Abstract
Severe sepsis is a life-threatening complication of infection and injury affecting more than 700,000 people in the United States each year. Two thirds of patients with severe sepsis will survive to be discharged. Survivors have high incidence of cognitive impairment, immune dysregulation, functional impairments with marked disability, and 5-year mortality rates of 82%. High-mobility group box 1 (HMGB1) is necessary and sufficient mediator of sepsis pathogenesis in experimental models of this syndrome. The spleen is a crucial organ in the immune response to severe infection, and splenocyte dysfunction occurs in sepsis survivors. We hypothesized that HMGB1 plays a key role in mediating the immune dysfunction of splenocytes in sepsis survivors. Mice that survived cecal ligation and puncture–induced sepsis develop persistent splenomegaly; furthermore, splenocytes derived from sepsis survivors had enhanced responses to lipopolysaccharide ex vivo. Administration of neutralizing anti-HMGB1 antibody to sepsis survivors attenuated development of splenomegaly and reversed splenocyte priming. Splenocytes exposed to HMGB1 and subsequently challenged with cognate ligands to Toll-like receptor 2 (TLR2,) TLR4, TLR9, and RAGE (receptor for advanced glycation end product) receptors had enhanced cytokine release as compared with splenocytes not previously exposed to HMGB1. Exposure of TLR2−/−, TLR9−/−, or RAGE−/− splenocytes to HMGB1 enhanced responses to other TLR receptor ligands; in contrast, HMGB1 failed to prime TLR4−/− splenocytes. These findings indicate that exposure to HMGB1 enhances splenocyte responses to secondary inflammatory challenges, a priming effect dependent on TLR4, and that anti-HMGB1 monoclonal antibody may be beneficial in sepsis survivors.
Keywords: HMGB1, splenocyte dysregulation, priming
INTRODUCTION
Severe sepsis is a potentially lethal syndrome of organ dysfunction that occurs following infection or injury. At least 700,000 people will develop severe sepsis in the United States; more than 500,000 of these patients will survive (1–3). Severe sepsis survivors have worsened quality of life (4) and increased morbidity and mortality (5, 6). Recent population-based studies have estimated a 5-year mortality of 82% among survivors (7). The pathogenesis of the late-occurring events underlying high morbidity and mortality in severe sepsis survivors is incompletely understood.
We recently observed that circulating high-mobility group box 1 (HMGB1) increases after onset of sepsis and remains elevated for at least 8 weeks in murine sepsis induced by cecal ligation and puncture (CLP). High-mobility group box 1 levels are directly related to the development of splenomegaly and an expansion of splenic granulocytes, B cells, and inflammatory (CD11b+, Ly-6CHigh) monocytes. Splenocytes derived from murine sepsis survivors are sensitized in response to HMGB1, showing an enhanced responses to lipopolysaccharide (LPS) and other inflammatory stimuli when challenged ex vivo. Neutralizing HMGB1 reverses several features of the proinflammatory state, including a reduction in splenomegaly, and improvement of proinflammatory sensitization of splenocytes. Furthermore, in vivo exposure to recombinant HMGB1 in the absence of other inflammatory stimulating agents is sufficient to induce sensitization of splenocytes (8).
High-mobility group box 1 activates monocytes and macrophages by binding to a family of receptors, including Toll-like receptor 2 (TLR2), TLR4, and TLR9, as well as the receptor for advanced glycation end products (RAGE) (9–11). Little is known about the mechanism underlying HMGB1 proinflammatory sensitization of splenocytes in sepsis survivors. Here, we studied HMGB1 priming of splenocytes leading to downstream activation of tumor necrosis factor (TNF) release.
METHODS
Mice
Adult BALB/c male 20 to 25 g mice from Charles River (Wilmington, Mass) were used for CLP experiments. Adult C57BL/6 male mice 20 to 25 g were used for the isolated splenocyte responses. Animals were housed in standard conditions (room temperature 22°C with a 12-h light-dark cycle) and had free access to standard chow and water. Animals were allowed to acclimate for at least 8 days before experiments. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Cecal Ligation and Puncture
Severe polymicrobial abdominal sepsis was induced by modified CLP as previously described (12, 13). In brief, the cecum was isolated and ligated below the ileocecal valve and then punctured once with a 22-gauge needle. Stool (approximately 1 mm) was extruded, the cecum returned to the abdominal cavity, and the wound closed with surgical clips. One dose of antibiotic (imipenemcilastatin, 0.5 mg/kg diluted in a 0.9% saline solution) was administered immediately after CLP as part of the 1 mL resuscitation fluid. We have established that this model has an expected 50% mortality by day 7 and little or no mortality thereafter (13). Sham-operated animals had the cecum exposed and then returned to the peritoneal cavity without further manipulation. Sham animals also received an antibiotic treatment and resuscitative fluid as described above. At the a priori established weekly time points, survivors were killed with CO2. Blood was collected by cardiac puncture and transferred to EDTA-coated tubes. Spleens were harvested in an aseptic environment and kept on ice until splenocyte isolation.
Spleen isolation and splenocyte treatment
Spleens from healthy C57BL/6 mice were collected under aseptic conditions. Splenocytes were harvested into a single cell suspension by infusing them with 500 µL of cold PBE (phosphate-buffered saline + 1% bovine serum albumin and 2 mM of EDTA) and transferred to a 40-µm cell strainer, where cells were mechanically detached and then washed with ice-cold PBE. Cell pellets were washed with 5 mL of PBE and pellets resuspended in 10 mL of lysis buffer (5PRIME, Hamburg, Germany) for 10 min and washed twice with cold PBE. Cells, 2 × 105, were transferred to U-bottom 96-well plates and diluted in 200 µL of RPMI supplemented with 10% fetal calf serum, penicillin 100 U/mL, and streptomycin (100 µg/mL; Gibco, Grand Island, NY), with or without rHMGB1 (10µg/mL), and incubated for 24 h, or 8 days, at 37°C, 5% CO2. Cells were then washed with warm PBE and resuspended in 200 µL of medium enriched with agonists to TLR2 (Pam2CSK4; InvivoGen, San Diego, Calif), TLR4 (LPS 25 ng/mL 0111.B4; Sigma-Aldrich, St Louis, Mo), TLR9 (CpG: ODN 1668 sequence 5′-tccatgacgttcctgatgct-3′ (20 mer); InvivoGen), or RAGE (S100A12; CircuLex, Nagano, Japan) for an extra 24 h. Supernatants were then collected and frozen at −20°C until analysis was done. Three to five mice were included at each time point.
Splenocytes were isolated fromTLR2−/−, TLR4−/−, TLR9−/−, and RAGE−/− mice on C57BL/6 background or matching wild-type (WT) mice and conditioned with or without HMGB1 for 24 h, as described above; splenocytes were then washed and exposed for 24 h to LPS.
Cytokine measurement
Mouse interleukin 2 (IL-2), IL-4, IL-6, interferon γ, TNF, IL-17a, and IL-10 were measured by flow cytometry–assisted bead assays (CBA; BD Biosciences, San Jose, Calif using a FACSArray instrument (BD Biosciences). All measurements were made in duplicate.
Statistical analysis
Data are expressed as mean ± SEM. Differences between means were determined using two-tailed Student t test. Survival analysis was performed using log-rank test. P < 0.05 was considered significant.
RESULTS
Splenocytes from sepsis survivors develop sustained proinflammatory priming
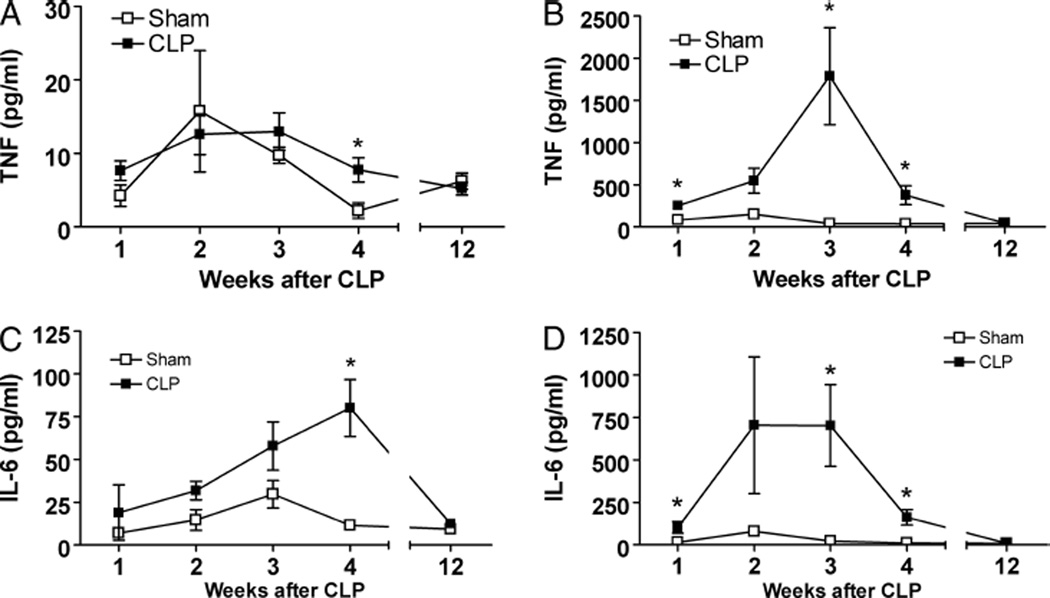
Splenocytes obtained from mice surviving sham surgery or CLP released constitutively low levels of TNF and IL-6. Although splenocytes collected 4 weeks after CLP showed a significant increase in TNF and IL-6 release, the levels were low throughout studied time points (Fig. 1, A and C). Ex vivo challenge of CLP-derived splenocytes with LPS significantly increased the release of TNF (Fig. 1B) and IL-6 (Fig. 1D). The priming effect peaked between weeks 2 and 3 post-CLP and persisted for at least 4 weeks after CLP. In contrast to TNF and IL-6 levels, release of IL-10, IL-2, IL4, IL-17, and interferon γ by splenocytes from sepsis survivors was not increased in the nonstimulated state and failed to increase after incubation with LPS (data not shown).
Fig. 1. Lipopolysaccharide-induced release of TNF and IL-6 is significantly increased in splenocytes derived from sepsis survivors.
Splenocytes, 2 × 105, from BALB/c sepsis survivors were incubated for 24 h with or without LPS (25 ng/mL). Supernatants were collected, and TNF and IL-6 concentrations measured by CBA. A, Nonstimulated levels of TNF were similar between sham and CLP survivors. B, In response to LPS stimulation, splenocytes derived from CLP mice had an highly significant increase of TNF for at least 4 weeks, in comparison to the sham group (n = 5 group). C, Similarly, levels of IL-6 remained low in nonstimulated splenocytes, whereas the concentration of IL-6 in supernatants from splenocytes stimulated with LPS was significantly increased (D). Data shown as mean ± SEM (n = 5 mice/group). *P < 0.05 vs. sham.
Sustained exposure to HMGB1 enhances splenocyte response to secondary inflammatory challenges
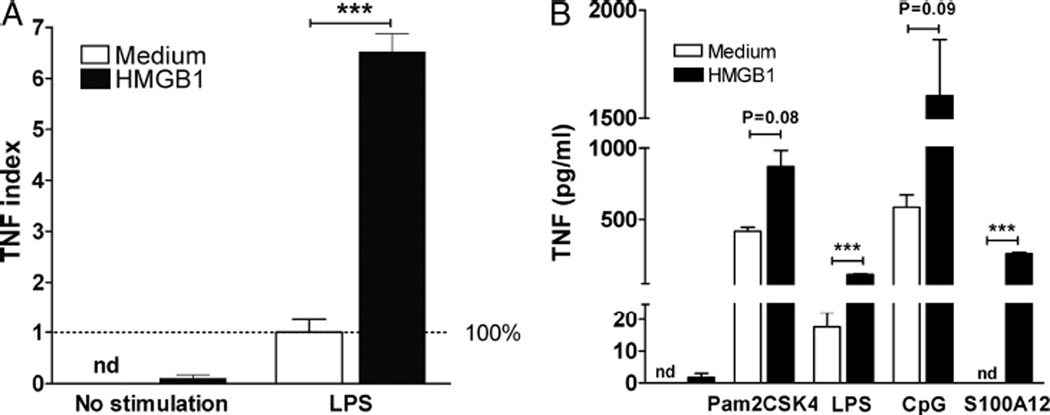
We and others have observed that, unlike other inflammatory cytokines that are released in the early hours after onset of sepsis, circulating HMGB1 increases later and remains elevated for several weeks after sepsis (7, 14). We hypothesized that HMGB1 mediates sensitization of splenocytes to secondary inflammatory challenges. Splenocytes from healthy mice were incubated for 8 days in medium with or without HMGB1 (10 µg/mL), washed, and then incubated with LPS (25 ng/mL) for 24 h. Tumor necrosis factor concentration in the supernatants of splenocytes that had been incubated with HMGB1 was increased 6.5-fold (Fig. 2A).
Fig. 2. Sustained exposure to HMGB1 enhances the response to secondary inflammatory challenges of splenocytes through TLR4 and RAGE.
A, Splenocytes, 2 × 105, from healthy BALB/c mice were incubated for 8 days in the presence or not of HMGB1 (10 µg/mL) in U-bottom 96-well plates. Cells were washed after 8 days and reincubated for 24 h with LPS (25 ng/mL). Supernatants were collected and concentration of TNF measured by CBA. Preincubation with HMGB1 did not change the TNF released in comparison to control cells. In contrast, TNF increased more than 6-fold in response to LPS in cells that had been preincubated with HMGB1. Broken line represents 100% of LPS concentration in LPS stimulated control cells. B, Splenocytes, 2 × 105, from healthy mice were incubated overnight in the presence or not of HMGB1 (10 µg/mL). After 24 h, cells were washed and stimulated with Pam2CSK4 (TLR2), LPS (TLR4), CpG (TLR9), or S100A12 (RAGE) for an additional 24 h. Tumor necrosis factor was measured in supernatants by CBA. Data shown as mean ± SEM (n = 5 mice/group). ***P < 0.001 vs. control. Other P values as expressed.
HMGB1 sensitizes splenocytes to pathogen-associated molecular patterns acting through TLR4 and RAGE
Previous studies have indicated that HMGB1 signals though TLR2, TLR4, TLR9, and RAGE. To examine the role of previous exposure to HMGB1 as a sufficient step to amplify the inflammatory response of splenocytes, we first isolated splenocytes from healthy mice. Splenocytes were preincubated for 24 h in the presence of HMGB1 (10 µg/mL) and then washed. Fresh medium containing either TLR2 ligand (Pam2CSK4, 10 ng/mL), a TLR4 ligand (LPS, 25 ng/mL), a TLR9 ligand (CpG, 5 µM/mL), or a RAGE ligand (S100A1210 ng/mL) was added, and cells incubated for an additional 24 h. Preincubation with HMGB1 significantly increased the release of TNF in response to TLR4 and RAGE agonists (Fig. 2B).
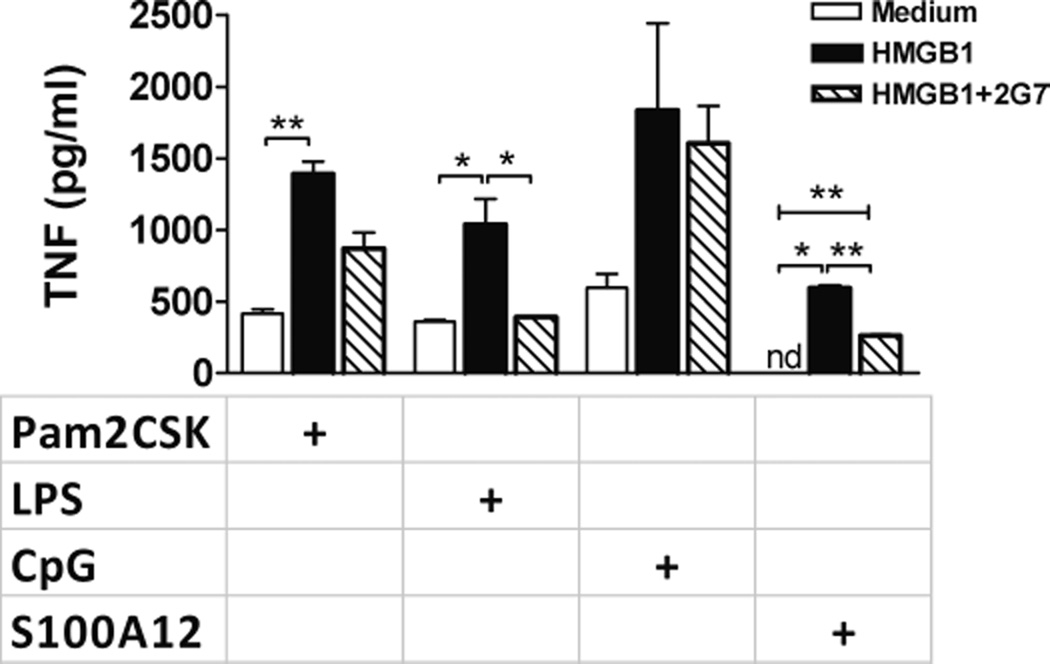
HMGB1 sensitization is reduced by anti-HMGB1 antibodies
Splenocytes from healthy mice were incubated for 24 h in the presence of HMGB1 or HMGB1 and the anti–HMGB1-neutralizing monoclonal antibody 2G7. Splenocytes were then washed and incubated for an additional 24 h with cognate ligands to TLR2, TLR4, TLR9, and RAGE. As expected from the experiments shown above, in splenocytes preincubated with HMGB1, we observed enhancement of immune activation to all pathogen-associated molecular patterns (PAMPs) studied. Nonetheless, when cells were coincubated with HMGB1 and 2G7, we observed a significant reduction in the inflammatory response to TLR4 and RAGE ligands in comparison to the group that had been pretreated with HMGB1 without antibody (Fig. 3). A trend was also found for a reduced response to a TLR2 ligand. In this 24-h-incubation–24-h-challenge model, no difference was found in the response to a TLR9 ligand across the three groups (Fig. 3).
Fig. 3. Anti-HMGB1 monoclonal antibody 2G7 reduces HMGB1 sensitization to TLR4 and RAGE agonists.
Splenocytes, 2 × 105, from healthy C57BL-6 mice were incubated in medium, in medium enriched with HMGB1, or with HMGB1+2G7. After 24 h, cells were washed and stimulated with Pam2CSK4 (TLR2), LPS (TLR4), CpG (TLR9), or S100A12 (RAGE) for an additional 24 h. Tumor necrosis factor was measured in supernatants by CBA. Data shown as mean ± SEM (sum of two experiments, each n = 3–4 mice/ group). *P < 0.05; **P ≤ 0.01.
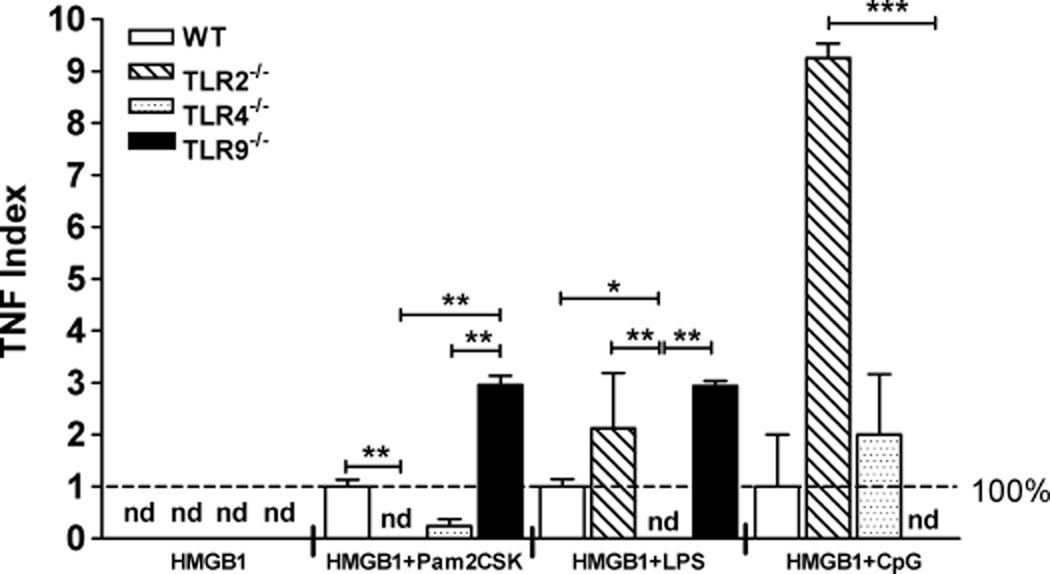
We then collected splenocytes derived from healthy C57BL/ 6 WT mice or splenocytes derived from TLR2−/−, TLR4−/−, TLR9−/−, or RAGE−/− mice on the C57BL/6 background. Splenocytes (2 × 105) were incubated for 8 days in the presence of HMGB1 (10 µg/mL), then washed and incubated for an additional 24 h with TLR2, TLR4, and TLR9 cognate ligands. Preincubation with HMGB1 did not enhance the baseline release of TNF in cells that were not secondarily challenged with PAMPs (Fig. 4). As expected, genetically manipulated splenocytes preincubated with HMGB1 failed to respond to the cognate ligands to the deleted TLRs. In contrast, TLR2−/− and TLR9−/− splenocytes displayed an enhanced response to ligands targeting other receptors. TLR2−/− splenocytes displayed an expanded response to ligands of TLR4 (2-fold) or TLR9 (9-fold) as compared with the response on WT cells to the same stimuli. TLR9−/− splenocytes had an increased response to TLR2 and TLR4 ligands (3-fold increase vs. WT). In contrast to our findings on TLR2−/− and TLR9−/− splenocytes, not only did TLR4−/− splenocytes fail to respond to a TLR4 ligand, but when challenged with a TLR2 or TLR9 ligand they also had a response inferior or similar to WT cells (Fig. 4).
Fig. 4. The priming effect of HMGB1 is TLR4-dependent.
For each experiment, 2 × 105 splenocytes from healthy C57BL/6 mice were incubated in the presence or absence of HMGB1 for 8 days. Cells were washed and subsequently incubated with Pam2CSK4 (TLR2), LPS (TLR4), CpG (TLR9), or S100A12 (RAGE) for an additional 24 h. Tumor necrosis factor was measured in supernatants by CBA. Broken line represents 100% of response in WT cell concentration in LPS-stimulated control cells (n = 6 mice/group). *P < 0.05; **P ≤ 0.01.
DISCUSSION
Severe sepsis is a life-threatening complication of infection and injury, and HMGB1 is an important inflammatory mediator in pathogenesis. High-mobility group box 1 levels are increased in patients with community-acquired pneumonia (14), severe burns (15, 16), sepsis (17), or pancreatitis (18). Recently, we reported that persistent HMGB1 elevation after CLP is sufficient to induce immune dysregulation (splenomegaly, leukocytosis, and splenocyte priming) lasting for at least 8 weeks after onset of sepsis (8). Suppressing HMGB1 levels in murine models of acute sepsis by pharmacological intervention reduces sepsis mortality (8, 19, 20). During murine endotoxemia, the spleen is the main organ source of systemic TNF, and splenic monocytes/ macrophages are the major producers of TNF (21).
Our current results show that HMGB1 is sufficient for the development of a magnified inflammatory response by splenocytes, mediating the response to systemic infections and sepsis. Our data suggest that prolonged exposure to HMGB1 induces functional changes facilitating a response to secondary immune stimuli. Furthermore, the priming effect depends on competent TLR4, as splenocytes derived from mice lacking these receptors failed to show priming when incubated for a prolonged period with HMGB1.
The immune response in sepsis survivors can be a continuum from enhanced inflammation to profound suppression (22–24). The role of anti-inflammatory cytokines in the inflammatory continuum is still unclear, although recent evidence suggests that IL-10 and soluble CD25 may be increased in nonsurvivors to severe sepsis during the first 7 days after onset of sepsis (25, 26). In murine sepsis survivors, we failed to detect increased serum IL-10. In addition, splenocytes isolated from these mice and stimulated ex vivo with LPS did not secrete measurable IL-10 (8). In the present study, IL-10 release was not different in vivo in mice surviving sepsis, or when cells were analyzed ex vivo. This suggests that HMGB1 priming has no effect on IL-10 production. It is possible that in survivors of severe sepsis HMGB1 may be a mediator of the initial overreactivity of the immune response, but later leading to late immunosuppression in sepsis survivors.
The present study identifies HMGB1 as a cytokine that is potentially relevant to understanding the pathophysiology of sepsis survivors. One of the conundrums of sepsis is that the cascade of events beyond the first hours after the triggering insult is poorly understood. Here, we correlate sustained exposure to HMGB1 with a sustained proinflammatory state. Moreover, blocking HMGB1 reduces splenocyte overt response to secondary challenges with PAMPs. Targeting HMGB1 may decrease the sustained proinflammatory state of splenocytes, a major source for inflammatory cytokines, and improve immune function in sepsis survivors.
Footnotes
This article was presented at the 36th Annual Conference on Shock as one of the finalists of the New Investigator Awards Competition, San Diego, California, June 1–4, 2013.
None of the authors has any conflict of interest to declare.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–135. [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 6.Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, Webb SA. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36(5):1523–1530. doi: 10.1097/CCM.0b013e318170a405. [DOI] [PubMed] [Google Scholar]
- 7.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14(1):R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdés-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, Ochani M, Li JH, Scheinerman JA, Katz DA, Levine YA, et al. Treatment with anti-HMGB1 nmAb normalizes leukocyte counts, splenomegaly and splenocyte cytokine response to endotoxin in murine sepsis survivors [published online ahead of print] J Intern Med. 2013;274(4):381–390. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 10.van Zoelen MAD, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of Toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1–induced inflammation in vivo. Shock. 2009;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey KJ, Diamond B. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18(1):930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal Ligation and Puncture. Curr Protoc Immunol. 2010;91:19.13.1–19.13.11. doi: 10.1002/0471142735.im1913s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L GenIMS Investigators. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35(4):1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 15.Lantos J, Földi V, Roth E, Wéber G, Bogár L, Csontos C. Burn trauma induces early hmgb1 release in patients: its correlation with cytokines. Shock. 2010;33(6):562–567. doi: 10.1097/SHK.0b013e3181cd8c88. [DOI] [PubMed] [Google Scholar]
- 16.Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association of high mobility group box-1 protein levels with sepsis and outcome of severely burned patients. Cytokine. 2011;53(1):29–34. doi: 10.1016/j.cyto.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant Increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33(4):359–363. doi: 10.1097/01.mpa.0000236741.15477.8b. [DOI] [PubMed] [Google Scholar]
- 19.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15(7–8):195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35(4):1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 21.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 25.Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19(2):113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Matera G, Puccio R, Giancotti A, Quirino A, Pulicari MC, Zicca E, Caroleo S, Renzulli A, Liberto MC, Focà A. Impact of interleukin-10, soluble CD25 and interferon-γ on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: a prospective observational study. Crit Care. 2013;17(2):R64. doi: 10.1186/cc12596. [DOI] [PMC free article] [PubMed] [Google Scholar]