Abstract
Mass bird mortality has been observed in North America after the introduction of West Nile virus (WNV), most notably massive die-offs of American crows (Corvus brachyrhynchos). In contrast, WNV epidemic activity in Europe has been characterized by very low incidences of bird mortality. As the general susceptibility of European corvids to strains of WNV remains in question, European jackdaws (Corvus monedula) were inoculated with WNV strains circulating currently in Greece (Greece-10), Italy (FIN and Ita09) and Hungary (578/10), as well as a North American (NY99) genotype with a demonstrated corvid virulence phenotype. Infection with all strains except WNV-FIN resulted in mortality. Viraemia was observed for birds inoculated with all strains and virus was detected in a series of organs upon necropsy. These results suggested that jackdaws could potentially function as a sentinel for following WNV transmission in Europe; however, elicited viraemia levels might be too low to allow for efficient transmission of virus to mosquitoes.
INTRODUCTION
West Nile virus (WNV; genus Flavivirus; family: Flaviviridae) is maintained in an enzootic cycle between mosquitoes and birds, but may also infect humans and horses, which serve as incidental dead-end hosts. WNV is endemic in many parts of Africa, Australia, the Middle East and Asia, and more recently emerged in North America in 1999, and has since rapidly spread across North America, Mexico, South America and the Caribbean. Since 2008, WNV has emerged as a serious veterinary and public health problem in central and south-eastern Europe, affecting countries such as Greece (Barzon et al., 2013b; Danis et al., 2011; Papa et al., 2010), Italy (Barzon et al., 2009, 2011, 2012, 2013a), Hungary, Austria (Bakonyi et al., 2013) and Romania (Sirbu et al., 2011).
Birds were considered to be less susceptible to West Nile disease until high mortality rates were recorded in flocks of young domestic geese in Israel in 1998 (Banet-Noach et al., 2003; Malkinson et al., 2002), after which WNV has been implicated in deaths of members of 326 species of birds in North America (De Filette et al., 2012). Among the passeriform birds, the family Corvidae is ranked as the most highly susceptible species to WNV (Wheeler et al., 2009) and, in particular, deaths among the American crow (Corvus brachyrhynchos) have been used to track the spread of the virus in North America (Eidson et al., 2005; Julian et al., 2002).
In contrast to the mass bird mortality observed in North America, sporadic isolated death events and low mortality rates have been reported among European birds, even during severe human and equine West Nile outbreaks (Calistri et al., 2010; Dauphin et al., 2004; Jourdain et al., 2007; Valiakos et al., 2011). Possible explanations for the lack of reporting of bird mortality could be that European birds are less susceptible to WNV-induced disease or that the North American strain of WNV exhibits an increased capacity for eliciting avian virulence. Other theories include poor detection of bird carcasses (due to their small size or scavenging) (Wobeser & Wobeser, 1992), limited monitoring of wild bird mortality or development of herd immunity due to local transmission spurred by avian migration through WNV endemic areas of the Middle East and Africa (Hubálek & Halouzka, 1999).
To date, the susceptibility of European jackdaws to WNV isolates circulating in Europe has not yet been addressed. In order to examine the role of a bird that is found ubiquitously across Europe as a natural reservoir for the transmission of WNV disease or as a potential sentinel for WNV activity, European jackdaws were inoculated with four different WNV isolates clinically relevant to Europe, including lineage 1 isolates Ita09 and FIN from Italy, as well as lineage 2 isolates from Greece and Hungary. The NY99 strain was used to assess and compare the susceptibility of the European birds to a strain known to be highly virulent in North American corvids, especially in American crows. Susceptibility was assessed in terms of mortality, median survival time, duration and magnitude of viraemia, and dissemination of virus to the different organs.
RESULTS
Morbidity and mortality
During a 9 day period, lethargy, low activity, anorexia and ruffled feathers were observed among some of the jackdaws inoculated with NY99, Greece-10, Ita09 and 578/10. Birds died within 24–48 h after onset of clinical symptoms. Among the five jackdaws that were inoculated with NY99 and Ita09 and followed for survival, three (60 %) died; among the five jackdaws inoculated with 578/10, two (40 %) died; and among the six jackdaws inoculated with Greece-10, three died (50 %). In contrast, all five birds inoculated with FIN survived the infection (Table 1). Comparison of the survival curves of the birds inoculated with the different virus strains revealed a significant difference between the survival curves of jackdaws infected with FIN compared with Ita09 (Fig. 1; P=0.05). Median day of death was 7 days post-infection (p.i.) for birds that succumbed due to infection with NY99, Ita09 and 578/10, and 8 days p.i. for birds that died due to infection with Greece-10 (Table 1).
Table 1.
Clinical profile of five or six European jackdaws infected with WNV strains NY99, Greece-10, FIN, Ita09 and 578/10
| Virus group | Mortality [n/N (%)] | Median day of death | Mean ± sd day of onset of viraemia | Mean ± sd peak serum viraemia (log10RNA copy number ml−1 | Mean ± sd day of peak viraemia | Mean ± sd duration of viraemia (days) |
|---|---|---|---|---|---|---|
| NY99 | 3/5 (60) | 7 | 2.0±0 | 6.2±1.9 | 4.0±0 | 5.2±1.0 |
| Greece-10 | 3/6 (50) | 8 | 2.0±0 | 5.9±1.3 | 3.3±1.0 | 6.0±1.2 |
| FIN | 0/5 (0) | NA | 3.2±1.0 | 4.0±0.7 | 4.0±1.3 | 6.4±0.8 |
| Ita09 | 3/5 (60) | 7 | 2.8±1.0 | 4.7±1.5 | 4.0±1.3 | 5.2±1.6 |
| 578/10 | 2/5 (40) | 7 | 2.0±0 | 5.0±1.3 | 4.0±2.2 | 6.4±1.5 |
NA, not applicable.
Fig. 1.
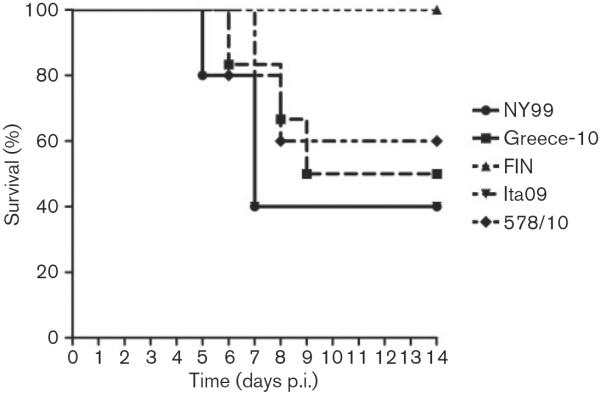
Survival of five or six European jackdaws, each inoculated with 2000 TCID50 WNV strains NY99, Greece-10, FIN, Ita09 or 578/10. Jackdaws were monitored daily for signs of disease up to 14 days p.i.
Viraemia profiles
We determined WNV viraemia profiles for the jackdaws inoculated with the five different virus strains and found that all birds developed viraemia within 96 h p.i (Table 1; Fig. 2). For NY99-infected birds, peak serum viraemia titres ranged from 4.1 to 9.6 log10RNA copies ml−1 (mean 6.2 log10RNA copies ml−1). Birds infected with the Greece-10 strain had peak serum viraemia titres ranging from 4.2 to 8.4 log10RNA copies ml−1(mean 5.9 log10RNA copies ml−1). For FIN-infected birds, peak serum viraemia titres ranged from 3.3 to 5.1 log10RNA copies ml−1(mean 4.0 log10RNA copies ml−1). Ita09-infected birds displayed peak serum viraemia titres ranging from 3.4 to 7.5 log10RNA copies ml−1 (mean 4.7 log10RNA copies ml−1). Among jackdaws infected with the 578/10 strain, peak serum viraemia titres ranged from 3.1 to 6.9 log10RNA copies ml−1 (mean 5.0 log10RNA copies ml−1).
Fig. 2.
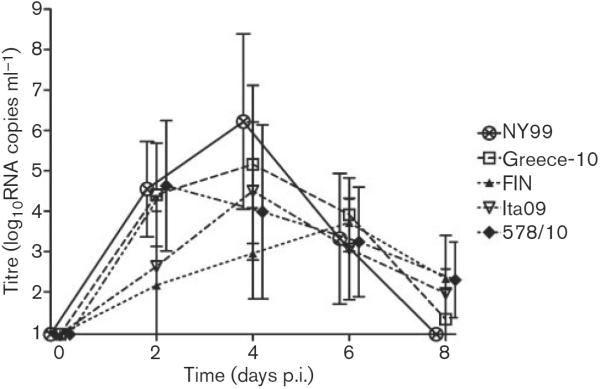
Serum viraemia profiles for WNV-infected European jackdaws after inoculation with 2000 TCID50 NY99 (n=5), Greece-10 (n=6), FIN (n=5), Ita09 (n=5) or 578/10 (n=5). Viral titres were determined by RNA copy numbers and are represented as geometric mean±SD. A detection limit of 0.95 log10RNA copies ml−1 was determined.
These data demonstrate that birds inoculated with NY99 and Greece-10 had the highest mean peak viraemia titres, followed by 578/10 and Ita09, with the lowest viraemia peaks for FIN-infected birds. Mean peak viraemia titres were significantly different between Greece-10 and FIN (P=0.02) and NY99 and FIN (P=0.03). Onset of viraemia occurred significantly later for FIN-infected birds compared with Greece-10 (P=0.02), NY99 and 578/10 (P=0.03, for both) (Table 1). The peak of viraemia was reached the earliest by birds inoculated with Greece-10, followed by the other viral strains (Table 1), but differences were not statistically significant (P=0.8). In terms of duration, viraemia lasted the longest for FIN and 578/10, followed by Greece-10, NY99 and Ita09 (Table 1), but these differences were also not statistically significant (P=0.4).
Determination of the viraemia profiles in terms of infectious virus (TCID50) titres was only successful for a limited set of birds. The infectious titres recorded successfully were for the birds with the highest peak viraemia titre (in terms of viral RNA) in each group. These titres were specifically 106.3 TCID50 ml−1 (109.6 RNA copies) for the NY99-infected bird with the highest peak viraemia, 105.3 TCID50 ml−1 for the Greece-10- and Ita09-infected birds (108.4 and 107.5 RNA copies, respectively), and 102.3 TCID50 ml−1 for the 578/10-infected bird (106.9 RNA copies).
Tissue tropism
Viral loads were determined in the heart, liver, spleen, kidney, bone marrow and brain for all birds. In order to assess the spread of virus to the different organs at the approximate peak of viraemia, two birds per group were euthanized on day 4 p.i. Virus was detected in all organs of these birds (Fig. 3), with the highest mean tissue viral titres found in the spleen and bone marrow (7.1 and 6.9 log10RNA copies g−1, respectively), followed by the liver, kidney and heart (5.8, 5.7 and 5.5 log10RNA copies g−1, respectively). The lowest tissue viral titres were found in the brain (3.8 log10RNA copies g−1). Between the different virus strains, higher tissue viral RNA titres were found in the organs infected by Greece-10 (7.0 log10RNA copies g−1), followed by 578/10 (6.4 log10RNA copies g−1), NY99 and Ita09 (5.4 and 5.3 log10RNA copies g−1, respectively), whilst FIN-infected birds had the lowest tissue viral RNA burden (4.8 log10RNA copies g−1). Viral titres in the organs were significantly different for Greece-10 compared with NY99 (P=0.05), FIN (P=0.004) and Ita09 (P=0.02), and in the organs infected with 578/10 compared with FIN (P=0.01).
Fig. 3.
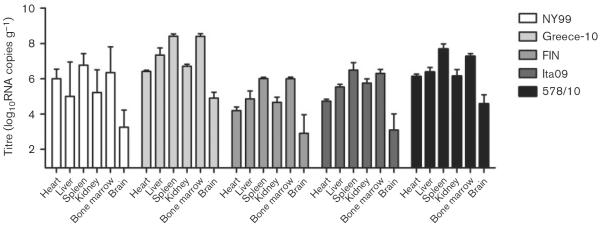
Tissue viral load, as determined by RNA copy numbers, in organs harvested from birds experimentally infected with different WNV strains and euthanized on day 4 p.i. (two per group). Viral titres are represented as geometric mean±SD. A detection limit of 0.95 log10RNA copies g−1 was determined.
For birds euthanized due to morbidity, virus was also found in all the organs (Fig. 4), with the tendency for the spleen, kidney and heart to contain the highest mean tissue viral RNA load (6.7, 6.6 and 6.2 log10RNA copies g−1, respectively), followed by the liver, brain and bone marrow (5.5, 5.4 and 5.2 log10RNA copies g−1, respectively). Viral RNA titres were highest in organs of birds infected by Greece-10 (6.2 log10RNA copies g−1), followed by 578/10 and NY99 (6.1 log10RNA copies g−1, for both), with the lowest titres exhibited by Ita09-infected birds (5.4 log10RNA copies g−1). However, these titres were not found to be significantly different (P=0.4).
Fig. 4.
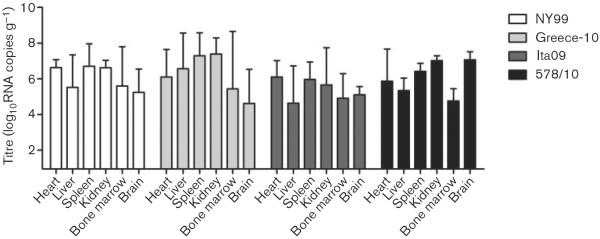
Tissue viral load, as determined by RNA copy numbers, in organs harvested from birds experimentally infected with different WNV strains and euthanized due to morbidity. NY99 (n=3): one bird euthanized on day 5 p.i., two birds on day 7 p.i.; Greece-10 (n=3): one bird euthanized on day 6 p.i., one bird on day 8 p.i. and one bird on day 9 p.i.; Ita09 (n=3): three birds euthanized on day 7 p.i.; and 578/10 (n=2): one bird euthanized on day 6 p.i. and one bird on day 8 p.i. Viral titres are represented as geometric mean±SD. A detection limit of 0.95 log10RNA copies g−1 was determined.
All birds that had survived infection by day 14 were euthanized and necropsied to determine whether virus could still be found in any of the organs (Fig. 5). Viral RNA was detected in all organs of the three Greece-10-infected survivor birds except in the bone marrow of one bird, whilst NY99-infected birds had a minimum of three positive organs (out of six), with the liver being completely negative for both birds. FIN-infected survivor birds had a minimum of two organs (out of six) positive for viral RNA, whilst the bone marrow was consistently negative for all five birds. The two birds that survived infection with Ita09 were both positive for viral RNA in five out of six organs, with the liver negative in one bird and the bone marrow negative in the other bird. Viral RNA was detected in all organs of the two 578/10-survivor birds except for in the liver of one bird. Between the different virus strains, mean viral RNA titres in the organs were significantly higher in Greece-10-infected birds compared with birds infected with FIN (P=0.03).
Fig. 5.
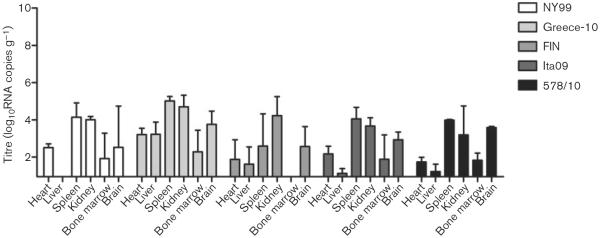
Tissue viral load, as determined by RNA copy numbers, in organs harvested from birds experimentally infected with different WNV strains and euthanized on day 14 p.i. NY99, two birds euthanized; Greece-10, three birds; FIN, five birds; Ita09, two birds; and 578/10, three birds. Viral titres are represented as geometric mean±SD. A detection limit of 0.95 log10RNA copies g−1 was determined.
Overall, mean tissue viral titres in the organs of the survivor birds were lower (2.7 log10RNA copies g−1) compared with the birds necropsied on day 4 (5.8 log10RNA copies g−1) or after illness/death (5.9 log10RNA copies g−1), with most virus persisting in the kidney and spleen (4.1 and 3.7 log10RNA copies g−1, respectively), followed by the brain and heart (3.0 and 2.3 log10RNA copies g−1, respectively), and the liver and bone marrow (1.7 and 1.6 log10RNA copies g−1, respectively).
Immunohistochemistry
Sections of organs of birds euthanized on day 4 p.i. were stained with polyclonal anti-WNV NS3 to determine replication of the virus in these tissues and rule out any positive quantitative real-time (qRT)-PCR detection as a result of spillover from blood (Table 2). Tissues most consistently positive for WNV antigen were the kidney (100 %), followed by the heart, liver, spleen (90 %) and bone marrow (80 %), with the brain only positive in 50 % of the cases. Antigen was most abundant in the spleen, followed by the kidney, bone marrow, heart and liver (Fig. S1, available in the online Supplementary Material). The abundance of viral antigen found in the positive brains was minimal. Overall, organs of birds most often positive, as well as most abundant for viral antigen, were those infected with Greece-10 and 578/10, followed by FIN and Ita09, with the lowest amount of antigen found in the organs of the two NY99-infected birds.
Table 2.
Immunohistochemical analysis of WNV antigen distribution in European jackdaws euthanized on day 4 p.i.
| Virus strain | Bird no. | Heart | Liver | Spleen | Kidney | Bone marrow | Brain | Total score per bird | Mean score per virus strain | No. of positive organs/bird |
|---|---|---|---|---|---|---|---|---|---|---|
| NY99 | 6 | ± | ± | ± | ± | + | ± | 7 | 6.5 | 6/6 |
| 7 | + | ± | − | + | − | ± | 6 | 4/6 | ||
| Greece-10 | 1 | + + | − | + + | + | + + | ± | 12 | 12.5 | 5/6 |
| 4 | + | + | + + | + | + + | ± | 13 | 6/6 | ||
| FIN | 6 | ± | ± | + | + | ± | ± | 8 | 7 | 6/6 |
| 7 | − | ± | + | + | ± | − | 6 | 4/6 | ||
| Ita09 | 1 | ± | + | + | + | − | − | 7 | 8.5 | 4/6 |
| 2 | + | + | + | + | + | − | 10 | 5/6 | ||
| 578/10 | 6 | + | + | + + | + | + | − | 11 | 10.5 | 5/6 |
| 7 | ± | + | + + | + | + | − | 10 | 5/6 | ||
| Score per organ | 15 | 14 | 21 | 19 | 16 | 5 | ||||
| No. of positive birds/organs | 9/10 | 9/10 | 9/10 | 10/10 | 8/10 | 5/10 |
Subjective determinations of the amount of antigen in each organ were made: −, negative; ±, minimal; +, moderate; + +, abundant. Each determination was given a score from 0 to 3: 0, negative; 1, minimal; 2, moderate; 3, abundant.
Viral antigen appeared to be slightly more abundant in birds euthanized due to morbidity (Table 3) as compared with the birds euthanized on day 4, with the spleen and kidney consistently positive for viral antigen (100 %), followed by the heart and brain (91 %), whilst the bone marrow (64 %) and liver (45 %) were the least often positive. In terms of antigen abundance, staining was most prominent in the heart, kidney and spleen, whilst lower amounts of antigen were present in the brain, bone marrow and liver (Fig. S1). Between the different virus strains, the abundance of viral antigen in the organs was very similar in Greece-10, 578/10 and Ita09-infected birds, and was also higher than the amount of antigen found in the organs of NY99-infected birds.
Table 3.
Immunohistochemical analysis of WNV antigen distribution in European jackdaws euthanized due to morbidity (days 5–9 p.i.)
| Virus strain | Bird no. | Heart | Liver | Spleen | Kidney | Bone marrow | Brain | Total score per bird | Mean score per virus strain | No. of positive organs/bird |
|---|---|---|---|---|---|---|---|---|---|---|
| NY99 | 1 | − | ± | + | ± | + | − | 6 | 7.7 | 4/6 |
| 3 | + | − | + | + | − | + | 8 | 4/6 | ||
| 5 | + + | − | + | + | − | + | 9 | 4/6 | ||
| Greece-10 | 5 | + | − | ± | + | − | + | 7 | 10.7 | 4/6 |
| 6 | + | + + | + + | + + | + + | ± | 15 | 6/6 | ||
| 8 | + + | − | + | + | ± | + | 10 | 5/6 | ||
| Ita09 | 3 | + | + | + | + + | + | + + | 14 | 10 | 6/6 |
| 4 | + | ± | ± | + | ± | ± | 8 | 6/6 | ||
| 5 | + | − | + | + + | − | ± | 8 | 4/6 | ||
| 578/10 | 4 | + | ± | + | + | ± | + + | 11 | 10.5 | 6/6 |
| 5 | + + | − | + | + | ± | + | 10 | 5/6 | ||
| Score per organ | 13 | 4.5 | 11 | 13.5 | 6 | 10 | ||||
| No. of positive birds/organs | 10/11 | 5/11 | 11/11 | 11/11 | 7/11 | 10/11 |
Subjective determinations of the amount of antigen in each organ were made: −, negative; ±, minimal; +, moderate; + +, abundant. Each determination was given a score from 0 to 3: 0, negative; 1, minimal; 2, moderate; 3, abundant.
DISCUSSION
In this study, European jackdaws appear to be susceptible to infection with European strains of WNV from both lineages 1 and 2, as well as the North American strain NY99. However, the same extent of susceptibility as shown by the American crow upon infection with NY99, with 100 % mortality rates and production of high viraemia titres reaching peaks >107 p.f.u. ml−1 (Brault et al., 2004, 2007; Komar et al., 2003; Nemeth et al., 2011; Weingartl et al., 2004), was not observed in this study. Herein, mortality rates ranged between 40 and 60 % for four out of five strains and the highest mean peak serum viraemia was ~6.2 log10RNA copies ml−1, reached by birds inoculated with NY99.
Viral RNA was detected in all organs on day 4 p.i. and at the time of euthanasia due to morbidity. Viral RNA titres were observed to be significantly higher on day 4 p.i. in the organs of birds inoculated with Greece-10 compared with NY99, FIN and Ita09, and in the organs of birds inoculated with 578/10 compared with FIN. Even though at this time point spillover of virus from blood most likely resulted in higher viral RNA titres in the organs, immunohistochemistry confirmed that at least the relative differences were the same, as the amount of viral antigen staining confirmed that the two Greece-10- and 578/10-inoculated birds did indeed have the most viral antigen present in their organs as a whole, suggesting that spillover of virus from the circulation did not generally influence these results. Interestingly, Greece-10 and 578/10 are both lineage 2 viruses with 99 % identity, possibly indicating a strain-related tropism. Nonetheless, by the time the birds were euthanized due to morbidity (between days 5 and 9), any virus-related significant differences were no longer observed in terms of viral RNA titres, although immunohistochemical staining suggested a tendency for viral antigen to be more abundant in organs of Greece-10, 578/10 and Ita09-infected birds.
In a previous study (Lim et al., 2013b), mice inoculated with WNV FIN displayed a slightly reduced mortality in comparison with the other WNV strains (also used here). In this study, however, the virulence phenotype of FIN has become much more pronounced, as birds inoculated with FIN suffered no mortality and had significantly lower peak viraemia titres as well as delayed onset of viraemia compared with the other groups. Even though viral virulence generally correlates well with high and prolonged viraemia (Kinney et al., 2006), in this case, viraemia was not significantly shorter in FIN-infected birds compared with the other virus strains, although onset of viraemia did occur significantly later.
Interestingly, the two birds inoculated with FIN and euthanized on day 4 were both positive for virus in the majority of their organs (qRT-PCR and immunohisto-chemistry) despite the absence of mortality seen in this group. This, in combination with the presence of viraemia observed in all birds of this group, suggests that even in birds that are not susceptible to lethal WNV infection, the virus is able to elicit viraemia and disseminate to the organs, including the brain. A similar observation was demonstrated in chickens, which experienced no mortality following infection with WNV, but exhibited titres as high as 5 log10p.f.u. ml−1 and virus could also be isolated from several organs, including the spleen and kidney (Senne et al., 2000).
The introduction of a T249P amino acid substitution (present in North American WNV) in the NS3 helicase of a low virulence strain of WNV has been demonstrated to result in increased virulence in American crows (Brault et al., 2007). The virus strains used in this study all contain a proline at this site, with the exception of FIN, which contains a threonine at this position (Lim et al., 2013b) (Table S1). Ita09, which is 99.7 % identical to the nucleotide sequence of FIN, did not display the same attenuation in jackdaws as FIN. It is therefore very likely that the attenuated phenotype of FIN demonstrated in this study is the result of this P249T substitution. Studies are ongoing to test the relevance of the T249P substitution present in the Italian backbone of WNV in both European and American corvids.
It has been shown that a blood titre of 106 p.f.u. ml−1 is required for transmission to feeding mosquitoes (van der Meulen et al., 2005). Additionally, viraemic titres >105 p.f.u. ml−1 were considered infectious for Culex pipiens (Turell et al., 2000) and Culex quinquefasciatus (Sardelis et al., 2001). This suggests that only the NY99-, Greece-10- and Ita09-infected birds that reached peak viraemia RNA titres >107.5 RNA copies ml−1 (which resulted in 105.3 TCID50 ml−1 upon virus isolation) would be likely to infect feeding mosquitoes, as the lowest cut-off of 105.0 p.f.u. ml−1 will result in ~105.2 TCID50 ml−1, according to a conversion factor of 1 TCID50 to 0.7 p.f.u. (Saunders, 2007). It is possible that the infectious viraemia titres of the other samples were below the detection limit of the assay. As the majority of the birds did not attain viraemia titres >107.5 RNA copies ml−1, it suggests that jackdaws would most likely not serve as efficient amplifying hosts in the transmission cycle of WNV in Europe. However, it is also possible that a higher peak of viraemia was missed due to sampling on intervening days. Similar results were observed in a study using a French strain (Fr2000) in carrion crows (Dridi et al., 2013), where the highest viral RNA titre recorded was equivalent to only 104 TCID50 ml−1, which is also below the infectious viral titre required for transmission to feeding mosquitoes.
None of the isolates used in this study has either caused massive die-offs of birds in the field or has spread swiftly across Europe as observed during the North American invasion by WNV (Hofmeister, 2011). Thus far, experimental studies using European strains of WNV and European birds have shown fairly low mortality rates (Dridi et al., 2013; Sotelo et al., 2011; Ziegler et al., 2013), with the highest mortality rate of 33 % observed following inoculation of carrion crows with the Fr2000 strain (Dridi et al., 2013). The study presented herein suggests that the susceptibility of European birds could be related to the WNV strain, as much higher mortality was observed in this study with alternative strains compared with the French strain. At the same time, host factors present in the jackdaw or other European avian species could also play an important role in susceptibility, as the mortality induced by the North American strain NY99, known to be highly virulent in corvids, was similar to that exhibited by Ita09 (60 %) in jackdaws. This mortality rate, as well as the viraemia levels, was also not comparable with those usually seen upon infection of American crows with NY99. This therefore suggests that host factors present in European jackdaws could potentially reduce their susceptibility to WNV.
However, a substantial proportion of the jackdaws did die as a result of infection, which therefore suggests that perhaps some birds in Europe are indeed succumbing to WNV infection in the field, but that the mortality rates of these birds are simply too low to be detected by the current monitoring systems present in Europe. It is also possible that natural infection via mosquito feeding could result in higher serum viraemia titres in jackdaws or that other ecological factors such as infection due to carcass scavenging by more susceptible birds could play a more important role in the transmission and maintenance of WNV in Europe. Nevertheless, this study shows that jackdaw mortality could be potentially useful for tracking WNV transmission in Europe.
METHODS
Source of virus and birds
Five different isolates of WNV were utilized (Table 4). The NY99-4132 strain (NY99; lineage 1a) (Brault et al., 2004) was isolated originally from the brain of an American crow and was passed two times in Vero cells before being used in this study. The lineage 2 Greek strain Nea Santa-Greece-2010 (Greece-10; GenBank accession number HQ537483.1) was isolated from a pool of C. pipiens mosquitoes (Papa et al., 2011) and passaged once in Vero cells. Two lineage 1a Italian strains, FIN (provided by Dr Vittorio Sambri, University of Bologna, Italy; two Vero E6 passages; GenBank accession number KF234080) and Ita09 (provided by Dr Luisa Barzon, University of Padova, Italy, one Vero E6 passage; GenBank accession number GU011992.2) were both isolated from a patient with neuroinvasive disease (Barzon et al., 2009). The Hungarian lineage 2 strain 578/10 (provided by Dr Tamás Bakonyi, Szent István University, Hungary, two passages on Vero E6 cells; GenBank accession number KC496015) was isolated from the brain of a horse that died of WNV neuroinvasive disease. Virus stocks used in this study (not including NY99 and Greece-10) were prepared by growing the viruses once in C6/36 insect cells (Table 1).
Table 4.
WNV strains used for susceptibility studies in European jackdaws
| Virus | Strain | Source | Passage history* | Location | Genetic lineage |
|---|---|---|---|---|---|
| NY99 | NY99-4132 | American crow (brain) | V2 | USA | 1a |
| Greece-10 | Nea Santa-Greece-2010 | Culex pipiens | V1 | Greece | 2 |
| FIN | FIN | Patient with neuroinvasive disease | V2, C1 | Italy | 1a |
| Ita09 | Ita09 | Patient with neuroinvasive disease | V1, C1 | Italy | 1a |
| Hungary | 578/10 | Horse (brain) | V2, C1 | Hungary | 2 |
Viruses were propagated in Vero (V) or C6/36 insect cells (C). Numbers following passage source represent the number of viral passages.
Jackdaws were captured using walk-in traps in the municipality of Rotterdam, The Netherlands. They were transported to indoor housing where they were kept in groups of seven or eight in isolators under negative pressure. Only seronegative birds were used in this study. All birds were cared for in animal holding facilities at the National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Detection of pre-existing WNV antibodies
To confirm that jackdaws had not been exposed previously to WNV, the birds were bled before experimental infection and serum was tested for neutralizing antibodies using TCID50 neutralization assays. Serum was heat-inactivated at 56 °C for 30 min, serially diluted twofold and incubated with an equal volume of virus (strain NY99, originally isolated from a dead Chilean flamingo at the Bronx Zoo in New York, obtained from the Health Protection Agency, Porton Down, UK; P5 on Vero E6 cells; GenBank accession number AF196835.2) to a final concentration of 100 TCID50 per 0.1 ml. Samples were incubated at 37 °C for 1 h and subsequently added to an 80 % confluent monolayer of Vero E6 cells in CELLSTAR 96-well plates (Greiner Bio-One). Plates were incubated at 37 °C for 5 days. Samples were read and a 100 % reduction in cytopathic effect (CPE) as compared with the serum-negative control was used for the determination of neutralization. Detection of any neutralizing activity to WNV in the serum of any bird precluded its use for experimental inoculation.
Experimental infection and sampling protocol
Jackdaws were subcutaneously inoculated in the thigh region with 2000 TCID50 virus in 0.1 ml Dulbecco's modified Eagle's medium (DMEM) containing no FBS. Jackdaws were injected with NY99 (n=7), Greece-10 (n=8), FIN (n=7), Ita09 (n=7) and 578/10 (n=7). Blood was collected from all birds (~0.1 ml) at 2 day intervals for a period of 8 days p.i. Coagulated blood was centrifuged at 1300 g for 5 min in MiniCollect vials (Greiner Bio-One) in order to separate serum, which was stored subsequently at −80 °C. All jackdaws were examined for signs of disease twice daily for 14 days following inoculation and euthanized under isoflurane anaesthesia upon display of clinical symptoms. Additionally, two birds per group were euthanized at day 4 p.i. for monitoring the dissemination of virus to the organs at the approximate time of peak viraemia, as well as all surviving birds at day 14 p.i.
Necropsies were performed on all euthanized birds and the following tissues were collected: heart, liver, spleen, kidney, bone marrow and brain. A small section of each tissue was collected, and subsequently weighed and homogenized using a metal bead in 1 ml DMEM containing antibiotics (100 U penicillin ml−1, 100 μg streptomycin ml−1). The remaining portion of the tissues was collected in formalin for use in immunohistochemical staining.
Determination of viral loads
To determine viral loads in the serum samples and tissue homogenates, we used qRT-PCR to measure viral RNA titres (serum and tissue) and TCID50 titration for the calculation of infectious virus titres (serum only). Briefly, RNA was isolated from 50 μl serum or 100 μl homogenized tissue using the MagNA Pure LC Total Nucleic Acid Isolation kit (Roche) and an automated nucleic acid robotic workstation (Roche) according to the manufacturer's instructions. RNA was eluted in 100 μl elution buffer (Roche) and stored at −80 °C until assayed. RNA copy numbers were quantified using unmodified primers as described previously (Lim et al., 2013a). The limit of detection of the assay was 0.95 log10RNA copies.
Infectious titres in the serum were determined by log10 titration of the serum samples on Vero E6 cells and calculating the TCID50 using the Spearman–Kärber method (Kärber, 1931; Spearman, 1908) after the determination of CPE 5 days p.i. Initial 1 : 10 dilution of serum resulted in a limit of detection of 101.75 TCID50 ml−1.
Immunohistochemistry
Sagittal organ paraffin sections (4 μm thick) were processed for peroxidase immunohistochemistry of virus non-structural protein markers. Sections were deparaffinized in xylene, rehydrated in descending concentrations of ethanol and incubated for 10 min in 3 % H2O2 diluted in PBS in order to block endogenous peroxidase activity. Antigen exposure was performed by 15 min incubation at 121 °C in citrate buffer (0.01 M, pH 6.0). Sections were incubated overnight at 4 °C with primary goat anti-WNV NS3 (1 : 100; R&D Systems) or goat serum (1 : 100; Dako) for isotype controls, and detected with secondary rabbit anti-goat IgGPO (Dako) antibody. Sections were counterstained with Mayer's haematoxylin, mounted with Kaiser's glycerol gelatin and analysed using a light microscope.
Statistical analyses
Survival curves were analysed using the log-rank (Mantel–Cox) test. All other statistical analyses were performed by Kruskal–Wallis ANOVA and any significant difference found was more closely analysed between the groups using the Mann–Whitney U test.
ACKNOWLEDGEMENTS
We thank Vittorio Sambri, Luisa Barzón, Giorgio Palù and Tamás Bakonyi for providing the low-passage isolates used in this study. We would also like to thank Tanja Schouten and Angela Gomersbach for their excellent technical assistance. We thank Jeroen Roose and Peter van Run for their technical assistance with the immunohistochemistry, and Thijs Kuiken for his assistance with the analysis of the histological staining. The research leading to these results has received complete funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under the project `VECTORIE' (EC grant agreement 261466). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Permission for trapping European jackdaws was obtained from the Ministry of Agriculture (registered under number FF/75A/2011/031). Experimental inoculations were performed under protocol number 122-12-12 with permission obtained from the Animal Ethics Committee of Erasmus Medical Centre. All efforts were made to minimize animal suffering.
REFERENCES
- Bakonyi T, Ferenczi E, Erdélyi K, Kutasi O, Csörgť T, Seidel B, Weissenböck H, Brugger K, Bán E, Nowotny N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165:61–70. doi: 10.1016/j.vetmic.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Banet-Noach C, Simanov L, Malkinson M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003;32:489–494. doi: 10.1080/0307945031000154080. [DOI] [PubMed] [Google Scholar]
- Barzon L, Franchin E, Squarzon L, Lavezzo E, Toppo S, Martello T, Bressan S, Pagni S, Cattai M, et al. Genome sequence analysis of the first human West Nile virus isolated in Italy in 2009. Euro Surveill. 2009;14:19384. [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Cusinato R, Cattai M, Franchin E, Pagni S, Martello T, Bressan S, Squarzon L, et al. Human cases of West Nile Virus infection in north-eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011;16:19949. [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Franchin E, Martello T, Lavezzo E, Squarzon L, Toppo S, Fiorin F, Marchiori G, et al. Clinical and virological findings in the ongoing outbreak of West Nile virus Livenza strain in northern Italy, July to September 2012. Euro Surveill. 2012;17:20260. [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Franchin E, Pagni S, Lavezzo E, Squarzon L, Martello T, Russo F, Nicoletti L, et al. Large human outbreak of West Nile virus infection in north-eastern Italy in 2012. Viruses. 2013a;5:2825–2839. doi: 10.3390/v5112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzon L, Papa A, Pacenti M, Franchin E, Lavezzo E, Squarzon L, Masi G, Martello T, Testa T, et al. Genome sequencing of West Nile Virus from human cases in Greece, 2012. Viruses. 2013b;5:2311–2319. doi: 10.3390/v5092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Huang CY, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri P, Giovannini A, Savini G, Monaco F, Bonfanti L, Ceolin C, Terregino C, Tamba M, Cordioli P, Lelli R. West Nile virus transmission in 2008 in north-eastern Italy. Zoonoses Public Health. 2010;57:211–219. doi: 10.1111/j.1863-2378.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Danis K, Papa A, Papanikolaou E, Dougas G, Terzaki I, Baka A, Vrioni G, Kapsimali V, Tsakris A, et al. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Euro Surveill. 2011;16:19951. [PubMed] [Google Scholar]
- Dauphin G, Zientara S, Zeller H, Murgue B. West Nile: worldwide current situation in animals and humans. Comp Immunol Microbiol Infect Dis. 2004;27:343–355. doi: 10.1016/j.cimid.2004.03.009. [DOI] [PubMed] [Google Scholar]
- De Filette M, Ulbert S, Diamond M, Sanders NN. Recent progress in West Nile virus diagnosis and vaccination. Vet Res. 2012;43:16. doi: 10.1186/1297-9716-43-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi M, Vangeluwe D, Lecollinet S, van den Berg T, Lambrecht B. Experimental infection of carrion crows (Corvus corone) with two European West Nile virus (WNV) strains. Vet Microbiol. 2013;165:160–166. doi: 10.1016/j.vetmic.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Eidson M, Schmit K, Hagiwara Y, Anand M, Backenson PB, Gotham I, Kramer L. Dead crow density and West Nile virus monitoring, New York. Emerg Infect Dis. 2005;11:1370–1375. doi: 10.3201/eid1109.040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister EK. West Nile virus: North American experience. Integr Zool. 2011;6:279–289. doi: 10.1111/j.1749-4877.2011.00251.x. [DOI] [PubMed] [Google Scholar]
- Hubálek Z, Halouzka J. West Nile fever – a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain E, Schuffenecker I, Korimbocus J, Reynard S, Murri S, Kayser Y, Gauthier-Clerc M, Sabatier P, Zeller HG. West Nile virus in wild resident birds, Southern France, 2004. Vector Borne Zoonotic Dis. 2007;7:448–452. doi: 10.1089/vbz.2006.0592. [DOI] [PubMed] [Google Scholar]
- Julian KG, Eidson M, Kipp AM, Weiss E, Petersen LR, Miller JR, Hinten SR, Marfin AA. Early season crow mortality as a sentinel for West Nile virus disease in humans, northeastern United States. Vector Borne Zoonotic Dis. 2002;2:145–155. doi: 10.1089/15303660260613710. [DOI] [PubMed] [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol. 1931;162:480–483. [Google Scholar]
- Kinney RM, Huang CY, Whiteman MC, Bowen RA, Langevin SA, Miller BR, Brault AC. Avian virulence and thermostable replication of the North American strain of West Nile virus. J Gen Virol. 2006;87:3611–3622. doi: 10.1099/vir.0.82299-0. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Koraka P, Osterhaus AD, Martina BE. Development of a strand-specific real-time qRT-PCR for the accurate detection and quantitation of West Nile virus RNA. J Virol Methods. 2013a;194:146–153. doi: 10.1016/j.jviromet.2013.07.050. [DOI] [PubMed] [Google Scholar]
- Lim SM, Koraka P, van Boheemen S, Roose JM, Jaarsma D, van de Vijver DA, Osterhaus AD, Martina BE. Characterization of the mouse neuroinvasiveness of selected European strains of West Nile virus. PLoS ONE. 2013b;8:e74575. doi: 10.1371/journal.pone.0074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson M, Banet C, Weisman Y, Pokamunski S, King R, Drouet MT, Deubel V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis. 2002;8:392–397. doi: 10.3201/eid0804.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth NM, Thomsen BV, Spraker TR, Benson JM, Bosco-Lauth AM, Oesterle PT, Bright JM, Muth JP, Campbell TW, et al. Clinical and pathologic responses of American crows (Corvus brachyrhynchos) and fish crows (C ossifragus) to experimental West Nile virus infection. Vet Pathol. 2011;48:1061–1074. doi: 10.1177/0300985811398249. [DOI] [PubMed] [Google Scholar]
- Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro Surveill. 2010;15:19644. doi: 10.2807/ese.15.34.19644-en. [DOI] [PubMed] [Google Scholar]
- Papa A, Xanthopoulou K, Gewehr S, Mourelatos S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin Microbiol Infect. 2011;17:1176–1180. doi: 10.1111/j.1469-0691.2010.03438.x. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. Virology – Principles and Application. Wiley; New York: 2007. [Google Scholar]
- Senne DA, Pedersen JC, Hutto DL, Taylor WD, Schmitt BJ, Panigrahy B. Pathogenicity of West Nile virus in chickens. Avian Dis. 2000;44:642–649. [PubMed] [Google Scholar]
- Sirbu A, Ceianu CS, Panculescu-Gatej RI, Vazquez A, Tenorio A, Rebreanu R, Niedrig M, Nicolescu G, Pistol A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16:19762. [PubMed] [Google Scholar]
- Sotelo E, Gutierrez-Guzmán AV, del Amo J, Llorente F, El-Harrak M, Pérez-Ramírez E, Blanco JM, Höfle U, Jiménez-Clavero MA. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: the red-legged partridge. Vet Res. 2011;42:11. doi: 10.1186/1297-9716-42-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The method of right and wrong cases (constant stimuli) with Gauss formulae. Br J Psychol. 1908;2:227–242. [Google Scholar]
- Turell MJ, O'Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- Valiakos G, Touloudi A, Iacovakis C, Athanasiou L, Birtsas P, Spyrou V, Billinis C. Molecular detection and phylogenetic analysis of West Nile virus lineage 2 in sedentary wild birds (Eurasian magpie), Greece, 2010. Euro Surveill. 2011;16:19862. [PubMed] [Google Scholar]
- van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637–657. doi: 10.1007/s00705-004-0463-z. [DOI] [PubMed] [Google Scholar]
- Weingartl HM, Neufeld JL, Copps J, Arszal PM. Experimental West Nile virus infection in blue jays (Cyanocitta cristata) and crows (Corvus brachyrhynchos) Vet Pathol. 2004;41:362–370. doi: 10.1354/vp.41-4-362. [DOI] [PubMed] [Google Scholar]
- Wheeler SS, Barker CM, Fang Y, Armijos MV, Carroll BD, Husted S, Johnson WO, Reisen WK. Differential impact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobeser G, Wobeser AG. Carcass disappearance and estimation of mortality in a simulated die-off of small birds. J Wildl Dis. 1992;28:548–554. doi: 10.7589/0090-3558-28.4.548. [DOI] [PubMed] [Google Scholar]
- Ziegler U, Angenvoort J, Fischer D, Fast C, Eiden M, Rodriguez AV, Revilla-Fernández S, Nowotny N, de la Fuente JG, et al. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Vet Microbiol. 2013;161:263–273. doi: 10.1016/j.vetmic.2012.07.041. [DOI] [PubMed] [Google Scholar]


