Abstract
The vast majority of cells delivered into the heart by conventional means are lost within the first 24 hours. Methods are needed to enhance cell retention, so as to minimize loss of precious material and maximize effectiveness of the therapy. We tested a cell-hydrogel delivery strategy. Cardiosphere-derived cells (CDCs) were grown from adult human cardiac biopsy specimens. In situ polymerizable hydrogels made of hyaluronan and porcine gelatin (Hystem®-C™) were formulated as a liquid at room temperature so as to gel within 20 minutes at 37°C. CDC viability and migration were not compromised in Hystem-C™. Myocardial infarction was created in SCID mice and CDCs were injected intramyocardially in the infarct border zone. Real time PCR revealed engraftment of CDCs delivered in Hystem-C™ was increased by nearly an order of magnitude. LVEF (left ventricular ejection fraction) deteriorated in the control (PBS only) group over the 3-week time course. Hystem-C™ alone or CDCs alone preserved LVEF relative to baseline, while CDCs delivered in Hystem-C™ resulted in a sizable boost in LVEF. Heart morphometry revealed the greatest attenuation of LV remodeling in the CDC + Hystem-C™ group. Histological analysis suggested cardiovascular differentiation of the CDCs in Hystem-C™. However, the majority of functional benefit is likely from paracrine mechanisms such as tissue preservation and neovascularization. A CDC/hydrogel formulation suitable for catheter-based intramyocardial injection exhibits superior engraftment and functional benefits relative to naked CDCs.
Keywords: acute myocardial infarction, biodegradable polymers, hyaluronan, cardiac stem cells
Introduction
Cardiovascular disease remains the leading cause of death and disability in Americans, claiming more lives each year than cancer, diabetes mellitus, HIV and accidents combined [1]. Ischemic heart disease is the predominant contributor to cardiovascular morbidity and mortality; ~1 million myocardial infarctions (MIs) occur per year in the United States while ~5 million patients suffer from chronic heart failure [2]. Death rates following MI have improved dramatically over the last four decades [3], but new approaches are nevertheless urgently needed for those patients who deteriorate and develop ventricular dysfunction [4]. Over the past ten years, stem cell transplantation has emerged as a promising therapeutic strategy for acute or chronic ischemic cardiomyopathy. Over the last six years, we have taken a unique cell therapy product, cardiosphere-derived cells (CDCs) from proof-of-concept animal studies [5–13] to a recently completed phase I clinical trial. Data from our clinical trial (CADUCEUS, NCT00893360 at clinicaltrials.gov) indicates that CDCs augment cardiac function and reduce scar size in mild to moderate ischemic cardiomyopathies [14]. However, CDCs face the same fate as most other cell types, that is extremely low retention rates in the heart shortly after delivery, which certainly cripples the efficiency and efficacy of cell therapies in general [15]. In fact, the vast majority of cells delivered into the heart by conventional means are lost within the first 24 hours [16]. Methods are needed to enhance retention.
The blooming of biomaterial and tissue engineering research opens the door for a new paradigm of cell therapy [17]. Injectable biomaterial gels are particularly appealing as they are amenable to minimally-invasive delivery and capable of enhancing cell engraftment by providing a temporary scaffold [18]. We have previously reported that injection of platelet gel alone or platelet gel spiked with CDCs ameliorates cardiac dysfunction in rats with myocardial infarction [19, 20]. Despite of its appearing autologous nature (i.e. derived from the same animals/patients who receive the gel), platelet gel usually contains numerous components that vary from patient to patient. Also, the gel needs to be freshly prepared from the patients in advance to injection and cannot be offered in an “off the shelf” fashion. Therefore, we started look for a commercially-available and chemically-defined biomaterial that can enhance the therapeutic benefit of our CDC products. One among various biomaterial choices, hyaluronan is a glycosaminoglycan component of the extracellular matrix of all connective tissues, making it an attractive scaffold [21]. Hyaluronan-based hydrogels can be formulated with varying gelation times depending on the concentrations of the individual monomers, making them suitable for catheter delivery and in situ polymerization. Hystem®-C™ (BioTime Inc.) is a hyaluronan-based hydrogel crosslinked using thiol-reactive poly(ethylene glycol) diacrylate and covalently linked to thiolated collagen to aid cell attachment. The base product is chemically-defined and nonimmunogenic and the collagen is porcine derived. It has been demonstrated that Hystem-C™ promotes tissue repair in various organ systems [22]. However, its utility in cardiac applications has yet to be explored.
In the present study, we developed and tested a cell-biomaterial strategy which embeds human CDCs within the Hystem-C™ hydrogel. In a mouse model of myocardial infarction, we compared the functional benefits of this CDC/hydrogel combination product with those seen using CDCs or hydrogel alone.
Methods
Human CDC culture
Percutaneous endomyocardial heart biopsies were obtained from the right ventricular aspect of the septum in patients during clinically-indicated procedures with IRB approval and informed consent from the patients. Cardiosphere-derived cells (CDCs) were derived as described [13]. Briefly, heart biopsies were minced into small fragments. After brief digestion with collagenase, the tissue fragments were cultured as "explants" on dishes coated with 20 µg/ml fibronectin (BD Biosciences). Within 1–2 weeks, stromal-like flat cells, and phase-bright round cells, emerged from the tissue fragments and became confluent. These cardiac-derived cells were harvested using 0.25% trypsin (Gibco), and then cultured in suspension as self-aggregated cardiospheres on poly-D-Lysine (20 µg/mL; BD Biosciences). CDCs were grown by seeding cardiospheres on fibronectin-coated dishes and passaged twice as described [13]. All cultures were incubated in 5% CO2 at 37°C, using IMDM basic medium (Gibco) supplemented with 20% FBS (Hyclone), 1% penicillin/streptomycin, and 0.1 mM 2-mercaptoethanol.
Flow cytometry analysis
The phenotype of CDCs was investigated by flow cytometry analysis. Briefly, cells were incubated with FITC, PE, or APC-conjugated antibodies against CD45, CD90, CD105, CD117 (c-kit), and DDR2 (ebiosciences Inc., San Diego, California) for 30 min. Isotype-identical antibodies served as negative controls. Quantitative analysis was performed using a CYAN-ADP flow cytometer with Summit 4.3 software (Beckman Coulter, Brea, California).
Hyaluronan-based hydrogel
Glycosan HyStem® (BioTime Inc., Alameda, CA) is a hyaluronan-based hydrogel crosslinked using thiol-reactive poly(ethylene glycol) diacrylate. Extracellular matrix (ECM) proteins such as collagen can be blended with hyaluronan to aid cell attachment. One such formulation utilizes thiolated denatured porcine collagen which can be covalently crosslinked to thiolated hyaluronan to create a cell-compatible hydrogel (Hystem®-C™) [23]. All materials were prepared according to the manufacturer’s instructions.
CDCs in hyaluronan-based hydrogel
Human CDCs were incorporated within the hydrogels during the crosslinking process prior to gelation. The final aqueous cell solution passed readily through a 30-gauge needle (used for injections in small animal studies) with no appreciable loss of material. Gelation occurred within 20 minutes of mixing all components. CDC viability within the Hystem and Hystem-C™ hydrogels was first examined in a 96 well format by an in vitro cell viability assay (Cell Counting Kit-8, Dojindo) at Day 4 and Day 7 after seeding. Since the final goal is to deliver CDCs that can survive and migrate out of the hydrogel to regenerate the infarcted myocardium, we next tested the in vitro migratory potential of CDCs incorporated within the two hydrogels (Hystem and Hystem-C™). A transwell plate setup allowed for cell migration through pores into the lower chamber where they could be detected. Calcein-labeled CDCs (10,000 cells/µL) were incorporated and fetal bovine serum (FBS) served as a chemoattractant in the lower chamber. As the CDCs migrated from the upper to the lower chamber, fluorescence (RFU) increased.
Animal model
Acute myocardial infarction was created in adult male SCID-beige mice (10–12 weeks old), as described [12, 13]. Briefly, after general anesthesia and tracheal intubation, mice were artificially ventilated with room air. A left thoracotomy was performed through the fourth intercostal space and the left anterior descending artery (LAD) was ligated with 9-0 prolene under direct visualization. The mice were then subjected to intramyocardial injections with a 30-gauge needle at two to four points in the infarct border zone. CDCs were added to the hydrogels immediately prior to injection, allowing for gelation to occur primarily in situ. For the acute retention study, CDCs were labeled with DiI and suspended at 10,000 cells/µL (total 1.5 ×105 CDCs) in PBS, Hystem™, or Hystem-C™ (n=4–5 mice for each group). All animals were sacrificed 24 hours after injection for qPCR and histological analysis of cell retention. For the long-term functional study, 4 groups were compared: 1) Control: intramyocardial injection of 15 µL PBS (n=8); 2) Hystem-C™ only: intramyocardial injection of 15 µL Hystem-C™ hydrogel (n=8); 3) CDCs only: intramyocardial injection of 1.5 ×105 CDCs in 15 µL PBS (n=8); 4) CDCs + Hystem-C™: intramyocardial injection of 1.5 ×105 CDCs in 15 µL Hystem-C™ hydrogel (n=8). Animals were followed for 3 weeks.
Cell engraftment assay by quantitative PCR
Animals were sacrificed and their hearts excised to obtain an actual measurement of the number of cells engrafted. Real-time PCR experiments using the human-specific repetitive Alu sequences were conducted [24]. The whole heart was weighed and homogenized. Genomic DNA was isolated from aliquots of the homogenate corresponding to 30 mg of myocardial tissue, using the DNAeasy minikit (Qiagen), according to the manufacturer’s protocol. The TaqMan® assay (Applied Biosystems) was used to quantify the number of transplanted cells with the human Alu sequence as template (Alu forward, 5'-CAT GGT GAA ACC CCG TCT CTA-3'; Alu reverse, 5'-GCC TCA GCC TCC CGA GTA G-3'; TaqMan probe, 5'-FAM-ATT AGC CGG GCG TGG TGG CG-TAMRA-3', Applied Biosystems). For absolute quantification of cell number, a standard curve was constructed with samples derived from multiple log dilutions of genomic DNA isolated from the same human CDC isolates that were used for the animal experiments. All samples were spiked with 50 ng of mouse genomic DNA to control for any effects this may have on reaction efficiency in the actual samples. All samples were tested in triplicates. The result from each reaction, number of human cells in 50 ng of genomic DNA, was expressed as the number of engrafted cells per heart, by first calculating the cell number in the total amount of DNA corresponding to 30 mg of myocardium and then extrapolating to the total weight of each heart.
Heart Morphometry
For morphometric analysis, mice were euthanized at 3 weeks and the hearts were explanted and frozen in OCT compound (n=3–5 mouse hearts per group). Cryo-sections every 100 µm (5 µm thickness) were prepared. Masson’s trichrome staining (6 sections per heart, collected at 400 ìm intervals) was performed as described [5]. Images were acquired with a PathScan Enabler IV slide scanner (Advanced Imaging Concepts, Princeton, NJ). From the Masson’s trichrome-stained images, morphometric parameters including viable tissue in infarct area and infarct wall thickness were measured in each section with NIH ImageJ software. Measurements were averaged for each heart.
Echocardiography
Mice underwent echocardiography 3 hrs (baseline) and 3 weeks after surgery using Vevo 770™ Imaging System (VISUALSONICS™, Toronto, Canada) (n=7–8 mice per group). With general anesthesia, the hearts were imaged two-dimensionally in long-axis views at the level of the greatest left ventricle diameter. Left ventricle ejection fraction (LVEF) was measured with VisualSonics V1.3.8 software from 2D long-axis views taken through the myocardial infarction area. The echocardiography images were obtained and analyzed blindly by an experienced study staff.
Histology
Mice were sacrificed 3 weeks after treatment. Hearts were sectioned in 5 µm sections and fixed with 4% paraformaldehyde (n=5 mice per group). The survival of implanted cells was observed by staining of human nuclei antigen (HNA; Millipore) in cells under fluorescence microscopy. Differentiation of cardiac stem cells into myocytes and endothelial cells was identified by immunostaining with monoclonal antibodies against α-sarcomeric actin (α–SA; Sigma) and von willebrand factor (vWF; Abcam), respectively, as described above. Vasculature was visualized by staining with alpha smooth muscle actin (αSMA; Sigma). Apoptotic cells were detected by TUNEL staining using the In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). Cell nuclei were stained with DAPI. Images were taken by a Leica TCS SP5 X confocal microscopy system.
Statistical analysis
All results are presented as mean ± SD. Statistical significance between two groups was determined using the 2-tailed unpaired t test and among groups by ANOVA followed by Bonferroni post hoc test. Differences were considered significant for p<0.05.
Results
CDCs in hyaluronan-based hydrogel
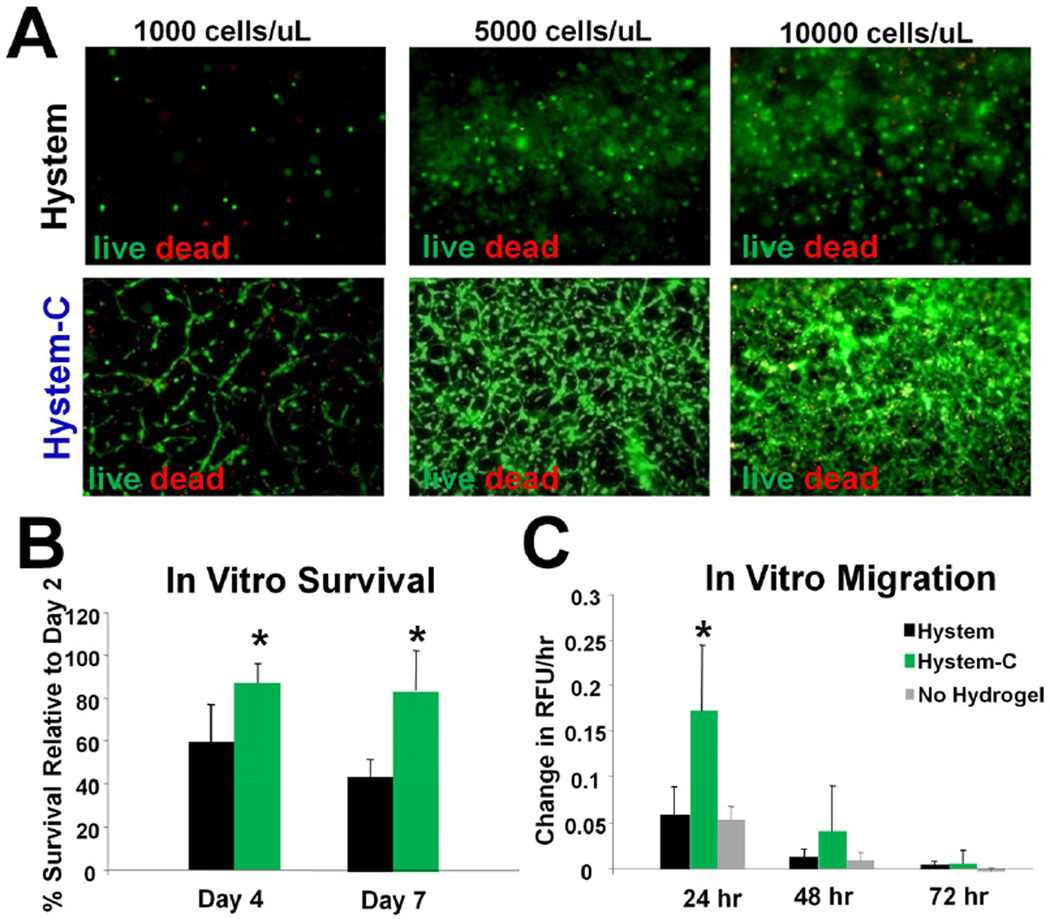
The morphology and mechanical properties of Hystem-C™ hydrogel were characterized previously [25]. The phenotype profiles (surface marker expression) of Human CDCs were characterized by flow cytometry analysis (Supplementary Figure 1). Consistent with our previous findings, CDCs revealed a distinctive phenotype with uniform expression of CD105, partial expression of c-Kit, CD90, and DDR2, and negligible expression of hematopoietic markers (e.g. CD45). CDCs were incorporated within the various hydrogels during the crosslinking process prior to gelation. The final aqueous cell solution passed readily through a 30-gauge needle (used for injections in small animal studies) with no appreciable loss of material and gelation occurred within 20 minutes. Fluorescent microscopic images revealed normal CDC morphology in Hystem-C™ while CDCs in Hystem™ remained round-shaped and non-spreading (Fig. 1A).CDC viability within the Hystem™ hydrogels was further examined quantitatively in a 96 well format by an in vitro cell viability assay. CDC viability relative to the Day 2 viability level in all three formulations was quantified at Day 4 and Day 7. This time point was chosen as it may take 1 week for the hydrogels to begin to biodegrade in vivo and it would be ideal to select a formulation which can sustain CDCs for that period of time. A significant difference in CDC viability between Hystem™ and the modified versions, Hystem-C™, is seen at both the time points, with the ECM-modified versions maintaining ~80% CDCs as viable while more than half of those embedded in Hystem™ do not survive (Fig. 1B). This indicates that the ECM molecules are essential for cell spreading and survival in the synthetic hydrogel.
Figure 1. CDC survival and migration in the hydrogel.
A, Representative fluorescent micrographs showing live (Calcien-AM: green) and dead (EthD: red) staining of CDCs cultured in Hystem™ and Hystem-C™ for 7 days. B, CCK-8 assay quantifying cell survival rates in Hystem™ (black bars) or Hystem-C™ (green bars) (n=3). C, Trans-well migration assay showing CDC migration rate in Hystem™ (black bars), Hystem-C™ (green bars) or Control (plain media; grey bars) (n=3). * indicates p<0.05 when compared to Hystem™ or no hydrogel control.
Since the final goal is to deliver CDCs that can survive and migrate out of the hydrogel to regenerate the infarcted myocardium, we next tested the in vitro migratory potential of CDCs incorporated within the three hydrogels and the non-hydrogel control. CDCs migrated out of the hydrogels as readily as did CDCs alone. Migration rate was maximal within the first 24 hours of the assay (Fig. 1C), at which time the rate of migration of CDCs through Hystem-C™ exceeded that in the ‘no hydrogel’ control, consistent with the notion that the incorporated ECM molecules within Hystem-C™ provide survival cues and facilitate cell function, in this case, migration. The rate of migration then decreased, as expected from gradual loss of the serum gradient. These data, considered along with the survival data shown previously, imply that Hystem-C™ promotes improved in vitro function.
Enhanced cell retention of CDCs delivered in hydrogel
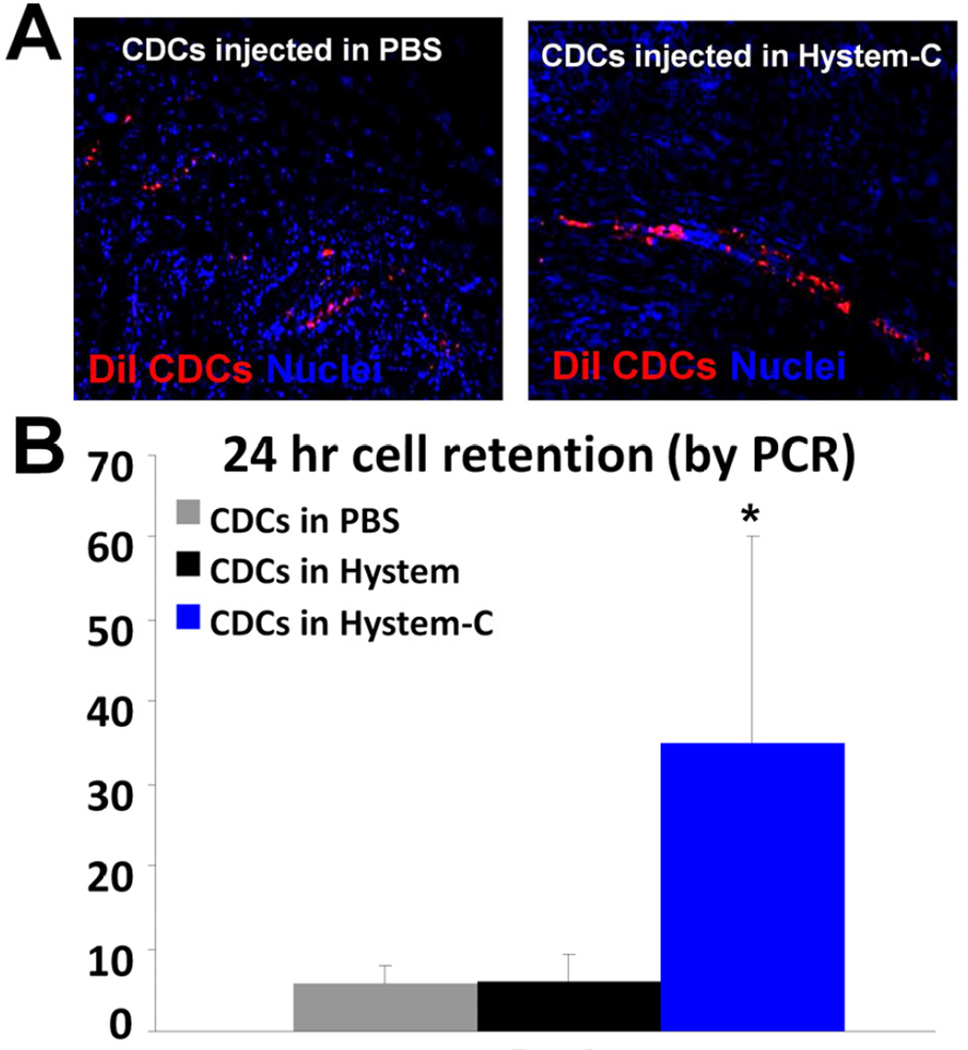
Myocardial infarction (MI) was created in SCID mice and cell products delivered intramyocardially (IM) through a 30-gauge needle. CDCs were added to the hydrogels immediately prior to injection, allowing for gelation to occur primarily in situ. CDCs, labeled with DiI and suspended at 10,000 cells/µL in PBS, Hystem™, or Hystem-C™, were delivered at two to four sites within the MI border zone (total 1.5 ×105 CDCs). 24 hours post-injection, hearts were collected for PCR quantification of engraftment (human Alu sequences) or histological examination. Few DiI-positive cells could be detected in the hearts received CDCs delivered in PBS (Fig. 2A). However, delivering cells in Hystem-C™ resulted in a sizable engraftment. qPCR further confirmed that CDCs delivered in Hystem-C™ exhibited far superior engraftment (~35%) relative to PBS or Hystem™ (Fig. 2B). These results suggest that CDC engraftment levels can be greatly enhanced by using a hydrogel carrier and provide strong motivation for the focus on long-term functional benefit. Given the favorable data on CDCs embedded in Hystem-C™, we focused on this hydrogel for subsequent in vivo experiments.
Figure 2. Enhanced cell engraftment by delivering CDCs in Hystem-C™.
A, Representative confocal images showing engraftment of DiI-labeled human CDCs (red) 24 hours after injection into post-MI mouse hearts. B, Quantitative PCR analysis of cell retention rates in the heart (n=4–5). * indicates p<0.05 when compared to “CDCs in PBS” or “CDCs in Hystem”.
Enhanced functional benefit of CDCs delivered in hydrogel
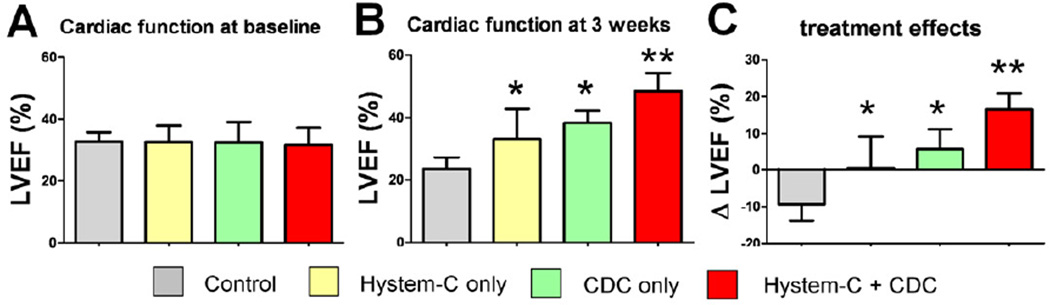
The most meaningful efficacy indicator of cell therapy, in practice, is the ability to produce functional benefit after transplantation into the injured heart. To test if the in vitro assets of CDCs in Hystem-C™ translate into greater functional benefit in the mouse MI model, we compared 4 treatment groups: Control, Hystem-C™ only, CDCs only and CDCs + Hystem-C™. Cardiac function was measured by echocardiography, and all images were interpreted blindly and independently. The LVEFs at baseline (i.e., two hours post-MI) were comparable, indicating similar ischemic injury among groups (Fig. 3A). Representative echocardiography images at 3 weeks are shown as Supplementary Figure 2. Over the 3 week time course of observation, LVEF deteriorated in the Control group. In contrast, Hystem-C™ only or CDCs only preserved LVEF, while CDCs + Hystem-C™ resulted in a net boost in LVEF (p<0.05 vs all other groups; Fig. 3B). The superiority of CDCs + Hystem-C™ is readily apparent upon inspection of the treatment effects, i.e. the changes of LVEF from baseline in each group (Fig. 3C). The highest cardiac function was seen in the CDCs + Hystem-C™ group.
Figure 3. Cardiac function.
Left ventricular ejection fraction (LVEF) measured by echocardiography at baseline (4 hr post MI) (A) and 3 weeks afterwards (B) in various treatment groups (n=7–8 mice per group). (C) Changes of LVEF from baseline to 3 weeks in each group. * indicates p<0.05 when compared to Control. ** indicates p<0.05 when compared to any other group.
Heart morphometry
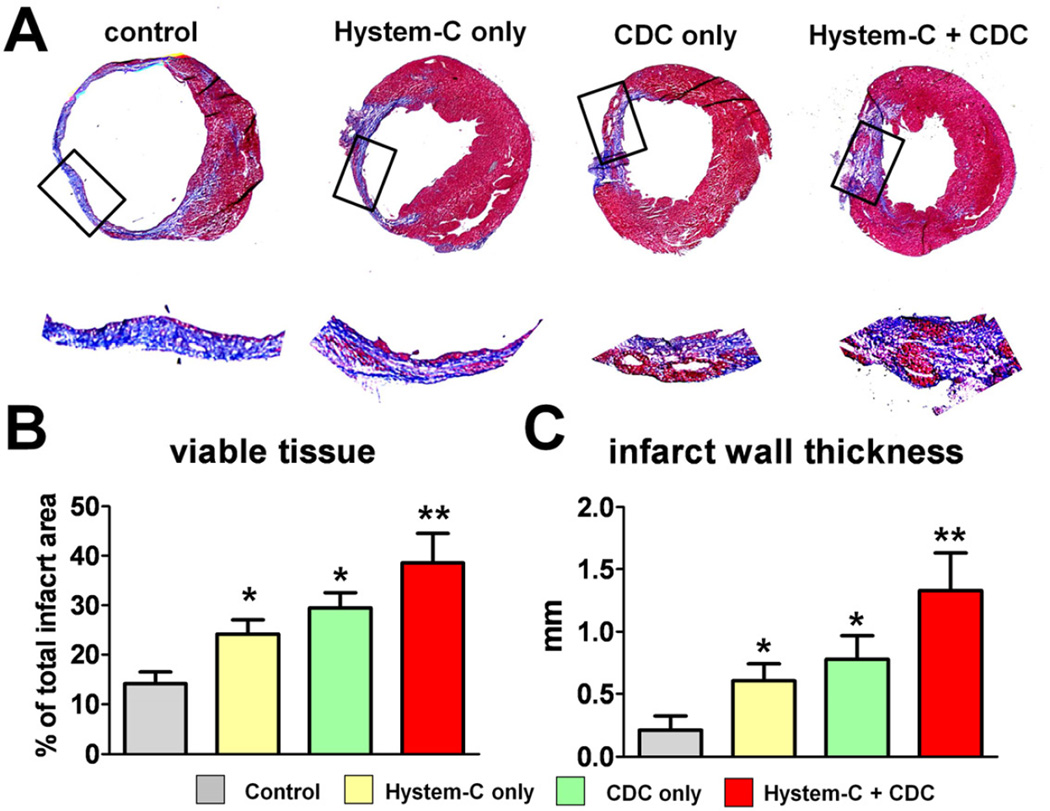
Masson’s trichrome staining clearly distinguished scar tissue (blue) from normal myocardium (pink) (Fig. 4A). Snapshots of the infarct region (black box area) revealed various degrees of infarct to normal ratios. Quantitative morphometry at 3 weeks showed severe LV chamber dilatation and infarct wall thinning in the control (vehicle-injected) hearts (Fig. 4A; “Control” panel). Consistent with our previous report [13], CDC-treated hearts (CDC only group) exhibited attenuated LV remodeling and less abnormal heart morphology (Fig. 4A; “CDC only” panel), with more viable tissue (Fig. 4B) and thicker infarcted walls (Fig. 4C). Hystem-C™-treated hearts (Fig. 4A; “Hystem-C only” panel) also exhibited some degree of heart morphology protection, which is consistent with previous findings that injectable biomaterials attenuate ventricular remodeling though Laplace’s Law. The best heart morphology and the most attenuation of adverse LV remodeling were seen from the CDCs + Hystem-C™ group (Fig. 4A; “Hystem-C + CDC” panel), with the highest amount of viable tissue and thickest infarct wall (Figs. 4B & C).
Figure 4. Heart morphometry analysis.
A, Representative Masson’s trichrome-stained myocardial sections 3 weeks after treatment. Scar tissue and viable myocardium are identified by blue and red color, respectively. The infarct area in the snap-shot box is magnified. B & C, Quantitative analysis and LV morphometric parameters of the Masson’s trichrome images (n=3–5 mice per group). * indicates p<0.05 when compared to Control. ** indicates p<0.05 when compared to any other group.
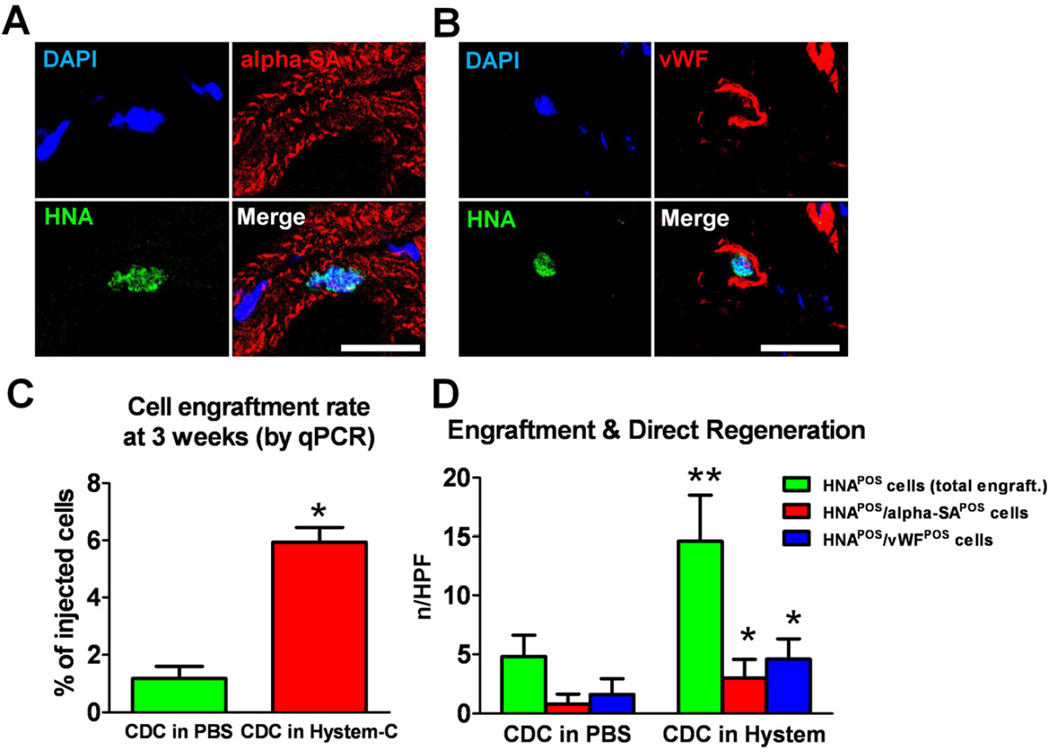
Engraftment and differentiation of CDCs delivered in hydrogel
The animals were sacrificed at 3 weeks after echocardiography. Hearts were excised for histological and PCR analysis of cell engraftment and differentiation. Transplanted CDCs could be detected by the expression of a human nuclei antigen (HNA; Figs. 5A & B green nuclei). Also, cardiac and endothelial differentiation of transplanted CDCs in Hystem-C™ were confirmed with HNAPOS/αSAPOS and HNAPOS/vWFPOS cells (Figs. 5A & B). This indicates that delivering CDCs in hydrogel did not compromise their differentiation capacity. qPCR analysis of cell engraftment at 3 weeks revealed huge decreases in cell numbers compared to 24 hr in both groups (Fig. 5C), as expected and previously described [5]. However, the engraftment superiority of CDCs delivered in hydrogel was sustained over the 3 weeks (Fig. 5C). Histological analysis confirmed that more human CDCs were evident in the CDCs + Hystem-C™ group as compared to CDCs only (Fig. 5D; green bars). With a similar differentiation ratio, the engraftment superiority further translates into more CDC-formed cardiomyocytes and endothelial cells in the CDCs + Hystem-C™ group (Fig. 5C; red and blue bars, respectively).
Figure 5. Long term engraftment and differentiation of CDCs delivered in hydrogel.
A & B, Representative confocal images showing cardiac (A) and endothelial (B) differentiation of CDCs delivered in Hystem-C™ 3 weeks after injection into the mouse hearts. Bars = 50 µm. C, Quantitative PCR analysis of cell engraftment rates in the mouse hearts 3 weeks post injection (n=3). D, Histological analysis of total engrafted CDCs (green bars) and newly-formed cardiomycytes (red bars) and endothelial cells (blue bards) from transplanted CDCs in the mouse hearts (n=5). * indicates p<0.05 when compared to “CDC in PBS”.
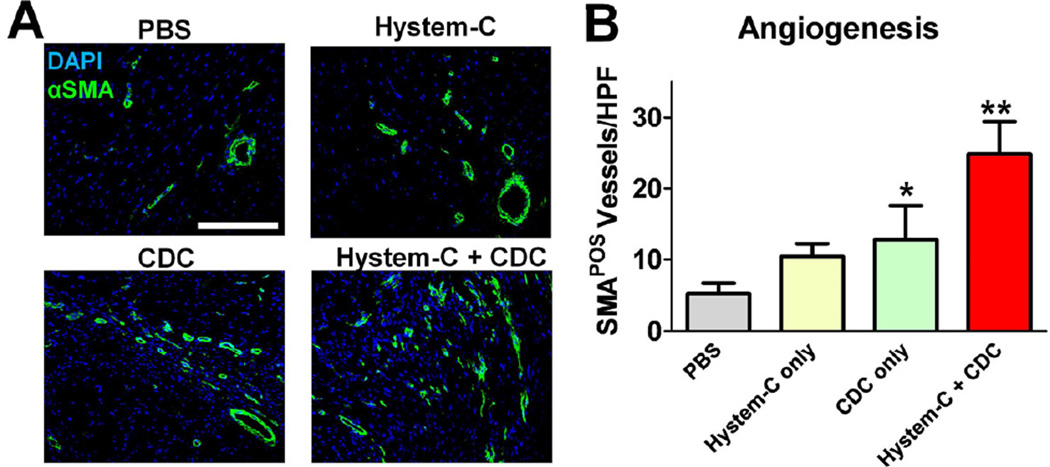
Enhanced neovascularization of CDCs delivered in hydrogel
Although direct differentiation of CDCs was observed (Fig. 5), the absolute numbers of engrafted and differentiated cells seemed insufficient to explain the salient therapeutic superiority (Fig. 3). Therefore, we sought alternative explanations, with a focus on indirect mechanisms such as paracrine effects. It has been shown that CDCs secreted various pro-angiogenic factors (e.g. VEGF, HGF, bFGF) [6]. Thus, we wondered if delivering CDCs in hydrogel promoted angiogenesis. Neovascularization was quantified by counting the SMA-positive lumen structures in the infarct area. Unlike the paucity of SMA-positive vessels in the Control-treated hearts, Hystem-C™ and CDC-treated hearts exhibited more vessels in the infarct area (Fig. 6A), indicating some degree of neovascularization potentiated by the biomaterial and cell therapy. However, the highest neovascularization was seen in the CDCs + Hystem-C™ group (Fig. 6B), nearly double that seen with CDCs alone.
Figure 6. Promotion of angiogenesis by CDC/hydrogel transplantation.
A, Representative confocal images showing alpha smooth muscle actin-positive vasculature in the hearts receiving various treatment products. B, Quantitation of alpha smooth muscle actin-positive vasculature in various groups (n=5 mice per group). * indicates p<0.05 when compared to Control. ** indicates p<0.05 when compared to any other group. Bar = 200 µm.
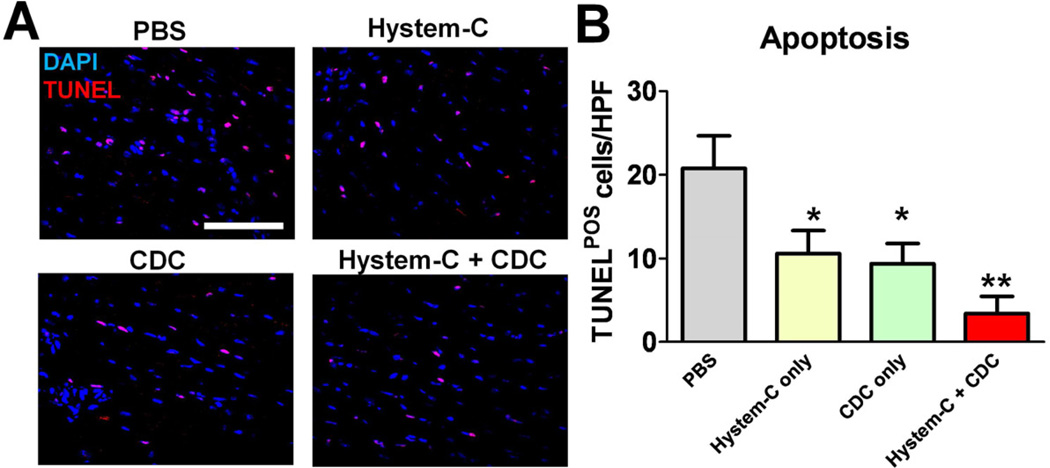
Enhanced tissue preservation of CDCs delivered in hydrogel
Coinciding with regeneration is tissue preservation. Previously we found CDC therapy decreases apoptosis in the post-MI heart, an activity possibly mediated by the anti-apoptotic factors (e.g. IGF) secreted by CDCs [6]. We wondered whether this tissue preservation effect was amplified by combination cell/biomaterial therapy rationalizing that more CDCs are engrafted and the biomaterial itself may also provide support to the injured myocardium. TUNEL staining revealed a reduction of apoptotic cells in the peri-infarct area (Fig. 7A; red nuclei) by CDCs or Hystem-C™ treatment as compared to Control. The least number of apoptotic cells was seen in the CDCs + Hystem-C™ group (Fig. 7B). One should note that at 3 weeks acute inflammation and cell death resulting from ischemia should have resolved. Therefore, the reduction of apoptotic cells is most likely a reflection of adverse remodeling attenuation over the 3 week time course. Nevertheless, our results suggest delivering CDCs in hydrogel maximize their tissue protection capabilities.
Figure 7. Reduction of apoptosis by CDC/hydrogel transplantation.
A, Representative confocal images showing TUNEL-positive nuclei in the hearts receiving various treatment products. B, Quantitation of TUNEL-positive cells in various groups (n=5 mice per group). * indicates p<0.05 when compared to Control. ** indicates p<0.05 when compared to any other group. Bar = 100 µm.
Discussion
In the present study, we tested the cardiac regenerative potential of a cell/biomaterial approach, which couples human cardiosphere-derived cells (CDCs), extensively characterized by our own lab and other groups [6–13, 26–34], with a biopolymer to create an engineered, universal-donor tissue product. Hyaluronan is a glycosaminoglycan component of the extracellular matrix of all connective tissues, making it an attractive scaffold [21]. It is also a crucial component during heart development [35]. Hyaluronan is currently used for a number of FDA-approved clinical applications, including dermal and intra-articular injections. Particularly, the success of the Hystem™ hydrogel for tissue repair has been demonstrated in various organ systems such as cartilage and neural repair [25, 36–38]. Overall, we found delivering CDCs in Hystem-C™ hydrogel maximized cell engraftment and therapeutic efficacy.
To initiate the study, we first compared various unmodified and modified Hystem™ hydrogels. The results indicated that ECM-modification significantly improved cell spreading, viability and activity in the hydrogel (Fig. 1). The RGD (Arg-Gly-Asp) sequences presented in those ECM motifs may play an essential role in anchoring cells via various membrane proteins such as integrins. Delivering CDCs in Hystem-C™ drastically increased acute cell retention rate by nearly an order of magnitude (Fig. 2). Interestingly, non-ECM modified hydrogel (Hystem™) did not help cell retention rate, i.e. retention rate was similar to cells delivered in PBS (Fig. 2B). This suggests that simple physical effects from the hydrogel are not sufficient to enhance cell retention. Rather, more complicated mechanisms (likely cell adhesion and cell-ECM interactions) are involved in the process. Another explanation is that the unmodified Hystem™ increases immediate cell retention (e.g. 10 min or 1 hour). However, since the cells have poor adhesion and survival rate in the matrix (Fig. 1), cell numbers incrementally shrink during the first 24 hours (via wash-out and/or cell death) and eventually retention rates are indistinguishable from the PBS group at 24 hours.
Next, we compared the functional benefit of various treatment groups in a mouse model of acute myocardial infarction. Saline injection did not exert any beneficial effects as LVEFs deteriorated over the 3 week time course (Fig. 3; grey bars). Consistent with our previous findings [13], CDC treatment robustly augmented cardiac function (Fig. 3; yellow bars). Hystem-C™ alone also exhibited beneficial effects, most likely resulted from thickening of the LV wall and reducing wall tension (by Laplace’s Law) [39]. An alternative explanation for the benefit is that the injected hydrogel provided a matrix for the recruitment of endogenous cardiovascular cells and/or progenitor cells [5]. The greatest functional benefit was seen in the Hystem-C™ + CDC group, which had the highest LVEFs at 3 weeks (Fig. 3B) and the most sizable treatment effects (Fig. 3C). Masson trichrome staining followed by quantitative heart morphometry confirmed the healthiest heart morphology from the CDCs + Hystem-C™ group (Fig. 4).
Multiple mechanisms may contribute to the functional superiority of CDCs + Hystem-C™ over CDCs alone. Likely much of the effect could be attributed to the significant improvement in cell retention (24 hr; Fig. 2) and engraftment (3 weeks; Fig. 5C) with the hydrogel. The enhancement of cell engraftment further translated into better functional benefits, through both direct differentiation into a cardiovascular phenotype (Figs. 5A, B & D) and indirect paracrine effects (i.e. more cells engrafted means more pro-angiogenic and anti-apoptotic factors secreted in the myocardium; Figs. 6 & 7) [6, 8]. Since the absolute numbers of engrafted cells were small at 3 weeks (Figs. 5C & D), we postulated that paracrine mechanisms play the major role in mediating endogenous repair. Indeed, the highest neovascularization (Fig. 6) and the lowest tissue apoptosis (Fig. 7) were seen in the CDCs + Hystem-C™ group.
Our study also has several limitations. We used open chest surgery and intramusclar injection to examine the feasibility of application in a small animal model. Minimally-invasive catheter delivery is favorable in clinical scenarios. Studies are on-going in the lab to confirm the feasibility of catheter delivery of CDCs + Hystem-C™. Also, the dose and timing of delivery are subject to optimization in large animal models.
Conclusion
We developed and tested a cell/biomaterial strategy which embeds CDCs within the Hystem-C™ hydrogel. This yields a therapeutic product with high engraftment and functional benefits superior to those of either component alone. Given that both CDC and hyaluronan-based hydrogels have entered human testing, our findings offer the possibility of rapid translation of the product to the clinic.
Supplementary Material
Supplementary Figure 1. Expression of surface makers in human CDCs. Typical expression of c-Kit, CD105, CD90, CD45, and DDR2 is shown. Green lines represent the isotype control population. Red lines represent the labeled population.
Supplementary Figure 2. Representative echocardiography images showing long-axis 2D diastolic and systolic heart images from the Control, Hystem-C only, CDC only, and Hystem-C + CDC groups. The endocardium is outlined with green (diastolic) or yellow (systolic) dash lines.
Acknowledgments
Funding source
This study was supported by Capricor, Inc., by the NIH (U54 HL081028) and TATRC (W81XWH-09-1-0644) to E.M. and by the Cedars-Sinai Board of Governors Heart Stem Cell Center. E.M. is the Mark S. Siegel Family Professor of the Cedars-Sinai Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
E.M. and L.M. are founders and equity holders in Capricor, Inc. The remaining authors report no conflicts.
References
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis DR, Kizana E, Terrovitis J, Barth AS, Zhang Y, Smith RR, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis DR, Smith RR, Marbán E. Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells. 2010;28:903–904. doi: 10.1002/stem.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Li T-S, Cheng K, Lee S-T, Matsushita S, Davis D, Malliaras K, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 14.Makkar R, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Czer LSC, et al. Intracoronary cardiosphere-derived cells for hear regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;10(379):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Kindi A, Ge Y, Shum-Tim D, Chiu RC. Cellular cardiomyoplasty: routes of cell delivery and retention. Front Biosci. 2008;13:2421–2434. doi: 10.2741/2855. [DOI] [PubMed] [Google Scholar]
- 17.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Karen LC, Andrew JV, Qizhi F, Richard ES, Hubert HF, Randall JL. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Cheng K, Shen D, Smith J, Galang G, Sun B, Zhang J, et al. Transplantation of platelet gel spiked with cardiosphere-derived cells boosts structural and functional benefits relative to gel transplantation alone in rats with myocardial infarction. Biomaterials. 2012;33:2872–2879. doi: 10.1016/j.biomaterials.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng K, Malliaras K, Shen D, Tseliou E, Ionta V, Smith J, et al. Intramyocardial injection of platelet gel promotes endogenous repair and augments cardiac function in rats with myocardial infarction. J Am Coll Cardiol. 2012;59:256–264. doi: 10.1016/j.jacc.2011.10.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64:1591–1596. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 23.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2007;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 24.Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, et al. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 25.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghila Rani KG, Kartha CC. Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth Factors. 2010;28:157–165. doi: 10.3109/08977190903512628. [DOI] [PubMed] [Google Scholar]
- 27.Chimenti I, Rizzitelli G, Gaetani R, Angelini F, Ionta V, Forte E, et al. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials. 2011;32:9271–9281. doi: 10.1016/j.biomaterials.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Gaetani R, Ledda M, Barile L, Chimenti I, De Carlo F, Forte E, et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc Res. 2009;82:411–420. doi: 10.1093/cvr/cvp067. [DOI] [PubMed] [Google Scholar]
- 29.Hodgkiss-Geere HM, Argyle DJ, Corcoran BM, Whitelaw B, Milne E, Bennett D, et al. Characterisation and cardiac directed differentiation of canine adult cardiac stem cells. Vet J. 2011;191:176–182. doi: 10.1016/j.tvjl.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Koninckx R, Daniëls A, Windmolders S, Carlotti F, Mees U, Steels P, et al. Mesenchymal stem cells or cardiac progenitors for cardiac repair? a comparative study. Cell Mol Life Sci. 2011;68:2141–2156. doi: 10.1007/s00018-010-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, et al. Controlled delivery of basic fibroblast crowth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aszódi A, Legate KR, Nakchbandi I, Fässler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 36.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toh WS, Lee EH, Guo X-M, Chan JKY, Yeow CH, Choo AB, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J, Chan A, Morad L, Kornblum HI, Guoping Fan, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Expression of surface makers in human CDCs. Typical expression of c-Kit, CD105, CD90, CD45, and DDR2 is shown. Green lines represent the isotype control population. Red lines represent the labeled population.
Supplementary Figure 2. Representative echocardiography images showing long-axis 2D diastolic and systolic heart images from the Control, Hystem-C only, CDC only, and Hystem-C + CDC groups. The endocardium is outlined with green (diastolic) or yellow (systolic) dash lines.