Abstract
Nonmyeloablative hematopoietic cell transplantation (HCT) has been used to treat patients with advanced or high-risk lymphoid malignancies. We studied 65 patients (median age 46 years) receiving an umbilical cord blood (UCB) graft after a single conditioning regimen consisting of cyclophosphamide (50 mg/kg) on day -6, fludarabine (40 mg/m2) daily on days -6 to -2, as well as a single fraction of total body irradiation (200 cGy) along with cyclosporine mycophenolate mofetil immunosuppression. Median time to neutrophil and platelet recovery was 7.5 days (range 0-32) and 46 days (range, 8-111), respectively. Cumulative incidences of grade II-IV, grade III-IV acute, and chronic graft-vs.-host disease were 57% (95%CI: 43-70%), 25% (95%CI: 14-35%), and 19% (95%CI: 9-29%), respectively. Transplant-related mortality at 3 years was 15% (95%CI, 5-26%). Median follow-up was 23 months. The progression free-survival, current progression-free survival and overall survival were 34% (95%CI, 21-47%), 49% (95%CI, 36-62%) and 55% (95%CI, 42-70%) at 3 years. Based on our data, we conclude that a nonmyeloablative conditioning regimen followed by UCB transplantation is an effective treatment for patients with advanced lymphoid malignancies who lack a suitable sibling donor.
Introduction
Nonmyeloablative hematopoietic cell transplantation (HCT) has been increasingly utilized in patients with advanced age, pre-existing co-morbidities or those patients previously treated with extensive alkylator-based chemotherapy or autologous transplantation [1-24]. These demographics are commonly seen in patients with advanced lymphoid malignancies, such as follicular and diffuse large cell lymphoma, chronic lymphocytic leukemia and Hodgkin lymphoma [8-23]. In addition, low- and intermediate-grade lymphoid malignancies are more often diagnosed in the fifth and sixth decade of life. A critical need, therefore, exists to identify new strategies to treat this patient population.
Depending on their ethnic background, 40-80% of the patients will not be able to find an acceptable adult stem cell donor. As a result, umbilical cord blood (UCB) has been increasingly used as an alternative source of stem cells for nonmyeloablative transplantation [1-7]. We have previously reported on the feasibility of nonmyeloablative UCB transplantation for adult patients with advanced hematological diseases [1,7,25]. However, there is still limited data on the efficacy of nonmyeloablative UCB transplantation for patients with lymphoid malignancies [23]. Here, we report the outcomes of 65 patients with low or intermediate grade lymphoid malignancies who underwent nonmyeloablative conditioning followed by UCB transplantation at our center between October 2001 and December 2006.
Patients and Methods
Patients
This is study included patients who had confirmed histological diagnoses of follicular small lymphoma (n=11), small lymphocytic lymphoma/chronic lymphocytic leukemia (n=9), mantle cell lymphoma (n=8), large cell B-cell lymphoma (n=11), anaplastic T-cell lymphoma (n=2), peripheral T-cell lymphoma, unspecified (n=1) and Hodgkin's lymphoma (n=23), and were eligible for UCB transplantation because they had no suitably matched related donor. Advanced disease was defined as disease failing at least 3 prior treatment regimens or autologous transplantation. High-risk disease was defined based on poor prognostic factors present at diagnosis. Thirty-eight patient have been previously reported in studies with different focus by our group [1,7,25-27].
The eligibility criteria for nonmyeloablative UCB transplantation has been previously described [1,7]. In summary, patients were eligible for nonmyeloablative therapy if they met any of the following criteria: age ≥ 45 years, pre-existing high-risk clinical features for treatment-related mortality (TRM) defined as serious organ dysfunction, invasive mold infection within 4 months prior to transplantation, Karnofsky Performance score ≤80, or history of extensive prior therapy (defined as ≥ 12 months of alkylator-based chemotherapy, ≥ 6 months alkylator-based chemotherapy plus extensive radiation, or history of autologous transplantation). Serious organ dysfunction was defined as: a hyperbilirubinemia > 2 times the upper limit of normal, elevated hepatic transaminases > 2 times the upper limit of normal, corrected carbon monoxide diffusing capacity (DLCO) ≤ 50% of predicted, or left ventricular ejection fraction (LVEF) < 45%. Patients > 70 years of age or having end stage organ dysfunction (DLCO > 30% predicted or LVEF < 35%), active serious infection, or human-immunodeficiency virus infection were not eligible. Patients pre-existing co-morbidities were scored according to the Hematopoietic Cell Transplant – specific Co-morbidity Index (HCT-CI)[28]. The patients reported in this study were treated in sequential protocols that were approved by the Institutional Review Board of the University of Minnesota and registered at http://www.clinicaltrials.gov as NCT00365287 (closed) and NCT00305682. All patients or legal guardians provided written informed consent in accordance to the Declaration of Helsinki prior to enrollment.
UCB unit selection algorithm
As previously described [1], UCB units were required to be matched at ≥ 4 of 6 HLA antigens based on antigen-level HLA-A and B and allele-level HLA-DRB1 typing. Matching at HLA-C, DQ and DP were not considered. Target cell dose was ≥ 3.0 × 107 total nucleated cell (TNC)/kg resulting in the selection of a second partially HLA matched UCB unit in 86% of the patients. In patients for whom a second UCB unit could be identified, the second unit had to be matched at ≥ 4 of 6 HLA antigens to the patient, as well as the first unit. Both units were required to have ≥ 1.5 × 107 TNC/kg. Over the duration of the study, single UCB unit grafts were required to have a minimum cryopreserved TNC dose ≥ 2.0 × 107/kg. ABO blood type matching was not considered in UCB unit selection [1,7].
Treatment
Preparative Regimen and Graft versus Host Disease (GVHD) Prophylaxis
All patients received a single dose of cyclophosphamide (CY) 50 mg/kg on day -6 intravenously (IV), fludarabine (FLU) 40 mg/m2 IV daily on days -6 to -2, and a single fraction of total body irradiation (TBI) 200 cGy without shielding on day -1, as previously described [1]. Equine anti-thymocyte globulin (ATG, ATGAM, Pharmacia, Kalamazoo, MI) at 15 mg/kg every 12 hours IV on days -3 to -1 was added in a subpopulation of patients (n=7) who had received less than two cycles of multi-agent chemotherapy within the 3 months prior to enrollment and who had no history of autologous transplantation. Immunosuppression consisted of mycophenolate mofetil (MMF) at 1 g intravenously or orally twice or thrice daily from day -3 to +30, as well as cyclosporine (CsA) twice daily starting on day -3 and continuing for at least 6 months, with target trough levels of 200-400 ng/ml.
UCB transplantation
Units were thawed using the method described by Rubinstein et al. [29]. On day zero, UCB units were infused after washing. In recipients of two partially HLA-matched units, infusion of the second unit was started as soon as the first unit was completely infused. The order of infusion was random, and not based on HLA matching or cell dose. Supportive care has been previously described [1,30].
Study Endpoints
The primary endpoint was progression-free survival (PFS). Secondary endpoints included the incidence of neutrophil recovery, (defined as ANC ≥ 500/μl by 42 days after transplantation), whole marrow chimerism (partial chimerism was defined as marrow reconstitution of 10-90% donor cells, and complete chimerism was defined as >90% as the level of accuracy in our chimerism lab is ± 5%), unsupported platelet recovery ≥50,000/μL at 6 months, acute GVHD at day 100, chronic GVHD at 1 year, presence of disease at 3 years, TRM at 3 years and overall survival (OS) at 3 years. We also performed an exploratory analysis to estimate the impact of the treatment of post-transplant relapse with manipulation of immunosuppression and/or chemotherapy and/or radiation therapy. For this purpose, patients who were progression-free since transplant plus patients who had relapsed or progressed after transplant but had achieved a second complete remission following intervention were included in a multi-state model approach to calculate the current PFS [31]. Current event times for neutrophil recovery were measured from the date of transplantation and were censored for death or disease progression before day 21 without neutrophil recovery. Primary graft failure was defined as lack of neutrophil recovery (ANC ≥ 500/μl) at day 42 or <10% marrow reconstitution of donor origin. Secondary graft failure was defined as severe neutropenia of >1 week duration without other etiology or autologous recovery after primary engraftment. Diagnosis of acute and chronic GVHD was based on standard clinical criteria with histopathologic confirmation where possible [32]
Statistical Analysis
For the purpose population description and analysis, patients were grouped with follicular small lymphoma and chronic lymphocytic leukemia (CLL) as follicular lymphoma/CLL, and those mantle cell lymphoma, large cell B-cell lymphoma, anaplastic T-cell lymphoma, and peripheral T-cell lymphoma, unspecified as large cell/mantle cell lymphomas. Hodgkin's lymphoma (HL) patients were their own group. Chemotherapy-sensitive disease was defined as patients in complete remission (CR), partial remission (PR), and minimal response. Resistant disease was defined as progressive disease and disease with stable tumor size since the last chemotherapy regimen. Current PFS was calculated using a multi-state model approach [31]. For current PFS analyses, relapse, progression and death were considered events. Variables related to patient, disease, and transplant characteristics were compared using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator.[33] For the purposes of survival analyses, death due to any cause was considered an event. Early deaths were counted as events. Data on surviving patients were censored at last follow-up for analyses of current PFS. Patients alive and in remission were censored at last follow-up. The cumulative incidence function was used to calculate probabilities and 95% confidence intervals (CI) of neutrophil and platelet recovery, acute and chronic GVHD, TRM and relapse.[33] For analyses of neutrophil and platelet recovery and GVHD, death without the event was the competing hazard. For TRM or relapse analyses, the competing event was relapse or TRM, respectively.
Statistical comparison of time-to-event curves was completed by Log-Rank test. Continuous factors, such as chimerism and cell dose, were compared between recipients of one and two units using the non-parametric Wilcoxon test [34]. Comparison of proportions was performed by the Chi-square test or Fisher's exact test if the expected number was ≤ 5. Multivariate analysis was not performed due to the small number of patients.
Results
Patients and Disease Characteristics
Between October 2001 and December 2006, 65 consecutive patients with low and intermediate grade lymphoid malignancies were treated with nonmyeloablative UCB transplantation at the University of Minnesota. Patient and graft characteristics are summarized in Table 1. Sixty-one percent of patients were male, with a median age of 46 years and median weight of 81kg. Eight of 35 patients who were ≥ 45 years of age who received a nonmyeloablative conditioning solely based on the age criteria. The median Karnofsky score at the time of transplantation was 90 (range, 70-100), with 3 patients being below 80. The HCT-CI was zero in 5, 1-2 in 18, and ≥ 3 in 27 patients. In 15 patients there was not enough information for the HCT-CI score to be calculated. Median infused TNC dose was 3.3 × 107/kg. Median CD34+ and CD3+ cell doses were 4.4 × 105/kg and 1.4 × 107/kg, respectively.
Table 1. Patient and graft characteristics.
| Number of patients | 65 |
|
| |
| Age in years, median (range) | 46 (6-68) |
|
| |
| Weight in kilograms, median (range) | 80.8 (22.1-124.3) |
|
| |
| Male | 40 (61%) |
|
| |
| Recipient CMV positive | 27 (42%) |
|
| |
| Histology | |
| Follicular lymphoma/CLL | 20 (31%) |
| Large cell/mantle cell lymphoma | 22 (34%) |
| Hodgkin's lymphoma | 23 (35%) |
|
| |
| Pre-transplant hematologic function | |
| White blood cell count, median (range) | 5,500 × 109/L (0.3-24) |
| Absolute neutrophil count, median (range) | 3,600 × 109/L (0.2-15) |
| Platelet count, median (range) | 171 × 109/L (13-422) |
|
| |
| Number of UCB units in the graft | |
| One UCB unit | 9 (14%) |
| Two UCB units | 56 (86%) |
|
| |
| HLA matching to recipients | |
| 6/6 or 6/6 + 6/6 | 3 (5%) |
| 6/6 + 5/6 | 3 (5%) |
| 5/6 or 5/6 + 5/6 | 14 (21%) |
| 5/6 + 4/6 | 20 (31%) |
| 4/6 or 4/6 + 4/6 | 25 (38%) |
|
| |
| Sex match (donor-recipient)* | |
| Matching | 19 (29%) |
| Mismatching | 46 (71%) |
|
| |
| ABO blood type (donor-recipient)* | |
| Matched | 10 (15%) |
| Mismatched | 54 (83%) |
| Missing | 1 (2%) |
|
| |
| Total nucleated cell dose (× 107/kg), median (range) | 3.3 (2.0-6.2) |
|
| |
| CD34+ cell dose (× 105/kg), median (range) | 4.4 (0.7-14.3) |
|
| |
| CD3+ cell dose (× 107/kg), median (range) | 1.4 (0.4-3.0) |
CMV, cytomegalovirus; CLL, chronic lymphocytic leukemia; UCB umbilical cord blood; HLA, human leukocyte antigen; kg, kilogram.
For recipients of two UCB units were considered mismatched if at least one of the units was mismatched.
Disease characteristics and treatment prior to UCB transplantation are summarized in Table 2. Patients received a median of 4 (range 1-9) prior treatment regimens before UCB transplantation. Forty-nine patients had received ≥ 3 prior treatment regimens. A majority of patients had chemotherapy-sensitive disease (82%) and normal LDH (normal range: 325-750 u/L) (66%) at transplantation with no significant difference among diagnostic subgroups. HL patients received a higher proportion of prior radiation therapy (65%) and autologous transplantation (78%), as compared to follicular lymphoma/CLL and large cell/mantle cell lymphomas. The disease status at the time of UCB transplantation for the follicular lymphomas was: complete remission in 1 patient, relapsed/persistent with chemotherapy sensitive disease 8 and chemotherapy refractory disease in 2 patients. For patients with Hodgkin's lymphoma disease status at the time of UCB transplantation was: complete remission in 6, relapsed/persistent chemotherapy sensitive disease in 15 and chemotherapy refractory in 2 patients. For patients with large cell lymphoma disease status at the time of UCB transplantation was: complete remission in 2, relapsed/persistent chemotherapy sensitive disease in 7 and chemotherapy refractory in 5 patients. For patients with mantle cell lymphoma disease status at the time of UCB transplantation was: complete remission in 1, relapsed/persistent chemotherapy sensitive disease in 6 and chemotherapy refractory in 1 patient. Lastly, For patients with CLL/SLL the disease status at the time of UCB transplantation was: RAI stage 0 in 1, stage I in 3, stage III in 1, stage IV in 2 and primary induction failure in 2 patients. At the time of transplantation after receiving salvage therapy, the median international prognostic index (FLIPI[35]) score was 2 (range, 1-3) for the follicular lymphoma patients, and whereas for revised international prognostic index for large cell B-cell lymphoma (R-IPI[36]) was “good” in 5, “very good” in 3 and not enough information to calculate in 3 patients. Patients with large cell/mantle cell lymphomas were more likely to have extra-nodal involvement, whereas patients with follicular lymphoma/CLL were more likely to have bone marrow involvement. Thirteen patients with large cell lymphoma (n=7) and HL (n=6) underwent an UCB transplant instead of autologous transplantation due to poor response to chemotherapy as evidenced by never achieving a CR or a remission of less than 6 months followed by poor response to chemotherapy (n=9), transformed large cell lymphoma (n=2) and large cell lymphoma in a patient with common variable immunodeficiency (n=1).
Table 2. Disease characteristics.
| Characteristics | Follicular lymphoma/CLL | Large cell/mantle cell lymphomas | Hodgkin's lymphoma | P value |
|---|---|---|---|---|
|
| ||||
| Number of patients | 20 | 22 | 23 | |
|
| ||||
| Chemosensitive* | ||||
| Yes | 13 (65%) | 18 (82%) | 22 (96%) | 0.07 |
| No | 7 (35%) | 4 (18%) | 1 (4%) | |
|
| ||||
| Number total previous chemotherapy regimens, median (range) | 4 (2-9) | 3 (1-6) | 4 (2-9) | 0.03 |
|
| ||||
| Bulky adenopathy* | ||||
| Yes | 2 (10%) | 2 (9%) | 1 (4%) | 0.31 |
| No | 18 (90%) | 18 (82%) | 22 (96%) | |
| Missing | 0 | 2 (9%) | 0 | |
|
| ||||
| Previous autologous hematopoietic stem cell transplantation | ||||
| Yes | 0 | 8 (36%) | 18 (78%) | < 0.01 |
| No | 20 (100%) | 14 (64%) | 5 (22%) | |
|
| ||||
| Prior radiation therapy | ||||
| Yes | 5 (25%) | 7 (32%) | 15 (65%) | 0.03 |
| No | 15 (75%) | 15 (68%) | 6 (26%) | |
| Missing | 0 | 0 | 2 (9%) | |
|
| ||||
| Bone Marrow involvement* | ||||
| Yes | 8 (40%) | 2 (9%) | 0 | < 0.01 |
| No | 12 (60%) | 20 (91%) | 22 (96%) | |
| Missing | 0 | 0 | 1 (4%) | |
|
| ||||
| LDH † | ||||
| Abnormal | 5 (25%) | 9 (41%) | 8 (35%) | 0.79 |
| Normal | 15 (75%) | 13 (59%) | 15 (65%) | |
|
| ||||
| Extra-nodal disease † ‡ | ||||
| Yes | 3 (15%) | 15 (68%) | 8 (35%) | < 0.01 |
| No | 17 (85%) | 7 (32%) | 15 (65%) | |
At the time of transplantation.
At the time of diagnosis.
Extra-nodal sites involved were soft tissue or bone (n=7); gastrointestinal (n=6), lung (n=5), skin (n=3), salivary gland (n=1), tonsils (n=1); liver (n=1), pancreas (n=1), and central nervous system (n=1).
CLL, chronic lymphocytic leukemia.
Hematopoietic recovery and chimerism
The incidence of neutrophil recovery was 89% (95%CI, 80-98%) by day 42, with a median time to neutrophil recovery of 7.5 days (range, 0-32). The cumulative incidence of platelet recovery was 82% (95%CI, 65-98%) at 180 days, with a median time to platelet recovery ≥ 50,000/μL of 46 days (range, 8-111). Neutrophil recovery was not influenced by the infused TNC, CD34 or CD3 cell doses, sex, ABO or HLA matching, and by the pre-transplant marrow cellularity, white blood cell, or neutrophil count. However, the cumulative incidence of neutrophil recovery was significantly higher for patients who had a platelet count pre-transplant above the median (97% [95%CI, 87-100] vs. 91% [95%CI, 66-92], p=.03). Median time to platelet recovery was influenced by the degree of HLA-matching. Patients who received only 6/6 HLA-matched unit(s) had a median time to platelet recovery of 37 days (range, 34-46), as compared to 44.5 days (range, 8-83) and 48 days (range, 24-111) for those who received at least one 5/6 or one 4/6 HLA-matched unit, respectively (p=.03).
Thirty patients (57%) had mixed chimerism (presence recipient and donor DNA) in the bone marrow at day 21, but 97% had achieved full chimerism (only donor DNA) by day 180. Among patients who received 2 UCB units, 28% had both donor units detectable in the bone marrow at day 21. As expected, all patients who received two UCB units had complete chimerism (only donor DNA) derived from a single UCB unit by 180 days.
Acute and Chronic GVHD
The incidences of grade II-IV and grade III-IV acute GVHD at day 100 were 57% (95%CI: 43-70%) and 25% (95%CI: 14-35%), respectively. The median time to the development of acute GVHD was 37 days and was not influenced by disease subgroups by the CD3 cell dose. Twelve patients had chronic GVHD, for an incidence of 19% at 24 months (95%CI: 9-29%). Incidences of acute and chronic GVHD were similar between lymphoma subgroups and for those who did or did not receive pre-transplant ATG (data not shown).
TRM
TRM at 3 years was 15% (95%CI, 5-26%) for the whole cohort. TRM at 3 years for patients with follicular lymphoma/CLL, large cell/mantle cell lymphomas and HL subgroups was 5% (95%CI, 0-14%), 24% (95%CI, 4-44%), and 13% (95%CI, 0-27%), respectively (p=.41). The TRM at 3 years for patients who had an HCT-CI score of 0 was zero, 1-2 was 18% (95%CI, 0-35%) and ≥ 3 was 17% (95%CI, 0-34), and this difference was not statistically significant (p=.46)
Relapse and Progression Free Survival
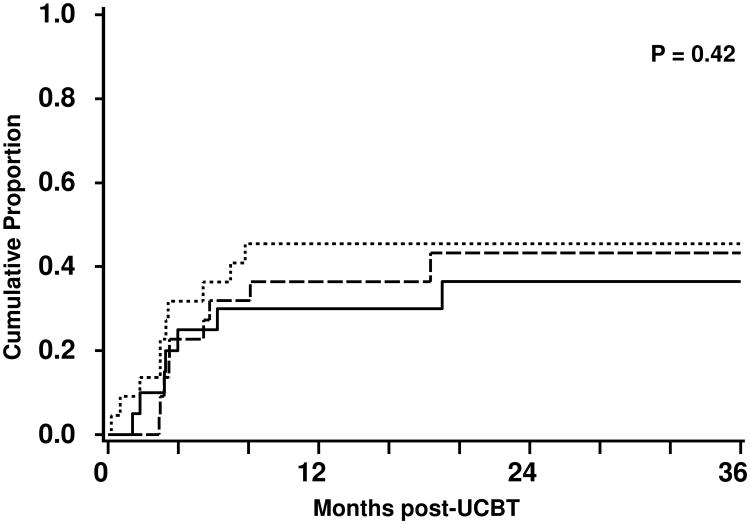
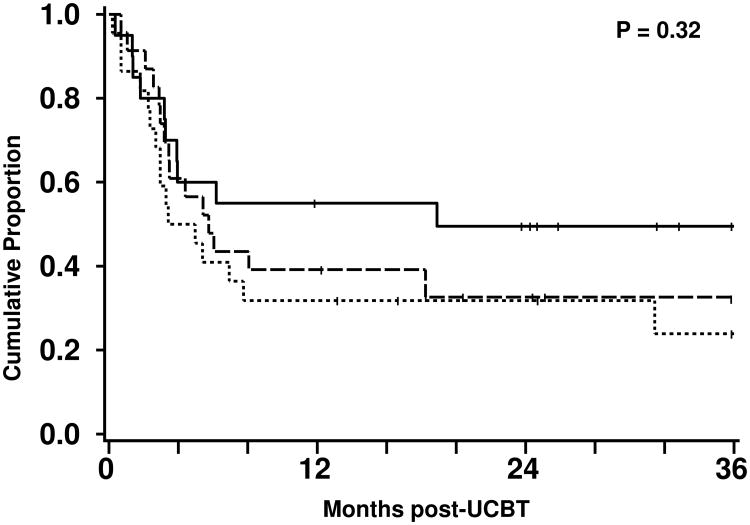
At a median follow-up in surviving patients of 23 months, the cumulative incidence of relapse of 42% (95%CI, 29-56%) at 3 years. The cumulative incidence of relapse for follicular lymphoma/CLL, large cell/mantle cell lymphomas and HL were 37% (95%CI, 15-58%), 45% (95%CI, 24-67%), and 43% (95%CI, 20-66%), respectively (Figure 1). The median time to progression was 90 (95%CI, 61-119) days and to relapse was 175 (95%CI, 119-370) days. The overall PFS was 34% (95%CI, 21-47%) and the PFS rates for follicular lymphoma/CLL, large cell/mantle cell lymphomas and HL were 50% (95%CI, 27-72%), 24% (95%CI, 4-44%), and 33% (95%CI, 12-53%), respectively (Figure 2).
Figure 1.
Cumulative incidence of relapse after nonmyeloablative UCB transplantation for patients with follicular lymphoma/CLL (—), large cell/mantle cell lymphoma (▪▪▪), and HL (---).
Figure 2.
Progression-free survival after nonmyeloablative UCB transplantation for patients with follicular lymphoma/CLL (—), large cell/mantle cell lymphoma (▪▪▪), and HL (---).
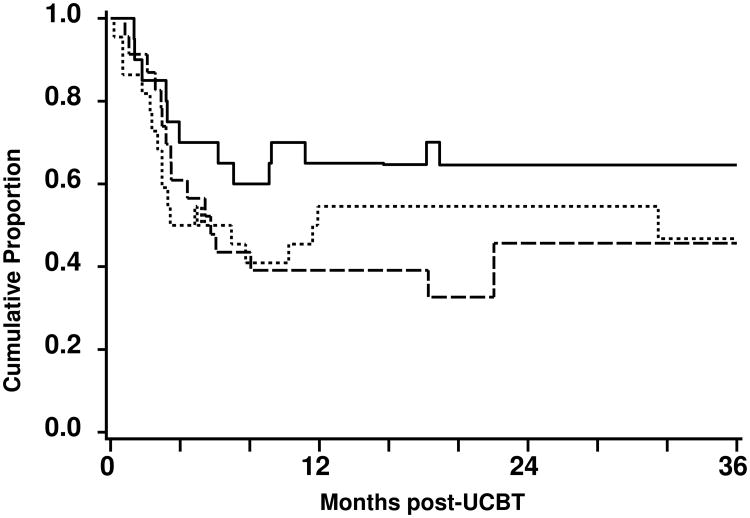
Of the 26 patients had disease progression or relapsed and 15 were treated with post-transplantation therapy (Table 3). The remaining 11 patients either declined further therapy or were clinically not suitable for treatment intervention (i.e. active acute GVHD). Nine patients achieved CR, whereas the remaining 6 patients progressed. The overall current PFS at 3 years was 49% (95%CI, 36-62%). The current PFS rates for follicular lymphoma/CLL, large cell/mantle cell lymphomas and HL were 68% (95%CI, 45-85%), 47% (95%CI, 26-67%), and 46% (95%CI, 23-68%), respectively (Figure 3). The rise and fall of the current PFS curves is due to successful induction of CR following treatment intervention for relapse or disease progression.
Table 3. Characteristics and outcomes of 15 patients who received treatment for relapse or progression after nonmyeloablative UCB transplantation.
| Patient | Histological Diagnosis |
Disease status at UCBT |
Number of Prior Therapies |
Time to relapse/ progression (days) |
Time to acute GVHD (days) |
Chimerism at the time of relapse/ progression |
Treatment at relapse/progression |
Time from relapse/ progression to treatment (months) |
Response to treatment |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Diffuse Large cell B-Cell | Partial Remission | 3 | 104 | 19 | 100% | taper immunosuppression* + rituximab† | < 1 | CR | Alive |
| 2 | Diffuse Large cell B-Cell | Partial Remission | 3§ | 6 | 51 | 82% | taper immunosuppression*,+ rituximab† | < 1 | PROG | Dead |
| 3 | Diffuse Large cell B-Cell | Partial Remission | 6 | 164 | 30 | 100% | rituximab† | 5 | CR | Alive |
| 4 | Diffuse Large cell B-Cell | Partial Remission | 3 | 120 | 31 | 100% | taper immunosuppression* + rituximab† | 5 | CR | Alive |
| 5 | Diffuse Large cell B-Cell | Partial Remission | 2§ | 21 | No | 100% | taper immunosuppression* + rituximab† | < 1 | PROG | Dead |
| 6 | Diffuse Large cell B-Cell | Partial Remission | 2 | 21 | 63 | NA | rituximab† + radiotherapy¶ | 3 | PROG | Dead |
| 7 | Diffuse Large cell B-Cell | Chemotherapy Refractory | 3 | 211 | No | 100% | taper immunosuppression* + rituximab† | 5 | CR | Alive |
| 8 | Mantle cell lymphoma | Chemotherapy Refractory | 2§ | 100 | No | 100% | taper immunosuppression* + chemotherapy + radio-immunotherapy | 2 | CR | Alive |
| 9 | Large cell T-Cell | Partial Remission | 2 | 90 | 35 | 97% | radiotherapy | 1 | PROG | Dead |
| 10 | Anaplastic T Cell | Partial Remission | 3 | 236 | No | 100% | taper immunosuppression* | < 1 | CR | Alive |
| 11 | Follicular B-cell | Partial Remission | 8 | 55 | 72 | 91% | rituximab† | 6 | CR | Alive |
| 12 | Follicular B-cell | Complete Remission | 4 | 188 | No | 100% | taper immunosuppression* + rituximab† | 3 | CR | Alive |
| 13 | Follicular B-cell | Partial Remission | 4 | 42 | 67 | 100% | taper immunosuppression* | 1 | PROG | Dead |
| 14 | Hodgkin Lymphoma | Complete Remission | 4§ | 106 | No | NA | taper immunosuppression* + chemotherapy + radiotherapy | 18± | CR | Alive |
| 15 | Hodgkin Lymphoma | Partial Remission | 9 | 88 | 15 | 100% | taper immunosuppression* + chemotherapy + radiotherapy | 6 | PROG | Dead |
UCBT, umbilical cord blood transplantation; GVHD, graft-vs.-host- disease; CR, complete remission; PROG, progression
Taper of cyclosporine A and/or steroids started one to four weeks of diagnosis of relapse/progression.
Rituximab was dosed at 375 mg/m2/week for 4 weeks
Patient who underwent prior autologous transplant
Interval to last salvage therapy
Palliative intent radiation therapy
Figure 3.
Current progression-free survival after nonmyeloablative UCB transplantation for patients with follicular lymphoma/CLL (—), large cell/mantle cell lymphoma (▪▪▪), and HL (---).
Survival
The median follow-up of the patients alive is 23 months (range, 3-62). The OS at 3 years was 55% (95%CI, 42-70) for all patients. The OS rates at 3 years for patients with follicular lymphoma/CLL, large cell/mantle cell lymphomas and HL were 69% (95%CI, 48-90), 54% (95%CI, 33-75), and 43% (5%CI, 18-69), respectively. The difference in OS among disease groups was not statistically significant (p=.37). Further univariate analysis showed survival was not influenced by HLA-matching, CD34+ cell dose, development of acute GVHD, presence of bulky tumor, chemotherapy sensitivity, LDH level, prior autologous transplantation, prior radiation therapy, presence of extra-nodal disease, bone marrow involvement, or number of prior cycles of chemotherapy. The median Karnofsky score of the surviving patients both at 100 days and at 1 year was 90% (range, 90-100%). The causes of death included disease recurrent (n=14), multiple organ failure (n=4), infection (n=4), respiratory insufficiency (n=3), and other (n=3).
Discussion
Umbilical cord blood is an attractive alternative source of HSC for transplantation as it is rapidly available, its ability to cross HLA barriers and relatively low incidence of GVHD if considered that most UCB transplants are mismatched. Umbilical cord blood has been the preferred source of unrelated HSC for transplantation in our institution since the early 2000's [1,7,30,37,38]. Some patients treated in our institution were referred to our institution after failing to find an unrelated donor after searching for an extended period of time. Our strategy of UCB graft selection was initiated in 2000 and allows for the utilization of two UCB units to compose a graft allowed us to find grafts for most adult patients [1,30]. This study demonstrates that patients with lymphoid malignancies treated with nonmyeloablative conditioning followed by UCB transplantation have a low TRM, with the added benefit that a sizable proportion of these patients enjoy prolonged PFS and OS. Moreover, despite the lack of donor lymphocytes, a proportion of patients who relapse after nonmyeloablative UCB transplantation may still be salvaged by manipulation of immunosuppressive therapy with or without additional chemotherapy or localized radiation therapy.
In the present study, neutrophil and platelet recovery was relatively rapid as compared to other reports of UCB transplantation after myeloablation [37,39,40]. Reason for this rapid hematological recovery is the nonmyeloablative intensity of our conditioning regimen that likely allows transient autologous reconstitution and a period of mixed chimerism. In our population of heavily pretreated patients, 90% of the patients had received chemotherapy within a few weeks of undergoing nonmyeloablative conditioning and 40% had received a prior autologous transplant; both of these factors are associated with a decreased risk of graft failure [1,41].
Consistent with previous reports by our group [1] and others[3,23], we observed that approximately two thirds of patients developed grades II-IV acute GVHD after nonmyeloablative UCB transplantation. Other studies, however, have reported incidences of acute GVHD below 40% after nonmyeloablative UCB transplantation.[2,4-6] This lower incidence is possibly to differences in patient selection, HLA matching and post-transplantation immunosuppressive regimens. Although studies involving adult donor stem cells for lymphoid malignancies have reported an incidence of acute GVHD lower than that reported here, it is important to note that alemtuzumab, an in vivo T-cell depleting agent, was frequently used as a part of the conditioning regimen [9,13,17-19,22]. In our cohort we found no predictors of the development of acute GVHD.
The TRM incidence of 15% observed in this study compares favorably with other series of nonmyeloablative transplantation that have reported rates of 10-45% for patients receiving UCB [2-6,23] or related and unrelated adult donor stem cells [13,16,18-20,22]. A lower TRM incidence has been associated with a low incidence of acute GVHD associated in series that administered ATG [12,14] or alemtuzumab [13,17,18] as part of the conditioning regimen. In this cohort we found no predictors of TRM, including the HCT-CI score, prior therapy or age.
In our study, one third of the patients were free of disease progression at 3 years. Similar studies of related and unrelated adult donor nonmyeloablative HCT for patients with lymphoid malignancies report a 2-4 year disease-free survival rate between 30 and 50% [8,10,11,13,16,18-22]. After nonmyeloablative related and unrelated donor HCT, up to 10-30% of patients may receive a donor lymphocyte infusion (DLI) as salvage therapy for persistent, relapsed or progressive disease [8,9,13,17,19]. Among patients receiving DLI, the response rate may be as high as 70% [19], with 30-50% achieving CR [8, 9, 13, 17, 19].
Interestingly, 9 of 26 patients who had disease progression or relapse in our cohort could be salvaged by tapering immunosuppression, rituximab, systemic chemotherapy, radiation therapy or a combination of these treatment strategies. Moreover, every CR achieved in this setting resulted in a durable outcome. Since these group patients had received a median of 3 (range 3-9) prior treatment regimens (Table 3), some had had a prior autologous transplant (n=4), and most (n=11) were in partial remission prior to UCB transplantation, we speculate that the graft-vs.-malignancy effect may have been reestablished after post-transplantation treatment and was contributing to the prolonged remissions. This is reflected in the current PFS of 49% at 3 years. The concept of current PFS was proposed to calculate the PFS for patient with chronic myelogenous leukemia who were treated with DLI and were restored to complete remission [31,42-44]. Recently, Thomson et al [24] reported a 42% current PFS for patients with Hodgkin's lymphoma who received a matched related or unrelated donor transplant after reduced intensity conditioning. In this series 21 of 38 patient relapsed, and 8 of 15 patients who received DLI responded, 7 with a complete remission; 3 later progressed. In our cohort complete remissions achieved after post-transplant treatment were sustained. It is interesting that the GVL effect may be reestablished after nonmyeloablative transplantation by manipulating the immune environment and/or chemotherapy. This may be of particular importance in the UCB setting where DLI is not available. The durability of the responses obtained after manipulation of the immunosuppression and/or additional therapy for relapses and progressions after UCB transplantation will be better determined by longer follow-up and larger numbers of patients.
In conclusion, nonmyeloablative UCB transplantation for patients with low and intermediate grade lymphoid malignancies is associated with low TRM and encouraging current PFS. Despite the unavailability of DLI, it is still possibly to salvage a significant proportion of patients and provide long term remissions by manipulating immunosuppression and/or additional systemic or local therapy. Our study demonstrates that nonmyeloablative UCB transplantation extends transplantation therapy to a much larger number of patients.
Acknowledgments
The authors acknowledge Dr. John Klein for his assistance on the multi-state model, and Michael Franklin for assistance in editing and preparing this manuscript.
This work was supported in part by grants from the National Cancer Institute PO1-CA65493 (J.E.W., T.E.D., P.B.M., J.S.M.) and the Children's Cancer Research Fund (J.E.W., T.E.D.).
References
- 1.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misawa M, Kai S, Okada M, et al. Reduced-intensity conditioning followed by unrelated umbilical cord blood transplantation for advanced hematologic malignancies: rapid engraftment in bone marrow. Int J Hematol. 2006;83:74–79. doi: 10.1532/IJH97.05124. [DOI] [PubMed] [Google Scholar]
- 4.Miyakoshi S, Yuji K, Kami M, et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res. 2004;10:3586–3592. doi: 10.1158/1078-0432.CCR-03-0754. [DOI] [PubMed] [Google Scholar]
- 5.Morii T, Amano I, Tanaka H, Takahashi T, Kimura H. Reduced-Intensity Unrelated Cord Blood Transplantation (RICBT) in Adult Patients with High-Risk Hematological Malignancies. Blood. 2005;106:444b. [Google Scholar]
- 6.Rocha V, Rio B, Garnier F, et al. Reduced Intensity Conditioning Regimen in Single Unrelated Cord Blood Transplantation in Adults with Hematological Malignant Disorders. An Eurocord-Netcord and SFGM-TC Survey. Blood. 2006;108:897a. [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez I, Sureda A, Caballero MD, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12:172–183. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Anderlini P, Saliba R, Acholonu S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin's disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35:943–951. doi: 10.1038/sj.bmt.1704942. [DOI] [PubMed] [Google Scholar]
- 10.Dean RM, Fowler DH, Wilson WH, et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy-refractory non-hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:593–599. doi: 10.1016/j.bbmt.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Dey BR, McAfee S, Sackstein R, et al. Successful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed hematologic malignancy following autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:604–612. doi: 10.1053/bbmt.2001.v7.pm11760148. [DOI] [PubMed] [Google Scholar]
- 12.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin's lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol. 2003;21:4407–4412. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 16.Kusumi E, Kami M, Kanda Y, et al. Reduced-intensity hematopoietic stem-cell transplantation for malignant lymphoma: a retrospective survey of 112 adult patients in Japan. Bone Marrow Transplant. 2005;36:205–213. doi: 10.1038/sj.bmt.1705027. [DOI] [PubMed] [Google Scholar]
- 17.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 18.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365:1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 19.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Sandemaier BM, et al. Hematopoietic Cell Transplantation After Nonmyeloablative Conditioning for Advanced Chronic Lymphocytic Leukemia. J Clin Oncol. 2005;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 22.Vigouroux S, Michallet M, Porcher R, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 23.Yuji K, Miyakoshi S, Kato D, et al. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant. 2005;11:314–318. doi: 10.1016/j.bbmt.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Thomson KJ, Peggs KS, Smith P, et al. Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin's lymphoma following autologous stem cell transplantation. Bone Marrow Transplant. 2008;41:765–770. doi: 10.1038/sj.bmt.1705977. [DOI] [PubMed] [Google Scholar]
- 25.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 26.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 31.Klein JP, Shu Y. Multi-state models for bone marrow transplantation studies. Stat Methods Med Res. 2002;11:117–139. doi: 10.1191/0962280202sm277ra. [DOI] [PubMed] [Google Scholar]
- 32.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 33.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Snedecor G, Cochran W. Statistical Methods. 8th. Iowa State University Press; 1989. [Google Scholar]
- 35.Montoto S, Lopez-Guillermo A, Altes A, et al. Predictive value of Follicular Lymphoma International Prognostic Index (FLIPI) in patients with follicular lymphoma at first progression. Ann Oncol. 2004;15:1484–1489. doi: 10.1093/annonc/mdh406. [DOI] [PubMed] [Google Scholar]
- 36.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 38.Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 41.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Craddock C, Szydlo RM, Klein JP, et al. Estimating leukemia-free survival after allografting for chronic myeloid leukemia: a new method that takes into account patients who relapse and are restored to complete remission. Blood. 2000;96:86–90. [PubMed] [Google Scholar]
- 43.Klein JP, Keiding N, Shu Y, Szydlo RM, Goldman JM. Summary curves for patients transplanted for chronic myeloid leukaemia salvaged by a donor lymphocyte infusion: the current leukaemia-free survival curve. Br J Haematol. 2000;109:148–152. doi: 10.1046/j.1365-2141.2000.01982.x. [DOI] [PubMed] [Google Scholar]
- 44.Klein JP, Szydlo RM, Craddock C, Goldman JM. Estimation of current leukaemia-free survival following donor lymphocyte infusion therapy for patients with leukaemia who relapse after allografting: application of a multistate model. Stat Med. 2000;19:3005–3016. doi: 10.1002/1097-0258(20001115)19:21<3005::aid-sim592>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]