with chronic granulomatous disease. Pediatr Blood Cancer. 2011;56(3):460–2. doi: 10.1002/pbc.22830. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Nishikomori R, Heike T. Diagnosis and treatment in anhidrotic ectodermal dysplasia with immunodeficiency. Allergol Int. 2012;61(2):207–17. doi: 10.2332/allergolint.12-RAI-0446. [DOI] [PubMed] [Google Scholar]
- 19.Temmerman ST, Ma CA, Zhao Y, et al. Defective nuclear IKKalpha function in patients with ectodermal dysplasia with immune deficiency. J Clin Invest. 2012;122(1):315–26. doi: 10.1172/JCI42534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doffinger R, Smahi A, Bessia C, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–85. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Ma CA, Liu S, et al. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–8. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 22.Mansour S, Woffendin H, Mitton S, et al. Incontinentia pigmenti in a surviving male is accompanied by hypohidrotic ectodermal dysplasia and recurrent infection. Am J Med Genet. 2001;99(2):172–7. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1155>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23•.Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for primary immunodeficiency. Frontiers Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [This paper provides the most updated classification of primary immunodeficiencies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim MS, Elenitoba-Johnson KS. The molecular pathology of primary immunodeficiencies. J Mole Diagn : JMD. 2004;6(2):59–83. doi: 10.1016/S1525-1578(10)60493-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangsinmankong N, Bahna SL, Good RA. The immunologic work-up of the child suspected of immunodeficiency. Ann Allergy Asthma Immunol. 2001;87(5):362–9. doi: 10.1016/S1081-1206(10)62915-8. quiz 70, 423. [DOI] [PubMed] [Google Scholar]
- 26•.Ochs HD, Hagin D. Primary immunodeficiency disorders: general classification, new molecular insights, and practical approach to diagnosis and treatment. Ann Allergy Asthma Immunol. 2014;112(6):489–95. doi: 10.1016/j.anai.2014.04.007. [This paper discusses the most recently discovered primary immunodeficiencies and a clinical approach to their diagnosis.] [DOI] [PubMed] [Google Scholar]
- 27.Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. The J Allergy and Clin Immunol Pract. 2013;1(6):545–56. doi: 10.1016/j.jaip.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematol / Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2012;2012:301–5. doi: 10.1182/asheducation-2012.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20(2):129–35. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 30.Huck K, Feyen O, Niehues T, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–8. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95(23):13765–70. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigaud S, Fondaneche MC, Lambert N, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444(7115):110–4. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 33.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 34••.Chou J, Ohsumi TK, Geha RS. Use of whole exome and genome sequencing in the identification of genetic causes of primary immunodeficiencies. Curr Opin Allergy Clin Immunol. 2012;12(6):623–8. doi: 10.1097/ACI.0b013e3283588ca6. [This paper provides a rational step-wise genomic approach for discovery of genes related to primary immunodeficiencies.] [DOI] [PubMed] [Google Scholar]
- 35••.Nijman IJ, van Montfrans JM, Hoogstraat M, et al. Targeted next-generation sequencing: a novel diagnostic tool for primary immunodeficiencies. J Allergy Clin Immunol. 2014;133(2):529–34. doi: 10.1016/j.jaci.2013.08.032. [This study demonstrates the use of next generation sequencing in complex immunodeficiency patients that can lead to their molecular diagnosis.] [DOI] [PubMed] [Google Scholar]
- 36••.Platt C, Geha RS, Chou J. Gene hunting in the genomic era: approaches to diagnostic dilemmas in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.08.021. PubMed PMID: 24100122. PubMed Central PMCID: 3976463. [This paper discusses the challenges in various approaches to molecular diagnosis of primary immunodeficiencies and the methods to overcome them.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Herz W, Bousfiha A, Casanova J-L, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for primary immunodeficiency. Frontiers in immunology. 2011:2. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parvaneh N, Casanova J-L, Notarangelo LD, et al. Primary immunodeficiencies: a rapidly evolving story. J Allergy Clin Immunol. 2013;131(2):314–23. doi: 10.1016/j.jaci.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 39.Bilgüvar K, Öztürk AK, Louvi A, et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467(7312):207–10. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi M, Scholl UI, Ji W, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci. 2009;106(45):19096–101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng SB, Buckingham KJ, Lee C, et al. Exome sequencing identifies the cause of a Mendelian disorder. Nat Genet. 2010;42(1):30–5. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell CJ, Dinwiddie DL, Miller NA, et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3(65):65ra4. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Saunders CJ, Miller NA, Soden SE, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Science translational medicine. 2012;4(154):154ra35. doi: 10.1126/scitranslmed.3004041. [This study describes the technique of rapid genome sequencing for molecular diagnosis in neonates in intensive care units.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. 2010;11(1):31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 45.Mardis ER. Next-generation DNA, sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 46.Higgins AW, Alkuraya FS, Bosco AF, et al. Characterization of apparently balanced chromosomal rearrangements from the developmental genome anatomy project. Am J Hum Genet. 2008;82(3):712–22. doi: 10.1016/j.ajhg.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mamanova L, Coffey AJ, Scott CE, et al. Target-enrichment strategies for next-generation sequencing. Nat Methods. 2010;7(2):111–8. doi: 10.1038/nmeth.1419. [DOI] [PubMed] [Google Scholar]
- 48.Dinwiddie DL, Saunders CJ, Farrow EG, et al. Structured genome-scale variant and clinical data reporting for meta-analysis in an era of genomic medicine. J Genomes and Exomes. 2013;2(3619-JGE-Structured-Genome-Scale-Variant-and-Clinical-Data-Reporting-for-Meta-2.pdf):31–42. English. [Google Scholar]
- 49.Kingsmore SF, Dinwiddie DL, Miller NA, et al. Adopting orphans: comprehensive genetic testing of Mendelian diseases of childhood by next-generation sequencing. Expert Rev Mol Diagn. 2011;11(8):855–68. doi: 10.1586/erm.11.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kingsmore SF, Saunders CJ. Deep sequencing of patient genomes for disease diagnosis: when will it become routine. Sci Transl Med. 2011;3(87):87ps23. doi: 10.1126/scitranslmed.3002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rehm HL. Disease-targeted sequencing: a cornerstone in the clinic. Nat Rev Genet. 2013;14(4):295–300. doi: 10.1038/nrg3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke W, Matheny Antommaria AH, Bennett R, et al. Recommendations for returning genomic incidental findings? We need to talk! Genet Med. 2013 doi: 10.1038/gim.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf SM. Return of individual research results and incidental findings: facing the challenges of translational science. Annu Rev Genomics Hum Genet. 2013;14:557–77. doi: 10.1146/annurev-genom-091212-153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf SM, Annas GJ, Elias S. Point-counterpoint. Patient autonomy and incidental findings in clinical genomics. Science. 2013;340(6136):1049–50. doi: 10.1126/science.1239119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christenhusz GM, Devriendt K, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet. 2013;21(3):248–55. doi: 10.1038/ejhg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenblatt DS. Who's on first in exome and whole genome sequencing? Is it the patient or the incidental findings? Mole Gene Metab. 2013;110(1–2):1–2. doi: 10.1016/j.ymgme.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Allyse M, Michie M. Not-so-incidental findings: the ACMG recommendations on the reporting of incidental findings in clinical whole genome and whole exome sequencing. Trends Biotechnol. 2013;31(8):439–41. doi: 10.1016/j.tibtech.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kingsmore SF. Incidental swimming with millstones. Sci Transl Med. 2013;5(194):194ed10. doi: 10.1126/scitranslmed.3006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biesecker LG. Secondary variants and human subjects research. Genet Med. 2013;15(2):157. doi: 10.1038/gim.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biesecker L. A health professional-centered approach to incidental findings. Hum Mutat. 2013;34(10):v. [Google Scholar]
- 61.Green RC, Lupski JR, Biesecker LG. Reporting genomic sequencing results to ordering clinicians: incidental, but not exceptional. JAMA :J Am Med Assoc. 2013;310(4):365–6. doi: 10.1001/jama.2013.41703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGuire AL, Joffe S, Koenig BA, et al. Point-counterpoint. Ethics and Genomic Incidental Findings Sci. 2013;340(6136):1047–8. doi: 10.1126/science.1240156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biesecker LG. Incidental variants are critical for genomics. Am J Hum Genet. 2013;92(5):648–51. doi: 10.1016/j.ajhg.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van de Vosse E, Haverkamp MH, Ramirez-Alejo N, et al. IL-12Rbeta1 deficiency: mutation update and description of the IL12RB1 variation database. Hum Mutat. 2013;34(10):1329–39. doi: 10.1002/humu.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cleary AM, Tu W, Enright A, et al. Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor beta 1 deficiency. J Immunol. 2003;170(1):597–603. doi: 10.4049/jimmunol.170.1.597. [DOI] [PubMed] [Google Scholar]
- 66.Rabbani B, Mahdieh N, Hosomichi K, et al. Next-generation sequencing: impact of exome sequencing in characterizing Mendelian disorders. J Hum Genet. 2012;57(10):621–32. doi: 10.1038/jhg.2012.91. [DOI] [PubMed] [Google Scholar]
- 67.Gonzaga-Jauregui C, Lupski JR, Gibbs RA. Human genome sequencing in health and disease. Annu Rev Med. 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilissen C, Hoischen A, Brunner HG, et al. Disease gene identification strategies for exome sequencing. Eur J Hum Genet. 2012;20(5):490–7. doi: 10.1038/ejhg.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;369(16):1502–11. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alangari A, Alsultan A, Adly N, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130(2):481–8. e2. doi: 10.1016/j.jaci.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badolato R, Prandini A, Caracciolo S, et al. Exome sequencing reveals a pallidin mutation in a Hermansky-Pudlak-like primary immunodeficiency syndrome. Blood. 2012;119(13):3185–7. doi: 10.1182/blood-2012-01-404350. [DOI] [PubMed] [Google Scholar]
- 73.Cullinane AR, Curry JA, Carmona-Rivera C, et al. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak syndrome type 9. Am J Hum Genet. 2011;88(6):778–87. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Salzer E, Daschkey S, Choo S, et al. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica. 2013;98(3):473–8. doi: 10.3324/haematol.2012.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Montfrans JM, Hoepelman AI, Otto S, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012;129(3):787–93. e6. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Yu X, Ichikawa M, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol. 2014;133(5):1400–9. 9, e1–5. doi: 10.1016/j.jaci.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conley ME, Dobbs AK, Quintana AM, et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85alpha subunit of PI3K. J Exp Med. 2012;209(3):463–70. doi: 10.1084/jem.20112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greil J, Rausch T, Giese T, et al. Whole-exome sequencing links caspase recruitment domain 11 (CARD11) inactivation to severe combined immunodeficiency. J Allergy Clin Immunol. 2013;131(5):1376–83. e3. doi: 10.1016/j.jaci.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Hara H, Wada T, Bakal C, et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18(6):763–75. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 80.Samuels ME, Majewski J, Alirezaie N, et al. Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia. J Med Genet. 2013;50(5):324–9. doi: 10.1136/jmedgenet-2012-101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen R, Giliani S, Lanzi G, et al. Whole-exome sequencing identifies tetratricopeptide repeat domain 7A (TTC7A) mutations for combined immunodeficiency with intestinal atresias. J Allergy Clin Immunol. 2013;132(3):656–64. e17. doi: 10.1016/j.jaci.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salzer E, Santos-Valente E, Klaver S, et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C delta. Blood. 2013;121(16):3112–6. doi: 10.1182/blood-2012-10-460741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuehn HS, Niemela JE, Rangel-Santos A, et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121(16):3117–25. doi: 10.1182/blood-2012-12-469544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byun M, Abhyankar A, Lelarge V, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med. 2010;207(11):2307–12. doi: 10.1084/jem.20101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Handsaker RE, Korn JM, Nemesh J, et al. Discovery and genotyping of genome structural polymorphism by sequencing on a population scale. Nat Genet. 2011;43(3):269–76. doi: 10.1038/ng.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jabara HH, Ohsumi T, Chou J, et al. A homozygous mucosa-associated lymphoid tissue 1 (MALT1) mutation in a family with combined immunodeficiency. J Allergy Clin Immunol. 2013;132(1):151–8. doi: 10.1016/j.jaci.2013.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinwiddie DL, Kingsmore SF, Caracciolo S, et al. Combined DOCK8 and CLEC7A mutations causing immunodeficiency in 3 brothers with diarrhea, eczema, and infections. J Allergy Clin Immunol. 2013;131(2):594–7. e1–3. doi: 10.1016/j.jaci.2012.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dinwiddie DL, Bracken JM, Bass JA, et al. Molecular diagnosis of infantile onset inflammatory bowel disease by exome sequencing. Genomics. 2013;102(5–6):442–7. doi: 10.1016/j.ygeno.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]