Abstract
The Centers for Disease Control and Prevention (CDC) Division of Select Agents and Toxins (DSAT) regulates laboratories that possess, use, or transfer select agents and toxins in the United States. DSAT also mitigates biosafety risks through the review of “restricted experiments,” which under the select agent regulations are experiments that pose heightened biosafety risks. From January 2006 through December 2013, DSAT received 618 requests from 109 entities to perform potentially restricted experiments. Of these requests, 85% were determined not to meet the regulatory definition of a restricted experiment, while 15% of the requests met the definition of a restricted experiment. Of the 91 restricted experiments proposed, DSAT approved 31 (34%) requests because the biosafety conditions proposed were commensurate with the experiments' biosafety risk. All 31 approved restricted experiments were for work with select toxins. DSAT did not approve 60 restricted experiment requests due to potentially serious biosafety risks to public health and safety. All 60 denied restricted experiments proposed inserting drug resistance traits into select agents that could compromise the control of disease. The select agents and toxins associated most frequently with requests that met the regulatory definition of a restricted experiment are Shiga toxin (n = 16), Burkholderia mallei (n = 15), Botulinum neurotoxin (n = 14), and Brucella abortus (n = 14). In general, all restricted experiment decisions are determined on a case-by-case basis. This article describes the trends and characteristics of the data associated with restricted experiment requests among select agents that have an impact on public health and safety (HHS only agents) or both public health and safety and animal health or products (overlap agents). The information presented here, coupled with the information published in the restricted experiment guidance document (www.selectagents.gov), is intended to promote awareness among the research community of the type of experiments that meet the regulatory definition of a restricted experiment as well as to provide a greater understanding of the restricted experiment review process.
The CDC Division of Select Agents and Toxins (DSAT) regulates laboratories that possess, use, or transfer select agents and toxins in the United States. DSAT also mitigates biosafety risks through the review of “restricted experiments,” which under the select agent regulations are experiments that pose heightened biosafety risks. This article describes the trends and characteristics of the data associated with restricted experiment requests among select agents that have an impact on public health and safety (HHS only agents) or both public health and safety and animal health or products (overlap agents). The information presented here, coupled with the information published in the restricted experiment guidance document (www.select agents.gov), is intended to promote awareness in the research community of the type of experiments that meet the regulatory definition of a restricted experiment and to provide a greater understanding of the restricted experiment review process.
The Federal Select Agent Program (FSAP) is a collaboration between the Centers for Disease Control and Prevention (CDC), Division of Select Agents and Toxins (DSAT), and the Animal and Plant Health Inspection Service (APHIS), Agriculture Select Agent Services (AgSAS), to regulate the possession, use, and transfer of select agents and toxins. The FSAP promotes laboratory safety and security to mitigate the inherent risks that accompany work with select agents. In addition, the program enhances the nation's oversight of the safety and security of select agents in a variety of ways, such as promulgating and enforcing the select agent regulations; maintaining a national database of registered entities and personnel; inspecting entities that possess, use, or transfer select agents; and ensuring that restricted experiments with select agents and toxins involving recombinant or synthetic DNA are reviewed to confirm that they are conducted under safe and secure conditions.
Restricted experiments1-3 are currently defined as:
(1) Experiments that involve the deliberate transfer of, or selection for, a drug (or chemical) resistance trait to select agents that are not known to acquire the trait naturally, if such acquisition could compromise the control of disease agents in humans, veterinary medicine, or agriculture (i.e., the transfer of drug resistant traits into select agents).
(2) Experiments involving the deliberate formation of synthetic or recombinant DNA containing genes for the biosynthesis of select toxins lethal for vertebrates at an LD50 <100 ng/kg body weight (i.e., nucleic acids that encode for select toxins).
These experiments are of particular concern because a product resulting from a restricted experiment has the potential to be directly misapplied by others to pose a threat to public health and safety, agricultural crops and other plants, animals, and/or the environment. In addition, the accidental release of a product of a restricted experiment may compromise the control or treatment of the disease agent in humans, animals, and/or plants.
Categories of experiments of concern, including those that meet the definition of restricted experiments in the select agent regulations, are addressed in the National Institutes of Health (NIH) Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).4 The purpose of the NIH Guidelines is to specify practices for the safe construction and handling of: (1) recombinant nucleic acid molecules; (2) synthetic nucleic acid molecules, including those that are chemically or otherwise modified but can base pair with naturally occurring nucleic acid molecules; and (3) cells, organisms, and viruses containing such molecules.
The types of experiments that are now specified as “restricted experiments” in the select agent regulations were first addressed in the NIH Guidelines. Their inclusion in the select agent regulations provides regulatory oversight for specific select agents, while the NIH Guidelines are applicable only to institutions that receive NIH funding for recombinant or synthetic nucleic acid molecules and conduct work with these molecules. For a complete description of the applicability of the NIH Guidelines, please see Section I-C of the Guidelines at: (http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html).4
The United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern of March 2012 establishes a regular review of research funded or conducted by the US government with certain high-consequence pathogens and toxins to determine the research's potential to be dual-use research of concern (DURC).5 The US Government Policy for Institutional Oversight of Life Sciences DURC (September 2014) addresses institutional oversight of DURC.6 These policies define DURC as “life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be directly misapplied to pose a significant threat with broad potential consequences to the public.”6 While the NIH Guidelines are limited to NIH-funded research and institutions, the DURC policies are applicable to US government funding agencies and institutions receiving US government funding. Although some select agent experiments are covered in the US DURC policy, the Federal Select Agent Program does not provide regulatory oversight of DURC policies or NIH Guidelines.
The select agent regulations state that an individual or entity may not conduct a restricted experiment with a select agent or toxin unless approved by and conducted in accordance with any conditions prescribed by the Health and Human Services (HHS) secretary or APHIS administrator. The HHS secretary has delegated the authority to approve or deny requests to conduct restricted experiments to DSAT. As part of the review, the CDC may consult with its Intragovernmental Select Agents and Toxins Technical Advisory Committee (ISATTAC). The committee is composed of federal government employees from CDC, NIH, the Food and Drug Administration (FDA), APHIS, USDA/Agricultural Research Service (ARS), USDA/CVB (Center for Veterinary Biologics), the Department of Homeland Security (DHS), the Department of Defense (DOD), and the Biomedical Advanced Research and Development Authority (BARDA), located in the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). The purpose of the ISATTAC is to assist CDC by reviewing restricted experiment requests and making recommendations pertaining to the list of select agents and toxins regulated by HHS.
This article describes the type of requests submitted to DSAT from 2006 through 2013 for review of potential restricted experiments concerning select agents and toxins regulated by HHS and the results of the biosafety and biosecurity assessments undertaken by DSAT to review these requests. HHS regulates select agents that have the potential to pose a severe threat to public health and safety and animal health (overlap agents). This review does not include requests associated with USDA-only select agents that pose a severe threat to animal health only or plant health and products only.
Materials and Methods
Data Collection Period
Potential restricted experiment requests submitted to DSAT from January 2006 through December 2013 were used in this analysis. This date range was selected because January 2006 began the first full calendar year following the publication of the HHS and USDA select agents and toxins final rule (published on March 18, 2005).1 The restricted experiment definition and provisions associated with restricted experiment requirements remained unchanged from March 18, 2005, through December 4, 2012. A subsequent final rule was published in the Federal Register on October 4, 2012, which modified the definition of restricted experiments by adding the following provisions: (1) experiments that “select” for drug resistance traits, (2) experiments generating “synthetic” nucleic acids that encode for a select toxin, and (3) the possession of a “product” of a restricted experiment.2,3
Definition: Restricted and Nonrestricted Experiment Requests
A “potential restricted experiment” is defined as an experiment that involves the transfer of a drug resistance trait into a select agent (eg, introduction of kanamycin resistance into Francisella tularensis) or an experiment involving the formation of nucleic acids that encode for a select toxin (eg, experiments using synthetic or recombinant nucleic acids for the biosynthesis of Botulinum neurotoxin). This definition was used until a technical review was performed to determine whether or not a request met the regulatory definition of a restricted experiment. An entity type was defined by 3 broad categories: (1) government (federal, state, or local agency); (2) academic (private or state academic institutions); and (3) private (for-profit or nonprofit corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity).
Technical Review
DSAT requested the following information from entities in order to conduct a technical review or risk assessment of each potential restricted experiment request:
1. Synopsis of the proposed experiment(s) and the intended objective(s);
2. Description of the nucleic acid insert (complete sequence information is not required) and the biological characteristics of the recombinant or synthetic product;
3. Description of the cloning/expression vector, if applicable;
4. Biosafety level, including a description of facility containment, equipment, and special practices to be used for the proposed experiment(s);
5. Identification and characteristics of the host organism used for molecular cloning;
6. Scientific references or supporting documentation, particularly with respect to the therapeutic usefulness of a proposed antibiotic used for selection purposes and the biosafety aspects of the proposed experimental product;
7. Synopsis of any planned animal or plant experiments (if applicable) or other relevant animal or plant work;
8. Description of the methods used for selection (eg, plasmid mediated or passive selection), to include all potential drug resistant products, including intermediate variants (if applicable);
9. Availability of alternative antibiotic marker genes that could be used—that is, to avoid the acquisition of drug resistance that could compromise the use of the drug to control disease agents in humans, veterinary medicine, or agriculture;
10. Description of the mechanism and specificity of the antimicrobial resistance (antibiotic, antifungal, and antiviral resistance) being conferred to include any cross-resistance to other therapeutically useful antimicrobials (if applicable); and
11. Estimated amount of toxin (recombinant or synthetic) to be produced (if applicable).
If a request required a technical review, DSAT convened the ISATTAC to provide recommendations. The ISATTAC's recommendations were considered by the DSAT director before a final determination was made. All restricted experiments that involved the deliberate introduction of a drug resistance trait were reviewed to determine if the drug resistance being introduced into the select agent was against a drug that is used therapeutically for treatment. If the drug resistance trait could compromise the control of disease, other considerations are taken into account—for example, are there other drugs available to treat the select agent infection? And if the drug is not used for treatment in the United States, is it used therapeutically outside the United States? In addition, experiments with specific select agent strains that have naturally acquired resistance to drugs that are used to treat the disease would not be considered restricted experiments.
Restricted experiments were approved only for the principal investigator at a specific entity conducting the experiment, and in some cases approval was based on the use of specific biosafety conditions. Other restricted experiments were not approved because of the heightened risk to public or animal health and safety.
Data Analysis
The following variables were abstracted from the restricted experiment requests submitted to DSAT during the study period: entity type submitting the request; select agent or toxin identified in the experiment; type of experiment proposed; drug-resistance trait transferred (if applicable); and final determination of the request (eg, restricted experiment approved, restricted experiment denied, experiment does not meet the regulatory definition of a restricted experiment, etc). Eleven incomplete submissions or requests that were withdrawn by the requesting entity were excluded from the analysis. Data were analyzed using Microsoft Excel (2010).
Restricted Experiment Violations
The Office of Inspector General of HHS (HHS OIG) has delegated authority to conduct investigations and impose civil money penalties against individuals or entities in violation of the select agent regulation (42 CFR 73). Data related to restricted experiment violations reported to the HHS Office of Inspector General and related civil money penalties were collected from internal DSAT compliance reports and the HHS Office of Inspector General website.7
Results
Requests for Technical Review
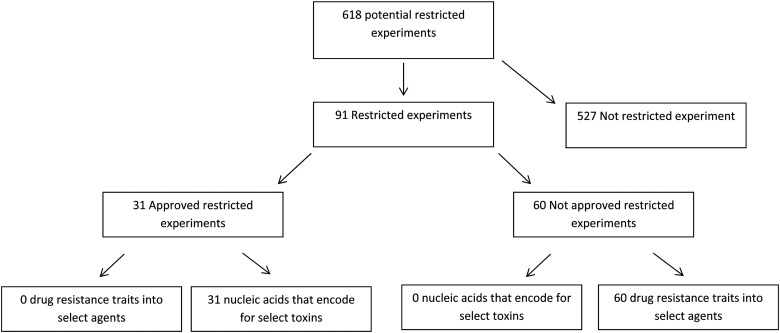
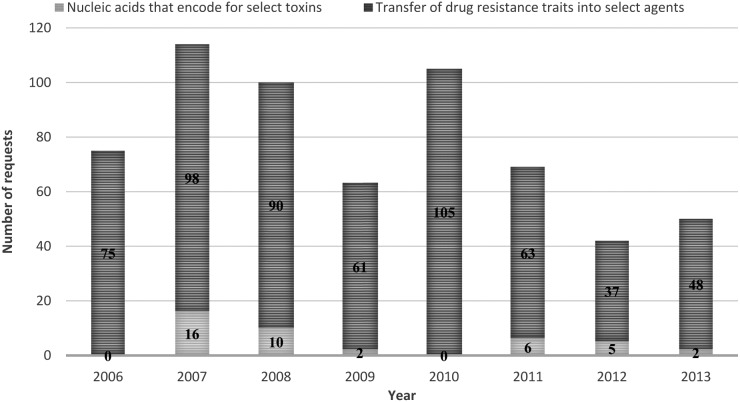
From 2006 through 2013, DSAT received and reviewed a total of 618 requests for potential restricted experiments (Figure 1). The majority of requests (n = 577, 93%) were for the review of experiments that involved the deliberate transfer of a drug resistance trait into select agents (Figure 2). The remaining requests were for experiments involving nucleic acids that encode for select toxins (Figure 2). An average of 77 requests (range = 47 to 114) were reviewed by DSAT per calendar year, with the highest number received in 2007 (n = 114). The total number of requests to review proposals declined from 2010 to 2013 (Figure 2).
Figure 1.
DSAT Review of Requests Submitted for Determination of Restricted Experiment Status, 2006-2013
Figure 2.
Requests Submitted for Determination of Restricted Experiment Status by Type of Experiment, 2006-2013. Requests were categorized as either experiments involving transfer of drug-resistant traits into select agents (dark gray stack) or experiments involving the formation of nucleic acids that encode for select toxins (light gray stack).
Requests for Restricted Experiments
The number of restricted experiment and nonrestricted experiment requests received by DSAT fluctuated over the study period (Figure 3). Of all the experiments reviewed, 85% (n = 527) did not meet the regulatory definition of a restricted experiment because the select toxin had an LD50 >100 ng/kg body weight or the drug-resistant trait did not compromise the control of disease. Only 15% (n = 91) of the total requests met the regulatory definition of a restricted experiment (Figure 3). DSAT received the highest number of requests that met the regulatory restricted experiment definition in 2007 and the lowest number in 2013. However, during any given year, restricted experiments made up 26% or less of the total requests received and reviewed by DSAT (Figure 3).
Figure 3.
Restricted Experiment Status Following DSAT Review of Requests Submitted for Determination of Restricted Experiment Status, 2006-2013. Requests were analyzed to determine the number of requests that met the regulatory definition of a restricted experiment (dark gray stack) and for those that did not meet the regulatory definition of a restricted experiment (light gray stack).
Requests by Experiment Type
Requests were categorized into 2 experiment types: (1) the proposal was for the transfer of a drug-resistant trait(s) into a select agent, or (2) the proposal was to form nucleic acids that encode for a select toxin. Of the 91 restricted experiment requests that met the regulatory definition of a restricted experiment, 34% (n = 31) were approved (Figure 1). All approved restricted experiments proposed the formation of nucleic acids that encode for select toxins. Sixty-six percent of the total requests were denied because the product of the experiments could compromise the control of the disease in humans and/or animals if the drug-resistant select agent were to be accidentally or intentionally released.
Entity Type Submitting Requests
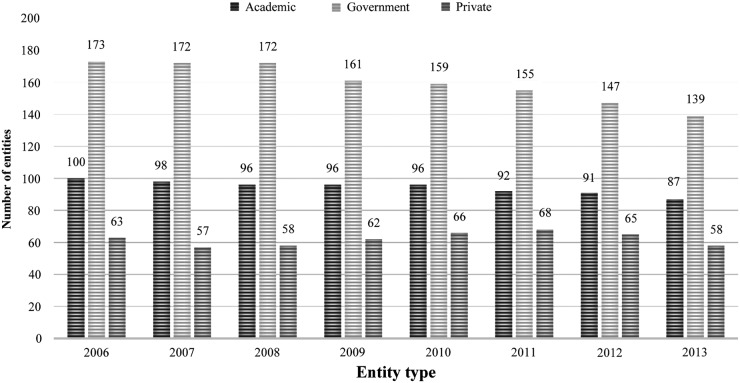
We analyzed the data to determine the number and type of entities that submitted requests to conduct potential restricted experiments. From 2006 to 2013, an average of 316 entities were registered with DSAT each year. Entity types registered each year were government entities (48%), academic entities (33%), and private entities (19%) (Figure 4). A total of 109 entities submitted requests to conduct potentially restricted experiments. Academic entities submitted 89 requests to review potential restricted experiments, accounting for 82% of the total requests submitted to DSAT. Government entities submitted 13% (n = 14) of all requests, followed by private entities with 5% (n = 6).
Figure 4.
Entity Types Registered with DSAT from 2006 until 2013. The total number of entities registered with DSAT from 2006 through 2013 ranged from 284 to 336 total registered entities. The entity types are categorized into academic (dark gray), government (federal, state, and local) (light gray), and private (medium gray).
We analyzed the data to determine the types of entities that submitted requests that met the regulatory definition of a restricted experiment. Thirty-two percent of the entities (n = 35/109) submitted requests that met the regulatory definition of a restricted experiment. Of these, 28 were academic entities, 4 were government entities, and 3 were private entities. Although private entities make up a small number of the total portfolio of DSAT-registered entities, 50% (n = 3/6) of private entities that submitted a request to DSAT met the regulatory definition of a restricted experiment, compared to 29% (n = 4/14) of government entities and 31% (n = 28/89) of the academic entities (data not shown).
Requests by Select Agent
The introduction of drug resistance traits into F. tularensis (22%, n = 117), Burkholderia pseudomallei (18%, n = 94), Burkholderia mallei (12%, n = 61), and Yersinia pestis (12%, n = 61) were the top 4 select agent experiments submitted for review (Figure 5A) that did not meet the definition of a restricted experiment. Collectively, these top 4 agents represented 64% of all requests that were not restricted experiments because the type of drug resistance traits transferred did not compromise the control of disease. Among the requests that met the regulatory definition of restricted experiment, the formation of nucleic acids encoding for Shiga toxin (18%, n = 16) and Botulinum neurotoxins (15%, n = 14) or experiments involving the introduction of drug resistance into B. mallei (17%, n = 15) and Brucella abortus (15%, n = 14) were the most frequent requests submitted for review (Figure 5B). Collectively, these top 4 select agents and toxins represented 65% of all requests that were restricted experiments. Requests to review experiments with B. mallei are in the top 4 for both restricted and nonrestricted experiments.
Figure 5.
(A) Nonrestricted Experiments by Agent. Requests that did not meet the restricted experiment definition were examined to identify the most common select agents or nucleic acids encoding for select toxins requested to review. (B) Restricted Experiments by Agent. Requests that did meet the restricted experiment definition were examined to identify the most common select agents or nucleic acids encoding for select toxins requested to review.
Types of Experiments Commonly Requested
All restricted experiment requests were reviewed individually based on biosafety risks associated with each experiment. We examined the types of drug resistance markers frequently requested in experiments with select agents. Table 1 shows common drug resistance markers that were requested for introduction into select agents categorized according to the definition of a restricted experiment. All requests were analyzed to determine the type of drug resistance traits proposed to be deliberately transferred into the select agent(s).
Table 1.
Examples of Restricted Experiments and Nonrestricted Experiments
| Select Agent | Nonrestricted Experiments | Restricted Experimentsa |
|---|---|---|
| Bacillus anthracis | erythromycin, spectinomycin, kanamycin | ciprofloxacin, chloramphenicol |
| Brucella abortus, Brucella melitensis, Brucella suis | ampicillin, chloramphenicol (in vitro only), kanamycin, hygromycin, spectinomycin, and zeocin | Gentamicin, chloramphenicol (in vivo)b |
| Burkholderia mallei | biapholos, kanamycin, nourseothricin, polymyxin B, streptomycin, tellurite, triclosan, and zeocin | chloramphenicol, erythromycin, gentamicin, trimethoprim, and tetracycline resistance |
| Burkholderia pseudomallei | ampicillin, biapholos, bleomycin, erythromycin, gentamicin, glyphosate, kanamycin, mercuric chloride, neomycin, nourseothricin, polymyxin B, spectinomycin, streptomycin, tellurite, triclosan, and zeocin | chloramphenicol, trimethoprim, and tetracycline |
| Francisella tularensis | ampicillin, cefprozil, erythromycin, hygromycin B, kanamycin, nourseothricin, ribostamycin, rifampicin, and spectinomycin | chloramphenicol |
| Rickettsia prowazekii | erythromycin and rifampin | chloramphenicol |
| Yersinia pestis | ampicillin, carbenicillin, and kanamycin | chloramphenicol |
| Clostridium spp. producing Botulinum neurotoxin | ampicillin, chloramphenicol, erythromycin, kanamycin, tetracycline, and erythromycin resistance | n/a |
Historically, approval to transfer these drug resistance traits into specific select agents has been denied. The ability to naturally acquire drug resistance traits was taken into consideration when determining which experiment met the regulatory definition of a restricted experiment.
Restricted Experiment Violations
During the study period, DSAT referred 13 cases involving select agent regulation violations to the HHS Office of Inspector General. Four of the 13 cases included restricted experiment violations. Of the 4 restricted experiment violation cases, 2 cases resulted in civil money penalties ranging from $40,000 to $1 million. The restricted experiment violations involved entities conducting restricted experiments that deliberately transferred drug resistance traits into select agents that could compromise the control of disease without seeking prior approval from DSAT.7
Discussion
From 2006 to 2013, DSAT received 618 requests to review potential restricted experiments from 109 entities. Of these requests, 93% were to review experiments that involved the deliberate transfer of a drug resistance trait into a select agent and 7% were for the review of experiments involving nucleic acids that encode for select toxins. Historically, 75% of the select agents and toxins list has been composed of bacteria and viruses, which could explain why DSAT reviewed more experiments with select agents compared to select toxins during this time.
Of the requests reviewed, 85% did not meet the regulatory definition of a restricted experiment because the requests were for select toxins that had an LD50 greater than 100ng/kg body weight or the transfer of the drug resistant trait into a select agent did not compromise the control of disease in humans. A possible explanation for this trend could be that entities were practicing due diligence by requesting DSAT review of the research prior to the experiments being conducted, which is a practice that the DSAT encourages. Another explanation may be that entities are overly cautious in assessing whether proposed experiments could be considered part of the regulatory definition of a “restricted experiment” to prevent any potential compliance action in case DSAT makes a different determination. The information presented in this article, coupled with the information published in the restricted experiment guidance document (www.select agents.gov), is intended to promote awareness among the research community of the type of experiments that meet the regulatory definition of a restricted experiment as well as to provide a greater understanding of the restricted experiment review process. In addition, DSAT provides outreach, including information about restricted experiments, to select agent stakeholders through exhibitions at national and international conferences in an effort to provide technical guidance and scientific consultation.
The Federal Select Agent Program recognized a gap in regulating restricted experiments involving passive selection, synthetic nucleic acids, and products resulting from a restricted experiment. Therefore, the restricted experiment definition was modified on October 4, 2012, with the intent to address these gaps. The data collected from this study were unaffected by the restricted experiment definition modifications, because DSAT did not receive any requests to conduct experiments that fell under the scope of the new restricted experiment provisions. However, DSAT will continue to assess experiments that could have a severe impact on public health and safety by performing technical reviews and gap assessments of the select agent regulations in general and the restricted experiment provisions in particular.
The total number of requests submitted to DSAT for review has declined since 2010. One explanation could be the decline in the total number of entities registered with DSAT. In 2006, there were 336 entities registered with DSAT compared to 284 entities registered in 2013 (Figure 4). Although the total number of entities declined from 2006 to 2013, the proportion of entity types remained constant over time. Finally, the decline observed could be influenced by the number and type of select agents and toxins that were removed from the select agent list effective December 4, 2012.
The majority of total requests to review experiments were from academic entities, which represented the second largest entity type registered with DSAT. This could be a result of the type of basic research commonly conducted at universities or the availability of funding opportunities to better understand the biology of select agents and toxins. Private entities make up less than 25% of all entity types registered with the select agent program, yet half of the requests submitted by private entities met the regulatory definition of a restricted experiment. All of the restricted experiment requests submitted by private entities were for select toxins, and the requesting entities either perform work with select toxins or manufacture licensed products that are obtained from select toxins.
The top 4 types of restricted experiments reviewed were experiments involving the formation of recombinant nucleic acids encoding for Shiga toxin and Botulinum neurotoxin in addition to experiments involving the introduction of drug resistance traits into B. mallei and B. abortus. Prior to December 4, 2012, all of the requests to review experiments involving Shiga toxin met the regulatory definition of a restricted experiment because (1) the experiments involved the deliberate formation of recombinant DNA containing genes for the biosynthesis of Shiga toxin, and (2) the Shiga toxin LD50 is less than 100ng/kg body weight. However, on December 4, 2012, Shiga toxin, despite its low LD50, was removed from the select agent list for a variety of reasons,2 including, but not limited to, the difficulty in producing or administering large quantities of toxin via the aerosol route, poor environmental stability, the lack of significant toxicity seen with oral exposure,10 and the observation that the worst effects seen with natural intoxication incidents are associated with other pathogenic factors from the Shiga toxin–producing strains of Escherichia coli that are not regulated as select agents.11 Epsilon toxin was also removed from the select agent list due to the absence of known human cases of disease, lack of human or nonhuman primate toxicity data, and insufficient new data to indicate that Clostridium perfringens epsilon toxin is a significant threat to public health and safety.2,12 Therefore, these 2 toxins and experiments involving nucleic acids that encode for their biosynthesis are no longer regulated. The data presented in this study involving Shiga toxin or Epsilon toxin were received and reviewed by DSAT prior to the removal of these agents from the select agent list.
Currently, cloning of recombinant or synthetic nucleic acids encoding for Botulinum neurotoxin meets the regulatory definition of a restricted experiment. Because the toxin itself possesses an LD50 less than 100 ng/kg, experiments involving the deliberate formation of synthetic or recombinant DNA containing genes for the biosynthesis of this neurotoxin are considered restricted experiments due to their potential threat to public health, and they must be reviewed by DSAT prior to conducting an experiment.
During this study period, all restricted experiment requests with select toxins have been approved because the restricted experiments proposed could be conducted safely under the biosafety conditions that meet the minimum safety guidelines prescribed for select toxins in the CDC/NIH publication Biosafety in Microbiological and Biomedical Laboratories.13
In addition to biosafety requirements, DSAT also considers site-specific security plans and assessments (42 CFR 73.11), personnel security risk assessments (42 CFR 73.11), and incident response plans and procedures (42 CFR 73.14) when registering an entity with DSAT. The outcome of this assessment is taken into consideration during the biosafety review of the restricted experiment request. Therefore, restricted experiment decisions are a result of careful consideration of entity, principal investigator, and biosafety factors and cannot be transferred to another entity or principal investigator planning similar work.
Inspections of registered facilities are conducted to confirm that entities are in compliance with the select agent regulations as well as to corroborate information included on the entity's select agent registration. During this study period, all restricted experiment requests that were not approved were due to biosafety risks only.
The most common experiment type requested for review was for the introduction of drug resistance into a select agent, specifically kanamycin resistance into B. mallei, B. pseudomallei, and F. tularensis (data not shown). Kanamycin resistance is commonly used in cloning experiments as a tool for selection. Since kanamycin is not used for the treatment or prevention of glanders or tularemia in humans or animals, the introduction of kanamycin resistance does not meet the regulatory definition of a restricted experiment as long as the gene encoding kanamycin resistance does not confer cross resistance to gentamicin. Gentamicin continues to represent a viable treatment option for tularemia, the disease caused by F. tularensis infections, and has been shown to have some efficacy against B. mallei.14,15 Therefore, a case in which the introduction of one drug resistance trait can induce cross resistance to other drugs may fall under the regulatory definition of a restricted experiment. For this reason, the Federal Select Agent Program encourages entities to review and disclose all drug resistance markers that are used in an experiment (including intermediate variants) to determine if a proposed experiment meets the regulatory definition of a restricted experiment prior to the experiment being conducted.
During the study period, all restricted experiments that introduced drug resistance traits that could compromise the control of disease were denied because either the biosafety approach or the experimental procedures proposed did not adequately mitigate the public health risks associated with deliberate or accidental release of these products. Accidental release of these products and the risks to public health were so severe that the knowledge gained by conducting the experiment in the strictest biosafety and security conditions was not commensurate with the risk to public health. In most, if not all of these cases, other drug resistance traits that did not compromise treatment or control of the select agent disease were available for use as selection markers (Table 1) or attenuated strains of the select agent were available to conduct the experiment.
DSAT, in consultation with the ISATTAC, performed thorough risk assessments of all restricted experiment requests to identify potential biosafety risks that are associated with each request. If approved, adherence to specific conditions such as biosafety level containment is required in order to conduct the restricted experiment. In addition, security plans, personnel security risk assessments, and incident response plans are considered by the Federal Select Agent Program before a final determination is given to the entity.
One limitation to this article is the absence of restricted experiment data from the Agricultural Select Agent Service (AgSAS) to observe trends across the Federal Select Agent Program. In addition, we did not perform predictive statistical analyses to identify statistically significant relationships between the frequency and types of restricted experiment requests submitted over time among entity types. This is the first publication that characterizes requests received by DSAT to review potential restricted experiments.
References
- 1.Select Agent Regulation. 42 CFR Part 73: Possession, Use, and Transfer of Select Agents and Toxins; Final Rule. Fed Regist March 18, 2005. https://oig.hhs.gov/authorities/docs/05/032905FRselectagents.pdf Accessed August19, 2015
- 2.Select Agent Regulation. 42 CFR Part 73: Possession, Use, and Transfer of Select Agents and Toxins; Biennial Review; Final Rule. Fed Regist October 5, 2012;77(194). http://www.gpo.gov/fdsys/pkg/FR-2012-10-05/pdf/2012-24389.pdf Accessed August19, 2015 [PubMed] [Google Scholar]
- 3.Select Agent Regulation. 7 CFR Part 121, 9 CFR Part 331: Agricultural Bioterrorism Protection Act of 2002; Biennial Review and Republication of the Select Agent and Toxin List; Amendments to the Select Agent and Toxin Regulations; Final Rule. Fed Regist October 5, 2012;77(194). http://www.gpo.gov/fdsys/pkg/FR-2012-10-05/pdf/2012-24434.pdf Accessed August19, 2015 [Google Scholar]
- 4.Department of Health and Human Services, National Institutes of Health. NIH Guidelines for Research Involving Recombinant or Synhetic Nucleic Acid Molecules (NIH Guidelines). November 2013. http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html Accessed August19, 2015
- 5.United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern (March 2012). http://www.phe.gov/s3/dualuse/Documents/us-policy-durc-032812.pdf
- 6.United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern. September 24, 2014. http://www.phe.gov/s3/dualuse/Documents/durc-policy.pdf Accessed August19, 2015
- 7.Office of Inspector General. US Department of Health and Human Services. Civil monetary penalties. Select agents and toxins. Updated August 11, 2014. http://oig.hhs.gov/fraud/enforcement/cmp/agents_toxins.asp Accessed August19, 2015
- 8.Ariza J, Bosilkovski M, Cascio A, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 2007;4(12):e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young EJ. Brucella species (brucellosis). In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 2d ed. New York: Elsevier; 2003:877-878 [Google Scholar]
- 10.Russo LM, Melton-Celsa AR, Smith MA, Smith MJ, O'Brien AD. Oral intoxication of mice with Shiga toxin type 2a (Stx2a) and protection by anti-Stx2a monoclonal antibody 11E10. Infect Immun 2014;82(3):1213-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 1999;37(3):497-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, et al. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect Immun 2005;73(11):7413-7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. December 2009. http://www.cdc.gov/biosafety/publications/bmbl5/ Accessed August19, 2015
- 14.Enderlin G, Morales L, Jacobs RF, Cross JT. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin Infect Dis 1994;19(1):42-47 [DOI] [PubMed] [Google Scholar]
- 15.Office of the Surgeon General. Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare. 2007. http://www.bordeninstitute.army.mil//published_volumes/biological_warfare/biological.html Accessed August19, 2015