Abstract
Particle engineering strategies remain at the forefront of aerosol research for localized treatment of lung diseases and represent an alternative for systemic drug therapy. With the hastily growing popularity and complexity of inhalation therapy, there is a rising demand for tailor-made inhalable drug particles capable of affording the most proficient delivery to the lungs and the most advantageous therapeutic outcomes. To address this formulation demand, nanoparticle agglomeration was used to develop aerosols of the asthma therapeutics, fluticasone or albuterol. In addition, a combination aerosol was formed by drying agglomerates of fluticasone nanoparticles in the presence of albuterol in solution. Powders of the single drug nanoparticle agglomerates or of the combined therapeutics possessed desirable aerodynamic properties for inhalation. Powders were efficiently aerosolized (~75% deposition determined by cascade impaction) with high fine particle fraction and rapid dissolution. Nanoparticle agglomeration offers a unique approach to obtain high performance aerosols from combinations of asthma therapeutics.
Keywords: Fluticasone, albuterol, combination therapy, dry powder, aerosols
1. Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are currently treated using either nebulizers, pressurized metered dose inhalers or dry powder inhalers (Dalby and Suman, 2003; Murnane et al., 2008b; Yang et al., 2008a). A major determinant of aerosol deposition in the respiratory tract is the aerodynamic size of particles and the polydispersity (Louey et al., 2004; Pilcer and Amighi, 2010; Pritchard, 2001). Inhaled drugs should ideally possess an aerodynamic diameter less than 5 µm for delivery into the ‘deep’ lung for local therapy or systemic absorption (Weers et al., 2010). Nanoparticles (<0.5 µm) are more likely to be exhaled, which may lead to dose variability (Shi et al., 2007). If delivered as a suspension, such small particles are also prone to particle growth due to Ostwald ripening and can suffer from uncontrolled agglomeration (Berkland, 2010). A major obstacle to inhaled therapeutics is the inability to efficiently deliver large quantities of a drug to the deep lung (Gillian, 2010).
Natural aerosols, in particular, spores from molds and fungi as well as soot and asbestos, have a size and structure that allows them to aerosolize efficiently into the lungs. They are composed of underlying nanostructures that join together to form microparticles. Following this rationale, nanoparticle agglomerates were designed by formulating nanometer-sized drug particles, then assembling them to micron-sized clusters with the desired aerodynamic diameter (e.g., 1 µm for treating distal airways or 3–5 µ m for treating upper airways) (Bailey et al., 2008; El Gendy et al., 2009; Plumley et al., 2009). By agglomerating nanoparticles under controlled process conditions, nanoparticle agglomerate dry powders can be tailored to the desired physical and chemical characteristics for aerosol delivery and dissolution (Aillon et al., 2010; El-Gendy and Berkland, 2009).
Asthma is a disease that is commonly treated with two types of aerosolized agents; bronchodilators (β2 agonists) and anti-inflammatory agents (steroidal compounds). Apart from acute asthma attacks, which are primarily treated with short acting β2 agonists, there is a strong need for chronic therapy to reduce inflammation and to avoid asthma exacerbations (Barnes, 2002). Therapeutic interventions using combinations of a β2 agonist and a glucocorticoid have emerged as an effective asthma management strategy to control persistent asthma (Rajeswari et al., 2006). The use of β2 agonists to prevent bronchial spasm and glucocorticoids to decrease inflammation is widely accepted (Westmeier and Steckel, 2008). Combination formulations have also been suggested to be more effective than a single drug due to synergistic effects in the same target cell in the lung epithelia. It appears rational, therefore, to combine both substances in one particle instead of formulating a combination product containing both drugs in a physical mixture (Adi et al., 2008; Nelson et al., 2003; Papi et al., 2007).
Combination products such as Advair and Symbicort are currently marketed. Advair combines fluticasone propionate and salmeterol xinafoate into one inhaler (Michael et al., 2000). Salmeterol (long acting β2 agonists) does not replace the need for rescue inhalers, such as albuterol, which are still necessary for immediate relief of asthma symptoms (Kamin et al., 2007; Salpeter et al., 2006). Symbicort is another combination product containing budesonide and formoterol. Fluticasone propionate is a synthetic corticosteroid used to treat asthma, allergic rhinitis (hay fever) and eosinophilic esophagitis (Murnane et al., 2008a; Rehman et al., 2004; Vatanara et al., 2009). Albuterol sulfate is a short-acting β2 adrenoreceptor agonist used for the relief of bronchospasm in conditions such as asthma and COPD, and is currently one of the most prescribed bronchodilators for the treatment of bronchial asthma (Ahmad et al., 2009; Xu et al., 2010).
Development of dry powder aerosols for delivering fluticasone and/or albuterol nanoparticle agglomerates as single anti-asthmatic therapies or in combination to achieve synergistic effect is herein described. The study illustrates the formulation of fluticasone nanoparticles using potentially acceptable surfactants that control the size and surface charge of the prepared nanoparticles. Also, albuterol nanoparticles free of excipients were engineered using different techniques. The nanoparticle suspensions were destabilized via ionic charge interactions using l-leucine. Combination drug formulations were prepared by adding albuterol aqueous solution to the fluticasone nanoparticle suspension followed by addition of l-leucine. The aerosol performance of these nanoparticles agglomerate formulations were fully characterized and compared to micronized stock drug.
2. Materials and methods
2.1. Materials
Fluticasone propionate (Flu) and albuterol sulfate (Albu) were generously provided by 3M. L-α-phosphatidylcholine (lecithin; Lec), cetyl alcohol (CA), l-leucine (Leu) and polyvinylpyrrolidone K90 (PVP) were purchased from Sigma Chemical Co., USA. Pluronic F-127 (PL, Mw ~12,220) was purchased from BASF, USA. Ethanol, acetone, potassium dihydrogen phosphate (KH2PO4), disodium hydrogen phosphate (Na2HPO4) and sodium chloride (NaCl) were purchased through Fisher Scientific, USA. Floatable dialysis membrane units (MWCO=10 kDa) were obtained from Spectrum Laboratories Inc., USA. Amicon Ultra Centrifugal filter units (MWCO=5 kDa) used for dissolution were purchased from Millipore, Co (Billerica, MA). Double-distilled water was used throughout the study, provided by an EASYpure® RODI (Barnstead International, USA).
2.2. Nanoparticle formulation
2.2.1. Preparation of fluticasone nanoparticle suspension
Nanoparticle suspensions of fluticasone propionate were prepared using antisolvent precipitation. Solutions of the drug in organic solvent (acetone or ethanol) were prepared at different concentrations and directly injected into water at a rate of 2.5 mL/min. A variety of solvent/ non-solvent ratios were precipitated under ultrasonication (probe-type sonicator, Fisher Scientific, Sonic Dismembrator) operating with an amplitude of 48% in an ice bath or under homogenization (probe-type homogenizer, Tissue tearor, Biospec Products, Inc.). Hydrophobic surfactants (cetyl alcohol and lecithin) were added to the drug solution while hydrophilic surfactants (PL F127, PVA and PVP K90) were dissolved in the aqueous phase.
2.2.2. Formulation of combination therapy
The combined formulation was prepared by adding albuterol sulfate dissolved in water to the precipitated fluticasone propionate nanosuspension during homogenization at 25,000 rpm. The two drugs were combined, at a ratio of 2:1 w/w, fluticasone propionate: albuterol sulfate (Papi et al., 2007; Westmeier and Steckel, 2008).
2.2.3. Fabrication of albuterol nanoparticle suspension
Albuterol sulfate nanoparticles were prepared by precipitation or by a top-down (attrition) method. Concerning the precipitation technique, solutions of albuterol in water were prepared and directly injected into ethanol or acetone at a rate of 2.5 mL/min. Various solvent/ non-solvent ratios were used under ultrasonication operating with an amplitude of 48% or under homogenization. In the top-down method, albuterol nanoparticles were prepared by ultrasonicating or homogenizing a suspension of albuterol in acetone or ethanol. The concentration of the drug in the anti-solvent was varied between 0.2 and 1 mg/mL. The ultrasonication or homogenization time was also varied.
2.3. Characterization of nanoparticle suspensions
The average size and polydispersities of the nanoparticle suspensions were determined by dynamic light scattering (Brookhaven, ZetaPALS, SA). The same instrument was used to determine the zeta potential of the nanoparticles in 1 mM potassium chloride solution. Three runs of 15 cycles were acquired, and the mean zeta potential was recorded. Measurements were taken at an angle of 90° to the incident light source. Some samples were frozen at −80 °C and lyophilized (FreeZone 1) for ~36 h at a temperature of −50 °C under vacuum (~0.02 millibar). Lyophilized powder was stored at room temperature for further characterization.
2.4. Agglomeration of nanoparticle suspensions
Nanoparticles were agglomerated via addition of an agglomerating agent. l-Leucine solution in water (2.5 mg/mL) was slowly injected into nanoparticle colloids during homogenization at 25,000 rpm for 30 s. The amount of l-leucine added was adjusted to a fluticasone: L-leucine ratio equal to 1:1 for agglomerating the fluticasone suspension and the combination suspension. An albuterol: l-leucine ratio of 1:1.5 was used for agglomerating the albuterol suspension.
The agglomerated suspensions were incubated with the agglomerating agent for three hours. Then, the size of the prepared nanoparticle agglomerates was measured in Isoton diluent using a Coulter Multisizer 3 (Beckman Coulter Inc.) equipped with a 100 µm aperture. The suspensions were kept overnight at room temperature to allow evaporation of organic solvent and then frozen at −80 °C. The frozen suspensions were transferred to the freeze dryer where drying lasted for ~3 days. Lyophilized powder was stored at room temperature for further characterization.
2.5. Particle size and morphology by transmission electron microscopy (TEM)
Lyophilized powders were resuspended in Isotonic solution and the particle size and size distribution was detected using a Coulter Multisizer 3. In addition, the size and morphology of the lyophilized nanoparticles and nanoparticle agglomerate powders were evaluated using JEOL 1200 EXII transmission electron microscope. Prior to imaging, carbon-coated grids (Electron Microscopy Sciences) were placed on a droplet of the suspensions on a glass microscope slide to permit the adsorption of the particles onto the grid. After this, the grid was blotted with a filter paper and air dried for 1 h.
2.6. Powder flow characteristics
Nanoparticle agglomerate dry powders were poured through a glass funnel from a height of 4 cm onto a level bench top. The angle that the side of the conical heap made with the horizontal plane was recorded as the angle of repose (tan θ = height / radius). In addition, bulk and tapped densities were determined. Then, the Hausner ratio (tapped density / bulk density) and Carr’s compressibility index (Ci) [(tapped density – bulk density)/ tapped density X 100%] were calculated (Kumar et al., 2001; Louey et al., 2004).
2.7. Evaluation of Aerosol performance of nanoparticle agglomerate dry powders
2.7.1. Measurement of theoretical mass mean aerodynamic diameter
The geometric particle size and tap density measurements were used for calculating the theoretical mass mean aerodynamic diameter (dae) of the nanoparticle agglomerates (El-Gendy et al., 2010b; Fiegel et al., 2008).
2.7.2. Aerodynamic size distribution by time-of-flight analysis
The aerodynamic diameter and size distributions of the nanoparticle agglomerate powders were determined by time-of-flight measurement (TOF) using an Aerosizer LD (Amherst Instruments, Hadely, MA, USA) equipped with a 700 µm aperture operating at 6 psi. For these studies, ~1 mg of the powder was added to the instrument disperser and data were collected for ~60 s under high shear force (~3.4 kPa). The instrument size limits were 0.10–200 µm and particle counts were above 100,000 for all measurements.
2.7.3. In vitro aerosol deposition of nanoparticle agglomerates by cascade impactor
An eight-stage Mark II Andersen Cascade Impactor (ACI, Tisch Environmental, Inc.) had stages with particle aerodynamic diameter specifications at a flow rate of 28.3 L/min as follows: pre-separator (10.00 µm), stage 0 (9.00 µm), stage 1 (5.80 µm), stage 2 (4.70 µm), stage 3 (3.30 µm), stage 4 (2.10 µm), stage 5 (1.10 µm), stage 6 (0.70 µm), stage 7 (0.40 µm) and the final filter (< 0.40 µm). Aerodynamic behavior of nanoparticle agglomerate dry powders was assessed using the ACI and compared with that of the two drugs as received.
The powder was delivered into the cascade impactor by placing capsules (gelatin type, size 3, generously provided from Capsugel®, NJ, USA) containing 5 ± 0.5 mg of powder into a Plastiape Monodose Inhaler RS01 Model 7. The capsule was punctured and the powder was drawn through the cascade impactor which was operated at a flow rate of 28.3 L/min for 4 s. Dry powder aerosols deposited on each of the nine stages of the impactor were quantified by HPLC. After actuation, the device, capsule, adapter, throat, all plates, stages and filter were washed into separate volumetric flasks using ethanol (for fluticasone alone or Flu/Alu combination) or phosphate buffered saline (pH 7.4 for albuterol). Appropriate sample dilutions were made prior to testing by HPLC. Each sample was tested in triplicate.
Concerning the combination formula, the powder deposited on stages was suspended in ethanol and was ultrasonicated in a bath-type sonicator (Branson 3510) for 30 min. Then, the solution was centrifuged (Beckman, Avanti ) at ~15,000 rpm for 30 min and the amount of fluticasone in the supernatant was determined using a reversed-phase HPLC method. As albuterol has a very slightly solubility in ethanol, the drug content in both supernatant and precipitate were detected by HPLC.
All ACI experiments were performed under controlled conditions (21 ± 2 °C, 50 ± 5% RH) in triplicate. The emitted dose (ED) was defined as the mass of drug delivered from the inhaler (i.e., total amount excluding the inhaler device and capsule) (Xu et al., 2010). The emitted fraction was determined as the percent of the emitted dose divided by the initial mass delivered into the impactor (Lechuga-Ballesteros et al., 2008; Shur et al., 2008; Yang et al., 2008b).
The fine particle fraction of the total dose (FPFTD) was calculated as the percentage of aerosolized particles that reached the lower seven stages of the impactor (corresponding to aerodynamic diameters below 5 µm; stage 2-filter), or the lower five stages (corresponding to aerodynamic diameters below 3 µm; stage 4-filter) (El-Gendy et al., 2010a).
The fine particle fraction of the emitted dose (FPFED) was determined from the cumulative mass distribution curve at 5 µm and 3 µm and was calculated as a function of the emitted dose. Additionally, mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were determined from the cumulative mass distribution curve (Pham and Wiedmann, 2001; Vanbever et al., 1999; Xu et al., 2010).
2.8. Solid-state characterization
2.8.1. Power X-Ray Diffraction (PXRD)
For analysis of crystallinity, X-ray diffraction analysis was performed using an XGEN-4000 (Scintag, Inc.). The powders were analyzed over the range of 5° to 45° (2θ) at 45.0 kV and 35.0 mA.
2.8.2. Differential scanning calorimetry (DSC)
DSC data of materials as received, nanoparticles and nanoparticle agglomerates were collected using a Q100 DSC from TA Instruments. For thermogram acquisition, sample sizes of 1 to 5 mg were weighed into aluminum hermetic pans. Measurement was carried out under inert conditions (nitrogen flow of 50 mL/min) with a scan rate of 10 °C/min from 25 to 350 °C.
2.8.3. Thermogravimetric analysis (TGA)
TGA was also performed using a Q50 TGA from TA Instruments. A platinum sample pan was loaded with 5 ± 0.5 mg of sample and heated from 25 to 350 °C at a rate of 10 °C/min under dry nitrogen flowing at rate of 40 mL/min. Data analysis was completed using Universal Analysis 2000 (Version 4.3A) software that was provided by TA Instruments.
2.9. Determination of process yield
The weight of dry powder for the prepared nanoparticle agglomerates was measured and the yield was calculated (El Gendy et al., 2009).
2.10. Determination of drug content uniformity of nanoparticle agglomerates
Fluticasone content in the dry powders was assessed by dispersing 1 mg of the lyophilized powder in 10 mL of ethanol. The dispersion was ultrasonicated in a bath-type sonicator for 30 min. Then the solution was centrifuged at ~15,000 rpm for 30 min to remove insoluble ingredients and the amount of fluticasone in the supernatant was determined using a reversed-phase HPLC method. For the combination formula, albuterol content was detected in both the pellet after dissolving in 10 mL PBS and in the supernatant using HPLC. Drug content in albuterol nanoparticle agglomerates was determined by dispersing 1 mg of the lyophilized powder in PBS. The dispersion was ultrasonicated in a bath-type sonicator for 15 min. Then the amount of albuterol was determined using a reversed-phase HPLC method. All experiments were performed in triplicate and drug content was calculated (El Gendy et al., 2009).
2.11. Dissolution studies
The dissolution of the prepared nanoparticles and nanoparticle agglomerates was determined under sink condition and compared with the dissolution of the drugs as received. The dissolution of fluticasone was carried out at 37 ± 0.5 °C in a 1 liter beaker. Lyophilized powder (~10 mg) was dispersed in 10 mL PBS (pH 7.4) and was suspended in a floatable dialysis membrane unit (Mw cut-off = 10 kDa). The unit was allowed to float in 500 mL of PBS and the whole assembly was stirred at a constant speed (100 rpm) using a magnetic stirrer (Barnstead, Thermolyne MIRAK™). At predetermined time intervals for a total period of 8 h, samples (1 mL) of the medium were withdrawn from the dialysis bag and replaced with fresh medium. Then, the samples were centrifuged for 30 min at ~13,000 rpm. The supernatant was removed and the pellet was dissolved in 1 mL of ethanol. Fluticasone content was determined using reversed-phase HPLC. In the case of the combination formula, both pellet and supernatant were analyzed for albuterol using a reversed-phase HPLC method. Studies were conducted in triplicate.
For albuterol dry powders, 5 mg was dispersed in 0.6 mL phosphate buffered saline (PBS, pH 7.4) and placed in a 5 kDa Ultra Centrifugal filter unit which was immersed inside a 10 mL centrifugal tube containing PBS solution to a final volume of 7 mL. All samples were incubated at 37 ± 0.5 °C and shaken at 100 rpm. One mL aliquots were taken at various time points up to 8 hours from the bulk solution and replaced with 1 mL of fresh PBS. The drug concentration was measured using reversed-phase HPLC. All experiments were performed in triplicate.
2.12. Quantitative analysis of the drug concentrations by HPLC
Drug content, dissolution, and ACI concentration on stages were carried out using reversed-phase HPLC methods. A Shimadzu HPLC system including a solvent delivery pump (Shimadzu LC-10AT), a controller (Shimadzu SCL-10A), SIL-10AxL autoinjector, and a SPD-10A UV detector was used in this study. Chromatograms were acquired and analyzed using Shimadzu Class VP 4.3 software. A long Zorbax SB C-18 column (Agilent C; 4.6 mm × 100 mm) with a particle diameter of 3.5 µm was used for separation. During the assay of fluticasone, the drug was eluted isocratically at a mobile phase flow rate of 1.2 mL/min and monitored with a UV detector operating at 238 nm. The mobile phase for the assay consisted of an acetonitrile and water mixture (65:35 v/v) (Asmus et al., 2004; Steckel and Muller, 1998). The run time for the assay was 10 minutes, and the retention time for fluticasone was 3.9 ± 0.2 min. For analyzing albuterol samples, an isocratic system was used with mobile phase of 90:10 v/v phosphate buffer (10 mM, pH3.5): acetonitrile at a flow rate of 0.3 mL/min and detection was performed at 225 nm (Kamin et al., 2007). The run time for the assay was 10 min, and the retention time for albuterol was 3.85 ± 0.4 min.
3. Results and discussion
3.1. Fluticasone nanopaticle suspensions made by precipitation
To obtain small nanoparticles from poorly water soluble fluticasone propionate, a non-solvent precipitation method was employed (Bilati et al., 2005). Selected surfactants were chosen from a list of excipients that may be appropriate for inhalation (Chougule et al., 2007; Pilcer and Amighi, 2010). Different suspensions were produced using water as anti-solvent and a drug concentration of 0.1% or 0.2% w/w dissolved in ethanol or acetone. Fluticasone particles prepared without surfactants were very large with high polydispersity. Mean diameters of fluticasone nanoparticles made with individual surfactants were larger than those with combined surfactants. Particle size tended to increase and colloidal stability was difficult to maintain as fluticasone concentration was increased. Nanoparticles precipitated from acetone were larger than those precipitated from ethanol. Nanoparticle size appeared to decease slightly when using ultrasonication rather than homogenization during the precipitation process (Supplementary Table 1).
The most successful fluticasone nanosuspension was prepared by precipitation from ethanol under ultrasonication (0.1% w/v Flu + 0.01% w/v PVP + 0.005% w/v Lec). The surfactant combination employed yielded a small drug particle size (~400 nm) and low polydispersity (0.132). The charged surface of the nanoparticles (~12 mV) allowed the potential to destabilize this colloid via interaction with an agglomerating agent (Table 1). This formula was chosen for the preparation of the fluticasone nanoparticle agglomerates and for the combination formulation with albuterol sulfate in solution.
Table 1.
Formulation composition and characterization of the selected fluticasone and albuterol nanoparticles (values = average ± S.D.; n =3).
|
Fluticasone nanoparticle composition (% w/w) |
Fluticasone | 1 | |
| Lecithin | 0.05 | ||
| PVP K90 | 0.1 | ||
| Solvent | ethanol | ||
| Process | ultrasonication | ||
|
Fluticasone nanoparticle Characterization |
Nanoparticle size (nm) | 403.8 ± 3 | |
| Polydispersity | 0.3 ± 0.01 | ||
| Zeta-potential (mV) | 12.4 ± 2 | ||
|
Albuterol nanoparticle composition (% w/w) |
Conc. (mg/mL) | 1 | |
| Process | Ha | ||
| non-solvent | acetone | ||
| Time (min.) | 15 | ||
|
Albuterol nanoparticle Characterization |
Nanoparticle size (nm) | 46 ± 2 | |
| Polydispersity | 0.1 ± 0.04 | ||
| Zeta-potential (mV) | 10 ± 1 | ||
H = homogenization process at 25,000 rpm
3.2. Albuterol nanoparticle suspensions made by two approaches
3.2.1. Production of nanoparticles by precipitation
Albuterol nanoparticles were first prepared by precipitation. A smaller nanoparticle size was produced when using acetone as non-solvent as opposed to ethanol. A decrease in particle diameter followed from a decrease of the drug concentration. The smallest nanoparticle size was obtained at a 2.5/25 water/acetone ratio at a drug concentration of 0.1 % w/v; however, the polydispersity was high with low yields for all precipitation trials (Supplementary Table 2).
3.2.2. Production of nanoparticles by attrition
Nanoparticles were also produced by fragmenting micronized drug particles using homogenization or ultrasonication. The homogenizer was superior to ultrasonication in the preparation of albuterol nanoparticles causing a significant decrease in size with low polydispersity (Supplementary Table 3). With these considerations in mind, the A7 nanoparticle formulation was selected which was prepared by homogenizing a suspension of the drug in acetone in a concentration of 1 mg/mL for 15 min (Table 1).
3.3. Agglomeration of the formulated nanoparticles
Colloidal suspensions of fluticasone nanoparticles, albuterol nanoparticles (A7) and fluticasone nanoparticles combined with albuterol in solution were destabilized using l-leucine to disrupt the electrostatic repulsion between particles (Young et al., 2002). The resulting nanoparticle agglomerates had a geometric size of ~3–5 µm (Table 2). After drying, powders showed a slightly broader size distribution (Fig. 1). In addition, the combination powders had a wider distribution compared to the single-drug formulations, perhaps due to large albuterol particles formed during freeze drying.
Table 2.
Particle size characteristics of the nanoparticle agglomerates (values = average ± S.D., n = 3).
| Characteristics | Formulations | ||
|---|---|---|---|
| Fluticasone NAa |
Albuterol NAb |
Flu/Albu combination c |
|
| Geometric particle size (µm) of NCe before lyophilization |
3.8 ± 1.1 | 3.9 ± 1.3 | 4.1 ± 1.5 |
| Geometric particle size (µm) of lyophilized NCd |
4.8 ± 1.0 | 5.4 ± 2.0 | 5 ± 0.9 |
| MADAe of lyophilized NCd | 2.1 ± 0.1 | 3.2 ± 0.1 | 1.9 ± 0.02 |
| MMADtf of lyophilized NCd | 0.98 ± 0.1 | 1.1 ± 0.01 | 0.8 ± 0.05 |
Fluticasone NC = 1: 0.05: 0.1: 1; Flu: Lec: PVP K90: Leu
Albuterol NC = 1: 1.5; Albu: Leu
Flu/Albu combination = 2: 1; Flu: Albu
NA = Nanoparticle agglomerates.
MADA = Median aerodynamic diameter obtained from Aerosizer.
MMADt = Theoretical mass mean aerodynamic diameter calculated from density measurements.
Fig. 1.
The particle size distributions of A) fluticasone nanoparticle agglomerates, B) Flu/Albu combination and C) albuterol nanoparticle agglomerates in suspension after agglomeration and resuspended after lyophilization.
The particle size of the nanoparticles and nanoparticle agglomerates was congruent with the structures observed in TEM micrographs. Fluticasone nanoparticles were slightly elongated with smooth surfaces and a particle size of ~400 nm (Fig. 2A). Nanoparticle agglomerates appeared as elongated nanoparticles that were agglomerated together into micron-sized clusters with a somewhat porous structure (Fig. 2B). The combination powders of fluticasone nanoparticles dried with albuterol in solution generally exhibited slightly larger particles with a rough surface. Presumably, albuterol in solution deposited on the rod-shaped fluticasone particles during drying (Fig. 2C). Small albuterol nanoparticles with a particle diameter less than 100 nm were (Fig. 3A) [/agglomerated into micron-sized particles (Fig. 3B).
Fig. 2.
Transmission electron micrographs of A) fluticasone nanoparticles B) fluticasone nanoparticle agglomerates and C) Flu/Albu combination.
Fig. 3.
Transmission electron micrographs of albuterol sulfate A) nanoparticles and B) nanoparticle agglomerates.
Powder properties for micronized drugs as received and nanoparticle agglomerates were also studied. Flowability and density characterization helped elucidate any differences in bulk powder properties (Table 3). Flowability indices were calculated from density differences and the angle of repose. The micronized drugs showed a larger angle of repose, greater tap density and higher values of the Hausner ratio and Carr’s index compared to the nanoparticle agglomerates (Fig. 4). This was probably the result of a reduction of cohesive forces in nanoparticle agglomerates compared to drug powders as received. l-Leucine may have also reduced surface energy in nanoparticle agglomerate dry powders (Shur et al., 2008).
Table 3.
Flowability characteristics of the nanoparticle agglomerates (values = average ± S.D., n = 3).
| Formula No. | Angle of repose (θ) |
Bulk density (g/cm3) |
Tapped density (g/cm3) |
Carr’s index (Ci %) |
Hausner ratio |
|---|---|---|---|---|---|
| Fluticasone as received |
32 ± 1 | 0.2 ± 0.1 | 0.3 ± 0.01 | 33 ± 0.03 | 1.5 ± 0.04 |
| Albuterol as received |
31 ± 0.4 | 0.3 ± 0.1 | 0.45 ± 0.02 | 33 ± 0.1 | 1.5 ± 0.1 |
| Fluticasone NAa |
24 ± 1 | 0.05 ± 0.02 | 0.06 ± 0.01 | 17 ± 0.1 | 1.2 ± 0.2 |
| Albuterol NAb | 23 ± 1 | 0.03 ± 0.03 | 0.04 ± 0.01 | 23 ± 0.1 | 1.3 ± 0.1 |
| Flu/Albu combination c |
29 ± 2 | 0.04 ± 0.1 | 0.05 ± 0.01 | 20 ± 0.04 | 1.3 ± 0.1 |
Fluticasone NA = 1: 0.05: 0.1: 1; Flu: Lec: PVP K90: Leu
Albuterol NA = 1: 1.5; Albu: Leu
Flu/Albu combination = 2: 1; Flu: Albu
Fig. 4.
Comparison in the density for the same mass between A) fluticasone powder as received, B) fluticasone nanoparticle agglomerates, C) Flu/Albu combination, D) albuterol nanoparticle agglomerates and E) albuterol powder as received.
3.4. Nanoparticle agglomerates yielded desirable aerosol characteristics
Theoretical mass mean aerodynamic diameters (daero) of the prepared nanoparticle agglomerates were calculated from the geometric particle size and tap density. The calculated daero (0.8–1.1 µm) was appropriate for increasing the probability of aerosol deposition in the alveolar region of the lungs. Aerosizer LD time-of-fight (TOF) measurements of nanoparticle agglomerate dry powders also showed particles in the respirable size range (2 – 3.2 µm) with relatively narrow size distribution (Table 2 and Fig. 5). The MAD value of the combination formula obtained by Aerosizer appeared to be close to that of the pure fluticasone nanoparticle agglomerates.
Fig. 5.
Aerodynamic size distributions of A) fluticasone nanoparticle agglomerates, B) Flut/Albu combination and C) albuterol nanoparticle agglomerates after lyophilization determined by time-of-flight analysis.
Cascade impactor analysis is the standard technique for in vitro characterization of dry powder aerosols. Fluticasone nanoparticle agglomerates mainly deposited on stages 3 and 4, while albuterol nanoparticle agglomerates favored deposition on stages 4 and 5 of the impactor. The combination formula exhibited size distribution similar to fluticasone nanoparticle agglomerates with some additional powder deposition on upper stages. This may be explained by the fact that once fluticasone nanoparticle agglomerates were dried in the presence of albuterol in solution, forces such as van Der Waals are sufficient to hold all particles together. This may suggest that fluticasone nanoparticle agglomerates were indeed a ‘carrier’ for much of the albuterol. Albuterol in the combination showed a different deposition profile than the fluticasone, although the albuterol deposition was improved compared to the albuterol as received. This may be due to the segregation of some larger albuterol particles from the fluticasone nanoparticle agglomerates.
Conversely, drug powders as received largely deposited on the mouthpiece, throat and the upper stages (Fig. 6). A larger percentage of fluticasone (27%) and albuterol (28%) powder as received remained in the capsule shell and device when compared to the nanoparticle agglomerates (9% for Flu NA, 11% for Albu NA and 18% for the combined formulation). This reflected the efficient aerosolization and high fine particle fraction of the nanoparticle agglomerates. In addition, the irregular shape of the nanoparticle agglomerates may decrease their contact area with device surfaces, thus resulting in superior dispersion properties.
Fig. 6.
The distribution of fluticasone nanoparticle agglomerates, Flu/Albu combination and albuterol nanoparticle agglomerates deposited on the stages of a cascade impactor at a flow rate of 28.3 L/min using a Monodose inhaler and compared to the drugs as received.
The aerosol performance was described according to emitted fraction (EF), emitted dose (ED), fine particle fraction (FPF), and mass median aerodynamic diameter (MMAD) (Table 4). The high EF (~75–90%) and ED of nanoparticle agglomerate dry powders obtained at the tested flow rate suggested efficient aerosolization of the nanoparticle agglomerates as compared to the micronized drugs as received. All prepared formulas offered high fine particle fraction with anticipated total lung deposition (FPFTD <5 µm) of about 74–88% and deep lung deposition (FPFTD<3 µm) of ~55–62%. No significant change in FPF of fluticasone was observed after combination with albuterol in solution, suggesting the fluticasone nanoparticle agglomerates were indeed a carrier for albuterol (Table 4). Analysis of the drug content on each stage indicated that some larger albuterol particles were segregated from the fluticasone nanoparticle agglomerates (Fig. 7). The drug powders as received gave significantly lower FPF compared to all prepared nanoparticle agglomerates.
Table 4.
Cascade impaction results of lyophilized nanoparticle agglomerates chemical analysis (values = average ± S.D., n = 3).
| Characteristics of the lyophilized powder |
Flu NAa | Albu NAb | Fluticasone in Flu/Albu combination c |
Albuterol in Flu/Albu combination |
Flu as received |
Albu as received |
|
|---|---|---|---|---|---|---|---|
| % EFd | 92 ± 10 | 89 ± 7 | 90 ± 12 | 86 ± 8 | 73 ± 12 | 72 ± 4 | |
| % FPFe | ≤ 5 | 88± 3 | 85 ± 5 | 84 ± 7 | 70 ± 3 | 55± 2 | 60 ± 5 |
| ≤ 3 | 62 ± 8 | 60 ± 6 | 51 ± 5 | 48 ± 11 | 20 ± 4 | 21 ± 4 | |
| % FPFe EDh |
≤ 5 | 81 ± 6 | 72 ± 4 | 74 ± 6 | 63 ± 2 | 17 ± 3 | 32 ± 7 |
| ≤ 3 | 52 ± 13 | 54 ± 4 | 50 ± 9 | 42 ± 12 | 7.5 ± 4 | 15 ± 2 | |
| MMADf | 2.8 ± 0.2 | 2.1 ± 0.1 | 3 ± 1 | 3.4 ± 0.5 | 5.2 ± 1 | 4.8 ± 0.4 | |
| GSDg | 2.4 ± 0.3 | 2.5 ± 0.1 | 2.2 ± 0.2 | 2.9 ± 1 | 1.9 ± 0.3 | 2.4 ± 0.3 | |
| EDh (mg) | 2.8 ± 1 | 2.9 ± 1 | 3.2 ± 2 | 1.5 ± 0.2 | 2.6 ± 0.4 | 2.2 ± 0.2 | |
Fluticasone NA = 1: 0.05: 0.1: 1; Flu: Lec: PVP K90: Leu
Albuterol NA = 1: 1.5; Albu: Leu
Flu/Albu combination = 2: 1; Flu: Albu
% EF = Percent emitted fraction.
FPF = Fine particle fraction.
MMAD = Mass median aerodynamic diameter obtained from cascade impactor.
GSD = Geometric standard deviation.
ED = Emitted Dose
Fig. 7.
The percent of fluticasone and albuterol in Flu/Albu combination deposited on the stages of a cascade impactor at a flow rate of 28.3 L/min using Monodose inhaler.
The mass median aerodynamic diameter (MMAD) was calculated from ACI data as the 50th percentile of the aerodynamic particle size distribution by mass. MMADs of the nanoparticle agglomerates ranged from 2–3.5 µm (Table 4). These values were very close to the Aerosizer data but slightly higher than the theoretical MMAD calculations as expected. In addition, the geometric standard deviation (GSD) was determined (El-Gendy et al., 2010a; Xu et al., 2010). GSDs for the selected nanoparticle agglomerates ranged between 2.1 and 2.5, suggesting an acceptable range of particle size (Table 4).
Nanoparticle agglomerate formulations reported here offered a highly efficient aerosol in comparison to previous reports. For example, Steckel et al. prepared fluticasone by milling and micronization and evaluated the powder using a multi-stage liquid impinger. The highest fine particle fraction (% FPF <5µm) was achieved when using a FlowCaps® inhaler without lactose (37.5 %) (Steckel et al., 2003). Louey et al. explored multiple methods to generate fluticasone particles and characterized the aerosol using an eight-stage cascade impactor with pre-separator. Low-resistance (Rotahaler) and high-resistance (Inhalator) inhalers were compared. The Inhalator yielded the highest FPF (11.95%) for the jet milled powder blended with lactose (Louey et al., 2004). Very fine aerosols of albuterol are more common in the literature. For example, Chiou et al. and Hu et al. formulated salbutamol sulfate using high gravity controlled precipitation and assessed these powders using an Aerolizer connected to a multi-stage liquid impinger. They achieved similar albuterol performance as was reported here, the best reported FPFloaded and FPFemitted was 76.5% and 83.7%, respectively (Chiou et al., 2007; Hu et al., 2008). Aerosol performance of fluticasone in combination with salmeterol seemed to revert more towards the values reported for fluticasone. Westmeier et al. formulated these actives together by precipitation in a micromixer. The aerodynamic behavior of the particles was assessed using a Next Generation Impactor. Even though a high flow rate of 100 L/min was used, an Aerolizer device still only produced a FPF of 36.4% when the actives were blended with lactose (Westmeier and Steckel, 2008).
3.5. Crystallinity and thermal properties
PXRD was performed to analyze the nanoparticles and nanoparticle agglomerates. The X-ray diffraction patterns showed sharp diffraction peaks suggesting that both fluticasone as received and nanoparticles were crystalline (Fig. 8) (Louey et al., 2004; Murnane et al., 2008a; Yang et al., 2008a). The pattern of fluticasone nanoparticle agglomerates showed features of l-leucine and fluticasone nanoparticles. There was a peak at ~7.5° in the pattern of the drug as received that did not appear in that of the nanoparticles or nanoparticle agglomerates. On the other hand, a peak at 9° was apparent in the patterns of fluticasone nanoparticles and nanoparticle agglomerates, perhaps due to a slight change in the crystal arrangement (Fig. 8). Nanoparticle agglomerates also had diffraction peaks at 12° and 27.5° that differed from the nanoparticle pattern, which suggested some overlap with l-leucine peaks. The intensity of the diffraction peaks of the nanoparticles and nanoparticle agglomerates was low due to the small particle size. The PXRD pattern of combination nanoparticle agglomerates illustrated the features of l-leucine, fluticasone nanoparticles and albuterol as received (Fig. 9). The results indicated that fluticasone in this formulation showed the same crystalline form of fluticasone as received.
Fig. 8.
Powder X-ray diffraction patterns: (a) l-leucine, (b) fluticasone as received, (c) fluticasone nanoparticles, and (d) fluticasone nanoparticle agglomerates.
Fig. 9.
Powder X-ray diffraction patterns: (a) l-leucine, (b) fluticasone as received, (c) fluticasone nanoparticles, (d) albuterol as received, and (e) Flu/Albu combination.
Both albuterol as received and albuterol nanoparticles showed crystalline character (Fig. 10) (Xu et al., 2010). The X-ray diffraction pattern of albuterol nanoparticles was different from that of albuterol as received as a result of the appearance of a peak at ~10° in the nanoparticle sample. This peak disappeared after agglomeration. The albuterol nanoparticle agglomerate pattern had features of l-leucine and albuterol as received indicating that the crystal form of nanoparticle agglomerates was likely the same crystal form as that of albuterol as received (Fig. 10).
Fig. 10.
Powder X-ray diffraction patterns: (a) l-leucine, (b) albuterol as received, (c) albuterol nanoparticles, and (d) albuterol nanoparticle agglomerates.
Fluticasone and albuterol nanoparticles and nanoparticle agglomerates were also analyzed using differential scanning calorimetry (Table 5 and Fig. 11–13). No characteristic melting peak was found in fluticasone as received or the prepared nanoparticles. Micronized fluticasone as received showed an endothermic degradation peak at 282.51 °C while the selected nanoparticles exhibited an exothermic degradation peak at 278.42 °C (Louey et al., 2004; Westmeier and Steckel, 2008). For lecithin, there was a sharp melting endotherm at 168.2 °C. A DSC scan of PVP K90 showed a shallow, broad endothermic peak at 75.29 °C with an enthalpy of −0.835 W/g. l-Leucine powders sublimed at 323.7 °C. The DSC curve of fluticasone nanoparticle agglomerates exhibited an endothermic peak of melting at 210.17 °C with an enthalpy change of −2.447 W/g followed by subsequent degradation of the compound; however, the characteristic peaks of the excipients were not apparent (Fig. 11A). This may be indicative of some re-crystallization of the drug during the agglomeration process or some overlap of excipient peaks.
Table 5.
DSC peak integrations for materials as received as well as nanoparticles and nanoparticle agglomerates.
| Samples | Peak Temp. (°C) |
Enthalpy (W/g) |
|---|---|---|
| Fluticasone powder as received | ------ | ------ |
| Fluticasone NPd | ------ | ------ |
| Fluticasone NAa | 210.17 | −2.447 |
| Albuterol powder as received | 206.49 | −2.209 |
| Albuterol NP | 206.49 | −2.2 |
| Albuterol NA b | 259.22 | −3.126 |
| Flu/Albu combination c | 228.65 | −3.273 |
| Lecithin powder as received | 168.2 | −2.76 |
| PVP K90 powder as received | 75.29 | −0.835 |
| l-leucine powder as received | 323.7 | −6.914 |
Fluticasone NA = 1: 0.05: 0.1: 1; Flu: Lec: PVP K90: Leu
Albuterol NA = 1: 1.5; Albu: Leu
Flu/Albu combination = 2: 1; Flu: Albu
NP = Nanoparticles
Fig. 11.
Differential scanning calorimetry thermograms for A) fluticasone nanoparticle agglomerates (NA), B) Flu/Albu combination and C) albuterol nanoparticle agglomerates (NA).
Combination nanoparticle agglomerates included fluticasone NP with albuterol in solution and l-leucine having been freeze dried from solution. The DSC thermogram exhibited an endothermic peak at 228.65 °C, Fig. 11B. This may be a result of some overlap of albuterol and excipient peaks. This peak was also similar to that of fluticasone nanoparticle agglomerates with a slight shift that may be indicative of the same crystalline form in both single and combination fluticasone nanoparticle agglomerate formulations.
The DSC data of albuterol as received showed an endothermic peak at 206.49 °C with an enthalpy change of −2.209 W/g followed by subsequent degradation of the compound (Fig 11C). It has been reported that this degradation could be explained by a possible dehydration of the alcoholic function (catalyzed by sulfuric acid present in the molecule) and then oxidation (Hadef et al., 2008; Raula et al., 2008; Xu et al., 2010). The DSC scans for both the drug as received and nanoparticles of albuterol were similar with no change in the melting points (Table 5). This indicated that creating nanoparticles by attrition may not affect the physical characteristics of albuterol. The sharpness of the peak seen in the nanoparticle formulation also suggested crystallinity. Albuterol in the nanoparticle agglomerates showed a shifted endothermic event that appeared at 259.22 °C with an enthalpy of −3.126 W/g. This may have resulted from some overlap with the sublimation peak of l-leucine that is usually observed at 323.7 °C (Raula et al., 2008). Thermogravimetric studies also support these findings (Supplementary Fig. 1 and Supplementary Text)
3.6. Fluticasone and albuterol nanoparticle agglomerate dissolution
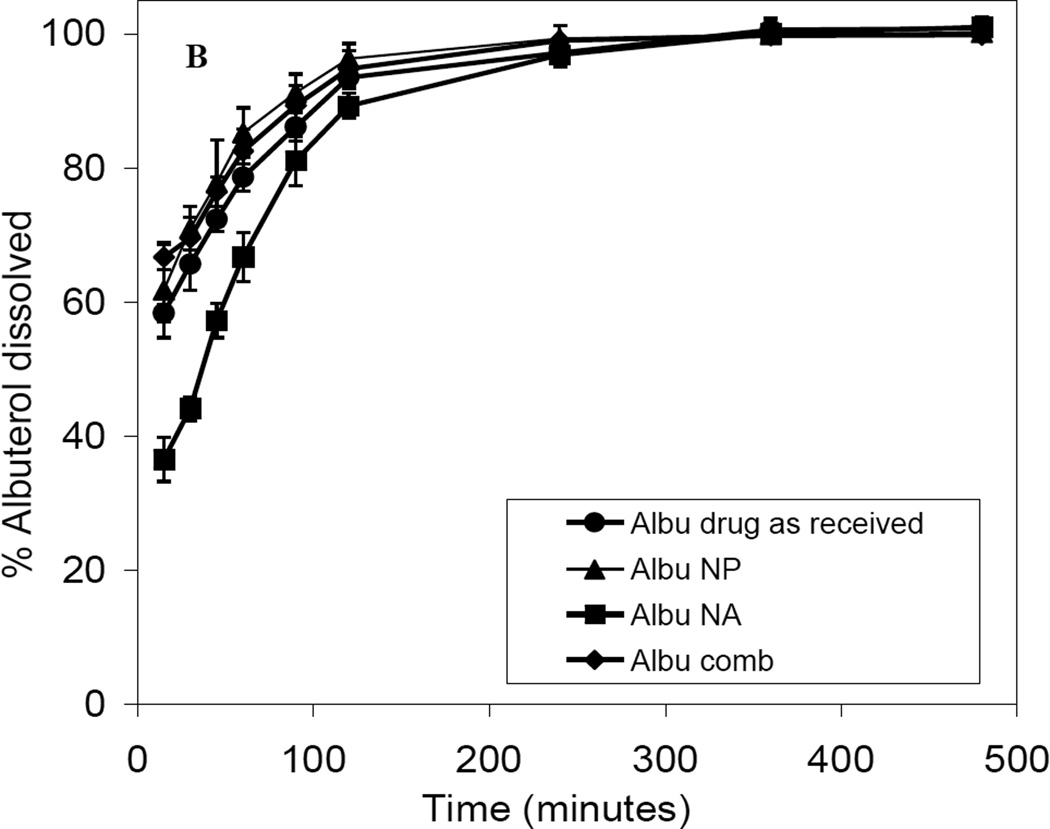
All nanoparticle agglomerates were produced with a high yield (~86–96 %) and with low batch variation (Table 6). All prepared nanoparticle agglomerates exhibited high drug content which ranged from 82% to 92% (Table 6), indicating negligible loss of drug during formation. The cumulative percentage of fluticasone dissolved from nanoparticle agglomerates after 8 h (Q8h) was found to be slower than that of the nanoparticles and faster than that of the drug as received (Table 6 and Fig. 12A). This was the expected result of increasing the surface area by decreasing the particle size. There was no significant difference in the fluticasone dissolution from the combination nanoparticle agglomerates and nanoparticle agglomerates containing only fluticasone. There was a slight delay in the dissolution of albuterol from nanoparticle agglomerates in the first 90 min followed by dissolution behavior similar to that of the micronized drug as received. On the other hand, no significant difference was observed in the dissolution performance of albuterol from nanoparticles, drug as received and combination therapy over 8 h (Fig. 12B). These results were likely due to high solubility of albuterol. Fluticasone nanoparticles did not affect the dissolution of albuterol in the combination formula.
Table 6.
Yield, content and dissolution behavior of fluticasone and albuterol nanoparticle agglomerates (values = average ± S.D., n = 3).
| Characteristics | Formulations | |||
|---|---|---|---|---|
| Fluticasone NAa |
Albuterol NAb |
Flu/Albu combination c | ||
| Fluticasone | Albuterol | |||
| Process yield of lyophilized NA (%) |
86 ± 7 | 96 ± 2 | 90 ± 3 | |
| % Drug content of Flu & Albu in the lyophilized NA |
92 ± 5 | 85.3 ± 7 | 90 ± 3 | 82 ± 6 |
| Q8hd NP | 96 ± 1 | 100 ± 0.4 | n/a | n/a |
| Q8h NA | 75 ± 7 | 100 ± 8 | 73 ± 10 | 100 ± 6 |
Fluticasone NC = 1: 0.05: 0.1: 1; Flu: Lec: PVP K90: Leu
Albuterol NC = 1: 1.5; Albu: Leu
Flu/Albu combination = 2: 1; Flu: Albu
Q8h = % Fluticasone (Flu) and albuterol (Albu) dissolved after 8 hours.
Fig. 12.
Dissolution profiles of A) fluticasone and B) albuterol in PBS (pH 7.4) from drug powder as received, nanoparticle formulation (NP), nanoparticle agglomerate formulation (NA) and Flu/Albu combination.
4. Conclusion
Inhaled dry powders represent a preferred formulation for first-line therapy treating asthma and chronic obstructive pulmonary diseases. Combination powders may provide simultaneous delivery to the same site of action increasing the potential synergistic effect of the drugs. A challenging task in engineering dry powders is achieving the aerosol particle size that can avoid the physiological barriers of the lung and deliver the drug to the appropriate lung region. Here, fluticasone and albuterol were fabricated into nanoparticle suspensions. Agglomeration of these suspensions produced micrometer-sized nanoparticle agglomerate aerosols with a large fine particle fraction (particles ~1–5 µm in diameter) and nanostructure for improving the dissolution rate of poorly water soluble fluticasone. The powders reported here offer a novel formulation for localizing potent drugs as single agents or in combination for the treatment of asthma and chronic obstructive pulmonary disease.
Supplementary Material
Thermogravimetric analysis curve (TGA) for A) fluticasone nanoparticle agglomerates (NA) and B) albuterol nanoparticle agglomerates (NA).
Acknowledgements
We would like to gratefully acknowledge Savara Pharmaceuticals for funding and 3M for providing fluticasone and albuterol. Additional lab funding was provided by the Coulter Foundation, the Higuchi Biosciences Center, the American Heart Association, and the NIH (R03 AR054035). In addition, we acknowledge the support of the NSF (CHE 0719464). We also thank Prof. C. Russell Middaugh (University of Kansas) for equipment use and The University of Kansas Microscopy Lab for TEM assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adi H, et al. Cospray dried antibiotics for dry powder lung delivery. Journal of pharmaceutical sciences. 2008;97:3356–3366. doi: 10.1002/jps.21239. [DOI] [PubMed] [Google Scholar]
- Ahmad F, et al. Techniques to develop and characterize nanosized formulation for salbutamol sulfate. Journal of Materials Science: Materials in Medicine. 2009;20:71–76. doi: 10.1007/s10856-008-3483-5. [DOI] [PubMed] [Google Scholar]
- Aillon KL, et al. Iodinated NanoClusters as an inhaled CT contrast agent for lung visualization. Molecular Pharmaceutics. 2010;7:1274–1282. doi: 10.1021/mp1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus MJ, et al. In vitro performance characteristics of valved holding chamber and spacer devices with a fluticasone metered-dose inhaler. Pharmacotherapy. 2004;24:159–166. doi: 10.1592/phco.24.2.159.33147. [DOI] [PubMed] [Google Scholar]
- Bailey MM, et al. Pure insulin nanoparticle agglomerates for pulmonary delivery. Langmuir. 2008;24:13614–13620. doi: 10.1021/la802405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. Scientific rationale for inhaled combination therapy with long-acting 2-agonists and corticosteroids. European Respiratory Journal. 2002;19:182. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- Berkland C. Next Steps for Pharmaceutical Nanotechnology. Journal of Pharmaceutical Innovation. 2010;5:70–71. [Google Scholar]
- Bilati U, et al. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Chiou H, et al. Production of salbutamol sulfate for inhalation by high-gravity controlled antisolvent precipitation. International Journal of Pharmaceutics. 2007;331:93–98. doi: 10.1016/j.ijpharm.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Chougule MB, et al. Development of Dry Powder Inhalers. Recent Patents on Drug Delivery & Formulation. 2007;1:11–21. doi: 10.2174/187221107779814159. [DOI] [PubMed] [Google Scholar]
- Dalby R, Suman J. Inhalation therapy: technological milestones in asthma treatment. Advanced drug delivery reviews. 2003;55:779–791. doi: 10.1016/s0169-409x(03)00077-2. [DOI] [PubMed] [Google Scholar]
- El-Gendy N, et al. Dry powdered aerosols of diatrizoic acid nanoparticle agglomerates as a lung contrast agent. International Journal of Pharmaceutics. 2010a;391:305–312. doi: 10.1016/j.ijpharm.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendy N, Berkland C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharmaceutical research. 2009;26:1752–1763. doi: 10.1007/s11095-009-9886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendy N, et al. Agglomerates of ciprofloxacin nanoparticles yield fine dry powder aerosols. Journal of Pharmaceutical Innovation. 2010b;5:79–87. [Google Scholar]
- El Gendy N, et al. Budesonide nanoparticle agglomerates as dry powder aerosols with rapid dissolution. Journal of pharmaceutical sciences. 2009;98:2731–2746. doi: 10.1002/jps.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiegel J, et al. Preparation and in vivo evaluation of a dry powder for inhalation of capreomycin. Pharm Res. 2008;25:805–811. doi: 10.1007/s11095-007-9381-6. [DOI] [PubMed] [Google Scholar]
- Gillian E. Pilot Study of Inhaled Aerosols Targeted via Magnetic Alignment of High Aspect Ratio Particles in Rabbits. Journal of Nanomaterials 2011. 2010 [Google Scholar]
- Hadef Y, et al. Thermal stability evaluation of doping compounds before GC-MS analysis by DSC. Journal of Thermal Analysis and Calorimetry. 2008;93:553–560. [Google Scholar]
- Hu T, et al. Preparation of inhalable salbutamol sulphate using reactive high gravity controlled precipitation. Journal of pharmaceutical sciences. 2008;97:944–949. doi: 10.1002/jps.21026. [DOI] [PubMed] [Google Scholar]
- Kamin W, et al. Physicochemical compatibility of fluticasone-17-propionate nebulizer suspension with ipratropium and albuterol nebulizer solutions. International Journal of Chronic Obstructive Pulmonary Disease. 2007;2:599. [PMC free article] [PubMed] [Google Scholar]
- Kumar V, et al. Compression, compaction, and disintegration properties of low crystallinity celluloses produced using different agitation rates during their regeneration from phosphoric acid solutions. AAPS PharmSciTech. 2001;2:E7. doi: 10.1208/pt020207. [DOI] [PubMed] [Google Scholar]
- Lechuga-Ballesteros D, et al. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci. 2008;97:287–302. doi: 10.1002/jps.21078. [DOI] [PubMed] [Google Scholar]
- Louey MD, et al. Aerosol dispersion of respirable particles in narrow size distributions using drug-alone and lactose-blend formulations. Pharmaceutical research. 2004;21:1207–1213. doi: 10.1023/b:pham.0000032991.74736.3e. [DOI] [PubMed] [Google Scholar]
- Michael Y, et al. The physico-chemical properties of salmeterol and fluticasone propionate in different solvent environments. International Journal of Pharmaceutics. 2000;200:279–288. doi: 10.1016/s0378-5173(00)00397-5. [DOI] [PubMed] [Google Scholar]
- Murnane D, et al. Crystallization and crystallinity of fluticasone propionate. Crystal Growth and Design. 2008a;8:2753–2764. [Google Scholar]
- Murnane D, et al. Investigations into the Formulation of Metered Dose Inhalers of Salmeterol Xinafoate and Fluticasone Propionate Microcrystals. Pharmaceutical research. 2008b;25:2283–2291. doi: 10.1007/s11095-008-9622-3. [DOI] [PubMed] [Google Scholar]
- Nelson HS, et al. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. Journal of allergy and clinical immunology. 2003;112:29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]
- Papi A, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356:2040–2052. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- Pham S, Wiedmann TS. Note: dissolution of aerosol particles of budesonide in Survanta, a model lung surfactant. J Pharm Sci. 2001;90:98–104. doi: 10.1002/1520-6017(200101)90:1<98::aid-jps11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. International Journal of Pharmaceutics. 2010;392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Plumley C, et al. Nifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategy. International Journal of Pharmaceutics. 2009;369:136–143. doi: 10.1016/j.ijpharm.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J. The influence of lung deposition on clinical response. Journal of Aerosol Medicine. 2001;14:19–26. doi: 10.1089/08942680150506303. [DOI] [PubMed] [Google Scholar]
- Rajeswari S, et al. Combined fluticasone propionate and salmeterol reduces RSV infection more effectively than either of them alone in allergen-sensitized mice. Virology Journal. 2006;3:32–41. doi: 10.1186/1743-422X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raula J, et al. Investigations on the humidity-induced transformations of salbutamol sulphate particles coated with L-leucine. Pharmaceutical research. 2008;25:2250–2261. doi: 10.1007/s11095-008-9613-4. [DOI] [PubMed] [Google Scholar]
- Rehman M, et al. Optimisation of powders for pulmonary delivery using supercritical fluid technology. European journal of pharmaceutical sciences. 2004;22:1–17. doi: 10.1016/j.ejps.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, et al. Meta-analysis: effect of long-acting -agonists on severe asthma exacerbations and asthma-related deaths. Annals of internal medicine. 2006;144:904. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- Shi L, et al. Biodegradable nanoparticle flocculates for dry powder aerosol formulation. Langmuir. 2007;23:10897–10901. doi: 10.1021/la7020098. [DOI] [PubMed] [Google Scholar]
- Shur J, et al. Cospray-dried unfractionated heparin with L-leucine as a dry powder inhaler mucolytic for cystic fibrosis therapy. J Pharm Sci. 2008;97:4857–4868. doi: 10.1002/jps.21362. [DOI] [PubMed] [Google Scholar]
- Steckel H, Muller BW. Metered-dose inhaler formulation of fluticasone-17-propionate micronized with supercritical carbon dioxide using the alternative propellant HFA-227. International Journal of Pharmaceutics. 1998;173:25–33. [Google Scholar]
- Steckel H, et al. In vitro characterization of jet-milled and in-situ-micronized fluticasone-17-propionate. International Journal of Pharmaceutics. 2003;258:65–75. doi: 10.1016/s0378-5173(03)00153-4. [DOI] [PubMed] [Google Scholar]
- Vanbever R, et al. Sustained release of insulin from insoluble inhaled particles. Drug Development Research. 1999;48:178–185. [Google Scholar]
- Vatanara A, et al. Precipitation of fluticasone propionate microparticles using supercritical antisolvent. DARU Journal of Pharmaceutical Sciences. 2009;17 [Google Scholar]
- Weers JG, et al. Pulmonary formulations: what remains to be done? Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2010;23:5–23. doi: 10.1089/jamp.2010.0838. [DOI] [PubMed] [Google Scholar]
- Westmeier R, Steckel H. Combination particles containing salmeterol xinafoate and fluticasone propionate: Formulation and aerodynamic assessment. Journal of pharmaceutical sciences. 2008;97:2299–2310. doi: 10.1002/jps.21154. [DOI] [PubMed] [Google Scholar]
- Xu Z, et al. Dry powder aerosols generated by standardized entrainment tubes from drug blends with lactose monohydrate: 1. Albuterol sulfate and disodium cromoglycate. Journal of pharmaceutical sciences. 2010;99:3398–3414. doi: 10.1002/jps.22107. [DOI] [PubMed] [Google Scholar]
- Yang JZ, et al. Fluticasone and budesonide nanosuspensions for pulmonary delivery: preparation, characterization, and pharmacokinetic studies. Journal of pharmaceutical sciences. 2008a;97:4869–4878. doi: 10.1002/jps.21380. [DOI] [PubMed] [Google Scholar]
- Yang ZY, et al. Production o ultrafine sumatriptan succinate particles for pulmonary delivery. Pharm Res. 2008b;25:2012–2018. doi: 10.1007/s11095-008-9586-3. [DOI] [PubMed] [Google Scholar]
- Young P, et al. Characterization of a surface modified dry powder inhalation carrier prepared by “particle smoothing”. Journal of pharmacy and pharmacology. 2002;54:1339–1344. doi: 10.1211/002235702760345400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thermogravimetric analysis curve (TGA) for A) fluticasone nanoparticle agglomerates (NA) and B) albuterol nanoparticle agglomerates (NA).