Abstract
Summary
In aging, the bone marrow fills with fat and this may lead to higher fracture risk. We show that a bone marrow fat measurement by magnetic resonance spectroscopy (MRS), a newer technique not previously studied in chronic kidney disease (CKD), is useful and reproducible. CKD patients have significantly higher bone marrow fat than healthy adults.
Introduction
Renal osteodystrophy leads to increased morbidity and mortality in patients with CKD. Traditional bone biopsy histomorphometry is used to study abnormalities in CKD, but the bone marrow, the source of osteoblasts, has not been well characterized in patients with CKD.
Methods
To determine the repeatability of bone marrow fat fraction assessment by MRS and water-fat imaging (WFI) at four sites in patients with CKD, testing was performed to determine the coefficients of reproducibility and intraclass coefficients (ICCs). We further determined if this noninvasive technique could be used to determine if there are differences in the percent bone marrow fat in patients with CKD compared to matched controls using paired t tests.
Results
The mean age of subjects with CKD was 59.8±7.2 years, and the mean eGFR was 24±8 ml/min. MRS showed good reproducibility at all sites in subjects with CKD and controls, with a coefficient of reproducibilities ranging from 2.4 to 13 %. MRS and WFI assessment of bone marrow fat showed moderate to strong agreement (ICC 0.6–0.7) at the lumbar spine, with poorer agreement at the iliac crest and no agreement at the tibia. The mean percent bone marrow fat at L2–L4 was 13.8 % (95 % CI 8.3–19.7) higher in CKD versus controls (p<0.05).
Conclusions
MRS is a useful and reproducible technique to study bone marrow fat in CKD. Patients with CKD have significantly higher bone marrow fat than healthy adults; the relationship with bone changes requires further analyses.
Keywords: Adipogenesis, Marrow fat, Spectroscopy, Water-fat imaging
Introduction
Chronic kidney disease (CKD) is an important public health problem affecting more than 26 million Americans. Fractures are two to four times more prevalent in patients with CKD compared to the general population [1, 2]. Renal osteodystrophy, an abnormal bone histomorphometry associated with CKD, is one component of CKD-mineral bone disorder (CKD-MBD) and is associated with significant morbidity [3–6]. In patients with renal osteodystrophy, there are extremes of histomorphometric findings—from high bone formation rates secondary to hyperparathyroidism where there is increased osteoblast and osteoclast cell numbers to adynamic bone disease with low bone formation rates and a paucity of cells. These extremes in bone remodeling also make a bone architecture different in healthy controls and make noninvasive diagnosis a challenge [7]. Currently, the gold standard diagnosis for renal osteodystrophy is bone biopsy. However, not all facilities have such capabilities, leading to the development of techniques that may offer an alternative to either bone biopsy or biomarkers. Similar to the need to validate circulating biomarkers, it is important to examine the reproducibility of new bone imaging techniques specifically in CKD patients prior to use in research or for clinical care.
CKD patients have a phenotype of premature aging with increased cardiovascular, fall, and fracture risk [8–10]. In the general population, there is an increase in bone marrow fat content with advanced age [11] and this correlates with decreased bone mineral density [12, 13]. Adipocytes and osteoblasts arise from common cellular precursors, mesenchymal stem cells (MSCs), suggesting that the preferential differentiation towards adipocyte lineage, assessed by increased bone marrow fat, may alter osteoblast differentiation from mesenchymal stem cells. Bone marrow fat can be quantified by magnetic resonance spectroscopy (MRS), which utilizes the resonance frequency of protons to differentiate fat from water; the ratio provides an assessment of chemical composition of bone marrow [14]. In animal studies, the volume of bone marrow fat by biopsy strongly correlates (r=0.9) with fat volume assessed by MRS [15]. MRS is currently used for quantifying the bone marrow fat in the normal population, with lumbar spine being the most studied site [16].
Bone marrow fat content can be also measured using water-fat imaging (WFI) methods such as the three-point Dixon method which covers a large area of bone and is more cost-effective than MRS [14]; therefore, we compared the reproducibility or the two techniques for the assessment of bone marrow fat content and whether there were differences in subjects with CKD compared to controls.
Methods
The study had four objectives: (1) to determine the reproducibility of bone marrow fat assessment by MRS in CKD subjects, (2) to determine if the bone marrow fat percentage in CKD patients is different at various skeletal sites (lumbar L2–L4 vertebrae, posterior iliac crest (the site of bone biopsy used to diagnose renal osteodystrophy), and tibia) (3) to compare the bone marrow fat assessment by MRS and using WFI-based technique (three-point Dixon method) at the above skeletal sites, and (4) to compare the percent bone marrow fat in CKD subjects to that in matched controls with normal kidney function at the lumbar vertebrae, the standard site for MRS, in a pilot study.
Inclusion/exclusion criteria
CKD subjects were recruited from the clinics at Indiana University. Inclusion criteria were age ≥45 years old, estimated GFR±45 ml/min/1.732 m2 using the four-parameter MDRD formula in the last 6 months, and elevated PTH above the normal range (±65 pg/ml) in the past year, confirming a diagnosis of CKD-MBD. Exclusion criteria were females who were pregnant or nursing, a prior history of tibial or lumbar fracture, general contraindications to MRI/MRS exam (e.g., IUD, pacemaker), bilateral lower extremity amputation, history of hematological malignancy, chemotherapy or radiation therapy, institutionalized, or unable to give consent. The healthy control participants were subsequently recruited from a centralized volunteer registry. They were matched for race, gender, and age ±10 years to the CKD subjects. All participants, both CKD subjects and controls, provided written informed consent, and all study procedures were approved by the Institutional Review Board at Indiana University School of Medicine.
Study procedures
For the intrapatient variability assessments, the first five CKD subjects and five controls underwent two MRS and WFI scans of the lumbar vertebrae, iliac crest, and tibia with repositioning between measurements. MRS of L2 to 4 vertebrae was performed in additional five subjects; after interim analyses, it was demonstrated that the lumbar spine was the most reproducible site. All subjects underwent a blood draw at the time of their MRS scan. The blood was collected, stored at −80 °C, and batch analyzed for intact PTH (ALPCO, Salem, NH). GFR was taken from the last clinically available assessment, all within the last 6 months.
Detailed imaging methods
Protons in water and fat molecules are subjected to different chemical environments. They produce signals with slightly different frequencies. If measured with a spectroscopy technique at 3 tesla, fat and water signal peaks are separated by about 440 Hz. If measured with multi-echo gradient echo techniques, the phase shift difference between fat and water is modulated by the delay of acquisition from the initial excitation or the echo time (TE). By modeling signal behavior with respect to TE, the ratio of fat and water signal can be determined. Such technique for WFI is known as the Dixon technique [14].
All MR examinations of the lumbar spine, iliac crest, and tibia (junction of upper one third and lower two thirds) were performed on a 3-T clinical MRI scanner (Magnetom TIM Trio; Siemens Medical Systems, Erlangen, Germany) using a phased array spine coil. Subjects were in supine position with knees supported by a form wedge to reduce spine curvature and motion by improving patient comfort. After initial localization, single-voxel MRS and three-point Dixon scans were performed on the L2–L4 lumbar vertebrae, iliac crest, and tibia to quantify the fat fraction of bone marrow.
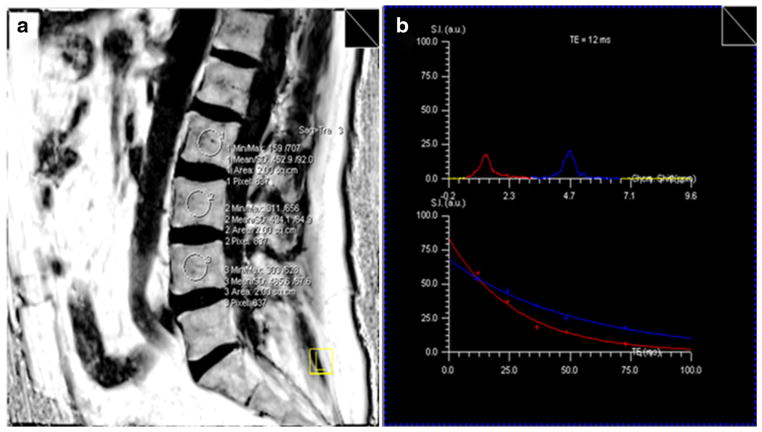
To determine the MRS measurement of percent bone marrow fat, multiple proton (1H) spectra of TE=12, 24, 36, 48, and 72 ms and repetition time (TR)=4 s were acquired with stimulated echo acquisition method (STEAM) from a 10×10×10 mm3 voxel in the center of the marrow region for each site. The signal intensity of water and fat peaks was quantified at each TE by integrating the corresponding spectral region. The fat% was calculated from the water and fat signal intensities after correction for T2 decay by fitting an exponential function to the signal change with TE as shown in Fig. 1. To determine the WFI measurement of percent bone marrow fat, a 3D multi-echo spoiled fast gradient echo sequence was used to acquire data at three TEs=2.46, 3.67, and 7.35 ms and TR= 10 ms. For the lumbar spine, a sagittal slab consists of 32 slices of 4 mm in thickness and a 25-cm field of view (FOV) was used to cover the entire lumbar spine. The acquisition matrix size was 256×192, and the flip angle (FA) was 15°. For the iliac crest and tibia, a transverse slab of 32 slices of 2 mm in thickness was used. The FOV and matrix size were 35 cm and 256×192 for the iliac crest and 17 cm and 128×102 for the tibia, respectively. Using the Dixon fat and water separation technique, corresponding fat-only, water-only, and fat fraction images were also generated. The 3D fat fraction images with thin slices were formatted to 10-mm slices to improve the signal-to-noise ratio (SNR). Regions of interest (ROIs) of 10×10 mm2 were then placed in the center of the L2, L3, and L4 lumbar vertebrae, right iliac crest, or right tibia on the reformatted fat fraction image to obtain the average fat percentage of the bone marrow.
Fig. 1.
MRS assessment of bone marrow fat. a The circles within the lumbar spine body on MRI image are the regions of interest (10×10×10 mm3 voxels in the bone marrow region). Within each region, the T2-weighted proton spectra of TE=12, 24, 36, 48, and 72 ms and TR=4 s were acquired with STEAM techniques. b The signal intensity of water and fat peaks were quantified at each TE. The fat% was calculated from the T2-corrected water and fat signal intensities
Statistical analysis
We enrolled ten CKD subjects and controls, but due to technical difficulties in scans of one subject and one control scan, only a total of eight pairs of scans could be used in the analysis. The coefficient of repeatability for percent bone marrow fat at each site by MRS and WFI was calculated. The coefficient of repeatability (COR) represents the value under which the difference between any two repeat measures on the same patient acquired under identical conditions should fall with 95 % probability [17]. Inter-method intraclass coefficients (ICCs) were calculated at each site and used to compare marrow fat content assessment by WFI and MRS at different skeletal sites. The ICC is the ratio of the between-method variation to the total variation [18]; higher ICCs (range from 0 to 1) represent better repeatability or agreement. Pearson’s correlations also were calculated to compare the bone marrow fat percentage at each site between WFI and MRS. Paired t tests were used to compare the percent bone marrow fat between the eight CKD subjects and controls at the lumbar sites. Results are expressed as mean±SD. A 5 % significance level was used for statistical tests. Analyses were performed using SigmaPlot (SigmaPlot, Chicago, IL) and SAS version 9.3 (SAS Institute, Cary, NC).
Results
Among the eight CKD subjects, five were female. Two subjects were African-American, and the majority (five subjects) were diabetic. The mean age of subjects in the study was 59.8±7.2 years, and mean eGFR was 24±8 ml/min/1.732 m2 (Table 1). The mean age of controls was 58.1±10.2 years, and each CKD patient-control pair was matched according to race and gender, as per the protocol. The mean body mass index (BMI) of subjects was 31.3+7.6 kg/m2 and was not significantly different from the mean BMI of controls (27.6 ±4.7 kg/m2, p=0.28). MRS showed good reproducibility at all sites in subjects with CKD and controls, including at the iliac crest with COR values ranging from 2.4 to 13 %. The tibia site, with the percent bone marrow fat greater than 80 %, offered the highest reproducibility (Table 2). MRS and WFI assessment of bone marrow fat showed moderate to strong agreement (ICC 0.6–0.7) at the lumbar sites, with poorer agreement at the iliac crest and no agreement at the tibia (Table 3). Pearson’s correlations for bone marrow fat percentage at L2, L3, and L4 by MRS versus MRI were 0.78 (p= 0.007), 0.83 (p=0.002), and 0.79 (p=0.007), respectively.
Table 1.
Characteristics of study subjects
| Subject | Age | Sex | eGFR (ml/min/1.732 m2) | Race | CKD cause | DM | PTH (pg/ml) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| 1a | 54 | M | 27 | African-American | DM | Yes | 174.5 | 35.7 |
| 2a | 57 | M | 20 | Caucasian | HTN | Yes | 84.7 | 21.9 |
| 3a | 65 | F | 22 | Caucasian | Stones | No | 156 | 32.0 |
| 4a | 66 | F | 29 | African-American | HTN | Yes | 271.1 | 39.3 |
| 5a | 72 | M | 40 | Caucasian | Stones | No | 79.5 | 24.8 |
| 6 | 50 | F | 20 | Caucasian | DM | Yes | 76.9 | 31.0 |
| 7 | 59 | F | 15 | Caucasian | PKD | No | 141 | 22.9 |
| 8 | 56 | F | 21 | Caucasian | GN | Yes | 149.6 | 42.2 |
HTN hypertension, DM diabetes mellitus, GN glomerulonephritis, PKD polycystic kidney disease
Underwent repeatability scanning, scanning at multiple sites, and both MRS and three-point Dixon scans
Table 2.
Marrow fat% at different sites in CKD patients and controls by MRS and coefficients of repeatability
| Skeletal site | CKD patients (n =5)
|
Controls (n=5)
|
||
|---|---|---|---|---|
| Mean±SD (%) | COR (%)a | Mean±SD (%) | COR (%)a | |
| L2 | 58.1±11.0 | 13 | 47.1±8.6 | 4.9 |
| L3 | 58.6±10.2 | 6.1 | 51.0±6.7 | 4.7 |
| L4 | 61.0±11.0 | 3.4 | 51.8±9.5 | 6.6 |
| Iliac crest | 59.4±14.6 | 5.5 | 52.4±8.5 | 7.1 |
| Tibia | 86.4±1.5 | 2.4 | 84.8±2.6 | 3.8 |
COR=coefficient of repeatability is the value under which the difference between any two repeat measurements on the same patient acquired under identical conditions should fall with 95 % probability
Table 3.
Comparison of MRS and WFI for bone marrow fat quantification using inter-method intraclass coefficients
| Skeletal site | Inter-method ICC
|
CKD | |
|---|---|---|---|
| Overall | Controls | ||
| L2 | 0.705 | 0.607 | 0.606 |
| L3 | 0.674 | 0.482 | 0.696 |
| L4 | 0.694 | 0.497 | 0.736 |
| Iliac crest | 0.285 | 0.083 | 0.330 |
| Tibia | 0.038 | 0.069 | 0 |
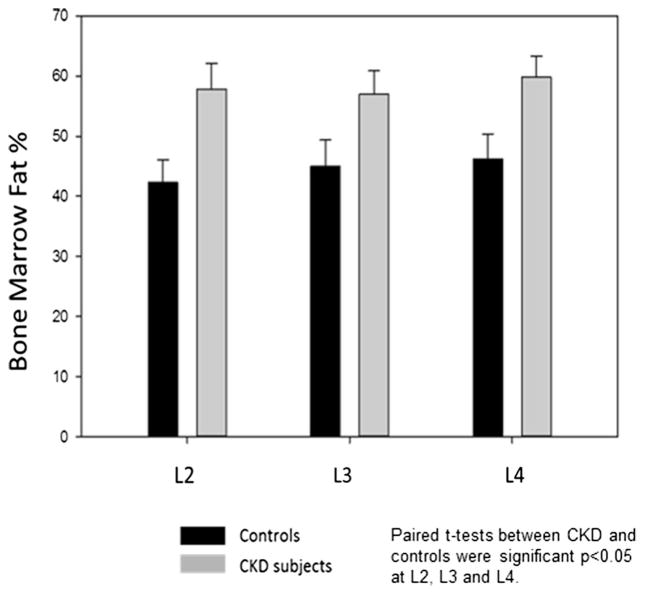
The mean bone percent marrow fat at L2 was 57.8±12.2 % in subjects with CKD versus 41.7±10.8 % in controls (p= 0.02). At L3, the bone marrow fat percentage was 56.9± 11.1 % in CKD versus 44.8±11.4 % in controls (p=0.02). Similarly, at L4, the percent bone marrow fat was 59.8±10.1 % versus 46.3±11.6 % (p=0.03) (Fig. 2). There was no significant correlation with PTH and the bone marrow fat percentage for either group. Similarly, there was no significant correlation of the BMI and the bone marrow fat percentage for either group with the exception of the L2 region in the control group.
Fig. 2.
Percent bone marrow fat by MRS in CKD patients compared to controls. CKD subjects (black bars) were compared to age-, race-, and gender-matched controls (gray bars) for MRS at L2, L3, and L4 sites (eight matched pairs, total N=16). At each site, the percent bone marrow fat in patients with CKD was less than that in control subjects, p<0.05
Discussion
In the present study, we compared single-voxel MRS to three-point Dixon WFI techniques for the assessment of bone marrow fat at multiple sites in patients with CKD. The results showed comparable variability in subjects with CKD compared to controls. MRS has high spectral resolution which ideally allows more complete fat and water separation and low spatial resolution because of its large voxel size. However, MRS is sensitive to various system imperfections which can distort the spectral peaks and baseline, causing quantification errors. In addition, MRS measurement is performed at a finite echo time (TE) which causes the results to be T2 weighted. While the T2 weighting can be corrected by extrapolating results obtained at multiple TEs, this process inevitably introduces an additional error. WFI, on the other hand, has much higher spatial resolution, but low spectral resolution. Its fat and water separation may not be accurate, especially when the spectral peak is more complicated with several overlapping smaller peaks. The Dixon technique also requires phase unwrapping, which sometimes does not work reliably, especially in regions of high magnetic field inhomogeneity. The main advantages of the MRI (WFI) technique are its efficiency and flexibility. A large region can be covered with a high resolution 3D acquisition. The fat fraction at multiple locations can be measured with retrospectively user-defined ROIs anywhere in the imaging volume. The single-voxel MRS technique, however, only produces the fat fraction from a single predetermined voxel. Previous studies in healthy populations assessing the lumbar spine showed Pearson’s correlations of 0.79 and 0.85 between the MRS and the Dixon technique [19, 20], similar to the correlation coefficients between 0.78 and 0.83 at L2–L4 between these two methods in our CKD subjects. However, the ICC is a superior method for assessing the agreement between methods, and in our study, the ICC of MRS compared to WFI for the assessment of bone marrow fat at the lumbar spine was reasonable (0.6–0.7), but not perfect. The results were poor for the iliac crest and tibia. This may reflect a heterogeneity of marrow fat content in different bones. Autopsy studies demonstrated the increased marrow fat deposition with aging occurs diffusely throughout the skeleton, although the proportion of fat replacement of the marrow varies at different skeletal sites [21]. Based on our studies in CKD subjects, we conclude that MRS is the preferable technique to detect the percent bone marrow fat and that the lumbar spine is the most reliable site for marrow fat measurements.
In subjects with CKD, we found that the bone marrow fat content assessed by MRS was 13.8 % (95 % CI 8.3–19.7) higher at L2–L4 compared to matched controls. The key limitations of our study were small sample size as well as the lack of a gold standard assessment of bone and adipose content of marrow such as could be obtained by a bone biopsy. However, despite the limited sample size in this pilot study, the difference of 13.8 % in lumbar bone marrow fat was significant. Griffiths et al. showed that the difference in bone marrow fat at the L2 vertebra by MRS between 17 osteoporotic males and 42 control participants with normal bone density was 7.78±7.8 % [22]. In the aging general population, the bone marrow fat by MRS correlates well with osteopenia and osteoporosis by DXA and vertebral fractures [12, 22, 23]. Although we did not performed bone density or fracture assessments, based on studies in the aging population, the 13.8 % difference in bone marrow fat percentage in patients with CKD compared to controls observed in the present study is likely clinically significant. Future studies are needed to study the bone marrow in conjunction with bone density, volume, and histomorphometric changes in humans with CKD.
Aging is associated with significantly increased adipogenesis in the bone marrow on iliac crest biopsies, and this increased adipocyte fraction was found to be inversely correlated with the trabecular bone volume [11, 21, 24]. With aging, there is a fat replacement of the vertebral marrow that is disproportionate to the loss of hematopoietic elements, leading to net marrow fat accumulation [24]. The similarities of these studies and our observation of increased marrow adipogenesis in CKD suggest that alterations of marrow composition may be another manifestation of the premature aging of CKD. The cause of increased adipogenesis in CKD and aging is unknown. In the past, adiposity was assumed to be secondary to fat simply “filling the holes left by trabecular loss” and thus a consequence rather than a cause of bone loss. However, more recent studies have found that adipogenesis in the marrow may inhibit osteoblast formation and/or increase osteoclastic activity due to both abnormal differentiation and impaired bone marrow niche [25, 26]. Understanding these mechanisms may identify the role of modulators of marrow adiposity to improve bone outcomes [26].
Patients with CKD have a wide range of findings on bone biopsy and high fracture rates [27, 28]. Over the years, the diagnosis and management of renal osteodystrophy has been focused on bone formation rates, as PTH was considered as the primary etiology of bone abnormalities in CKD. However, despite years of lowering PTH, a fracture risk has not substantially changed [29]. Furthermore, fractures in CKD are associated with both low and high levels of PTH [30, 31]. Therefore, there is a need to understand the importance of non-PTH-mediated effects on the bone [32]. In the present study, we found increased fat adiposity in the bone marrow of patients with CKD compared to age-, sex-, and race-matched controls. Whether this leads to an abnormal MSC cell number of differentiation is unknown. Further studies correlating these observations with bone biopsy in patients with CKD are warranted. The results of the present study demonstrate that MRS is a reproducible technique to study changes in bone marrow fat, facilitating the conduct of such studies.
Conclusions
Bone marrow fat assessment by MRS remains to be the best available technique to study changes in bone marrow fat at various skeletal sites in CKD patients, with the lumbar spine being the most reproducible site. We further demonstrated that the percentage of marrow adiposity is higher in the lumbar spine of those with moderate to late stage CKD compared to those with normal kidney function. The bone consequences of this increased adiposity in the marrow of CKD patients require further study.
Acknowledgments
Dr. Moorthi was supported by a career development award from the American Society of Bone and Mineral Research (ASBMR). Additional funding for the support of this study was from the Indiana Clinical and Translational Sciences Institute funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (UL1TR001108).
Footnotes
Conflicts of interest None.
Contributor Information
R. N. Moorthi, Email: rmoorthi@iu.edu, Division of Nephrology, Indiana University School of Medicine, Indianapolis, IN 46202, USA
W. Fadel, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN 46202, USA. Richard M. Fairbanks School of Public Health, Indianapolis, IN 46202, USA
G. J. Eckert, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN 46202, USA. Richard M. Fairbanks School of Public Health, Indianapolis, IN 46202, USA
K. Ponsler-Sipes, Division of Nephrology, Indiana University School of Medicine, Indianapolis, IN 46202, USA
S. M. Moe, Division of Nephrology, Indiana University School of Medicine, Indianapolis, IN 46202, USA. Roudebush Veterans Administration Medical Center, Indianapolis, IN 46202, USA
C. Lin, Department of Radiology, Indiana University School of Medicine, Indianapolis, IN 46202, USA
References
- 1.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 2.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G Kidney Disease: Improving Global O. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 4.Piraino B, Chen T, Cooperstein L, Segre G, Puschett J. Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol. 1988;30(2):57–62. [PubMed] [Google Scholar]
- 5.Gerakis A, Hadjidakis D, Kokkinakis E, Apostolou T, Raptis S, Billis A. Correlation of bone mineral density with the histological findings of renal osteodystrophy in patients on hemodialysis. J Nephrol. 2000;13(6):437–443. [PubMed] [Google Scholar]
- 6.London GM, Marchais SJ, Guerin AP, Boutouyrie P, Metivier F, de Vernejoul MC. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19(9):1827–1835. doi: 10.1681/ASN.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KDIGO. Clinical practice guidelines for the management of CKD-MBD. Kidney Int. 2009;76(S113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 8.West SL, Jamal SA, Lok CE. Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27(6):2384–2388. doi: 10.1093/ndt/gfr620. [DOI] [PubMed] [Google Scholar]
- 9.Wagner J, Jhaveri KD, Rosen L, Sunday S, Mathew AT, Fishbane S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol Dial Transplant. 2014;29(1):146–151. doi: 10.1093/ndt/gft352. [DOI] [PubMed] [Google Scholar]
- 10.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(5):2200–2205. doi: 10.1111/j.1523-1755.2000.00394.x. [DOI] [PubMed] [Google Scholar]
- 11.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 12.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18(5):641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang GY, Lv ZW, Tang RB, Liu Y, Peng YF, Li W, Cheng YS. Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clin Radiol. 2010;65(5):377–381. doi: 10.1016/j.crad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Hu HH, Kan HE. Quantitative proton MR techniques for measuring fat. NMR Biomed. 2013;26(12):1609–1629. doi: 10.1002/nbm.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichardo JC, Milner RJ, Bolch WE. MRI measurement of bone marrow cellularity for radiation dosimetry. J Nucl Med. 2011;52(9):1482–1489. doi: 10.2967/jnumed.111.087957. [DOI] [PubMed] [Google Scholar]
- 16.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183(6):1761–1765. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 18.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Gong X, Weiss J, Jin Y. Comparison among T1-weighted magnetic resonance imaging, modified dixon method, and magnetic resonance spectroscopy in measuring bone marrow fat. J Obes. 2013;2013:298675. doi: 10.1155/2013/298675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regis-Arnaud A, Guiu B, Walker PM, Krause D, Ricolfi F, Ben Salem D. Bone marrow fat quantification of osteoporotic vertebral compression fractures: comparison of multi-voxel proton MR spectroscopy and chemical-shift gradient-echo MR imaging. Acta Radiol. 2011;52(9):1032–1036. doi: 10.1258/ar.2011.100412. [DOI] [PubMed] [Google Scholar]
- 21.Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8(3):157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236(3):945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 23.Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241(3):831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 24.Dunnill MS, Anderson JA, Whitehead R. Quantitative histological studies on age changes in bone. J Pathol Bacteriol. 1967;94(2):275–291. doi: 10.1002/path.1700940205. [DOI] [PubMed] [Google Scholar]
- 25.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4(3):290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26(6):1368–1376. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74(6):721–731. doi: 10.1038/ki.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair SS, Mitani AA, Goldstein BA, Chertow GM, Lowenberg DW, Winkelmayer WC. Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2013;8(8):1336–1342. doi: 10.2215/CJN.10901012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47(1):149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 32.Moorthi R, Moe S. Recent advances in the non-invasive diagnosis of renal osteodystrophy. Kidney Int. 2013;84:886–94. doi: 10.1038/ki.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]