Abstract
Objective
Minnelide is a water-soluble pro-drug of triptolide, a natural product. The goal of this study was to evaluate the effectiveness of Minnelide on ovarian cancer growth in vitro and in vivo.
Methods
The effect of Minnelide on ovarian cancer cell proliferation was determined by real time electrical impedance measurements. Multiple mouse models with C200 and A2780 epithelial ovarian cancer cell lines were used to assess the efficacy of Minnelide in inhibiting ovarian cancer growth.
Results
Minnelide decreased cell viability of both platinum sensitive and resistant epithelial ovarian cancer cells in vitro. Minnelide with carboplatin showed additive effects in vitro. Minnelide monotherapy increased the survival of mice bearing established ovarian tumors. Minnelide, in combination with carboplatin and paclitaxel, improved overall survival of mice.
Conclusions
Minnelide is a promising pro-drug for the treatment of ovarian cancer, especially when combined with standard chemotherapy.
Keywords: Ovarian cancer, Minnelide, Triptolide, Chemotherapy
Introduction
Ovarian cancer has the highest mortality rate of all of the malignancies of the female genital tract. In the United States 21,980 patients were diagnosed with ovarian cancer in 2014 and 14,270 patients died of their disease [1]. The 5 year survival rate for ovarian cancer is 25% for advanced stage disease, which is when 85% of patients are diagnosed. The poor survival is due to a combination of factors including a lack of effective screening and prevention techniques, late stage at diagnosis and lack of treatment options once a patient has failed initial therapies. The current standard first line treatment for ovarian cancer is a combination of a taxane and platinum agent. While 80% of patients will respond to first line treatment, 20% of patients will be platinum resistant and not respond to standard therapy. There are a number of second line agents approved for the treatment of recurrent ovarian cancer including liposomal doxorubicin, topotecan and gemcitabine; however progression free survival times are generally <12 months [2]. Given these relatively low response rates there is a need for new drug discovery and more effective ways to treat ovarian cancer.
Triptolide, a diterpenoid triepoxide isolated from the plant Tripterygium wilfordii Hook F, has shown efficacy against several different cancer types including pancreatic, melanoma, neuroblastoma and gastric [3–5]. Because triptolide is not water soluble, the physical properties of this natural product have limited its clinical development. To overcome solubility problems, triptolide was chemically modified to prepare a prodrug which was named Minnelide [6,7]. Minnelide or 14-O-phosphonooxymethyltriptolide disodium salt is a white powder which is converted into the parent compound triptolide in the presence of alkaline phosphatase which is common in all tissues in the body [8]. The conversion of triptolide to Minnelide has been shown previously both in vitro and in vivo [6]. This compound has been found to be effective against pancreatic cancer as well as osteosarcoma and non-small cell lung cancer both in vitro and in vivo [6,9,10]. Since both pancreatic and ovarian cancers metastasize in the peritoneum, we sought to determine the efficacy of Minnelide in inhibiting ovarian cancer growth.
Materials and methods
Reagents
Minnelide was synthesized in the Department of Medicinal Chemistry, University of Minnesota as described by Chugh et al. [6]. Carboplatin was purchased from Calbiochem and Paclitaxel from Sigma Aldrich.
Cell lines and cell culture
Platinum sensitive (A2780) and platinum resistant (C200) ovarian cancer cell lines were obtained from Thomas Hamilton, Fox Chase Cancer Center, Philadelphia. Both cell lines were cultured at 37 °C in the presence of 5% CO2 in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% Fetal Bovine Serum (FBS), 2.0 mM glutamine, penicillin and streptomycin. The C200 cells were grown in the presence of 0.5 µg/mL of human insulin.
Cell viability and proliferation assays
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT as described before [11].
Electrical impedance measurement using the xCELLigence system
xCELLigence experiments were performed according to published protocols from Roche. C200 and A2780 cells were grown and expanded in tissue culture flasks. After reaching approximately 80% confluence, the cells were harvested by trypsin/EDTA. Aliquots of 100 µL of culture media were added to each well of the E-plate 16. After the background impedance was measured for 1 h, 7000 cells were added to each well along with different concentrations of Minnelide, carboplatin or a combination of Minnelide and carboplatin. Following a 30 minute incubation period at room temperature the E-plate 16 was placed into the xCELLigence system. Changes in electrical impedance were determined every 10 min for 48 h and expressed as ‘cell index’.
Orthotopic ovarian cancer tumor model
Six week-old, female, athymic mice (National Cancer Institute)were injected intraperitoneally with 5 × 106 A2780 or C200 cells transduced with GFP. Tumors were allowed to establish for 7–12 days before starting treatment. Animals were randomized into groups of 10 at the time of treatment with chemotherapeutic compounds. Minnelide was prepared using saline as the solvent. Experiments were performed and animals sacrificed according to the regulations of the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Histopathology and determination of apoptotic index and proliferation
Residual tumor tissues collected from the control and treated mice were fixed in neutral formalin and then sectioned. Sections were then processed for immunohistochemical detection of Caspase-3 to determine changes in apoptosis in various treatment groups. Images were captured at random from different areas of the sections and then processed. Images were binearized and thresholded to remove noise. Binearized images were used to count the number of cells positive for Caspase-3 by image processing as described in a previous publication [12]. Parallel sets of sections were used for histology and stained for vessel density using anti-mouse CD31-HRP conjugate. These same tissue samples were also subjected to immunohistochemical detection of Ki-67 which was quantified in a manner similar to that described above for Caspase-3.
Western blot analysis
A2780 (80% confluent) were treated with either saline or 25 nM of Minnelide for 24 h. Following treatment, cell lysates were prepared using the RIPA Buffer (radioimmunoprecipitation assay) with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific). Western blot was used to determine changes in protein levels and PARP cleavage (poly ADP ribose polymerase). PARP, HSP70 and NFκβ specific antibiodies were purchased from Cell Signaling Technology. NFκβ and beta-Actin antibodies were purchased from Santa Cruz Biotechnology.
Statistical analysis
Overall survival (OS) of the mice was calculated from treatment initiation to death or censored at the end of the experiment (100 days). OS was summarized using Kaplan–Meier methods and compared by treatment group using log rank tests; median survival and 95% confidence intervals (CI) are presented. p-Values less than 0.05 were considered statistically significant.
Results
Minnelide decreases cell viability of ovarian cancer cells in vitro
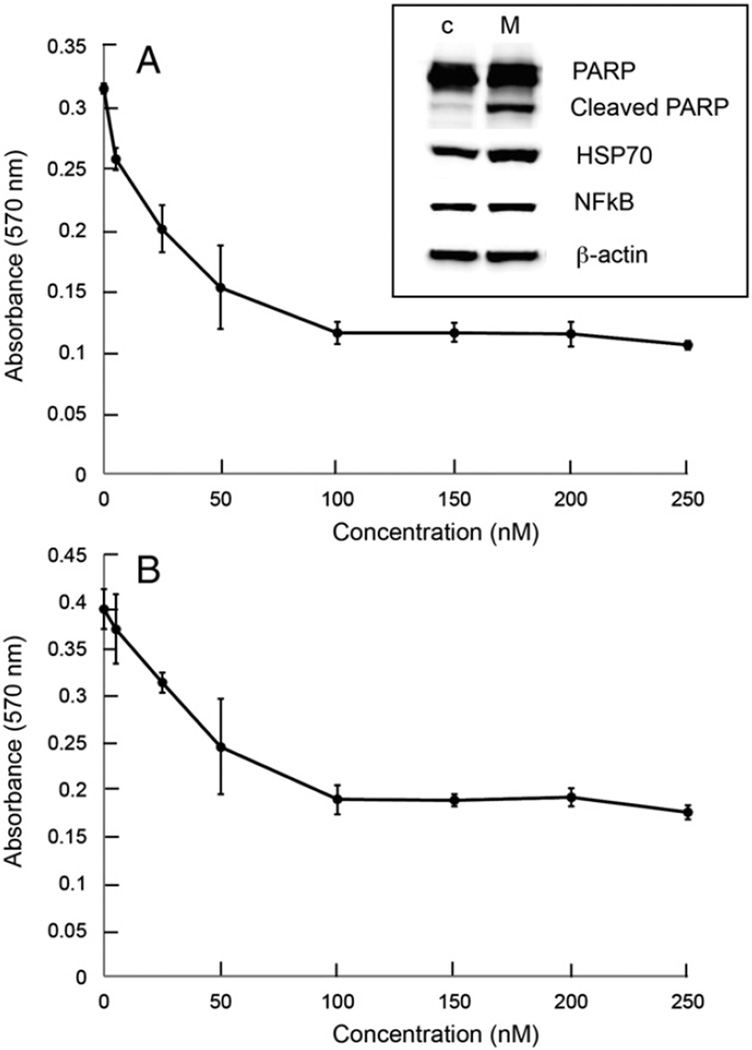
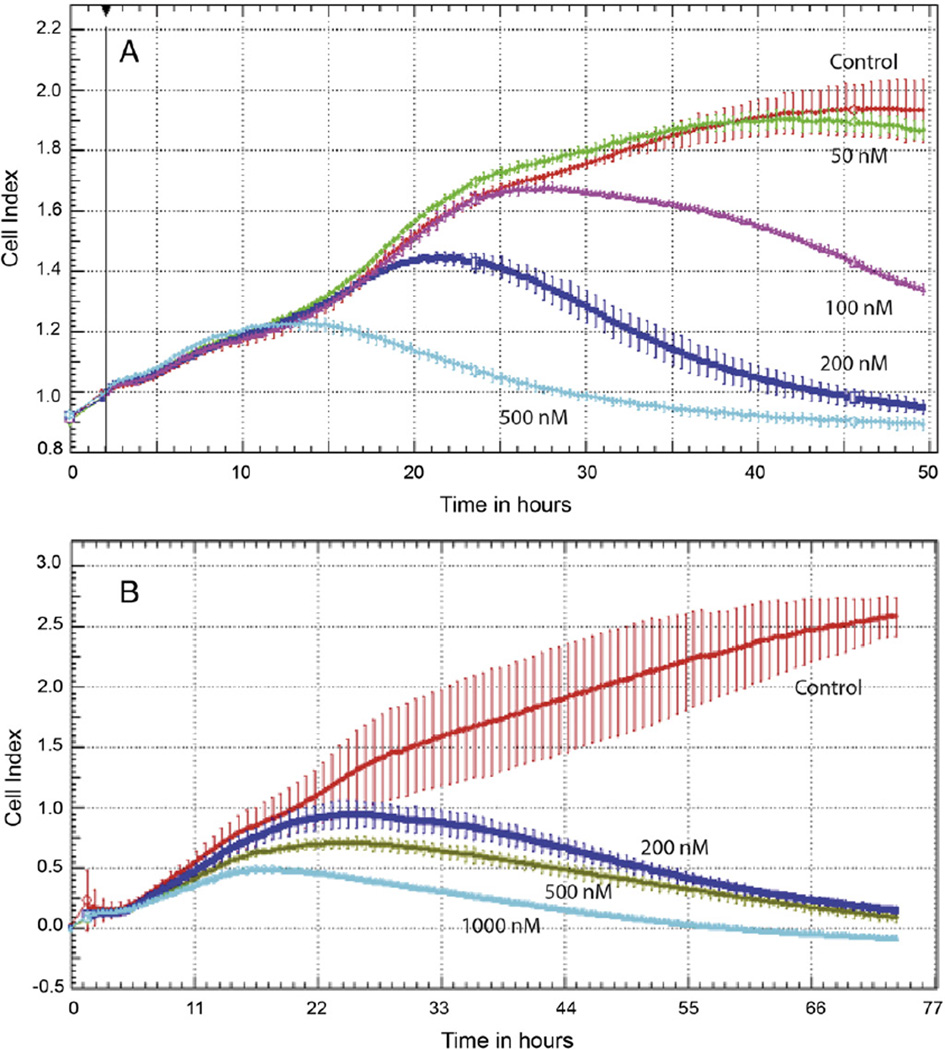
In the initial experiment, cell viability was determined following treatment with Minnelide. Both A2780 and C200 cells were inhibited in a concentration dependent manner between 10 and 250 nM (Fig. 1). To assess the efficacy of Minnelide on proliferation of ovarian cancer cell lines, we treated A2780 with 50 nM, 100 nM, 200 nM and 500 nM of Minnelide and C200 cells with 200 nM, 500 nM and 1000 nM of Minnelide. Preliminary studies had shown that C200 cells display a multi-drug resistance phenotype and given this we determined that the C200 cells would likely need higher doses of Minnelide to achieve responses. Changes in cell index were determined every 10 min over a period of 48 h (A2780) or 70 h (C200) (Fig. 2). Each value is a mean of quadruplicate cultures and vertical bars show standard deviation. Control cells (red line) grew from the initial cell index of 0.9 to 1.9 and 2.5 in A2780 and C200 respectively, during the two days in culture. Minnelide treatment showed concentration dependent inhibition of ovarian cancer cell growth at nM concentrations. At 500 nM, ovarian cancer cells were completely inhibited. A2780 cells stopped growing after exposure to Minnelide for approximately 20 h (Fig. 2A). C200 cells are resistant to platinum even at 200 µM concentration. C200 cells however required longer exposure time to Minnelide. Cell index decreased only after 30 h of continuous exposure to the drug (Fig. 2B).
Fig. 1.
Inhibition of ovarian cancer cell viability by Minnelide. A) A2780 ovarian cancer cell line. Cell lysates were prepared from control (C) and A278-cells treated with 25 nM Minnelide and used to perform Western blot analysis for PARP, cleaved PARP, HSP70 and NFκβ; B) C200, platinum resistant ovarian cancer cell line was treated with Minnelide for 48 h. Viability was determined by MTT assay (absorbance of 570 nm). Each value is a mean of triplicate cultures ± SD.
Fig. 2.
Real time changes in ovarian cancer cell proliferation. Electrical impedance measurements (cell index) were used to determine the effect of Minnelide on cell proliferation. Changes in cell index were recorded every 10min for a total of 48 h. Each value is a mean of triplicate wells ± SD. A) Platinum sensitive cancer cell line, A2780. B) Platinum resistant ovarian cancer cell line, C200.
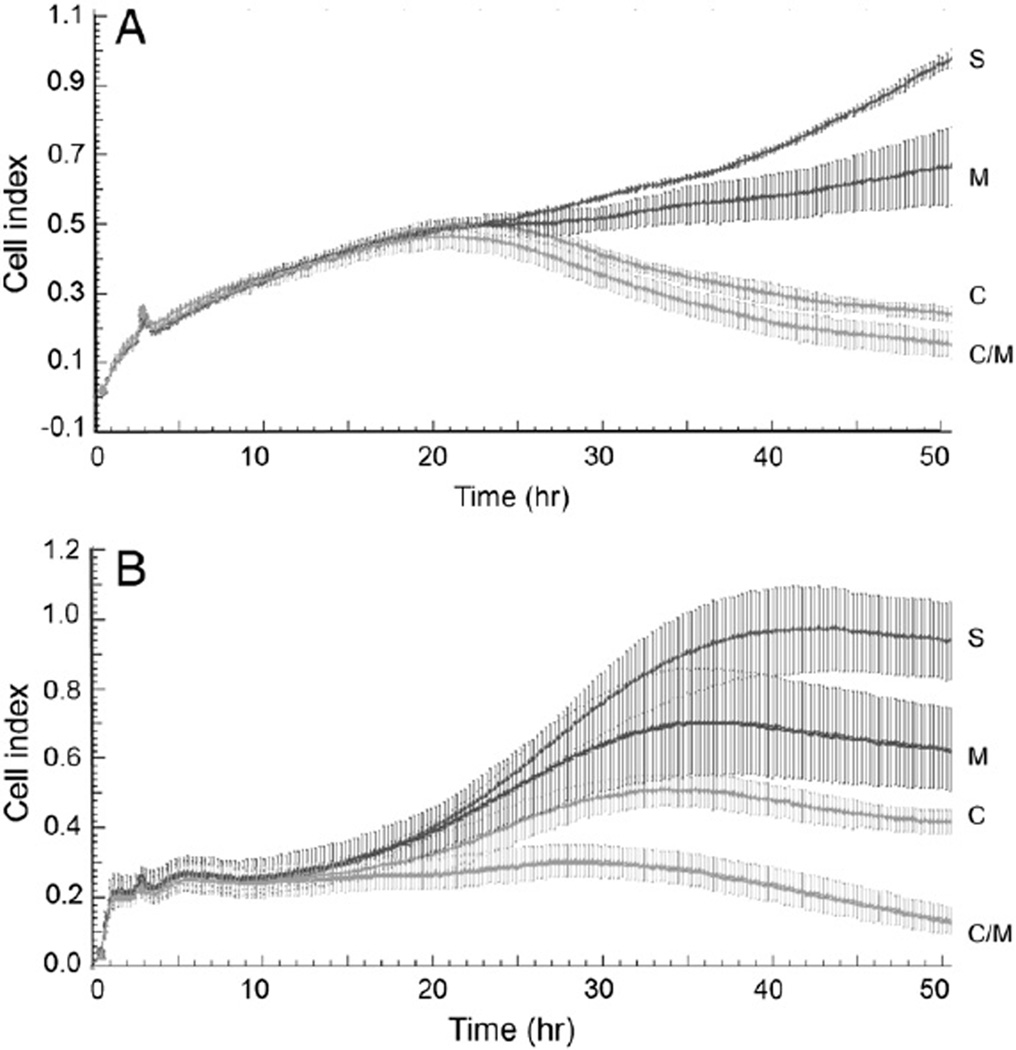
We then explored the potential for Minnelide to potentiate the cytotoxicity of carboplatin. Data in Fig. 3 show the effect of combination treatment on cell proliferation. Platinum sensitivity increased in the presence of Minnelide. A2780 cells treated with 50 µM carboplatin showed moderate inhibition by monotherapy. Presence of Minnelide (25 nM) alone decreased the cell index from 0.92 to 0.67 at the end of the experiment. Carboplatin (50 nM) treatment decreased the cell index to 0.24. Combination treatment with Minnelide and carboplatin (25 nM and 50 nM respectively) decreased the cell index to 0.15. C200 cells were treated with a higher concentration of carboplatin (100 nM) but the same dose of Minnelide (25 nM). Minnelide alone decreased cell index from 0.92 to 0.62. Carboplatin treatment reduced cell index to 0.36 and combination treatment resulted in further reduction in cell index (0.13).
Fig. 3.
Real time changes in ovarian cancer cell proliferation with the effect of combination treatment with Minnelide and carboplatin. Electrical impedance measurements (cell index) were recorded every 10 min over a period of 48 h. Error bars represent SD. A) Platinum sensitive cell line, A2780. S = saline; C = carboplatin 50 µM; M = Minnelide (25 nM); C/M = combination of carboplatin (50 µM) + Minnelide (25 nM). B) Platinum resistant cell line, C200. S = saline; C = carboplatin 100 µM; M = Minnelide (25 nM); C/M = combination of carboplatin (100 µM) + Minnelide (25 nM). C200 cells were treated with a higher concentration of carboplatin given their platinum resistance.
Minnelide increases survival in orthotopic mouse models of platinum sensitive cells
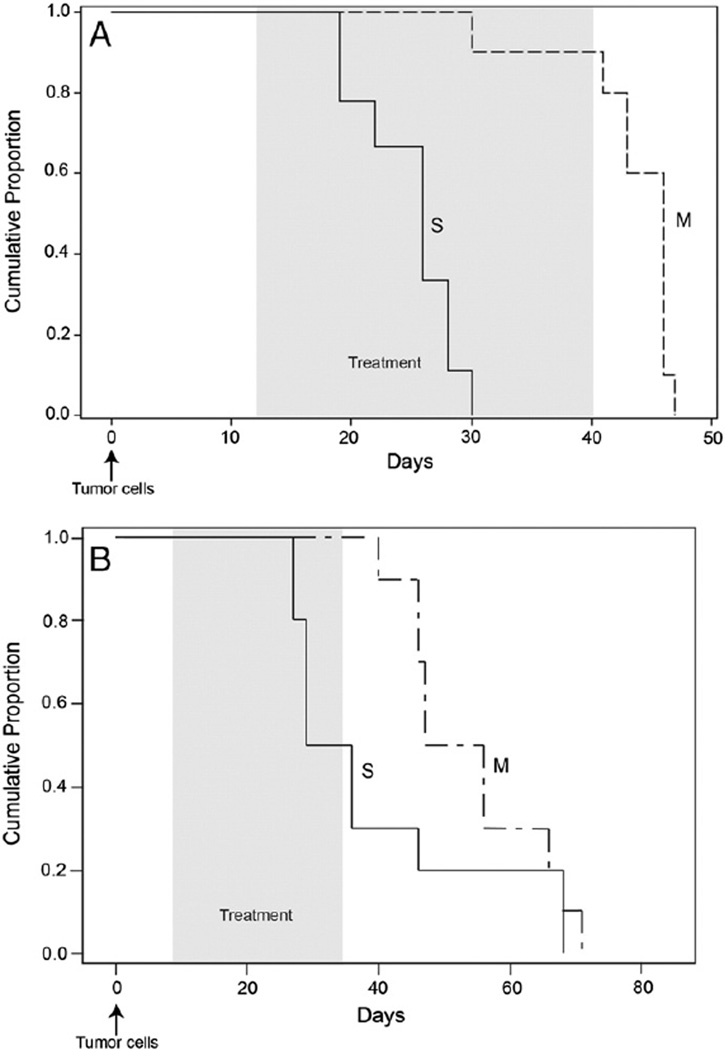
For our first orthotopic mouse model, 5 × 106 A2780 cells were injected intraperitoneally into athymic mice and tumors were allowed to establish for 12 days post-injection. Mice were then treated with 0.42 mg/kg of Minnelide or 0.2mL of saline daily for 28 days. Mice were sacrificed when they were moribund according to the animal protocols in place at the University of Minnesota. In addition, median overall survival (OS) was greater for the Minnelide treated mice (46 days [95% CI: 30–46]) compared to the control mice (26 days [95% CI: 19–28]; Fig. 4A, p < 0.0001).
Fig. 4.
Inhibition of ovarian cancer tumor growth by Minnelide in vivo. A) Kaplan–Meier survival curve shows the overall survival of A2780 tumor bearing mice treated with Minnelide (0.42 mg/kg daily). Overall survival was significantly increased in treatment vs. control mice (26 days [95% CI: 19–28] vs. 46 days [95% CI: 30–46], p < 0.0001). B) Kaplan–Meier survival curve of C200 tumor bearing mice treated with Minnelide (0.6 mg/kg daily). Overall survival was significantly increased in treatment vs. control mice (51.5 days [95% CI: 40–66 days] vs. 32.6 days [95% CI: 27–46 days], p = 0.0001). Shaded areas represent treatment period and arrows represent tumor cell injection (intraperitoneal). S = saline; M = Minnelide. Ten animals were used in each group.
Minnelide increases survival in orthotopic model of platinum resistant ovarian cancer cells
Since Minnelide showed efficacy against platinum resistant cell lines, we injected 5 × 106 C200 cells intraperitoneally and allowed the tumor to establish for 7 days. At this time mice were randomized to receive either saline (0.2 mL) or Minnelide (0.6 mg/kg daily). Mice were euthanized when they were moribund. Median OS of control mice was significantly shorter (32.6 days [95% CI: 27–46 days]) than the OS of Minnelide treated mice (51.5 days [95% CI: 40–66 days]; Fig. 4B; p = 0.0001).
Minnelide in combination with carboplatin and paclitaxel therapy
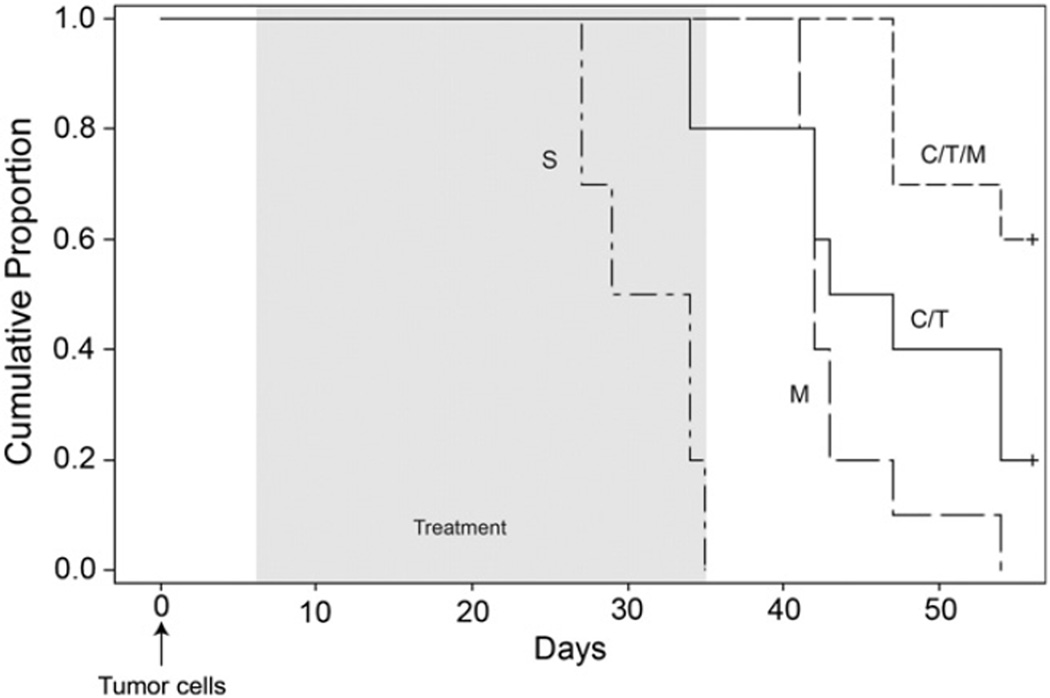
Because the in vitro studies showed that the combination of Minnelide and standard chemotherapeutic agents was effective, we injected 5 × 106 C200 platinum resistant cells intraperitoneally into 6 week old athymic female mice and tumors were allowed to establish for 7 days. Following this mice were randomized to one of 4 groups; control (saline), Minnelide (0.6 mg/kg), carboplatin and paclitaxel (20 mg/kg and 10 mg/kg respectively, twice a week) and Minnelide plus carboplatin and paclitaxel (0.6 mg/kg Minnelide daily, carboplatin 20 mg/kg twice a week and paclitaxel 10 mg/kg twice a week). All mice received treatment for 28 consecutive days. OS was significantly improved in mice treated with Minnelide in combination with carboplatin and paclitaxel (p < 0.0001; Fig. 5). Specifically, median OS for the control group was 31.5 days (95% CI: 27–34), for the Minnelide treated group it was 42 days (95% CI: 41–43), for the carboplatin and paclitaxel group it was 45 days (95% CI: 34–54) and for the Minnelide plus carboplatin and paclitaxel group it was 70 days (95% CI: 47–93).
Fig. 5.
Minnelide in combination with carboplatin and Taxol improves inhibition of tumor growth in vivo. Animals were randomized into 10 per group and then treated with either saline (S), Minnelide (M — 0.6 mg/kg, daily), carboplatin and Taxol (C/T, 20 mg/kg and 10 mg/kg respectively, twice a week) or Minnelide plus carboplatin and Taxol (0.6 mg/kg daily and 20 mg/kg and 10 mg/kg twice weekly) for 28 days. Kaplan–Meier survival curve for overall survival of C200 tumor bearing mice. Overall survival for control of 31.5 days (95% CI: 27–34), Minnelide of 42 days (95% CI: 41–43), carboplatin and paclitaxel of 45 days (95% CI: 34–54) and Minnelide plus carboplatin and paclitaxel of 70 days (95% CI: 47–93; p < 0.0001). An arrow shows the time of tumor cell injection (intraperitoneal) and the shaded area represents the treatment period.
Combination treatment increases apoptosis and decreases proliferation
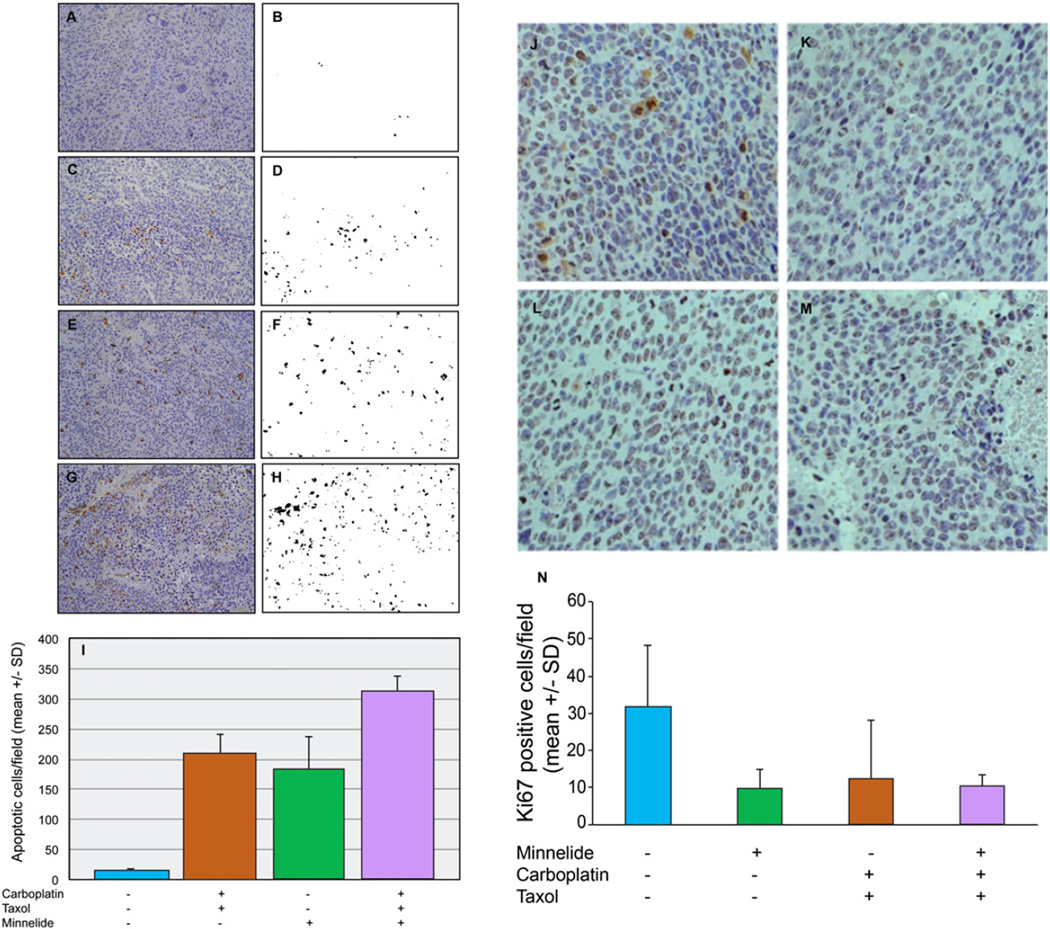
Representative binearized images of Caspase-3 positive cells are shown side by side with original photomicrograph in Fig. 6A–H. Control tumors showed an average of 13 apoptotic cells per field (SD 7.0). Minnelide treated tumors showed an average of 183 Caspase-3 positive cells per field (SD 93.7). Minnelide treatment therefore increased the apoptotic cells by about 14-fold when compared to control tumors. Carboplatin plus paclitaxel treatment showed a similar increase in apoptotic cells. Carboplatin and paclitaxel treated tumors showed a 16-fold (208 apoptotic cells/field, SD 57.6) increase in apoptosis. The combination of carboplatin and paclitaxel with Minnelide treatment resulted in an additive increase in apoptosis. Combination treatment group showed an average of 308 Caspase-3 positive cells per field (SD 44.5). There was a 23-fold increase in apoptosis when compared to control tumors. These studies suggest that the addition of Minnelide to standard chemotherapy with carboplatin and paclitaxel increases apoptosis in ovarian tumors which could account for the improvement seen in the survival of animals treated with the three drug combination.
Fig. 6.
Combination treatment increases apoptosis in orthotopic tumors. Residual tumors obtained from mice at the end of treatment were fixed in formalin and sectioned. Caspase-3 staining was used to identify apoptotic cells. Panels A,C,E and G show representative tumor sections stained for Caspase-3 on a Hematoxylin and Eosin stained slide (brown color). A = saline; C = Minnelide; E = carboplatin and Taxol; G = combination treatment with Minnelide + carboplatin + Taxol. Caspase-3 positive cells were quantified by morphometric analysis. Cells with brown color were filtered by thresholding and the images were binearized (panels B,D,F,H). Reindeer morphometric analysis was carried out as per a previously published method [18]. The histogram shows the average number of apoptotic cells per field from each treatment group. Three sections from different depths of tumor tissues were used for analysis. Error bars represent SD. Representative images of Ki-67 stained tumor tissues are show in panels J–M. J = control, K = Minnelide, L = carboplatin and paclitaxel and M = combination of Minnelide, carboplatin and paclitaxel. Panel N shows a histogram of Ki-67 staining per high power field. The histogram shows the average number of Ki-67 positive cells per high power field from each treatment group. Four sections from different depths of tumor tissues were used for analysis. Error bars represent SD.
Photomicrographs of Ki-67 positive cells are shown in Fig. 6J–N. Control tumors showed an average of 31.7 Ki-67 positive cells/hpf (SD 16.5). Minnelide treated tumors showed an average of 9.75 Ki-67 positive cells/hpf (SD 4.99, p = 0.048). Carboplatin and paclitaxel treated tumors showed an average of 12.25 Ki-67 positive cells/hpf (SD 15.9, p = 0.048). The combination of carboplatin, paclitaxel and Minnelide treated tumors showed an average of 10.0 Ki-67 positive cells/hpf (SD 3.46, p = 0.023). These studies suggest that Minnelide treatment leads to decreased proliferation in treated tumors.
Discussion
Previous studies have shown that Minnelide, a water soluble analog of triptolide, is effective in pancreatic, gastric and osteosarcoma [9,10,3, 5,13]. We have found that Minnelide is an active agent against ovarian cancer both in vitro and in vivo.
Chugh et al. have shown Minnelide to be highly active and even curative for early pancreatic tumors [6]. Others have shown that Minnelide exhibits anti-proliferative effects in non-small cell lung cancer by promoting apoptosis in vitro and in vivo [10]. Further, Minnelide has been shown to reduce tumor burden and metastasis in an orthotopic model of osteosarcoma [9]. While our results are less promising than those found in pancreatic cancer, they remain encouraging. The difference in the tumor responses (complete in the pancreas versus partial in the ovary) is likely due to differences in tumor biology and genetics and is an area of further research. Ductal pancreatic cancers are driven by oncogenic KRAS, EGFR signaling and P53 mutations [14]. More than 52% of pancreatic cancers are positive for oncogenic KRAS, whereas only 15% of Type I ovarian cancers (low grade serous and mucinous) have mutated KRAS [15]. A2780 and C200 cells are derived from high-grade serous adenocarcinoma (type II tumors), in which the majority are associated with p53 mutation but infrequently with oncogenic KRAS. Perhaps, KRAS mutation is necessary for Minnelide sensitivity in vivo. Other reasons that Minnelide may be less effective in the ovarian cancer model include the method of drug uptake into ovarian tumors, how the drug is metabolized, as well as the pro-drug to drug conversion. Ongoing studies are investigating these pathways to determine Minnelide's method of action. We used only serous cell lines for our studies, therefore further study is ongoing to determine the responses of non-serous histological subtypes of ovarian cancers to Minnelide.
Although only a partial response is observed with Minnelide alone, it appears effective when used with standard chemotherapy. There are previous reports of triptolide, the parent compound of Minnelide, enhancing the cytotoxic activity of carboplatin both in vitro and in vivo [16]. In our current study we are able to show that Minnelide can enhance the effects of standard chemotherapy with carboplatin and paclitaxel and that Minnelide induces apoptosis in an orthotopic model of ovarian cancer.
The mechanism by which Minnelide is able to induce apoptosis and improve the sensitivity to standard chemotherapy agents is largely unknown and was not directly addressed in the current study. There appear to be several mechanisms of action by which triptolide/Minnelide induce cell death. Studies have shown that triptolide leads to cell death via inhibition of heat shock protein 70 (hsp70) [4]. The exact mechanism by which HSP70 is downregulated has been shown to be upregulation of miR142-3p leading to inhibited cell proliferation and apoptosis. It was also shown that Minnelide induced the expression of miR142-3p in vivo [16]. Others have shown that Minnelide downregulates levels of pro-survival proteins including HSP, cMYC and the NFκB pathway [9, 17–19]. Several studies have shown that TNF-related apoptosis-inducing ligand (TRAIL) induced apoptosis is another possible mechanism of action for triptolide/Minnelide [18,20]. Given these varying effects of triptolide/Minnelide it is likely that there are several mechanisms working in conjunction to inhibit cell proliferation.
Our studies show that Minnelide effectively inhibited proliferation of both platinum sensitive and resistant ovarian cancer cell lines. Minnelide treatment prolonged the survival of mice bearing established ovarian cancers. When combined with standard chemotherapy, carboplatin and paclitaxel, Minnelide further increased survival. Our findings suggest that further pre-clinical evaluation of Minnelide is needed to determine the optimal setting for this drug in the treatment of ovarian cancer. Given the positive results found in the pancreatic literature, Minnelide is currently being tested in a Phase I trial. With the promising response observed in pre-clinical studies of ovarian cancer, clinical investigation of this drug is warranted.
HIGHLIGHTS.
Minnelide is an effective agent against epithelial ovarian cancer in preclinical models.
Given the promising in vitro and in vivo results, Minnelide should progress to further studies in epithelial ovarian cancer.
Footnotes
The research reported in this publication was supported by the NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. This research was also supported in part by the Minnesota Ovarian Cancer Alliance (MOCA).
Conflict of interest statement
Dr. Saluja is the only author with a conflict of interest in that he has Minneamrita, stock options and acts as a consultant for Minnelide.
References
- 1.National Cancer Institute SEER Program. http://seer.cancer.gov/statfacts/html/ovary.html.
- 2.National Comprehensive Cancer Network Treatment Guidelines. http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. [Google Scholar]
- 3.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 4.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 5.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–290. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, et al. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4(156ra139):1–10. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stella V, Zygmunt JJ, George GI, Safadi MS. Water-soluble prodrugs of hindered alcohols. 6,451,776 B2. US Patent. 2002 Sep 17;
- 8.Krebs HA. Chemical composition of blood plasma and serum. Annu Rev Biochem. 1950;19:409–430. doi: 10.1146/annurev.bi.19.070150.002205. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Thayanithy V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett. 2013;335:412–420. doi: 10.1016/j.canlet.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousalova I, Banerjee S, Sangwan V, Evenson K, McCauley JA, Kratzke R, et al. Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PloS One. 2013;8(10):1–14. doi: 10.1371/journal.pone.0077411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing Y, Lu H, Wu K, Subramanian IV, Ramakrishnan S. Inhibition of ovarian cancer by RGD-P125A-endostatin-Fc fusion proteins. Int J Cancer. 2011;129:751–761. doi: 10.1002/ijc.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild R, Ramakrishnan S, Sedgewick J, Griffioen AW. Quantitative assessment of angiogenesis and tumor vessel architecture by computer assisted digital image analysis: effects of VEGF-toxin conjugate on tumor microvessel density. Microvasc Res. 2000;59(3):368–376. doi: 10.1006/mvre.1999.2233. [DOI] [PubMed] [Google Scholar]
- 13.Clawson KA, Borja-Cacho D, Antonoff MB, Saluja AK, Vickers SM. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–249. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22(3):304–327. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auner V, Kriegshauser G, Tong D, Horvat R, Reinthaller A, Mustea A, et al. KRAS mutation analysis in ovarian cancer samples using high sensitivity biochip assay. BMC Cancer. 2009;9(111):1–8. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westfall SD, Nilsson EE, Skinner MK. Role of triptolide as an adjunct chemotherapy for ovarian cancer. Chemotherapy. 2008;54:67–76. doi: 10.1159/000112419. [DOI] [PubMed] [Google Scholar]
- 17.Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor kappaB activation in gastric cancer. Oncogene. 2001;20:8009–8018. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- 18.Lee KT, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis inducing ligand (TRAIL)-induced apoptosis by inhibition of NFkappaB activation. Exp Mol Med. 2002;34:462–468. doi: 10.1038/emm.2002.64. [DOI] [PubMed] [Google Scholar]
- 19.Qui D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Sangwan V, Banerjee S, Chugh R, Dudeja V, Vickers SM, et al. Triptolide sensitizes pancreatic cancer cells to the TRAIL-induced activation of the death receptor pathway. Cancer Lett. 2014;21:S0304–S3835. doi: 10.1016/j.canlet.2014.03.016. http://dx.doi.org/10.1016/j.canlet.2014.03.016 [(14)00161-X, epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]