Abstract
OBJECTIVES
To determine safety and tolerability of lowering blood pressure in older adults with lacunar stroke.
DESIGN
Cohort study.
SETTING
The Secondary Prevention of Small Subcortical Strokes (SPS3) Trial, which compared the efficacy of two systolic blood pressure (SBP) targets (<130 mmHg and 130–149 mmHg) for secondary stroke prevention.
PARTICIPANTS
Of 3,020 SPS3 participants, 494 aged 75 and older at baseline were used in these analyses.
MEASUREMENTS
Rates of side effects related to lowering SBP and clinical outcomes, including stroke recurrence and vascular death, were examined.
RESULTS
Older participants achieved SBP levels similar to those of younger participants (mean SBP of 125 mmHg and 137 mmHg in lower and higher SBP target groups, respectively). At least once during the approximately 3.5 years of follow-up, 21% reported dizziness, and 15% reported lightheadedness when standing; the only significant difference between the younger and older groups was unsteadiness when standing (23% vs 32% respectively, P < .001). There was no difference according to treatment group. In younger adults, recurrent stroke was less likely in the lower than the higher SBP group (hazard ratio (HR) = 0.77, 95% confidence interval (CI) = 0.59–1.01) but not in older participants (HR = 1.01, 95% CI = 0.59– 1.73), although the interaction was not significant (P = .39). The lower SBP target was associated with a significant reduction in vascular death in older participants (HR = 0.42, 95% CI = 0.18–0.98), with a significant interaction between age and SBP group (P = .049).
CONCLUSION
Except for unsteadiness when standing, there was no difference according to age in individuals with lacunar stroke with respect to side effects potentially related to lowering blood pressure. Although the lower SBP target was not associated with lower likelihood of recurrent stroke, these exploratory analyses suggested a possible benefit related to vascular death.
Keywords: blood pressure management, hypertension, ischemic stroke, stroke prevention, lacunar stroke
Hypertension is a powerful independent risk factor for stroke, and reducing blood pressure (BP) is an effective intervention to prevent stroke.1–5 Although they are at greatest risk of stroke,6 data from several studies suggests that BP is less well controlled in older adults.7–9 Under-treatment may be related to concerns about side effects or interactions, both of which may be more common in older than younger adults.10,11
Several trials have demonstrated that hypertension treatment in older adults can be undertaken with minimal risk.12–15 Furthermore, the Hypertension in the Very Elderly Trial (HYVET) showed a 30% reduction in stroke (P = .06) and 21% reduction in mortality (P = .02) with BP lowering.13 Given that only 7% of participants in HY-VET had a history of stroke, the safety and effectiveness of BP control in older adults with established cerebral small vessel disease could not be studied.
Elderly adults are the fastest-growing segment of the population in developed countries and will contribute significantly to a growing stroke burden. This underscores the need for evidence-based information on targeted risk factor management for this population. The Secondary Prevention of Small Subcortical Strokes (SPS3) Trial was undertaken to define efficacious therapies for prevention of recurrent stroke and cognitive decline in individuals with symptomatic lacunar (subcortical) stroke.16 There was a nonsignificant reduction in recurrent stroke associated with SBP lowering (hazard ratio (HR) = 0.81, 95% confidence interval (CI) = 0.64–1.03) and a nonsignificant interaction with age (<65 vs ≥65; P = .53).17 The objectives of the present analyses were to examine the safety and tolerability of lowering BP in the older subgroup of participants in the SPS3 trial.
METHODS
Design and Sample
SPS3 was a randomized, multicenter clinical trial conducted between March 2003 and April 2011. Details of the study design and execution have been published elsewhere.16–19 Eligible individuals had experienced a symptomatic lacunar stroke within the 6 months before enrollment, verified using magnetic resonance imaging (MRI). Individuals with prior cortical stroke, ipsilateral carotid stenosis greater than 50%, or major cardioembolic sources were excluded. There was no upper age exclusion. Participants were randomized, in a two-by-two factorial design to an antiplatelet intervention and two targets of SBP control: higher (130–149 mmHg) and lower (<130 mmHg). Outcomes included recurrent stroke (all strokes); myocardial infarction; and death, including all death, vascular death, and nonvascular death. Definitions have been published elsewhere,16 but vascular death was defined as death attributable to an ischemic or hemorrhagic stroke or sudden death attributed to cardiac ischemic or after a well-documented vascular event. All events were subject to blinded adjudication. The institutional review boards of all participating centers approved the SPS3 study, and all participants provided written informed consent.
Procedures
All participants were seen at least monthly for the initial 3 months after randomization and quarterly thereafter. BP was measured in the sitting and standing positions at each follow-up visit to assess for postural hypotension (defined as a decrease in BP from sitting to standing position of systolic >20 mmHg or diastolic >10 mmHg). A standard set of questions was asked to assess for medication side effects and adverse events and to detect outcome events.
If participants refused or were unable (related to side effects or outcome events) to continue with their randomly assigned treatments, they were designated as “inactive” for that treatment. Participants whose SBP could not be lowered to their assigned target or who experienced intolerable side effects of antihypertensive drugs despite trying multiple agents were designated as “failure to achieve target.” All participants were followed to a common end-study date, regardless of active, inactive, or failure to achieve target status and outcome events.
Data Analyses
For purposes of this exploratory analysis, the older subgroup was defined a priori as participants aged 75 and older at study entry20 and compared with those younger than 75. Baseline characteristics and clinical outcomes are presented according to age group. Frequencies and percentages are presented for categorical measures and means and standard deviations for quantitative measures. Chi-square tests of general association and independent-sample t-tests were used as appropriate for categorical and quantitative variables, respectively. The occurrences of safety outcomes were quantified using odds ratios and 95% CIs. Time to efficacy outcomes were quantified using HRs and 95% CIs. Differences in SBP target efficacy between the younger and elderly groups were evaluated by testing for significant interactions in Cox proportional hazards models. All tests of significance were two-sided, and unadjusted P-values are presented. Because of multiple comparisons, an alpha level of less than .01 was selected to indicate statistical significance for main effect; a more-liberal significance level of .05 was used to evaluate statistical significance of interaction terms. SAS version 9.2 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
RESULTS
Of the 3,020 participants, 494 (16%) were aged 75 and older at SPS3 study entry, 288 (58%) of whom were aged 75 to 79, 153 (31%) aged 80 to 84, and 53 (11%) aged 85 and older. Older participants were more likely to be hypertensive (P = .004) and less likely to be current smokers (P < .001) and have diabetes mellitus (P < .001) (Table 1). Older participants were more likely to have multiple old subcortical infarcts according to MRI imaging (44.5%) than younger participants (38.7%) (P = .02) and more-severe white matter hyperintensities (38.4% vs 18.6%, P < .001). Although modified Rankin Scale scores at study entry were similar between age groups, a significantly smaller proportion of older adults scored greater than 90 on the Cognitive Abilities Screening Index (CASI) (28.8%) than of younger individuals (47.6%) (P < .001). There were no differences in baseline characteristics according to treatment group within either age group (P > .01; data not shown).
Table 1.
Baseline Demographic and Risk Factor Profile According to Age
| Variable | ≥75, n = 494 | <75, n = 2,526 | P-Value |
|---|---|---|---|
| Age, mean ± SD | 79.9 ± 3.8 | 60.1 ± 8.4 | <.001 |
| Male, n (%) | 268 (54.3) | 1,634 (64.7) | <.001 |
| Race and ethnicity, n (%) | |||
| Non-Hispanic white | 288 (58.3) | 1,250 (49.5) | <.001 |
| Hispanic | 163 (33.0) | 753 (29.8) | |
| Black | 31 (6.3) | 461 (18.3) | |
| Other or multiple | 12 (2.4) | 62 (2.5) | |
| Medical history, n (%) | |||
| Current smoking | 26 (5.3) | 591 (23.4) | <.001 |
| Hypertension | 384 (77.7) | 1,804 (71.4) | .004 |
| Diabetes mellitus | 133 (26.9) | 973 (38.5) | <.001 |
| Hyperlipidemia | 231 (46.8) | 1,240 (49.1) | .34 |
| Prior subcortical stroke or transient ischemic attacka | 70 (14.2) | 378 (15.0) | .65 |
| Ischemic heart disease | 51 (10.3) | 266 (10.5) | .89 |
| Family history of stroke | 128 (27.1) | 741 (31.1) | .08 |
| Selected laboratory values, mean ± SD | |||
| Estimated glomerular filtration rate | 66.0 ± 14.7 | 83.0 ± 18.3 | <.001 |
| Random glucose, mg/dLa | 114.5 ± 37.8 | 127.8 ± 57.5 | <.001 |
| Glycosylated hemoglobin, %b | 7.3 ± 1.4 | 8.4 ± 2.3 | <.001 |
| Total cholesterol, mg/dLa | 182.0 ± 40.8 | 189.1 ± 48.2 | .001 |
| Low-density lipoprotein cholesterol, mg/dLa | 106.8 ± 34.7 | 113.4 ± 40.7 | <.001 |
| High-density lipoprotein cholesterol, mg/dLa | 49.6 ± 20.9 | 44.6 ± 17.8 | <.001 |
| Triglycerides, mg/dLa | 137.5 ± 76.7 | 169.8 (119.5) | <.001 |
| Magnetic resonance imaging and magnetic resonance angiography findings, n (%) | |||
| Multiple subcortical infarcts | 218 (44.5) | 972 (38.7) | .02 |
| White matter hyperintensities | |||
| None to mild | 131 (27.2) | 1,365 (54.7) | <.001 |
| Moderate | 116 (19.9) | 668 (26.8) | |
| Severe | 185 (38.4) | 464 (18.6) | |
| Intracranial stenosis >50% | |||
| Anterior circulation | 44 (9.5) | 225 (9.5) | .99 |
| Posterior circulation | 67 (14.4) | 245 (10.3) | .009 |
| Functional status | |||
| Barthel Index, mean ± SDc | 93.1 ± 12.7 | 95.9 ± 9.0 | <.001 |
| Modified Rankin Scale score 0–1, n (%)d | 322 (65.2) | 1,689 (66.9) | .47 |
| Depressed, n (%) | 31 (13.1) | 267 (22.8) | .001 |
| Cognitive Abilities Screening Instrument score >90, n (%)a,e | 139 (28.8) | 1,189 (47.6) | <.001 |
Missing data.
Participants with diabetes mellitus only.
Range 0 to 100, higher scores indicating better functional ability.
Normal to near-normal recovery.
Range 0 to 100, higher scores indicating better cognitive function.
SD = standard deviation.
Safety and Tolerability of Lowering SBP
Average follow-up time was 3.5 ± 1.9 years for the older group and 3.7 ± 2.1 years for the younger group (P = .04). Mean baseline SBP and time to achieve target was not significantly different between age cohorts (Table 2). Average achieved SBP for the older group (excluding the initial 6 months) was 125 ± 16 mmHg for the lower SBP target and 137 ± 15 mmHg for the higher SBP target, the same the younger participants achieved. Approximately 75% of each group were within their assigned SBP target at more than 50% of their quarterly visits, most participants in both groups self-reported adherence as excellent or good, and older participants were as likely to be always active in their assigned treatment arm (73%) as their younger counterparts (76%) (P = .39). Looking separately at the two SBP targets, older adults assigned to the lower SBP target were less likely to be actively managed to achieve their assigned target than those in the younger group (71.8% vs 77.4%; P = .04). The older group was prescribed fewer antihypertensive medications, on average, than the younger group, although the differences were significant only at the end-study visit (P = .003). This was true for every class of medication with the exception of angiotensin receptor blockers (ARBs), with a higher percentage of the older group prescribed ARBs at baseline, Year 1, and the end-study visit, although differences were not significant (P = .54; Figure S1).
Table 2.
Management of Systolic Blood Pressure (SBP) According to Age
| Variable | ≥75, n = 494 | <75, n = 2,526 | P-Value |
|---|---|---|---|
| Assigned SBP group, n (%) | |||
| Lower target (<130 mmHg) | 248 (50.2) | 1,253 (49.6) | |
| Higher target (130–140 mmHg) | 246 (49.8) | 1,273 (50.4) | |
| SBP at study entry, mean ± SD | 144.4 ± 20.0 | 142.7 ± 18.6 | .09 |
| SBP at end-study visit, mean ± SD | 130.3 ± 17.3 | 131.9 ± 16.9 | .07 |
| SBP at quarterly follow-ups, mean ± SDa | |||
| Lower target (<130 mmHg) | 125.2 ± 15.8 | 125.1 ± 14.4 | .64 |
| Higher target (130–140 mmHg) | 137.1 ± 14.6 | 137.1 ± 14.4 | .95 |
| Months to achieve SBP target, mean ± SD | 5.6 ± 6.1 | 5.9 ± 7.3 | .39 |
| Quarterly visits within assigned target, %, n (%) | |||
| ≤25 | 50 (10.4) | 226 (9.2) | .31 |
| >25–49 | 74 (15.4) | 354 (14.3) | |
| >50–74 | 168 (34.9) | 879 (35.6) | |
| ≥75 | 190 (39.4) | 1,009 (40.9) | |
| Antihypertensive medication adherence excellent or good at >75% of quarterly visits, n (%) | 423 (92.4) | 2,128 (91.4) | .50 |
| Always active in SBP treatment group, n (%) | 360 (72.9) | 1,913 (75.7) | .39 |
| Taking antihypertensive medications at study entry, n (%) | 433 (87.7) | 2,124 (84.1) | .04 |
| Number of antihypertensive medications, mean ± SD | |||
| Baseline | 1.6 (1.1) | 1.7 (1.2) | .19 |
| Year 1 visit | 2.0 (1.4) | 2.1 (1.4) | .17 |
| End-study visit | 1.9 (1.3) | 2.1 (1.5) | .003 |
| Years of follow-up, mean ± SD | 3.5 ± 1.9 | 3.7 ± 2.1 | .04 |
Excluding initial 6 months.
SD = standard deviation.
Percentages of symptoms possibly related to BP lowering were examined according to treatment group within the two age groups, and the treatment groups were merged and compared according to age (Table 3). The percentages represent participants who reported the symptoms at least once over the approximately 20 follow-up visits. When examined according to treatment group, the percentage of participants with postural hypotension recorded at least once over the course of the study was significantly higher in the higher SBP target group than in the lower SBP target group for both age cohorts. There were no other significant differences according to treatment group for either age group. Merging the treatment groups, older participants were more likely to report symptoms possibly related to BP lowering, although only unsteadiness when standing was significantly higher (32% vs 23%, respectively; P < .001). There was no difference in the percentage of older and younger participants with at least one episode of postural hypotension recorded over the course of the study (both 59%, P = .82). Serious complications of hypotension were defined as events such as orthostatic syncope or falls associated with hypotension that required urgent medical evaluation or treatment, were life threatening, or resulted in permanent health consequences. The number of serious complications of hypotension events was small, with only seven (1.4%) in the older group and 31 (1.2%) in the younger group. An examination according to treatment group showed no significant differences in serious complications of hypotension for the older or younger cohort. Combining the target groups resulted in a nonsignificantly higher rate of all complications in the older (0.39% per participant-year) than the younger group (0.32% per participant year) (P = .67).
Table 3.
Safety of Lowering Systolic Blood Pressure (SBP) According to Age and Treatment Group
| Variable | ≥75 |
<75 |
P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall, (n = 494) | Higher Target, n = 246 | Lower Target, n = 248 | P-Value | Overall, n = 2,526 | Higher Target, n = 1,273 | Lower Target, n = 1,253 | P-Value | ||
| Participants with ≥1 episodes of postural hypotension recorded during trial, n (%) | 290 (59) | 159 (65) | 131 (53) | .008 | 1,497 (59) | 785 (62) | 712 (57) | .01 | .82 |
|
| |||||||||
| Symptoms possibly associated with lowering SBP (ever reported), n (%) | |||||||||
|
| |||||||||
| Unsteadiness when standing | 159 (32) | 77 (33) | 82 (34) | .72 | 571 (23) | 278 (23) | 293 (24) | .36 | <.001 |
|
| |||||||||
| Blurred vision when standing | 33 (7) | 19 (8) | 14 (6) | .34 | 155 (6) | 84 (7) | 71 (6) | .32 | .64 |
|
| |||||||||
| Dizziness when standing | 104 (21) | 51 (22) | 53 (22) | .90 | 524 (21) | 253 (21) | 271 (22) | .29 | .86 |
|
| |||||||||
| Lightheadedness when standing | 76 (15) | 45 (19) | 31 (13) | .07 | 382 (15) | 191 (16) | 191 (16) | .88 | .87 |
|
| |||||||||
| Palpitations when standing | 18 (5) | 4 (2) | 14 (17) | .03 | 77 (3) | 44 (4) | 33 (3) | .23 | .48 |
|
| |||||||||
| Serious complications of hypotension, n (% per person-year) | 7 (0.39) | 2 (0.23) | 5 (0.53) | .29 | 31 (0.32) | 13 (0.27) | 18 (0.37) | .36 | .67 |
|
| |||||||||
| Orthostatic syncope | 3 (0.17) | 1 (0.11) | 2 (0.21) | .58 | 13 (0.13) | 4 (0.08) | 9 (0.18) | .18 | .73 |
|
| |||||||||
| Stroke associated with hypotension | 1 (0.06) | 0 (0) | 1 (0.11) | >.99 | 2 (0.02) | 1 (0.02) | 1 (0.02) | .99 | .44 |
|
| |||||||||
| Myocardial infarction associated with hypotension | 0 (0) | 0 (0) | 0 (0) | N/A | 0 (0) | 0 (0) | 0 (0) | N/A | N/A |
|
| |||||||||
| Fall with injury secondary to hypotension | 1 (0.06) | 0 (0) | 1 (0.11) | >.99 | 2 (0.02) | 0 (0) | 2 (0.04) | >.99 | .44 |
N/A = not applicable.
Safety was further examined in the older participants to see whether adverse events were more likely in very old participants (Table S1). Although very old participants (≥85) had more episodes of postural hypotension and reported more symptoms related to lowering blood pressure, except for blurred vision when standing (P = .002), none of the differences were statistically significant.
Effectiveness of Lowering SBP
Pooling the two SBP treatment groups, older participants were more likely to experience intracranial hemorrhage (HR = 2.31, 95% CI = 1.10–4.83), disabling or fatal stroke (HR = 1.94, 95% CI = 1.19–3.17), and death (HR = 3.38, 95% CI = 2.52–4.54) than younger participants. There were no differences in recurrent stroke (ischemic and hemorrhagic; HR = 1.29, 95% CI = 0.95– 1.75) or myocardial infarction (HR = 0.92, 95% CI = 0.47–1.80) between the two age groups.
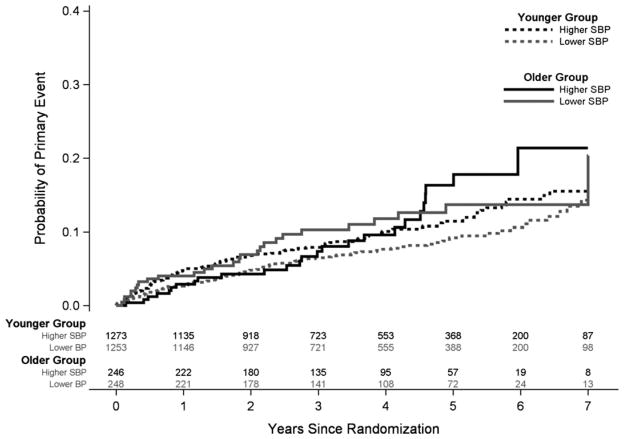
As seen in Table 4, in younger adults, recurrent stroke was less likely in the lower than the higher SBP group (hazard ratio (HR) = 0.77, 95% confidence interval (CI) = 0.59–1.01) but not in older participants (HR = 1.01, 95% CI = 0.59–1.73), although the interaction was not significant (P = .39) (Figure 1). The lower SBP target was associated with significantly fewer disabling and fatal strokes in older participants (HR = 0.40, 95% CI = 0.17–0.96), which was not present in the younger group (HR = 1.03, 95% CI = 0.63–1.67); the interaction with age was nonsignificant (P = .19). The lower SBP target was also associated with significantly fewer vascular deaths in the elderly participants (HR = 0.42, 95% CI = 0.18–0.98) but not the younger group (HR = 1.17, 95% CI = 0.68–2.01) (P = .049). This difference in vascular death was further examined in Cox models adjusting for sex, race and ethnicity, diabetes mellitus, hypertension, ischemic heart disease, hyperlipidemia, and smoking status. In the adjusted model, the interaction between age and SBP target group remained statistically significant (P = .03), and a consistent association between lower SBP target and fewer vascular deaths (HR = 0.39, 95% CI = 0.16–0.92) was observed in older participants. An examination of the interaction for treatment effect and age group was not significant for myocardial infarction (P = .75) or all-cause mortality (P = .29).
Table 4.
Estimates of Hazard Ratio for Efficacy Outcomes Measures According to Systolic Blood Pressure (SBP) Treatment Group for Older and Younger Participants
| Outcome | ≥75 |
<75 |
||||||
|---|---|---|---|---|---|---|---|---|
| Higher SBP (130–149 mmHg) | Lower SBP (<130 mmHg) | HR (95% CI) | P-Value | Higher SBP (130–149 mmHg) | Lower SBP (<130 mmHg) | HR (95% CI) | P-Value | |
|
|
|
|||||||
| n (%/p-y) | n (%/p-y) | |||||||
| Stroke | ||||||||
| All | 26 (3.07) | 27 (3.07) | 1.01 (0.59–1.73) | .98 | 126 (2.72) | 98 (2.09) | 0.77 (0.59–1.01) | .06 |
| Ischemic or unknown | 19 (2.24) | 24 (2.73) | 1.23 (0.67–2.24) | .50 | 112 (2.41) | 88 (1.88) | 0.78 (0.59–1.03) | .08 |
| Intracranial hemorrhage | 7 (0.83) | 3 (0.34) | 0.41 (0.11–1.59) | .20 | 14 (0.16) | 10 (0.10) | 0.71 (0.32–1.60) | .41 |
| Disabling or fatal | 17 (2.01) | 7 (0.80) | 0.40 (0.17–0.96) | .04 | 32 (0.69) | 33 (0.71) | 1.03 (0.63–1.67) | .91 |
| Myocardial infarction | 6 (0.69) | 5 (0.53) | 0.77 (0.23–2.52) | .66 | 34 (0.70) | 31 (0.64) | 0.91 (0.56–1.48) | .71 |
| Death | ||||||||
| All | 40 (4.53) | 37 (3.89) | 0.83 (0.53–1.29) | .41 | 61 (1.24) | 69 (1.40) | 1.13 (0.80–1.59) | .49 |
| Vasculara | 17 (1.93) | 8 (0.84) | 0.42 (0.18–0.98) | .05 | 24 (0.49) | 28 (0.57) | 1.17 (0.68–2.01) | .58 |
| Nonvascular | 15 (1.70) | 18 (1.89) | 1.08 (0.54–2.14) | .83 | 20 (0.41) | 22 (0.45) | 1.10 (0.60–2.01) | .77 |
| Uncertain | 8 (0.91) | 11 (0.16) | 1.22 (0.49–3.03) | .67 | 17 (0.34) | 19 (0.38) | 1.11 (0.58–2.14) | .75 |
Interaction between SBP target group and age, P = .049.
p-y = person-year.
Figure 1.
Cumulative probability estimates of the rate of all recurrent strokes, according to treatment group and age group. P for interaction = .39. SBP = systolic blood pressure.
DISCUSSION
This analysis of older adults (mean age 80) with recent lacunar stroke enrolled in the SPS3 Trial demonstrates that it is possible to lower SBP safely in elderly adults with established small vessel disease. The majority were already receiving treatment for hypertension at study entry, but through careful execution of the SPS3 protocol, SBP was reduced to a mean of 125 mmHg in the lower SBP group and 137 mmHg in the higher SBP group. Regardless of treatment group, older participants were no more likely than their younger counterparts to experience serious adverse effects related to BP lowering.
Several recent trials have investigated optimal BP targets in elderly adults and have similarly shown that SBP can be lowered safely in older adults.12–15 The HYVET Study was able to lower SBP safely to a mean of 144 mmHg in participants aged 80 and older at study entry.13,21 The Japanese trial to assess optimal SBP in individuals with hypertension aged 65 to 85 (JATOS) reported SBP of 136 mmHg in the strict treatment group and 146 mmHg in the mild treatment group after 2 years of treatment, with no differences in adverse events between the two treatment groups.12 Similar SBP values were safely achieved in the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study for participants aged 70 to 84, with 137 mmHg in the strict control group and 142 mmHg in the moderate control group after 3 years of follow-up.14
The mean SBP achieved in the lower group in older participants in SPS3 was lower than previously reported targets. To guide safe management of SBP into the randomly assigned higher and lower targets, an algorithm was developed and distributed to the sites.22 Within the context of a clinical trial, SBP was lowered slowly over time (mean of 6 ± 6 months to achieve target), and participants were followed at least monthly to ascertain side effects, assess adherence, and titrate medications until they achieved target SBP and quarterly thereafter. Almost 75% were within their assigned SBP target for more than 50% of their study visits, confirming that it is feasible and safe to achieve even lower targets than those previously used in older adults. Furthermore, the rate of serious complications of hypotension in SPS3 was not significantly different according to age or treatment group. Safety was not significantly different among the older subgroups, although the numbers in these older subgroups were small, and the rate of serious complications was low.
This exploratory analysis found that lowering SBP to a mean of 125 mmHg (lower SBP target) did not result in fewer recurrent strokes than lowering it to a mean of 137 mmHg (higher SBP target) in the older participants. Results from previous studies have been inconsistent in the association between lowering SBP and stroke reduction.12–15 The HYVET study reported a 30% lower rate of stroke (fatal or nonfatal) and a 21% lower rate of death from any cause associated with an achieved mean SBP of 144 mmHg than a mean of approximately 158 mmHg in the placebo group.13 In contrast, the JATOS trial reported no differences in a composite outcome (cardiovascular disease and renal failure) and death between the two SBP groups.12 Similarly, the VALISH study did not find significantly fewer composite cardiovascular events outcome associated with the strict control (HR = 0.89, 95% CI = 0.60–1.34).14
The recent guidelines recommend lowering SBP to less than 150 mmHg in elderly adults.23,24 It is not known whether these guidelines should be applied to older adults with established small vessel disease, such as the population studied in SPS3. The above studies that contributed to the guideline evidence were different from SPS3 in target population and features of study design. Only 4% to 7% of participants in the other trials had a history of stroke, and the percentage with diabetes mellitus ranged from 7% to 13%. In contrast, all older participants in SPS3 had had at least one lacunar stroke and almost one-third had diabetes mellitus. The above studies tested specific antihypertensive medications for lowering BP, whereas the goal of SPS3 was to test two specific targets and not medications. Although the results of this analysis suggest that there is no benefit in overall stroke reduction for an achieved average SBP as low as 125 mmHg in people with established small vessel disease, there was also no harm associated with this target. Furthermore, the results suggest a possible benefit related to vascular death. An age by treatment group interaction demonstrated 58% lower mortality from vascular causes in the lower SBP group for the older cohort (which was significant) and no association in the younger cohort. This lower vascular mortality in elderly adults is consistent with findings from the HYVET trial, which demonstrated a lower rate of cardiovascular death in the main trial (HR = 0.77, 95% CI = 0.60–1.01) in the elderly group assigned to BP lowering than in those receiving standard care and in the HYVET extension (HR = 0.19, 95% CI = 0.04–0.87).13,21
The exploratory nature of the analyses and the small sample of older participants suggest that caution should be taken in drawing conclusions related to BP lowering in elderly adults with lacunar stroke. The lack of interactions between SBP targets and age may suggest similar effectiveness for the lower SBP target in the older and younger groups but could also reflect insufficient power to detect interactions. Furthermore, these older stroke survivors were participants in a clinical trial and, by nature of agreeing to participate, may have been healthier than the general population of older adults with lacunar stroke. Participants were carefully selected to ensure that they would be adherent to the study procedures, which could be taxing, particularly on older participants, but a comparison with elderly adults (≥80) participating in a population-based study of stroke conducted in Dijon, France, with approximately 25% of stroke classified as lacunar infarcts25 suggests that the older SPS3 subgroup was similar to the general population with lacunar stroke, with a similar prevalence of hypertension (78% in SPS3, 73% in Dijon cohort) and an even greater prevalence of diabetes mellitus and hyperlipidemia (27% and 47%, respectively) than in the Dijon cohort (16% and 27%, respectively).
To summarize, lowering SBP in older adults with lacunar stroke was well tolerated. The lack of significant differences in subjective side effects potentially related to lowering blood pressure, except for unsteadiness when standing, between the younger and older adults suggests that lowering blood pressure to these levels is as safe in elderly as in younger individuals. Given a clinical indication in which an older adult with lacunar stroke would benefit from a lower BP treatment goal, a lower level of SBP could be targeted, with usual monitoring for orthostatic symptoms.
Supplementary Material
Acknowledgments
This study used data from the SPS3 Study, which was funded by National Institute of Neurological Disorders and Stroke Grant 2 U01 NS38529–04A1 (PI: Oscar R. Benavente). All coauthors had some portion of their salary funded for their contributions to this multisite study.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Author Contributions: White C.L.: conceived of the study, conducted the background literature search, and wrote the first draft of the manuscript. Szychowski J.M. and Peri K.: conducted the analysis and wrote the statistical methods section. Pergola P.E., Field T.S., Talbert R., Lau H., and Benavente O.R.: revised the manuscript and provided input on interpretation of the data.
Sponsor’s Role: None.
Additional Supporting Information may be found in the online version of this article:
Table S1. Examination of Safety within Older Subgroups.
Figure S1. Antihypertensive medications at baseline, Year 1, and end-study visits stratified according to age.
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 2.Gueyffier F, Boissel JP, Boutitie F, et al. Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators. Stroke. 1997;28:2557–2562. doi: 10.1161/01.str.28.12.2557. [DOI] [PubMed] [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: A systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681–1692. doi: 10.1016/S0140-6736(11)60516-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 7.Borzecki AM, Glickman ME, Kader B, et al. The effect of age on hypertension control and management. Am J Hypertens. 2006;19:520–527. doi: 10.1016/j.amjhyper.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 10.Brescacin L. Is it possible to apply secondary stroke prevention guidelines to very old populations? Cardiovasc Hematol Disord Drug Targets. 2011;11:9–16. doi: 10.2174/187152911795945150. [DOI] [PubMed] [Google Scholar]
- 11.Sanossian N, Ovbiagele B. Prevention and management of stroke in very elderly patients. Lancet Neurol. 2009;8:1031–1041. doi: 10.1016/S1474-4422(09)70259-5. [DOI] [PubMed] [Google Scholar]
- 12.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31:2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 13.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 14.Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension Study. Hypertension. 2010;56:196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang X, Liu L, et al. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J. 2011;32:1500–1508. doi: 10.1093/eurheartj/ehr039. [DOI] [PubMed] [Google Scholar]
- 16.Benavente OR, White CL, Pearce L, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: The SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White CL, Szychowski JM, Roldan A, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis. 2013;22:764–774. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alamowitch S, Eliasziw M, Algra A, et al. Risk, causes, and prevention of ischaemic stroke in elderly patients with symptomatic internal-carotidartery stenosis. Lancet. 2001;357:1154–1160. doi: 10.1016/S0140-6736(00)04332-4. [DOI] [PubMed] [Google Scholar]
- 21.Beckett N, Peters R, Tuomilehto J, et al. Immediate and late benefits of treating very elderly people with hypertension: Results from active treatment extension to hypertension in the very elderly randomised controlled trial. BMJ. 2012;344:d7541. doi: 10.1136/bmj.d7541. [DOI] [PubMed] [Google Scholar]
- 22.Pergola PE, White CL, Szychowski JM, et al. Achieved blood pressures in the Secondary Prevention of Small Subcortical Strokes (SPS3) study: Challenges and lessons learned. Am J Hypertens. 2014;27:1052–1060. doi: 10.1093/ajh/hpu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 25.Bejot Y, Rouaud O, Jacquin A, et al. Stroke in the very old: Incidence, risk factors, clinical features, outcomes and access to resources—a 22-year population- based study. Cerebrovasc Dis. 2010;29:111–121. doi: 10.1159/000262306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.