Abstract
Engineered skeletal muscle holds promise as a source of graft tissue for the repair of traumatic injuries such as volumetric muscle loss. The resident skeletal muscle stem cell, the satellite cell, has been identified as an ideal progenitor for tissue engineering due to its role as an essential player in the potent skeletal muscle regeneration mechanism. A significant challenge facing tissue engineers, however, is the isolation of sufficiently large satellite cell populations with high purity. The two common isolation techniques, single fiber explant culture and enzymatic dissociation, can yield either a highly pure satellite cell population or a suitably large number or cells but fail to do both simultaneously. As a result, it is often necessary to use a purification technique such as pre-plating or cell sorting to enrich the satellite cell population post-isolation. Furthermore, the absence of complex chemical and biophysical cues influencing the in vivo satellite cell “niche” complicates the culture of isolated satellite cells. Techniques under investigation to maximize myogenic proliferation and differentiation in vitro are described in this article, along with current methods for isolating and purifying satellite cells.
Keywords: Skeletal muscle tissue, Cell population, Enzymatic dissociation
Introduction
The human body naturally repairs basic skeletal muscle injuries, but traumatic damage often requires medical intervention [1,2]. The most prevalent such injury is volumetric muscle loss (VML), trauma overwhelming the normal muscle repair mechanism and resulting in permanent functional impairment and physical deformity [2]. Currently, clinical VML treatments have limitations including donor site morbidity and graft tissue scarcity [1]. Therefore, tissue engineers seek an alternative strategy to fabricate skeletal muscle graft tissue. The state-of-the-art technique involves placing myogenic cells on a biomimetic scaffold that can direct differentiation and provide structural support, followed by in vitro conditioning through chemical and mechanical stimuli to promote maturation of the construct tissue. This review discusses current challenges in obtaining the cellular component necessary for engineering skeletal muscle and presents several techniques for addressing these issues, paying particular attention to: 1) satellite cell isolation methods, 2) purification of isolated cells, and 3) optimization of in vitro culture conditions.
Due to its tissue-specific regenerative potential, the satellite cell has been identified as the ideal progenitor cell for skeletal muscle tissue engineering [3,4]. In the early 1960’s Mauro originally described the resident skeletal muscle stem cell, the satellite cell, by its cellular location beneath the basal lamina of a skeletal muscle fiber [5]. Since then, a great deal of research has attempted to elucidate the specific roles satellite cells play in skeletal muscle, particularly in muscle maturation, adaptation, and repair. During post-skeletal development, the satellite cell normally remains quiescent; however, satellite cell activity can be induced in response to muscle injury. In fact, recent studies demonstrate that satellite cells are indeed necessary for skeletal muscle to undergo regeneration [6–9]. These studies and others that evaluate satellite cell function utilize an important marker of satellite cells: paired box protein-7 or Pax7, encoded by the PAX-7 gene. Pax7 itself is a transcription factor that plays an integral role in myogenesis and regulation of muscle precursor cell proliferation. It is considered the requisite marker for satellite cell identification, and Pax7 genetic manipulation is utilized to assess specific myogenic function. Pax7 satellite cells, however, are not the only cell type that displays myogenic potential [3,10,11]. For example, mesoangioblasts, pericytes, Pax3, SK-34, CD45+/Sca1+, muscle side population, and PW1+/Pax7 interstitial cells have all been implicated in contributing to the myogenic cell pool. However, formation of functional skeletal muscle in vivo utilizing most of these progenitors appears to require some level of Pax7 expression [12–14]. Ultimately, the complexity of the myogenic pool allows for diversity in tissue engineering strategies. Isolating Pax7-positive satellite cells for tissue engineering applications, however, is complicated by the heterogeneity and diversity of cells in this larger myogenic population.
Skeletal muscle tissue engineers choose to focus on isolating Pax7 satellite cells because of the proven regenerative potential of this specific population. While the variety of cell types detailed above display some degree of myogenic potential, the satellite cell is identified as the primary source of regeneration in damaged skeletal muscle [3,15]. Quiescent satellite cells are activated in response to injury and progress toward a committed myogenic lineage, with a sub-population returning to quiescence to maintain the progenitor pool. Those satellite cells induced to a myogenic lineage are often referred to as myogenic precursor cells or myoblasts, characterized by their expression of the transcription factors myogenic differentiation 1 (MyoD) and myogenic regulatory factor 5 (Myf5) [16]. Following myoblast proliferation and differentiation, the myoblasts fuse with damaged fibers and promote regeneration.
In addition to extensive research into the satellite cell itself, a great deal of emphasis has focused on the microenvironment or “stem cell niche” which has been shown to be highly specific to and influential for satellite cell behavior and myogenic function [17,18]. The niche constituents include the satellite cell itself along with the surrounding extracellular matrix, vascular and neural networks, surrounding cells, and various diffusible molecules. This dynamic environment presents a unique challenge for engineered tissues in vitro because of the constant communication between the satellite cell and its niche in vivo. The growth factors and signaling pathways dictating cellular regeneration are in constant flux, increasing the complexity of emulating myogenesis in vitro. Therefore, understanding the intrinsic and extrinsic signaling factors that influence the niche has been a priority for developing therapeutics in regenerative medicine [19], and similarly, strategies for muscle tissue engineering must aim to replicate the ideal conditions for skeletal muscle tissue repair.
Since the first skeletal muscle tissue engineering experiments by Vandenburgh and colleagues in 1988 [20], the field has rapidly advanced to include a variety of techniques to enhance myogenesis and tissue regeneration. Although technologies include either scaffold materials ranging from acellularized tissues [21,22], to collagen and fibrin hydrogels [23,24] or a scaffold-free approach [25,26], the vast majority of these techniques utilize satellite cells as the cell source muscle engineering. The complex satellite cell biology shows that isolating and culturing satellite cells presents a unique challenge. In particular, engineered skeletal muscle tissue requires a large number of isolated satellite cells with high purity. Engineered tissues to date have produced minimal force in comparison to healthy muscle, and the suspected cause of this disparity is decreased muscle fiber content and maturity [25,27,28]. As such, it is essential to maximize the myogenesis in engineered skeletal muscle tissue by isolating a pure satellite cell population. This process should result in both increased fiber content and improved functionality. Unfortunately, current methods for isolating satellite cells cannot yield both the population size and myogenic purity required by tissue engineers. It is instead necessary to combine these isolation techniques with additional purification methods, followed by controlled proliferation and induction conditions, and these approaches are described in detail in this review.
Satellite Cell Isolation
Single fiber explant culture
First described in 1986 by Bischoff and colleagues, techniques to explant and isolate individual muscle fibers have since been modified to isolate rat satellite cells [29]. In the original work, digestion of a dissected muscle and tendon in crude collagenase type I allowed liberation of individual myofibers. This procedure was found to be most successful after the fractionation of the collagenase, which enhanced the ability to remove the basal lamina from fibers and exposed the satellite cells. Because the separated fibers did not adhere to a standard polystyrene tissue culture substrate, a coating of either collagen or clotted chicken plasma was applied. This discovery opened up an entire field of study on the use of similar substrates to promote adhesion of the satellite cells during the tissue engineering of skeletal muscle. Separation of individual fibers in this manner induced cellular signals of muscle injury, prompting satellite cell proliferation up to 4 days in vitro. Satellite cells remained attached to their respective fibers, however, so further study was needed to isolate the satellite cells from their basal lamina niche.
A modification of the Bischoff system by Rosenblatt and colleagues yields a pure population of both rat and mouse satellite cells from single fiber explants (Figure 1) [30]. Following enzymatic digestion and separation, each fiber is plated individually with the aid of a dissecting microscope. Central to this approach, satellite cells dissociate from their fibers and adhere to the tissue culture plate. Matrigel, an extract of basement membrane proteins derived from Engelbreth-Holm-Swarm mouse sarcoma cells, was used as a substrate coating in this case because it allowed both satellite cell attachment and subsequent removal of the fiber. As a result, when the fibers are removed from the plate after 3–4 days in vitro, a highly myogenic adherent cell population remains for further expansion and study. Using this method, up to 300 satellite cells can be obtained per fiber, with potential for approximately 300-fold expansion to 100,000 myogenic cells [30].
Figure 1. Single fiber explant culture for isolation of satellite cells.
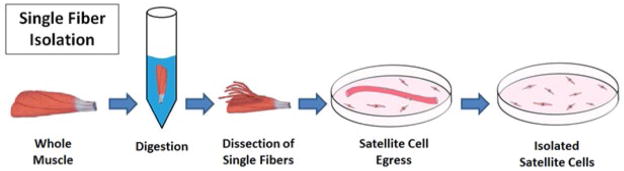
This technically challenging and labor-intensive isolation method yields a highly pure, but relatively small satellite cell population. The desired muscle is dissected and enzymatically dissociated until individual myofibers can be removed under a microscope. These fibers are then cultured for several days, allowing for egress of satellite cells onto the culture substrate. When the myofiber is removed, the isolated satellite cell population remains.
Since its introduction and subsequent modification, the single fiber method for isolating satellite cells has remained relatively unchanged. Conboy and colleagues published a much more detailed procedure in 2010, but the basic steps are consistent with previously published papers [31]. This approach is generally accepted to yield the most pure satellite cell populations, approximately 95% or higher [30] but has serious limitations in that dissecting individual muscle fibers is a highly precise and time-consuming task. Furthermore, although only individual fibers are needed for the final explant culture step, this protocol dictates removal of the entire muscle to avoid fiber damage. Because of its ability to yield satellite cells in vitro with high purity, single fiber isolations serve as powerful tool for the study of satellite cell biology, but the relatively low overall cell yield from such a large muscle volume make this technique sub-optimal for tissue engineering applications.
Enzymatic dissociation
To increase overall yield, many researchers use an enzymatic digestion of whole muscles to isolate satellite cells (Figure 2) [32,33]. This technique was also pioneered in the rat by Bischoff and colleagues [34], with significant modifications by Rando and Blau in the mouse [33]. Using this approach, muscle tissue is dissected and dissociated into a single cell suspension. Initially, all visible tendon and connective tissue is removed from the harvested muscle to maximize final myogenic purity. It is common for researchers to use a combination of enzymes to specifically digest the various skeletal muscle extracellular matrix components and “release” cells of interest. However, a rigorous enzymatic digestion process will also liberate a population of fibroblasts, leading to a diluted satellite cell suspension. Current methods have identified various combinations of trypsin, pronase, dispase, and several collagenases as optimal mixtures [34–36], and a study by Tebbets and colleagues highlights this variety in proteolytic enzymes used across the field [37]. Several studies have compared the relative efficacy of these enzymes [38–40]. It is believed that a mixture of collagenase and dispase minimally affects cell surface antigens, allowing for easier future enrichment of isolated cells [38]. On the other hand, pronase may inhibit survival and proliferation of non-myogenic cells and yield a more pure isolated satellite cell population [39]. Ultimately, direct comparison suggests that all of these enzymes are suitable for liberation of satellite cells, assuming one uses the correct duration of enzymatic digestion [40]. Following digestion, debris is removed from the suspension by filtration, and isolated cells are seeded for proliferation and differentiation.
Figure 2. Enzymatic dissociation for isolation of satellite cells.
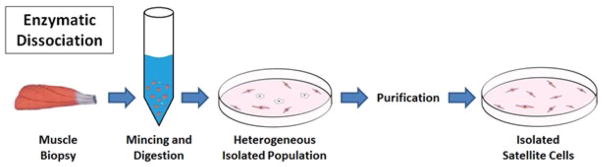
This isolation method can yield a large satellite cell population suitable for tissue engineering purposes, but requires additional purification to remove non-myogenic cells. A muscle biopsy is mechanically and enzymatically digested to yield a single cell suspension. The resulting heterogeneous population contains a mixture of satellite cells, fibroblasts, hematopoietic cells, and several other types. As a result, it is often necessary to enrich the myogenic satellite cell population through the purification methods described in this review.
Selection of the proper substrate adhesion protein is important at this stage in promoting satellite cell attachment. Collagen, polylysine, fibronectin, matrigel, and laminin are commonly used as adhesion proteins [28,35]. Several studies have compared the efficacy of different adhesion proteins in preferentially promoting satellite cell adhesion, proliferation, and differentiation [41–46] (Table 1). As these compiled results show, no specific adhesion protein has been identified as ideal for culturing satellite cells, but it is clear that the adhesion proteins described in table 1 (fibronectin, matrigel, gelatin, and laminin) promote increased attachment and proliferation relative to uncoated polystyrene tissue culture plastic or alternative proteins (polylysine and collagen types 3 or 4) [35,41].
Table 1.
Comparison of adhesion proteins for isolated satellite cells.
| Publication | Adhesion Proteins | Optimal Attachment | Optimal Proliferation | Optimal Differentiation |
|---|---|---|---|---|
| Wilschut et al. 2010 [46] | Matrigel, Gelatin, Collagen-1, Fibronectin, Laminin | Fibronectin, Laminin | Matrigel, Laminin | Matrigel, Laminin |
| Boonen et al. 2009 [45] | Matrigel, Collagen-4, Laminin, Poly-D-lysine | - | Matrigel | Laminin, Poly-D-Lysine |
| Maley et al. 1995 [43] | Gelatin, Collagen-4, Fibronectin, Laminin | - | - | Matrigel |
| Doumit et al.1992 [41] | Gelatin, Collagen-3, Fibronectin | - | Gelatin | Gelatin |
| Dodson et al. 1990 [44] | Gelatin, Collagen-1, Collagen-4, Fibronectin, Laminin, Poly-L-lysine, Poly-D-lysine | Fibronectin | Gelatin | Gelatin |
Note: Overall, fibronectin, matrigel, gelatin, and laminin seem ideal for culturing satellite cells, and these four adhesion proteins are most commonly used. From a tissue engineering perspective, matrigel has limited clinical relevance because it is derived from a cancer cell line.
Following isolation, characterizing the purity of the final cell population is essential when engineering skeletal muscle. While flow cytometry, described in detail below, certainly presents a high-throughput technique for identifying the various cells in a heterogeneous population, the use of cytochemistry for identifying myogenic and non-myogenic cells is a more traditional approach. Using markers of satellite cell activation, it is possible to stain isolated cells for canonical proteins that identify not only their myogenicity but also their state in the regenerative process. It is known that quiescent and activated satellite cells express Pax7 [17,47]. Following activation, identified by Myf5 upregulation and MyoD expression, the satellite cell becomes a myogenic progenitor or myoblast. By examining cultured satellite cells for Pax7, Myf5 and MyoD, it has been determined that removal of satellite cells from their niche during isolation immediately leads to their activation, and that a minimal quiescent population remains in vitro [35]. MyoD staining can therefore be used to identify proliferating satellite cells as soon as 24 hours post-isolation [35]. As regeneration continues, fusion of myoblasts and terminal differentiation occurs, accompanied by expression of myogenin, desmin, and c-met. Desmin, in particular, has often been utilized as an indicator of proliferating myogenic cells at time points ranging from 30 hours to several days post-isolation [33,35]. After myotube formation, typically occurring around 7 days post-isolation but varying with seeding density and onset of differentiation, neonatal or embryonic myosin heavy chain isoforms can be used to identify nascent muscle. By following these previously established markers to identify myogenic cells within the overall isolated population, it is possible to evaluate the overall success and purity of the isolation process.
Using such characterization methods, it has been determined that the final cell suspension isolated via the enzymatic dissociation method is significantly less myogenically pure than the single fiber isolation method. The isolates are contaminated by a small population of hematopoietic and neural cells, and connective tissue fibroblasts form the majority of the non-myogenic population. This fibroblast fraction plays an essential role in myogenesis through a reciprocal interaction with the proliferating satellite cells, but in large numbers can inhibit regeneration [8,48]. On the other hand, fibroblasts proliferate more rapidly than satellite cells [4,35], and this non-myogenic population can potentially overwhelm proliferating myogenic cells if too numerous initially. As a result, knowing the starting cell populations and monitoring the interaction between isolated satellite cells and fibroblasts is essential to understanding growth of muscle tissue during skeletal muscle tissue engineering. For reference, yields of approximately 10,000 satellite cells per 100 mg of digested muscle have been reported, with potential proliferation to 10 million cells [32]. Due to the ease at which significant volumes of muscle can be harvested, the potential for obtaining relevant numbers of satellite cells for tissue engineering is much larger with this method than through the single fiber isolation method. As a result, tissue engineers utilize this enzymatic dissociation method preferentially for high cell volume requirements. However, because of the lower myogenic purity, supplemental purification steps are often necessary to increase regenerative potential of the isolated satellite cells, and such purification methods are discussed in the following section.
Purification
Pre-plating
One of the most common methods for rapidly purifying satellite cells from a muscle homogenate is a selective pre-plating method published by Richler and Yaffe [49]. Following enzymatic dissociation, this serial pre-plating technique has been used to enrich the isolated myogenic cell population. Instead of seeding isolated cells directly onto a substrate coated with adhesion protein, the cell suspension is distributed onto uncoated tissue culture plastic for a period ranging from 15 minutes to 24 hours [33,37]. Fibroblasts and epithelial cells present in the muscle homogenate rapidly adhere to the tissue culture plastic, and the satellite cells remaining in suspension can be collected in the non-adherent cell population [32,49]. Using multiple iterations of pre-plating to enrich the non-adherent cells, a purified myogenic cell population can be obtained prior to the final seeding. Previous work has demonstrated increased purification with each subsequent step finding a 94% myogenic purity following six pre-plate steps over the course of 6 days [50].
The technical simplicity of using pre-plating as a purification step following enzymatic dissociation is certainly an advantage. However, during the pre-plating process, some myogenic cells inevitably attach either to the tissue culture plastic or to the adherent fibroblasts and are lost. Careful control of the pre-plate time is thus necessary to balance retention and purification. The major drawback of this technique is its time consuming nature, with the potential for myogenic and non-myogenic cell populations to vary significantly in the final cell suspension. In addition, the purity of isolated cells has certainly been increased, but the degree of improvement is inconsistent, and final purity remains unknown without additional analysis of cellular content. For tissue engineers, knowing that final purity is essential for consistent tissue fabrication. Fluorescence activated cell sorting (FACS) and magnetic activated cell sorting (MACS) have been used to evaluate the success of the pre-plating process, leading researchers to consider whether they can be used to skip pre-plating and immediately sort instead [51,52].
FACS - Fluorescence Activated Cell Sorting
Among the sorting techniques that may be applied to the isolation of satellite cells, FACS shows promise due to its efficiency in sorting large populations of cells. This technique has been used extensively as both a diagnostic and research tool to obtain information rapidly about heterogeneous cell populations including cell number, relative cell size, and cell viability. FACS operates through laser-based identification of fluorophore-conjugated cell markers and applied electrical charges to physically separate individual cells. The use of FACS in satellite cell purification, however, is complicated by the absence of a definitive surface marker. Early work on myoblast isolations by the Blau laboratory demonstrated that semi-pure primary mouse myoblasts could be isolated using FACS and antibodies to α7 Integrin alone [24]. Recent work has focused on improving the purity of isolated satellite cells by using a combination of markers to identify and isolate satellite cells from other muscle-derived cells, as described in table 2 [39].
Table 2.
Prospective surface markers for isolation of satellite cells.
| Publication | Sorted Population | Positive Markers | Negative Markers | Heterogeneous Markers |
|---|---|---|---|---|
| Pasut et al. 2012 [53] | Satellite Cells | α7-Integrin, CD34 | CD45, CD31, CD11b, Sca-1 | - |
| Bosnakovski et al. 2008 [57] | Pax7-ZsGreen Satellite Cells | CD29, CD34 | CD45, CD105, PDGFRα, c-Kit | CXCR4 |
| Montarras et al. 2005 [83] | Pax3GFP/+ Satellite Cells | CD34 | CD45, Sca-1 | - |
| Sherwood et al. 2004 [54] | Myogenic Progenitors | β1-Integrin, CXCR4 | CD45, Sca-1, Mac-1 | - |
| Asakura et al. 2002 [56] | Satellite Cells | CD34 | CD45, Sca-1 | - |
| Muscle Side Population Cells | - | - | CD45, Sca-1 | |
| Jankowski et al. 2001 [58] | Proliferating Muscle-Derived Stem Cells | - | CD45, c-Kit | CD34, Sca-1 |
Note: Due to the heterogeneity of the myogenic pool, FACS targeting different cell populations has identified various surface marker combinations. The most consistent trend is the use of CD34 as a positive marker, with CD45 and Sca-1 as negative markers. In several cases, these and other markers were identified on a unique subset of the isolated population, and they are labelled as heterogeneous markers as a result.
Recently, Pasut et al. demonstrated successful FACS isolation of satellite cells using a combination of positive markers α7 Integrin and CD34 with negative markers CD45, CD31, CD11b, and Sca-1 [53]. Likewise, Sherwood et al. demonstrated similar FACS isolation of myogenic cells using positive markers CXCR4 and β1-integrin and negative markers CD45, Sca-1, and Mac-1, and cell purity was confirmed by growing the sorted cells into myotubes in cell culture [54]. Because of the heterogeneity of the satellite cell population, with certain sub-populations displaying unique surface marker profiles, various additional marker combinations specific to satellite cells have been identified [55,56]. Consistent with the work described above, Bosnakovski et al identified CD34 as a positive marker and CD45 as a negative marker for satellite cells sorted from a Pax7- ZsGreen+ mouse [57]. Bosnakovski’s work also found that only half of the sorted satellite cells expressed CXCR4, in partial disagreement with Sherwood’s work. This finding again suggests the presence of unique sub-populations within the larger satellite cell pool, potentially complicating the sorting process. A similar heterogeneity within the satellite cell pool also occurs with injury to the muscle prior to isolation, evident when sorting yields Sca-1 positive, rather than negative, satellite cells. Similar variation in satellite cell surface markers has been demonstrated when examining CD34 and Sca-1 expression in myogenic cells in vitro [58]. In particular, distinct sub-populations, both positive and negative for CD34 and Sca-1, were found within the heterogeneous myogenic cell population over a period of 6 days in vitro. When examined together, these studies illustrate the difficulties in purifying satellite cells presented by the heterogeneous surface marker profiles across the overall isolated population.
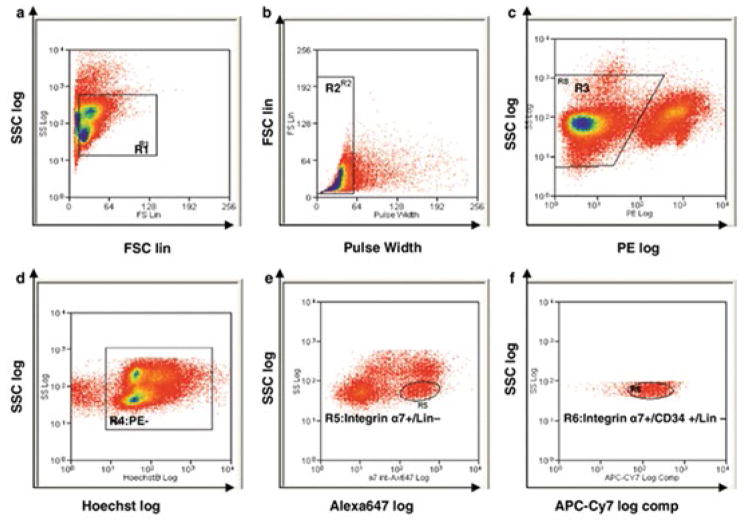
Ultimately, FACS has proven successful in purifying myogenic cells from a heterogeneous cell population isolated in a mouse model, illustrated in figure 3. Such plots also demonstrate the considerable technical expertise required to differentiate the sub-populations typically overlapping each other. Although researchers have utilized a wide variety of surface markers, the common trend across these strategies is the use of CD34 as a positive marker and CD45 and Sca-1 as negative markers. However, as tissue engineers seek to use larger animal models, study of these heterogeneous surface markers present in the myogenic pool will be required for each new species. Again, while satellite cell sorting with FACS has proven effective, there remains a significant drawback to the use of FACS in muscle tissue engineering: the potential damage to or alterations of cell proliferation during the sorting process. The electrical charge applied to separate cells requires a substantial voltage, and though the satellite cells typically survive this perturbation, it is thought that gene expression may be altered [59]. Specific studies to examine the effects of FACS on satellite cell viability and subsequent proliferation in vitro have not been conducted, so it remains to be seen if sorted cells are still suitable for tissue engineering purposes. The use of FACS in isolating human embryonic stem cells, however, led to decreased viability [60], and destabilization of the membrane was observed in sorted spermatozoa [61]. Unless such damaging effects can be avoided in the case of satellite cells, skeletal muscle tissue engineers may choose not to use FACS to purify isolated cells and instead use it only for characterization.
Figure 3. Example methodology for FACS purification of satellite cells (adapted from Pasut, et al. 2012 [53]).
Dot plots representing the sequential gating strategy used to identify satellite cells from a heterogonous muscle sample based on: (a) Side Scatter (SSC) and Forward Scatter (FSC), (b) Doublets discrimination, (c) PE- gating to remove CD45 and CD11b blood lineage cells, Sca-1 mesenchymal progenitors, and CD31 endothelial cells, (d) Hoechst staining for live (Hoechst+) and dead (Hoechst−) cells, (e) Integrin α7+ and Sca-1- (Lin−) gating, and finally (f) Integrin α7+ and CD34+ gating to sort the population defined as satellite cells. As the plots above illustrate, unique populations are rarely completely distinct, and substantial technical expertise is necessary to design such gating strategies properly.
MACS - Magnetic-Activated Cell Sorting
Magnetic-activated cell sorting (MACS) offers an alternative method for satellite cell sorting. Commonly utilized in immunology, neuroscience, and cancer research, MACS operates by incubating cells with antibodies for specific markers of interest conjugated to magnetic microbeads. Because it similarly relies on sorting by antigen expression, MACS suffers from the same primary limitation as FACS: the lack of a definitive surface marker prevents direct sorting of satellite cells, and some combination of markers must instead be used. Research by the Blau laboratory demonstrated that semi-pure mouse myoblast populations may be isolated using MACS technology to select for α7 Integrin+ cells, with similar purification levels as found with FACS [52]. Although MACS has been used in some cases to purify CD56 positive satellite cells [62], direct comparison of pre-plating and MACS indicated much greater purification using the pre-plating technique [51]. From these studies, it is clear that multiple markers are likely needed isolate a highly pure population.
An additional complication associated with MACS sorting is the magnetic labelling. While the antibody-marker conjugation used in FACS is certainly a modification of the target cells, with antibody binding potentially activating or blocking signaling pathways associated with the target surface receptor [59], the attachment of magnetic microbeads is a far more significant alteration [63]. From a tissue engineering standpoint, concerns arise regarding implantation of cells that may retain attached or internalized microbeads. As a result, MACS may be limited for application in tissue engineering to negative sorting, the removal of cell types other than that of interest, resulting in a semi-pure cell population. This technique has been demonstrated by using MACS to remove CD45+ cells from a cell isolate [64]. Until methods for magnetically sorting satellite cells has been improved and concerns about magnetic microbead retention have been resolved, MACS may not provide a realistic cell sorting approach for tissue engineering.
Proliferation and Induction in vitro
Media and growth factors
Because of the difficulties associated with isolating and purifying satellite cells, it is essential to maximize their myogenic potential in vitro. In addition, isolated satellite cells can only be expanded to a limited extent, because myogenic potential rapidly decreases after a period of two weeks in vitro [30,65]. This behavior can at least partially be explained by the fact that fibroblasts and other non-myogenic cells in the isolated population double every 18 hours, much more rapidly than the approximately 24 hours for satellite cells [4,35]. Several studies have demonstrated the ability to expand isolated muscle progenitor cells in vitro for up to 50 population doublings [33,66]. However, examination of myogenic potential over this expansion period demonstrated a steady decline in Pax7, MyoD, and desmin expression, beginning at over 90% and decreasing to approximately 55% by the third passage [67]. Furthermore, immunocytochemistry for 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU) showed 60% positive staining at the time of isolation but steadily decreasing values with each subsequent passage, indicating declining activation and proliferation of isolated satellite cells with increased time in vitro. With this understanding of in vitro satellite cell behavior, it is essential to maximize proliferation of isolated satellite cells before myogenic potential drops or faster proliferating populations begin to take over the culture. Thus, comprehensive study has determined optimal conditions for promoting proliferation and inducing differentiation of satellite cells. As one would expect, proper media formulation plays a key role. Because of the highly metabolic nature of skeletal muscle, a high serum content ranging from 10–15% is essential to provide the factors required for proliferation [29,32,41,42]. It is worth noting that serum lot to lot variations can profoundly alter satellite cell proliferation [41], so it is important to pre-test different lots to obtain optimal growth. To avoid the costs and variability associated with serum, several serum-free media have been developed specifically for enhancing satellite cell proliferation and differentiation [68]. In a similar manner, addition of soluble growth factors is essential for recreating in vivo myogenesis and maximizing proliferation of isolated satellite cells. Several families of growth factors have been consistently associated with increased satellite cell activation and proliferation in vitro, including fibroblast growth factor (FGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), and platelet derived growth factor (PDGF) [42,43,69,70]. In particular, IGF-I is integral to skeletal muscle growth and hypertrophy in vivo through the Akt signaling pathway [71]. When supplied to isolated satellite cells in vitro, IGF-I extends replicative life span and increases proliferation, again through the Akt signaling pathway [72]. Any improvements in proliferation rates or proliferative lifespan as a result of these culture conditions are vital to skeletal muscle tissue engineers seeking to repair large volume defects with a limited isolated satellite cell population.
For tissue engineers, the ability to induce uniform satellite cell differentiation is just as important as maximizing proliferation. As described in the introduction above, activated satellite cells may fuse into elongated myotubes during myogenesis prior to repairing damaged fibers or restoring lost function. As a result, without differentiation and fusion, tissues engineered from isolated satellite cells lack the ability to regenerate damaged skeletal muscle. To address this need, the generally accepted method for promoting differentiation is alteration to the culture medium, primarily focusing on the serum content and soluble growth factors, as in the case of improved proliferation. Whereas optimal proliferation requires high serum levels, inducing differentiation involves drastically reducing the media serum content to levels at 2% or lower [32,41,42,73]. Changing the serum type from fetal bovine serum to horse serum has a similar effect [41,42]. Again, lot to lot variations in the serum chosen have the potential to significantly alter differentiation. Interestingly, the same growth factors implicated in improved satellite cell proliferation, especially IGF and FGF, may be used to induce differentiation [43,69,70,74]. These methods for controlling proliferation and inducing differentiation through medium formulation are quite well established, and assuming a sufficiently large and pure population can be obtained, and offer tissue engineers several tools for increasing the myogenic potential of isolated satellite cells in vitro.
Biophysical stimuli
Recently, increasing attention has focused on recreating biophysical cues in vitro to promote satellite cell proliferation and differentiation, in concert with controlling media and growth factors. The complex nature of the satellite cell niche in vivo [17,18], with signaling not only from chemical factors but also through physical stimuli acting on the ECM and surrounding microenvironment, suggests the need for similar conditions in vitro. The beneficial effects of gelatin, fibronectin, and laminin as adhesion proteins are described above, and this interaction illustrates the importance of the physical cell culture microenvironment. The substrate onto which these adhesion proteins are deposited, and onto which the isolated satellite cells ultimately attach, has also been engineered to mimic native skeletal muscle with promising results. Instead of using stiff substrates such as polystyrene or glass, culture of isolated satellite cells on more compliant hydrogels promoted myogenic differentiation and allowed formation of advanced sarcomeric structure [75,76]. These hydrogels were engineered to match the stiffness of healthy skeletal muscle in vivo, with Young’s Moduli of 12 kPa. Even a slight variation in gel stiffness to values above 20 kPa or below 5 kPa significantly reduced differentiation. From these results, it is clear that the biophysical stimuli transduced through the extracellular microenvironment play a key role in regulating satellite cell behavior in vitro. It naturally follows that researchers have applied additional mechanical stimuli seeking to further recreate the in vivo environment, commonly through custom bioreactors. These systems can range from aligned, cylindrical microwells designed to promote alignment and fusion into myotubes [77], to dynamic bioreactors for providing cyclic strain either through fluid flow or mechanical stretch [27,78]. The efficacy of these bioreactors has led especially to improved satellite cell differentiation, but it has also brought attention to the importance of 3D tissue culture. Skeletal muscle tissue engineers are clearly seeking to recreate as much of the in vivo satellite cell niche as possible in vitro, but these efforts are inherently limited when using a flat, 2D tissue culture surface. The potential benefits of using a 3D culture system include improved satellite cell attachment and viability and myotube alignment [77,79]. It is expected that naturally derived acellularized ECM or collagen hydrogel scaffolds provide the support and 3D architecture necessary for satellite cell growth and development, while also avoiding potential biocompatibility issues [22,76,80]. At the same time, such scaffolds can shield the developing engineered tissue from the desired loading environment if degradation does not occur at the proper rate [81]. To avoid such complications, several labs have developed scaffold-free, 3D culture systems for engineering skeletal muscle tissues and implanting these tissues into small animal models [23,25,82]. Such advances show promising results, both in terms of recapitulating myogenesis in vitro and restoring lost muscle function in vivo. Overall, the improved understanding of culture conditions through both chemical and physical stimuli has aided skeletal muscle tissue engineers in improving the myogenic potential of isolated satellite cells in vitro.
Future Directions
Considerable effort has been dedicated to isolating and purifying satellite cells, as described above. Because of recent advances, several powerful tools are available to tissue engineers. Single fiber explant culture isolations yield small populations with high purity, whereas enzymatic dissociation isolates a mixture of myogenic and non-myogenic cells with a need for additional purification. Pre-plating is the most common method for purification and has proven effective but ultimately lacks a quantitative measure of the final myogenic cell population. FACS and MACS have also been considered, with FACS potentially providing the ability to both sort and characterize isolated cells. Further understanding of satellite cell surface markers may make FACS an increasingly viable alternative; however, the need for labelling and the potential for alteration during the sorting process ultimately limit FACS and MACS.
As researchers seek to translate engineered skeletal muscle technologies from small animal models to larger animals and eventually humans, more efficient and reliable isolation methods will become essential. Techniques for increasing myogenic cell proliferation and inducing differentiation in vitro by controlling culture conditions and seeking to recapitulate the satellite cell niche are being developed to utilize isolated satellite cells maximally, but the scaling up process could exceed these capabilities. It is expected that rapid development will continue in the areas covered in this review since they will help translate tissue engineering of skeletal muscle into a viable clinical treatment.
Acknowledgments
Financial Support
Funded internally by the University of Michigan.
References
- 1.Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 2.Grogan BF, Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19:S35–S37. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 3.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman JM, Tyraskis A, Maghsoudlou P, Urbani L, Totonelli G, et al. Skeletal muscle tissue engineering: which cell to use? Tissue Eng Pt B-Rev. 2013;19:503–515. doi: 10.1089/ten.TEB.2013.0120. [DOI] [PubMed] [Google Scholar]
- 5.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7- positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 10.Judson RN, Zhang RH, Rossi FM. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? FEBS Journal. 2013;280:4100–4108. doi: 10.1111/febs.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 12.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7- dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 13.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka KK, Hall JK, Troy AA, Cornelison DDW, Majka SM, et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 16.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannérec A, Parlakian A, Gomes ER, Sassoon DA, Mitchell KJ, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 19.Wagers AJ. The Stem Cell Niche in Regenerative Medicine. Cell Stem Cell. 2012;10:362–369. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev B. 1988;24:166–174. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 21.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Pt A. 2014;20:705–715. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–7484. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhas M, Engelmayr J, George C, Fontanella AN, Palmer GM, et al. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci U S A. 2014;111:5508–5513. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PH, Vandenburgh HH. Skeletal muscle atrophy in bioengineered skeletal muscle: a new model system. Eng Pt A. 2013;19:2147–2155. doi: 10.1089/ten.TEA.2012.0597. [DOI] [PubMed] [Google Scholar]
- 25.Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, et al. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET) Sci Rep. 2013;3:1420. doi: 10.1038/srep01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams ML, Kostrominova TY, Arruda EM, Larkin LM. Effect of implantation on engineered skeletal muscle constructs. J Tissue Eng Regen Med. 2013;7:434–442. doi: 10.1002/term.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach AD, Stern-Straeter J, Beier JP, Bannasch H, Stark GB. Engineering of muscle tissue. Clin Plast Surg. 2003;30:589–599. doi: 10.1016/s0094-1298(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 28.Kosnik PE, Faulkner JA, Dennis RG. Functional development of engineered skeletal muscle from adult and neonatal rats. Tissue Eng. 2001;7:573–584. doi: 10.1089/107632701753213192. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 31.Conboy MJ, Conboy IM. Preparation of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:149–163. doi: 10.1007/978-1-60761-063-2_10. [DOI] [PubMed] [Google Scholar]
- 32.Blau HM, Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981;78:5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974;180:645–661. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- 35.Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1997;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- 36.Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2005;290:C609–C615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- 37.Tebbets J, Péault B, Crisan M, Lu A, Zheng B, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nature Protocols. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 38.Yablonka-Reuveni Z, Day K. Skeletal muscle stem cells in the spotlight: The satellite cell. Regenerating the heart. Stem Cell Biology and Regenerative Medicine. 2011:173–200. [Google Scholar]
- 39.Danoviz ME, Yablonka-Reuveni Z. Skeletal Muscle Satellite Cells: Background and Methods for Isolation and Analysis in a Primary Culture System. Methods Mol Biol. 2012;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee EJ, Choi J, Hyun JH, Cho KH, Hwang I, et al. Steroid Effects on Cell Proliferation, Differentiation and Steroid Receptor Gene Expression in Adult Bovine Satellite Cells. Asian Australas J Anim Sci. 2007;20:501–510. [Google Scholar]
- 41.Doumit ME, Merkel RA. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell. 1992;24:253–262. doi: 10.1016/0040-8166(92)90098-r. [DOI] [PubMed] [Google Scholar]
- 42.McFarland DC, Doumit ME, Minshall RD. The turkey myogenic satellite cell: optimization of in vitro proliferation and differentiation. Tissue Cell. 1988;20:899–908. doi: 10.1016/0040-8166(88)90031-6. [DOI] [PubMed] [Google Scholar]
- 43.Maley MA, Davies MJ, Grounds MD. Extracellular matrix, growth factors, genetics: their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Exp Cell Res. 1995;219:169–179. doi: 10.1006/excr.1995.1217. [DOI] [PubMed] [Google Scholar]
- 44.Dodson MV, Mathison BA, Mathison BD. Effects of medium and substratum on ovine satellite cell attachment, proliferation and differentiation in vitro. Cell Differ Dev. 1990;29:59–66. doi: 10.1016/0922-3371(90)90024-q. [DOI] [PubMed] [Google Scholar]
- 45.Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:C1338–C1345. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- 46.Wilschut KJ, Haagsman HP, Roelen BA. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res. 2010;316:341–352. doi: 10.1016/j.yexcr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Shadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Phil Trans R Soc B. 2011;366:2297–2306. doi: 10.1098/rstb.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- 50.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, et al. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YG, Moon JH, Kim J. A comparative study of magnetic-activated cell sorting, cytotoxicity and preplating for the purification of human myoblasts. Yonsei Med J. 2006;47:179–183. doi: 10.3349/ymj.2006.47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on α7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- 53.Pasut A, Oleynik P, Rudnicki MA. Isolation of muscle stem cells by fluorescence activated cell sorting cytometry. Methods Mol Biol. 2012;798:53–64. doi: 10.1007/978-1-61779-343-1_3. [DOI] [PubMed] [Google Scholar]
- 54.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Chapman MR, Balakrishnan KR, Li J, Conboy MJ, Huang H, et al. Sorting single satellite cells from individual myofibers reveals heterogeneity in cell-surface markers and myogenic capacity. Integr Biol. 2013;5:692–702. doi: 10.1039/c3ib20290a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, et al. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 59.Beliakova-Bethell N, Massanella M, White C, Lada SM, Du P, et al. The effect of cell subset isolation method on gene expression in leukocytes. Cytometry A. 2014;85:94–104. doi: 10.1002/cyto.a.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fong CY, Peh GS, Gauthaman K, Bongso A. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) Stem Cell Rev. 2009;5:72–80. doi: 10.1007/s12015-009-9054-4. [DOI] [PubMed] [Google Scholar]
- 61.Maxwell WM, Johnson LA. Chlortetracycline analysis of boar spermatozoa after incubation, flow cytometric sorting, cooling, or cryopreservation. Mol Reprod Dev. 1997;46:408–418. doi: 10.1002/(SICI)1098-2795(199703)46:3<408::AID-MRD21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 62.Martin NR, Passey SL, Player DJ, Khodabukus A, Ferguson RA, et al. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials. 2013;34:5759–5765. doi: 10.1016/j.biomaterials.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Servida F, Soligo D, Caneva L, Bertolini F, de Harven E, et al. Functional and morphological characterization of immunomagnetically selected CD34+ hematopoietic progenitor cells. Stem Cells. 1996;14:430–438. doi: 10.1002/stem.140430. [DOI] [PubMed] [Google Scholar]
- 64.O’Connor MS, Carlson ME, Conboy IM. Differentiation rather than aging of muscle stem cells abolishes their telomerase activity. Biotechnol Prog. 2009;25:1130–1137. doi: 10.1002/btpr.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renault V, Thornell LE, Butler-Browne G, Mouly V. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37:1229–1236. doi: 10.1016/s0531-5565(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 66.Gaster M, Beck-Nielsen H, Schrøder HD. Proliferation conditions for human satellite cells. The fractional content of satellite cells. APMIS. 2001;109:726–734. doi: 10.1034/j.1600-0463.2001.d01-139.x. [DOI] [PubMed] [Google Scholar]
- 67.Machida S, Spangenburg EE, Booth FW. Primary rat muscle progenitor cells have decreased proliferation and myotube formation during passages. Cell Prolif. 2004;37:267–277. doi: 10.1111/j.1365-2184.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernyhough ME, Bucci LR, Feliciano J, Dodson MV. The Effect of Nutritional Supplements on Muscle-Derived Stem Cells in vitro. Int J Stem Cells. 2010;3:63–67. doi: 10.15283/ijsc.2010.3.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 70.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 71.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J Biol Chem. 2000;275:35942–35952. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- 73.Cheng CS, El-Abd Y, Bui K, Hyun YE, Hughes RH, et al. Conditions that promote primary human skeletal myoblast culture and muscle differentiation in vitro. Am J Physiol Cell Physiol. 2014;306:C385–C395. doi: 10.1152/ajpcell.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neal D, Sakar MS, Ong LL, Harry Asada H. Formation of elongated fascicle-inspired 3D tissues consisting of high-density, aligned cells using sacrificial outer molding. Lab on a chip. 2014;14:1907–1916. doi: 10.1039/c4lc00023d. [DOI] [PubMed] [Google Scholar]
- 78.Dennis RG, Smith B, Philp A, Donnelly K, Baar K. Bioreactors for guiding muscle tissue growth and development. Adv Biochem Engin/Biotechnol. 2009;112:39–79. doi: 10.1007/978-3-540-69357-4_3. [DOI] [PubMed] [Google Scholar]
- 79.Cimetta E, Flaibani M, Mella M, Serena E, Boldrin L, et al. Enhancement of viability of muscle precursor cells on 3D scaffold in a perfusion bioreactor. Int J Artif Organs. 2007;30:415–428. doi: 10.1177/039139880703000509. [DOI] [PubMed] [Google Scholar]
- 80.Mertens JP, Sugg KB, Lee JD, Larkin LM. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen Med. 2014;9:89–100. doi: 10.2217/rme.13.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]
- 82.VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A. 2014;20:2920–2930. doi: 10.1089/ten.tea.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]