Abstract
Background
Ample evidence suggests a dose-response relationship between increasing weight and level of asthma risk or reduced asthma control. To establish reversibility, several randomized controlled trials (RCTs) have recently been published to investigate the impact of weight management on asthma. This systematic review synthesizes evidence from these RCTs on the effects of weight management (weight loss, weight maintenance, maintenance of lost weight, or weight gain prevention) interventions on asthma outcomes in both adult and pediatric populations.
Methods
We searched Medline, CINAHL, PsychInfo, and Cochrane for studies published between 1950 and November 2014. Two researchers independently rated the included studies using the quality assessment tool for RCTs as outlined in the 2013 Obesity Treatment Guideline. Discrepancies were resolved by consensus after discussion between the raters and, if needed, with the senior author.
Results
Four RCTs in adults and 3 in children and adolescents were included. The adult studies seem to consistently support the benefit of substantial weight loss, but a threshold effect may exist such that only weight loss beyond a minimal amount will likely lead to clinically important improvement in asthma outcomes. Three of them suggest that the threshold may lie between 5-10% of weight loss. RCTs in youth suggest that modest calorie reductions alone or combined with increased physical activity, or even a healthy normocaloric diet, may lead to improved asthma outcomes. However, most RCTs reviewed were limited by small sample size, short intervention durations, and short follow-up periods.
Conclusion
Trial evidence shows the promise of weight loss interventions for asthma control in adults and youth. More adequately-powered, long-term RCTs are needed to elucidate the role of weight loss and other weight management interventions in asthma control and prevention. Definitive data are needed to guide clinical and public health practice to effectively address the dual epidemic of obesity and asthma.
Keywords: Randomized controlled trial, Weight, Asthma, Adults, Children, Adolescents
Introduction
Obesity and asthma are two major epidemics and their prevalence has increased concurrently in recent decades in the US [1-3]. Since a possible association between obesity and asthma was first reported in the 1980s [4], many cross-sectional and prospective observational studies in adults, children, and adolescents from diverse populations have been published, and a number of reviews have summarized the evidence [5-9].
Comorbid obesity and asthma are now recognized as a distinct phenotype; in some cases obesity may cause incident asthma whereas in other cases obesity alters pre-existing asthma to be more difficult to control and complicates its management, partly because of blunted effectiveness of inhaled corticosteroids. Evidence also suggests a dose-response relationship between increasing body mass index (BMI) and risk of incident asthma [10-12] and degree of reduced control in prevalent asthma [13]. It is hypothesized that multifactorial mechanisms involving mechanical, inflammatory, immunologic, hormonal, and genetic factors may link obesity and asthma [11,14]. To establish causality between obesity and asthma, adequate evidence is needed to address whether interventions to prevent or treat obesity lower the risk of asthma onset in high-risk individuals and/or improve disease outcomes in people already with asthma. Observational and quasi-experimental studies dominate the literature on weight loss in asthma [15,16], although several randomized controlled trials (RCTs) have recently been published.
We conducted a systematic review to provide an up-to-date evaluation of the published RCTs on the effects of weight management (defined as weight loss, weight maintenance, maintenance of lost weight, or weight gain prevention) interventions on asthma outcomes in both adult and pediatric populations.
Methods
An electronic literature search extending back to 1950 was conducted using Medline (PubMed), CINAHL, PsychInfo, and Cochrane in November 2014. The terms used to search titles and abstracts were (overweight or obese or obesity) and (asthma or wheeze or wheezing) and (weight or BMI or body mass index or waist or fat). Table 1 details the search strategy. Cross-referencing from the articles found was used to complete the search. Inclusion in the systematic review required that a study be original research with human subjects published in English, be an RCT of any type of weight management intervention, and specify a priori at least one asthma outcome as the primary outcome. Table 2 shows the complete inclusion and exclusion criteria for selection of publications.
Table 1.
Search strategy.
| Search Strategy | Number of references |
|---|---|
| Pubmed (overweight[TIAB] OR obese[TIAB] OR obesity[TIAB]) AND (asthma[TIAB] OR wheeze[TIAB] OR wheezing[TIAB]) AND (weight[TIAB] OR BMI[TIAB] OR body mass index[TIAB] OR waist[TIAB] OR fat[TIAB]) Limit to Clinical Trial |
82 |
| CINAHL ((TI (overweight OR obese OR obesity)) OR (AB (overweight OR obese OR obesity))) AND ((TI (asthma OR wheeze OR wheezing)) OR (AB (asthma OR wheeze OR wheezing))) AND ((TI (weight OR BMI OR body mass index OR waist OR fat)) OR (AB (weight OR BMI OR body mass index OR waist OR fat))) Limit to English Language, Randomized Controlled Trial, Human |
2 |
| PsychInfo ((TI(overweight OR obese OR obesity)) OR (AB(overweight OR obese OR obesity))) AND ((TI(asthma OR wheeze OR wheezing)) OR (AB(asthma OR wheeze OR wheezing))) AND ((TI(weight OR BMI OR body mass index OR waist OR fat)) OR (AB(weight OR BMI OR body mass index OR waist OR fat))) Limit to Human, English; Exclude interview, Retrospective Study; Literature Review; Focus Group, Mathematical Model |
81 |
| Cochrane ((overweight):ti,ab OR (obese):ti,ab OR (obesity):ti,ab) AND ((asthma):ti,ab OR (wheeze):ti,ab OR (wheezing):ti,ab) AND ((weight):ti,ab OR (BMI):ti,ab OR (body mass index):ti,ab OR (waist):ti,ab OR (fat):ti,ab) Limit to Trials |
47 |
Table 2.
Criteria for selection of publications.
| Inclusions | Exclusions | |
|---|---|---|
| Population | Overweight and/or obese participants of any age with diagnosed or self-reported asthma or wheezing | Animals studies |
| Population not overweight or obese at baseline | ||
| Intervention | Behavioral, pharmacological, or surgical interventions for weight loss, maintenance of lost weight, weight maintenance, or weight gain prevention. | Intervention not focused on weight loss, maintenance of lost weight, weight maintenance, or weight gain prevention |
| With the following characteristics: | ||
| Duration: short term (≤6 mos), intermediate (>6 mos and ≤12 mos), long term (>12 mos) | ||
| Delivery: group and/or individual | ||
| Frequency of contact: any | ||
| Comparator | Usual care | None |
| No or minimal intervention | ||
| Attention control intervention | ||
| Alternative active intervention | ||
| Outcome | One or more of the following asthma related outcomes as primary outcome(s): | Results are not reported according to randomized treatment or treatment groups |
| Asthma or wheezing symptoms | ||
| Lung function | ||
| Asthma medication use | ||
| Asthma-specific quality of life | ||
| Asthma-specific health care utilization | ||
| One or more of the following weight related outcomes: | ||
| Weight (kg, lbs, %) | ||
| Body fat measures (BMI, BMI z-score, waist circumference, waist-hip ratio, % body fat) | ||
| Weight loss maintenance | ||
| Percent reduction of excess weight | ||
| Timing | Follow-up period: any length | None |
| Setting | Any clinical or research setting | None |
| Study Design | RCTs | Systematic reviews/Meta-analyses |
| Post hoc analyses of large RCTs if analyses of original randomized comparisons are included | Non-RCT original studies | |
| Sample size: any size | RCTs but with results not compared according to randomized treatment assignments. | |
| Dropout rate ≥40 percent at the study-defined primary endpoint | ||
| Studies with <10 subjects per treatment arm | ||
| Publication Type | Published original research studies | Systematic reviews/Meta-analyses |
| Unpublished literature | ||
| Unpublished industry-sponsored trials | ||
| Other unpublished data | ||
| FDA Medical and Statistical reviews | ||
| Theses | ||
| Studies published only as abstracts | ||
| Letters | ||
| Commentaries and opinion pieces | ||
| Non-systematic reviews | ||
| Language | Full-text articles must be available in English | Non-English |
Two researchers (NL and LX) independently rated all the RCTs included in the systematic review using the quality assessment tool for controlled intervention studies as outlined in the 2013 Obesity Treatment Guideline by the American Heart Association (AHA), American College of Cardiology (ACC), and Obesity Society [17]. The included studies’ scientific soundness was rated using 14 questions, and based on the answers, one of three overall quality ratings was assigned: good, fair, or poor. Any discrepancies were resolved by consensus after discussion between the raters and, if needed, with the senior author (JM).
The review protocol was registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO #CRD42014014352).
Results
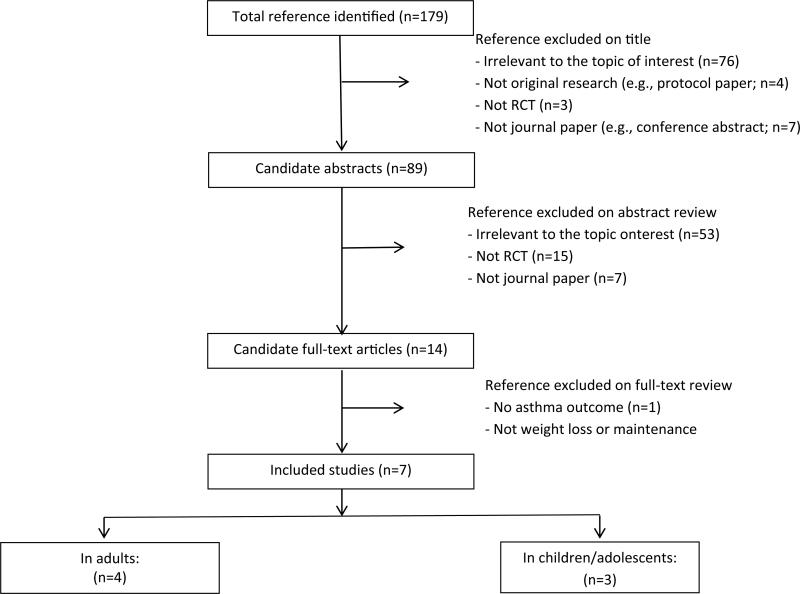
Of 179 references identified, 172 were excluded based on the review of titles (n=90), abstracts (n=75), and full text (n=7) (Figure 1). Seven RCTs were included, 4 in adults and 3 in children/adolescents (Tables 3 and 4). Five of the 7 RCTs were rated good quality, 1 rated fair quality, and 1 rated poor quality.
Figure 1.
PRISMA Diagram Showing Selection of Articles (as of August 11 2014).
Table 3.
Characteristics of RCTs for weight loss or maintenance interventions in adults with asthma.
| Study Cited, Design, Primary Outcome, Setting, Quality Rating | Inclusion Criteria, Group Size, Baseline Characteristics, Retention at Primary Assessment Time Point | Intervention Groups, Component Details | Treatment Duration, Total Intervention Contacts, Intervention Adherence | Results |
|---|---|---|---|---|
| Stenius-Aarniala et al., 2000 [18] RCT, ITT (interpreted by reviewer) PEF was a primary outcome (interpreted by reviewer) Finland, Medical center Duration: 1 year Quality: Good |
Inclusion criteria: ages 18-60 yrs, 30 ≤ BMI ≤ 42, with asthma diagnosed with a spontaneous diurnal variation or a bronchodilator response of 15% or more, non-smoker or having stopped smoking for ≤ 2 yrs before age 50. | G1: Intervention Diet during first 8 weeks: a very low calorie dietary preparation (1760 kJ/day [420 kcal/day] containing daily allowances of all essential nutrients) Medical: received normal medical care throughout the study Behavior: received education about asthma and allergy via group sessions (details unknown); measured daily morning and evening pre- and post-bronchodilator PEF during the dieting period and thereafter during the 2 weeks before each group meeting. G2: Control Diet: no intervention Medical: same as G1 Behavior: same as G1 |
Intervention: 14 weeks Contacts: G1: 12 group sessions on same education about asthma and allergy as G2 G2: 12 0.5-hour group sessions at the same intervals as G1, during which time themes about asthma and allergy chosen by the group were discussed freely. |
At the end of the 14-week weight loss intervention Weight change, %, mean G1: −14.5 G2: −0.3 (calculated by reviewer) P NR Weight change, kg, mean (range) G1: −14.2 (−22.1, −7.7) G2: −0.3 (NR) P NR PEF (% of predicted) change, mean (95% CI) G1: 4.5 (−0.9, 9.8) G2: −0.3 (−4.6, 4.1) P=0.16 FEV1 (% of predicted) change, mean (95% CI) G1: 5.3 (2.2, 8.4) G2: −1.5 (-−5.1, 2.2) P=0.006 FVC (% of predicted) change, mean (95% CI) G1: 1.9 (−0.7, 4.4) G2: −5.6 (−9.6, −1.5) P=0.002 Dyspnea symptom, median change on a 100 mm VAS G1: −13 (variance NR) G2: −1 (variance NR) P=0.02 Daily dose of an inhaled bronchodilator, median change G1: −1.2 (variance NR) G2: −0.1 (variance NR) P=0.03 At 1 year (whether primary time point NR) Weight change, %, mean G1: −11.3 G2: 2.2 P NR Weight change, kg, mean (range) G1: −11.1 (−22.5, −1.1) G2: 2.3 (NR) P NR PEF (% of predicted) change, mean (95% CI) G1: 5.6 (−0.2, 11.4) G2: −0.6 (−5.8, 4.7) P=0.11 FEV1 (% of predicted) change, mean (95% CI) G1: 4.9 (−0.5, 10.3) G2: −2.7 (−5.9, 0.5) P=0.02 FVC (% of predicted) change, mean (95% CI) G1: 2.0 (−1.4, 5.4) G2: −5.6 (−8.2, −3.1) P=0.001 Dyspnea symptom, median change on a 100 mm VAS G1: NR G2: NR P=0.12 Daily dose of an inhaled bronchodilator, median change G1: NR G2: NR P=0.08 |
| N=38 | ||||
| Group n's G1: 19 G2: 19 | ||||
| Age, yrs, mean (range) G1: 49.7 (34-60) G2: 48.3 (23-60) |
Intervention adherence: Two participants found the consistency or taste of the dietary preparation intolerable but followed a low energy diet. Details NR. | |||
| Females % G1: 68.4 G2: 84.2 |
||||
| White %: NR | ||||
| BMI, kg/m2, mean (range) G1: 35.8 (31.3-39.4) G2: 36.7 (32.8-41.8) |
||||
| The groups were similar for baseline characteristics except rhinitis. | ||||
| Retention %: G1: 100 G2: 100 |
||||
| Scott et al., 2013 [19] RCT, per protocol completer analysis Primary outcome is not indicated. Australia, Medical center Duration: 10 weeks Quality: Fair |
Inclusion criteria: adults, overweight and obese (28 ≤ BMI ≤ 40), with asthma defined by doctor's diagnosis and documented history of airway hyperresponsiveness, non-smoking | G1: Dietary intervention Diet: an intake of 3700–4900 kJ/day (885–1170 kcal/day), including two meal replacements provided free of charge Behavior: behavior modification and motivational strategies. Participants maintained a daily food dairy during the intervention. G2: Exercise intervention Physical activity: a goal of increasing daily steps by 10% each week to a target of 10,000 steps per day. A 12-week gym membership and weekly 1-hour group personal training (both aerobic activities and resistance training) sessions. Participants attended gym ≥3 times/week. Behavior: participants set goal with dietitian, wore a pedometer daily, and completed a daily physical activity diary during the intervention. G3: Combined dietary and exercise intervention Diet: same as G1 Physical activity: same as G2 Behavior: same as G1 + G2 |
Intervention: 10 weeks Contacts: G1: weekly counselling with a dietitian: 7 1-hour clinic visits and 4 10-min phone consultations G2: weekly 1-hour group training sessions G3: same as G1 + G2 |
At 10 weeks Weight change, %, mean (SD) G1: −8.5 (4.2) G2: −1.8 (2.6) G3: −8.3 (4.9) Weight change, kg, mean (SD) G1: −8.4 (4.4) G2: −1.8 (2.7) G3: −8.2 (5.1) G1 and G3 significantly different from G2 (P=0.001) ACQ change, mean (SD) G1: −0.6 (0.5) G2: −0.3 (0.5) G3: −0.5 (0.7) G1 and G3 significantly improved ACQ score; however, between group comparison is NS (P=0.343). AQLQ change, median (IQR) G1: 0.9 (0.4, 1.3) G2: 0.49 (0.03, 0.78) G3: 0.5 (0.1, 1.0) G1, G2, and G3 significantly improved AQLQ score; however, between group comparison is NS (P=0.212). TLC change, L, median [IQR] G1: −0.03 (−0.33, 0.14) G2: 0.13 (−0.02, 0.28) G3: 0.06 (−0.06, 0.33) G2 and G3 significantly improved TLC compared with G1 (P=0.037 for overall group difference) A 5-10% weight loss resulted in clinically significant improvements to ACQ in 58% and AQLQ in 83% of participants. Women had a significantly greater reduction in sputum percentage neutrophils compared with men (P < 0.05); however, men and women experienced a similar degree of improvement to ACQ and AQLQ. |
| N=46 Female %: 54.3 | ||||
| Group n's G1: 18 G2: 14 G3: 14 |
Intervention adherence: Diet: energy change, kJ, median (IQR) G1: −4261 (−5896, −2632) G2: −232 (−3254, −464) G3: −5584 (−7384, −4624) G1 and G3 significantly different from G2 |
|||
| Age, yrs, mean (SD) G1: 44.7 (14.7) G2: 42.2 (11.5) G3: 33.9 (11.5) |
||||
| White %: NR | Physical activity: total MET minutes/week change, medium (IQR) G1: 495 (−825, 3473) G2: 2651 (389, 7428) G3: 2267 (691, 4812) G2 significantly increased total physical activity. |
|||
| BMI, kg/m2, mean (SD) G1: 34.7 (4.0) G2: 32.8 (2.5) G3: 32.7 (3.4) |
||||
| ACQ score, mean (SD) G1: 1.24 (0.57) G2: 1.00 (0.71) G3: 1.36 (0.63) |
||||
| AQLQ score, median (IQR) G1: 5.8 (5.4, 6.1) G2: 6.1 (5.0, 6.3) G3: 5.8 (4.8, 6.1) |
||||
| All groups had a similar baseline age, sex, body composition, asthma status, and sputum inflammatory profile. | ||||
| Retention %: G1: 83 G2: 71 G3: 93 |
||||
| Dias-Junior et al., 2014 [20] RCT, ITT and per protocol population analysis (patients who lost >10% of body weight) ACQ was a primary outcome Brazil, Medical center Duration: 6 months Quality: Good |
Inclusion criteria: ages 18-65 yrs, BMI ≥ 30, with severe asthma according to GINA criteria after a 3-month run-in period, with a smoking history of less than 10 pack-years | G1: Intervention Diet: low caloric intake Medical: use of sibutramine (10 mg/day) and orlistat (≤120 mg/day) G2: Control No intervention |
Intervention: 6 months Contacts: G1: bimonthly in-person consultations where participants saw the same investigator to have their use of inhaler checked, compliance with medication assessed, and receive education on asthma. G2: NR |
At 6 months (ITT analysis) BMI, kg/m2, mean (SE) G1: 36.71 (1.37) G2: 36.85 (1.06) P < 0.001 for G1 and NS for G2 within groups P=0.015 for the comparisons between groups. ACQ score, mean (SE) G1: 2.25 (0.28) G2: 2.90 (0.16) P=0.001 for G1 and NS for G2 within groups P=0.044 for the comparisons between groups. FVC, L, mean (SE) G1: 2.98 (0.12) G2: 2.48 (0.12) P=0.004 for G1 and NS for G2 change within groups P=0.017 for the comparisons between groups. ITT analysis showed that the intervention group significantly lost weight (mean weight loss 7.88 kg; 7.5% of baseline weight), improved asthma control as measured by ACQ, and increased FVC. ACQ reached clinically significant improvements (>0.5) in 11 of the 12 intervention patients who lost >10% of their baseline weight. |
| N=33 | ||||
| Group n's G1: 22 G2: 11 Age, yrs, mean (SE) G1: 42 (15.75) G2: 44 (24) P=0.825 |
||||
| Intervention adherence: NR |
||||
| Females % G1: 90.9 G2: 100 P=0.542 White %: NR BMI, kg/m2, mean (SE) G1: 39.68 (1.31) G2: 37.29 (1.07) P=0.243 ACQ score, mean (SE) G1: 3.02 (0.19) G2: 2.91 (0.25) P=0.718 |
||||
| Retention %: G1: 100 G2: 100 |
||||
| Ma et al., 2014 [22] RCT, ITT ACQ was a primary outcome USA, Medical center Duration: 12 months Quality: Good |
Inclusion criteria: ages 18-70 yrs, BMI >30, with uncontrolled persistent asthma confirmed through a multi-stage screening process (i.e., electronic asthma registry queries, completion of the ACT, pre- and post-bronchodilator spirometry, and if necessary, medical chart reviews by an asthma specialist) | G1: Intervention Diet: healthy eating with moderate calorie reductions (by 500-1000 kcal/day, but daily total calories ≥1200 kcal) Physical activity: a goal of ≥150 minutes/week of moderate-intensity physical activity Behavior: self-management skills (e.g., self-monitoring, action planning, and problem solving); Same as G2 G2: Enhanced usual care control Diet: no intervention Physical activity: no intervention Behavior: received usual care enhanced with a pedometer, a body weight scale, a list of routinely offered weight management services at the participating clinics, and a standard asthma self-management educational DVD |
Intervention: 12 months Contacts: G1: 13 weekly in-person group sessions over 4 months, 2 monthly in-person individual sessions, ≥3 bimonthly phone consultations over 6 months G2: none Intervention adherence: Physical activity: leisure-time MET minutes/week change, mean (SE) G1: 418.2 (110.6) G2: 178.8 (109.1) P=0.05 Intervention attendance: Mean (SD) % of 13 weekly group session attended: 77.1% (26.7%) % participants who attended both monthly individual sessions: 63.6% % participants who received ≥3 phone consultations: 49% |
At 12 months Weight change, kg, mean (SE) G1: −4.0 (0.8) G2: −2.1 (0.8) P=0.01 |
| Weight change, %, mean (SE) G1: −4.1 (0.7) G2: −2.1 (0.7) P=0.005 | ||||
| N=330 Age, yrs, mean (SD): 47.6 (12.4) Female %: 70.6 White %: 49.7 BMI, kg/m2, mean (SD): 37.5 (5.9) ACQ score, mean (SD): 1.4 (0.8) |
ACQ change, mean (SE) G1: −0.3 (0.1) G2: −0.2 (0.1) P=0.92 |
|||
| Group n's G1: 165 G2: 165 |
Weight loss of >10% was associated with a large 1-sample effect size, Cohen's d=0.76, for ACQ change and with 3.78 (95% CI: 1.72-8.31) times the odds of achieving clinically significant reductions on ACQ as stable weight. | |||
| Between group P values are NS for all baseline characteristics above. | ||||
| Retention %: G1: 86 G2: 89 |
The intervention effects on ACQ change and weight loss at 12 months were comparable among men and women. |
ACQ: Asthma control questionnaire; ACT: Asthma control test; BMI, Body mass index; CI, Confidence interval; FEV1: Forced expiratory volume in one second; FVC, Forced vital capacity; GINA, Global initiative for asthma; ITT, Intent-to-treat; IQR, Interquartile range; MET, Metabolic equivalent task; NR, Not reported; NS, Not significant; PEF, Peak expiratory flow; RCT, Randomized controlled trial; SD, Standard deviation; SE, Standard error; TLC, Total lung capacity; VAS, Visual analogue scale.
Table 4.
Characteristics of RCTs for weight loss or maintenance interventions in children/adolescents with asthma.
| Study Cited, Design, Primary Outcome, Setting, Quality Rating | Inclusion Criteria, Group Size, Baseline Characteristics, Retention at Primary Assessment Time Point | Intervention Groups, Component Details | Treatment Duration, Total Intervention Contacts, Intervention Adherence | Results |
|---|---|---|---|---|
| Jensen et al., 2013 [24] RCT, ITT Systemic and airway inflammation, lung function, and ACQ were primary outcomes. Australia, Medical center Duration: 10 weeks Quality: Good |
Inclusion criteria: ages 8-17 yrs, BMI z-score 21.64 SD score, with asthma diagnosed by a physician. N=32 Group n's G1: 16 G2: 16 Age, yrs, mean (SD) G1: 11.5 (2.1) G2: 12.4 (2.4) P NS Females % G1: 23.1 G2: 53.3 P NS White %: NR BMI z-score, median [IQR] G1: 2.1 (1.9, 2.3) G2: 2.2 (1.8, 2.4) P NS ACQ score, median [IQR] G1: 1.14 (0.43, 1.57) G2: 0.57 (0.29, 0.86) P=0.026 Retention %: G1: 81.3 G2: 93.8 |
G1: Weight loss intervention Diet: a 500 kcal/day energy reduction from individually calculated age- and gender- appropriate energy requirements. Behavior: identification and resolution of barriers; goal setting; self-monitoring was encouraged. G2: Wait-list control Delayed intervention |
Intervention: 10 weeks Contacts: G1: a total of 11 in-person or phone contacts (7 in-person counselling sessions in week 1, 2, 3, 4, 6, 8, and 10 and phone contacts in alternative weeks) G2: none Intervention adherence: NR |
At 10 weeks BMI z-score change, median (IQR) G1: −0.2 (−0.4, −0.1) G2: 0.0 (−0.1, 0.0) P=0.014 |
| ACQ change, median (IQR) G1: −0.4 (−0.7, 0.0) G2: 0.1 (0.0, 0.6) P=0.004 | ||||
| CRP change, mg/L, median (IQR) G1: −0.4 (−0.5, 0.4) G2: 0.7 (−0.1, 1.9) P=0.037 |
||||
| There was no significant change in dynamic lung function, within or between groups. Static lung function, ERV increased significantly within the intervention group (L, median [IQR], 0.7 [0.0, 1.0]), but not significantly different from the control (P=0.355). Airway and systemic inflammation did not change within the intervention group. | ||||
| El-Kader et al., 2013 [25] RCT, whether ITT NR Measures of systemic inflammation were primary outcomes (interpreted by reviewer) Saudi Arabia, Medical center Duration: 8 weeks (interpreted by reviewer) Quality: Poor |
Inclusion criteria: ages 12-18 yrs, 30≤BMI≤35, with bronchial asthma N=80 Female %: 47.5 Age, yrs, mean (SD): 13.86 (3.21) White %: NR Group n's G1: 40 G2: 40 Weight, kg, mean (SD) G1: 65.32 (4.17) G2: 63.52 (5.48) P NR Retention %: NR |
G1: Weight reduction intervention Diet: low calorie diet that provides an energy deficit of about 250 kcal/day;15% as protein, 30-35% as fat, and 50-55% as carbohydrate Physical activity: aerobic exercises monitored by a physical education expert Medical: medical treatment G2: Control Diet: no intervention Physical activity: no intervention Medical: Same as G1 |
Intervention: 8 weeks (interpreted by reviewer) Contacts: G1: aerobic exercises, every other day, 4 sessions a week, for 8 weeks; diet and medical contacts NR G2: none Intervention adherence: NR |
At the end of the study BMI, kg/m2, mean (SD) G1: 27.15 (2.38) G2: 32.14 (2.16) P < 0.05 TNF-alpha, pg/mL, mean (SD) G1: 3.56 (1.12) G2: 4.31 (1.41) P < 0.05 IL-6, pg/mL, mean (SD) G1: 1.85 (0.76) G2: 2.30 (0.75) P < 0.05 IL-8, pg/mL, mean (SD) G1: 12.14 (3.72) G2: 15.65 (4.11) P < 0.05 Leptin, ng/mL, mean (SD) G1: 26.98 (4.50) G2: 31.02 (4.84) P < 0.05 Adiponectin, μg/mL, mean (SD) G1: 14.72 (3.21) G2: 10.76 (2.85) P < 0.05 Compared to baseline, the intervention group significantly decreased values of TNF-alpha, IL-6, IL-8, Leptin, and BMI and increased adiponectin (all P < 0.05). The changes in the control group were not significant. |
| Luna-Pech et al., 2014 [26] RCT, ITT PAQLQ[S] was a primary outcome (interpreted by reviewer) Mexico, Medical center Duration: 28 weeks Quality: Good |
Inclusion criteria: ages 12-16 yrs, BMI ≥ 95th percentile of the CDC BMI-for-age growth charts, with asthma according to GINA, in a stable phase of asthma, in the pubertal sage of the Tanner scale (stage 2-3), skin prick testing for allergy positive for at least one allergen, FEV1 >80% from the predicted value for age and height according to the ATS guidelines. | G1: Intervention Diet: normocaloric diet based on normal requirements for height; 10-15% proteins, 50-60% carbohydrates, 25-30% fat, with a daily meal pattern of breakfast (25% of daily caloric intake), lunch (30%), snack (15-20%), and dinner (25-30%). Behavior: same as G2, plus recommendations on variations in the menu based on exchange lists. G2: Control (free diet) Diet: no intervention Behavior: at bi-weekly follow-up visits, staff established an action plan with participants in case of worsened asthma symptoms. |
Intervention: 28 weeks Contacts: G1: bi-weekly follow up visits with normocaloric diet assessed G2: bi-weekly follow up visits (no diet was assessed) Intervention adherence at 28 weeks: Energy, kcal/day, mean (SD) G1: 2231 (231) G2: 3243 (278) P=0.001 |
At 28 weeks BMI z-score, mean (SD) G1: 1.66 (0.2) G2: 2.12 (0.3) P < 0.01 |
| Weight change, kg, mean (SD) G1: −2.5 (1.3) G2: 1.6 (1.3) P < 0.03 Change in PAQLQ[S] scores: [numbers NR] G1 achieved a significant improvement in PAQLQ[S] scores compared with controls, and this difference was achieved both in the overall score and all its subdomains (P < 0.002 for all). | ||||
| N=58 Female %: 49 |
||||
| Group n's G1: 26 G2: 25 |
||||
| Carbohydrate, % of total caloric intake, median (IQR) G1: 51 (11) G2: 68 (32) P=0.001 |
Compared with G2, G1 had significantly fewer acute asthma events requiring short-acting β-agonists (17 vs. 39, P < 0.02) and nighttime awakenings (11 vs. 26, P < 0.001). | |||
| Age, yrs, mean (SD) G1: 14 (0.7) G2: 14 (0.3) P NS |
||||
| BMI z-score, mean (SD) G1: 2.18 (0.3) G2: 2.17 (0.2) P NS |
Fat, % of total caloric intake, median (IQR) G1: 30 (3) G2: 42 (15) P=0.01 |
|||
| Retention %: 88 | Protein, % of total caloric intake, median (IQR) G1: 15 (4) G2: 10 (3) P=0.09 |
ACQ, Asthma control questionnaire; ATS, American thoracic society; BMI, Body mass index; CDC, Centers for Disease Control and Prevention; CRP, C-reactive protein; ERV, Expiratory reserve volume; FEV1: Forced expiratory volume in one second; GINA, Global initiative for asthma; IQR, Interquartile range; NR; Not reported; NS, Not significant; PAQLQ[S], Standardized Pediatric asthma quality of life questionnaire; SE, Standard deviation.
RCTs of weight loss interventions in adults
Table 3 summarizes the main characteristics and results of the 4 RCTs in adults, all of which focused on weight loss. The first weight loss trial in asthma was conducted in Finland by Stenius-Aarniala et al. who randomly assigned 38 obese adults with objectively confirmed asthma to a 14-week intervention or a control group [18]. The intervention group received a very low calorie dietary preparation, which contained 1760 kJ (420 kcal) of energy and daily allowances of all essential nutrients, for 8 weeks, and attended 12 group sessions for weight reduction and asthma/allergy education. The control group attended 12 group sessions for asthma/allergy education only. Both groups received routine medical care and self-monitored daily morning and evening pre- and post-bronchodilator peak expiratory flow (PEF). All participants were followed for one year. Intervention participants had a mean of 14.2 (range, 7.7-22.1) kg or 14.5% (variance estimate not reported) loss of baseline weight vs. 0.3 (range not reported) kg or 0.3% weight loss among controls at the end of the weight reduction program, and 11.1 (1.1-22.5) kg or 11.3% weight loss vs. 2.3 kg or 2.2% weight gain at month 12. Significant improvements were observed in forced expiratory volume in one second (FEV1: between-group mean difference, 7.6% predicted; 95% confidence interval [CI], 1.5% to 13.8%; P=0.02) and forced vital capacity (FVC: 7.6% predicted; 3.5% to 11.8%; P=0.001) at 12 months, but not in PEF (6.2% predicted; -1.4% to 13.7%; P=0.11). Also significant were the improvements in dyspnea severity (median change based on a visual analogue scale, intervention, -13 mm [variance estimate not reported]; control; -1 mm; P=0.02) and in rescue medication daily doses (intervention, -1.2; control, -0.1; P=0.03) at the end of the intervention, but not at 12 months. Significant improvements were also observed in the health status symptom domain (distress caused by specific respiratory symptoms: -12; -22 to -1; P=0.04) and total scores (-10; -18 to -1; P=0.02) as measured by the St George's respiratory questionnaire at 12 months. Serum concentrations of sodium, potassium, calcium, and magnesium did not significantly differ between groups over the 12-month study period.
Scott et al. compared a dietary restriction intervention, an exercise intervention, and a combined intervention over 10 weeks in 46 overweight or obese Australian adults with asthma (defined by physician diagnosis and documented history of airway hyperresponsiveness) [19]. The dietary intervention group was prescribed a daily calorie intake of 3700-4900 kJ (885-1170 kcal), including two meal replacements provided free of charge and one main meal and snacks provided by participants. Participants also completed weekly counselling with a dietitian. The exercise group received a membership to a gymnasium where they went at least 3 times each week and attended weekly 1-hour group trainings. Participants also wore a pedometer daily and had a goal to increase daily steps by 10% each week to a target of 10,000 steps per day. The combined group completed both dietary and exercise interventions concurrently. At 10 weeks mean (SD) weight loss was 8.5% (4.2%) in the dietary intervention group, 1.8% (2.6%) in the exercise group, and 8.3% (4.9%) in the combined group. The dietary and combined groups had significantly improved asthma control as measured by the Juniper Asthma Control Questionnaire (ACQ: mean [SD] change, dietary, -0.6 [0.5]; combined, -0.5 [0.7]; exercise, -0.3 [0.5]), and all three groups improved on the Juniper Asthma Quality of Life Questionnaire (AQLQ: median [interquartile range, IQR] change, dietary, 0.9 [0.4, 1.3]; combined, 0.5 [0.1, 1.0]; exercise, 0.5 [0, 0.8]). The authors explored and found no gender differences in these main outcomes. The between-group differences in changes of most spirometric results and inflammation markers were not statistically significant, except that total lung capacity improved significantly in the exercise (median [IQR] change, 0.13 [-0.02, 0.28]) and combined interventions (0.06 [-0.06, 0.33]), compared with the dietary intervention (-0.03 [-0.33, 0.14]; P=0.037 for overall group difference). In addition, subgroup analysis showed that participants with 5-10% weight loss achieved significant improvements in FVC, AQLQ, and ACQ, with 58% and 83% achieving clinically important improvements in ACQ and AQLQ (≥0.5 for both), respectively, vs. 20% and 40% among those with <5% weight loss. The authors suggested that 5-10% weight loss be recommended in the clinical management of overweight and obese adults with asthma.
In a Brazilian study by Dias-Junior et al. [20], 33 obese adults with severe uncontrolled asthma (defined by specialist diagnosis, documented history of airway hyperresponsiveness within the previous 5 years, ≥1 exacerbation requiring systemic corticosteroids in the last year, and use of high doses of inhaled corticosteroids) were randomly assigned in a 2:1 ratio to 6 months of treatment with a low-calorie diet (energy intake unspecified) and sibutramine (10 mg per day) and orlistat (maximum dose of 120 mg per day) or to a control group. Participants also attended bimonthly asthma education. At 6 months, intervention participants had significantly reduced BMI (mean [standard error/SE], 36.71 [1.37] vs. 39.68 [1.31] kg/m2 at baseline) and improved ACQ scores (2.25 [0.28] vs. 3.02 [0.19]), with 11 of the 12 participants who had >10% weight loss achieving clinically important improvement in ACQ. BMI and ACQ changes were insignificant in the control group. The between-group difference was statistically significant for changes in both BMI (P=0.015) and ACQ (P=0.044), but not for changes in most spirometric results (except FVC), airway hyperresponsiveness, nitric oxide fraction in exhaled air (FeNO), and other airway and systemic inflammatory markers. The authors suggested that changes in airway inflammation might not explain the effect of weight loss on asthma outcomes in obese adults with severe asthma.
Our research group conducted the largest RCT of weight loss in asthma to date. We compared the Breathe Easier through Weight Loss Lifestyle (BE WELL) intervention with usual care in a major US ambulatory care system among 330 adults with objectively confirmed obesity and uncontrolled asthma [21,22]. Adapted from the efficacious Diabetes Prevention Program lifestyle intervention [23], the BE WELL intervention dually targeted modest (7-10%) weight loss and increased physical activity (daily minimum of 150 minutes of moderate-intensity physical activity). The 12-month program had 13 weekly group sessions over a 4-month period and 2 monthly individual sessions, followed by bimonthly or more frequent individual phone consultations depending on participant needs, preferences, and availability for the remainder 6 months. All intervention and control participants received usual care enhanced with a pedometer, a weight scale, information about existing weight management services at the participating clinics, and a standard asthma education DVD. Compared with controls, the intervention group achieved significantly greater weight loss at 12 months (mean [SE], -4.0 [0.8] kg or -4.1% [0.7%] vs. -2.1[0.8] kg or -2.1% [0.7%]) and increased weekly leisure-time physical activity levels (metabolic equivalent task [MET] minutes: 418.2 [110.6] vs. 178.8 [109.1]). Both groups had modestly improved asthma control, but the between-group difference (ACQ changes: intervention, -0.3±0.1 vs. control, -0.2±0.1) was not significant. Subgroup analyses of all participants regardless of randomization showed that at least 10% weight loss was associated with a Cohen's d effect of 0.76 and with 3.78 (95% CI, 1.72-8.31) times the odds of achieving clinically important reductions on ACQ as stable weight (i.e., <3% loss or gain from baseline). Thus, weight loss of at least 10% may be required to produce clinically meaningful improvement in asthma control. The between-group difference was insignificant for other clinical asthma outcomes (e.g., MiniAQLQ, spirometric results, medication acquisition, and healthcare encounters). Furthermore, the intervention effects on ACQ change and weight loss at 12 months were comparable among men and women.
RCTs of weight management interventions in children and adolescents
Three RCTs of weight management interventions have been published in children and adolescents (Table 4). Jensen et al. compared a 10-week diet-induced weight loss intervention with a wait-list control group in 28 obese Australian youth aged 8-17 years with BMI z-scores ≥ 1.64 and physician diagnosed asthma [24]. The intervention targeted a 500 kcal/day reduction from the age- and gender-appropriate energy requirements. Intervention participants received dietitian counseling in person and by phone during the 10 weeks. Parents were invited to attend the in-person sessions as well to help facilitate their child's dietary change and monitoring. Compared with controls, the intervention group had significantly reduced BMI z-scores (median [IQR] change, -0.2 [-0.4, -0.1] vs. 0 [-0.1, 0]; P=0.014), improved ACQ scores (-0.4 [-0.7 to 0] vs. 0.1 [0 to 0.6]; P=0.004), and decreased C-Reactive Protein (-0.4 [-0.5, 0.4] vs. 0.7 [-0.1, 1.9], P=0.037) at 10 weeks. Changes in other airway and systemic inflammatory markers (e.g., FeNO, leptin, eosinophils, neutrophils) and spirometric results did not differ significantly by group.
In El-Kader et al.'s study in Saudi Arabia, 80 obese youth with a mean (SD) age of 14 (3) years who had a BMI between 30-35 kg/m2 and bronchial asthma (case definition not found in the article) were randomly assigned to an 8-week weight loss intervention including both diet and exercise regimens or a control group [25]. The intervention group was prescribed a low calorie diet, which provided an energy deficit of about 250 kcal per day and balanced macronutrients, and an 8-week training program with four sessions of aerobic exercise per week. Intervention participants had significantly reduced BMI (mean [SD], 27.15 [2.38] post intervention vs. 32.31 [2.46] kg/m2 at baseline) and inflammation (leptin: 26.98 [4.50] vs. 31.43 [5.47] ng/ml; IL-6: 1.85 [0.76] vs. 2.19 ± [0.81] pg/ml; IL-8: 12.14 [3.72] vs. 15.66 [4.63] pg/ml). Changes in BMI and the inflammatory biomarkers were insignificant in the control group. However, the authors did not report the between-group differences in these outcomes or any clinical asthma outcomes.
Luna-Pech et al. randomly assigned 51 obese Mexican adolescents aged 12-16 years who had a BMI ≥ 95th percentile (per the Centers for Disease Control and Prevention BMI-for-age growth charts) and objectively confirmed asthma to a 28-week normocaloric diet intervention (n=26) or a control group (n=25) [26]. Both groups attended biweekly follow-up visits to review dietary recalls, perform PEF, assess asthma control, and develop an action plan in case of worsened asthma symptoms. In addition, the intervention group received advice from a certified nutritionist on normocaloric diet according to the adolescent's height and physical activity levels and on menu planning based on an equivalent exchange system. Compared with controls, intervention participants had significantly reduced weight (mean [SD], -2.5 [1.3] vs. 1.6 [1.3] kg; P=0.03) and improved asthma-related quality of life (P<0.002 for overall scores and all 3 subdomains; estimates not found in the article). Compared with controls, the intervention group also had significantly fewer acute asthma events requiring short-acting β-agonists (17 vs. 39, P<0.02) and nighttime awakenings (11 vs. 26, P<0.001) at 28 weeks. The authors noted that normocaloric dietary interventions should be more suitable than very low calorie diets for obese pubertal adolescents with asthma.
Discussion
This systematical review focused on RCTs of weight management interventions in adults and youth with asthma. The 4 RCTs in adults with asthma support the benefit of substantial weight loss, but a threshold effect may exist such that only weight loss beyond a minimal amount would lead to clinically important improvement in asthma outcomes. Secondary findings from 3 of the RCTs [19,20,22] suggest that the threshold may lie between 5-10% of weight loss, which needs to be confirmed in future study. The 3 RCTs in obese youth with asthma suggest that modest calorie reductions alone or combined with increased physical activity, or even a healthy normocaloric diet, may be beneficial on asthma outcomes in this population.
The trial results strengthen and extend the evidence from observational and quasi-experimental weight loss in asthma studies. A number of non-RCT studies in adults have shown that substantial weight reductions through bariatric surgery [27-33] or very low calorie diets [34,35] are associated with significantly improved asthma outcomes, including overall control, symptoms, lung function, medication use, quality of life, and health care utilization. Fewer non-RCT studies have been conducted in children or adolescents with asthma [36,37]. One single-arm study showed that an interdisciplinary intervention consisting of medical, nutritional, exercise, and psychological therapy led to significant weight loss as well as improved lung function, systemic inflammation, and asthma severity in obese adolescents with asthma [36]. Another study reported that a school-based program consisting of nutrition, weight management, and asthma education significantly improved participants’ self-efficacy (i.e., perceived ability to manage asthma), quality of life, and self-care related to asthma; however, no objective asthma outcomes were measured [37].
Most of the RCTs included in this review were limited by small sample sizes, short intervention durations, and short follow-up periods. different from all the other weight loss RCTs in adults with asthma, the BE WELL intervention tested by Ma et al. lasted 12 months (vs. 10 weeks to 6 months) and used a comprehensive approach encompassing moderately calorie-restricted healthy eating, moderate-intensity physical activity, and evidence-based behavioral self-management strategies, as recommended in the latest (2013) Obesity Treatment Guideline [17]. As the largest RCT of weight loss in asthma, the BE WELL study showed that adults with uncontrolled asthma can safely participate in lifestyle interventions proven for cardiometabolic benefits but that modest improvements in weight and activity level are not likely to significantly change asthma outcomes consistently within the population treated [22]. Ma et al. posited that to achieve clinically important improvement in asthma among adults it may require at least 10% weight loss, which would be greater than the accepted modest amounts of 3-5% for diabetes prevention and cardiovascular risk factor control [22]. While somewhat encouraging, the trial evidence on weight loss in children and adolescents with asthma is even more limited than in adults. More adequately-powered, well-designed, and well-conducted RCTs are clearly needed. The gaps in knowledge identified through this review highlight several specific future research directions.
First, the proposition that more than modest weight loss may be required for clinically meaningful improvement in asthma among adults is provocative and certainly warrants further investigation. Studies are needed to identify the minimum threshold of clinically significant weight loss in asthma and what factors may affect the threshold chosen (e.g., clinical and sociodemographic characteristics of the target population and the specific asthma outcomes of interest). In addition, if such threshold cannot be achieved by pragmatic lifestyle interventions alone (the BE WELL intervention, for example), then stepped-up obesity treatment regimens such as medically supervised very low calorie diet, weight loss drug therapy, or bariatric surgery should be evaluated.
Second, the underlying mechanisms of the influence of obesity and weight loss on asthma remain an enigma. Although many of the RCTs reviewed examined the impact of weight management interventions on asthma outcomes (e.g., spirometric results, airway and systemic inflammation) in addition to asthma control and asthma-related quality of life, the findings were exploratory and largely inconsistent. The postulated mechanisms for improved asthma status after weight loss include improvements in gastroesophageal reflux symptoms, airway and systemic inflammation, oxidative stress, and lung function [16,38,39]. These hypotheses need to be tested in well-designed mechanistic studies. Research to investigate the threshold effect as noted above should concurrently explore the role of varying degrees of weight loss in lung mechanics, airway physiology, immune response, and inflammation as well.
Third, subpopulation studies are needed to help elucidate the effect of weight loss in asthma and potential mechanisms. Emerging data suggest the possibility of at least two distinct asthma phenotypes in obese adults depending on the age of asthma onset and the presence of atopy [29,40]. The later-onset (≥12 years of age), typically nonatopic asthma phenotype that is predominantly found in women has been previously shown to respond more favorably to weight loss [29]. In addition, some observational studies suggest a stronger relationship between obesity and certain asthma manifestations in females than in males [41-43]. However, the only two RCTs reviewed that reported intervention effects by gender found no differences [19,22]. Baseline obesity status (e.g., obese class I, II, and III) may be another factor that could modify the effects of weight management interventions among asthmatic patients, but none of the 7 RCTs examined this. Thus, future weight loss trials focused on subpopulations (e.g., females with nonatopic, later-onset asthma and people with different baseline obesity status) are needed.
Fourth, more research is needed to define the most suitable weight management approach for obese children and adolescents with asthma. An expert committee recommended that pubertal development should be evaluated in the prevention, assessment, and treatment of overweight and obesity in children and adolescents [44]. Luna-Pech et al. recommended that a healthy normocaloric dietary approach should be used in obese pubertal adolescents with asthma to ensure normal development [26]. Recent systematic reviews focusing on dietary patterns (not weight management) in asthma found good epidemiologic evidence supporting potentially protective effects of Mediterranean diet on child asthma and wheeze [45,46]. Thus, future RCTs need to rigorously test the efficacy of a Mediterranean diet or similarly healthy dietary pattern with or without weight management strategies in pediatric populations with asthma. This is particular important because healthy eating habits formed at a young age have not only potential short-term benefits on asthma outcomes but also lifelong benefits on general health.
Fifth, the potential independent and synergistic effects of a healthy diet and weight loss are equally worthy of investigating in adults with asthma. Evidence on the effect of healthy dietary patterns in this population is mixed [45], although at least one short-term RCT showed improved FEV1 and FVC values as well as time to asthma exacerbation following increased fruit and vegetable intake [47]. We recently completed the first RCT of the Dietary Approaches to Stop Hypertension (DASH) eating pattern in adults with uncontrolled asthma [48], with results forthcoming.
Last, research on weight management interventions other than weight loss and on the management as well as prevention of asthma is needed. Even though we purposely defined weight management to broadly include weight loss, weight maintenance, maintenance of lost weight, and weight gain prevention in our search strategy, all but one of the included RCTs tested weight loss interventions only, whereas Luna-Pech et al. [26] evaluated a normocaloric diet intervention which nevertheless led to weight loss as opposed to weight gain in the control group. Further, no RCTs have examined any type of weight management interventions for the prevention of asthma.
This systematic review was limited to studies published in English only. At least one RCT on the topic of interest but published in a foreign language was not included [49]. Also, the small number of studies and the heterogeneity of the interventions and outcomes precluded a meta-analysis. Despite these limitations, this is the most up to date synthesis of RCTs on weight management in asthma for all age groups. It reveals that overall the available trial evidence is weak but nevertheless supportive of the benefit of weight loss in adults and youth with asthma, although a minimum of 5-10% weight loss may be required to reach clinical significance at least in obese asthmatic adults. Future work in the several specifically recommended areas will be important to elucidate the role of weight management in asthma control and prevention, thereby guiding clinical and public health practice to effectively address the dual epidemic of obesity and asthma.
Acknowledgments
Funding Source
This research was supported by grants R01HL094466 and R34HL108753 from the National Heart, Lung, and Blood Institute and internal funding from the Palo Alto Medical Foundation Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Citation: Nan Lv, Xiao L, Ma J (2014) Weight Management Interventions in Adult and Pediatric Asthma Populations: A Systematic Review. J Pulm Respir Med 5: 232. doi:10.4172/2161-105X.1000232
Declaration of Interest
NL, LX, JM declare that they have no conflict of interest.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001--2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 3.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, et al. Vital & health statistics Series 3, Analytical and epidemiological studies / [US Dept of Health and Human Services, Public Health Service, National Center for Health Statistics] 2012. National surveillance of asthma: United States, 2001-2010. pp. 1–67. [PubMed] [Google Scholar]
- 4.Seidell JC, de Groot LC, van Sonsbeek JL, Deurenberg P, Hautvast JG. Associations of moderate and severe overweight with self-reported illness and medical care in Dutch adults. Am J Public Health. 1986;76:264–269. doi: 10.2105/ajph.76.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep. 2013;13:434–442. doi: 10.1007/s11882-013-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ME, Collins CE, Gibson PG, Wood LG. The obesity phenotype in children with asthma. Paediatr Respir Rev. 2011;12:152–159. doi: 10.1016/j.prrv.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Boulet LP. Asthma and obesity. Clin Exp Allergy. 2013;43:8–21. doi: 10.1111/j.1365-2222.2012.04040.x. [DOI] [PubMed] [Google Scholar]
- 8.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther. 2013;26:455–458. doi: 10.1016/j.pupt.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 11.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 12.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman ED, Bousquet J, Busse WW, Clark TJ, Gul N, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy. 2008;63:932–938. doi: 10.1111/j.1398-9995.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 15.Moreira A, Bonini M, Garcia-Larsen V, Bonini S, Del Giacco SR, et al. Weight loss interventions in asthma: EAACI evidence-based clinical practice guideline (part I). Allergy. 2013;68:425–439. doi: 10.1111/all.12106. [DOI] [PubMed] [Google Scholar]
- 16.Eneli IU, Skybo T, Camargo CA., Jr Weight loss and asthma: a systematic review. Thorax. 2008;63:671–676. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 20.Dias-Júnior SA, Reis M, de Carvalho-Pinto RM, Stelmach R, Halpern A, et al. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43:1368–1377. doi: 10.1183/09031936.00053413. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Strub P, Camargo CA, Jr, Xiao L, Ayala E, et al. The Breathe Easier through Weight Loss Lifestyle (BE WELL) Intervention: a randomized controlled trial. BMC Pulm Med. 2010;10:16. doi: 10.1186/1471-2466-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Jr., et al. Behavioral Weight Loss and Physical Activity Intervention in Obese Asthma Adults: A Randomized Trial. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201406-271OC. DOI: 101513/AnnalsATS201406-271OC [Epub online 2014 Dec 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43:775–784. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- 25.Abd El-Kader MS, Al-Jiffri O, Ashmawy EM. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. Afr Health Sci. 2013;13:682–688. doi: 10.4314/ahs.v13i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, et al. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int Arch Allergy Immunol. 2014;163:252–258. doi: 10.1159/000360398. [DOI] [PubMed] [Google Scholar]
- 27.Macgregor AM, Greenberg RA. Effect of Surgically Induced Weight Loss on Asthma in the Morbidly Obese. Obes Surg. 1993;3:15–21. doi: 10.1381/096089293765559700. [DOI] [PubMed] [Google Scholar]
- 28.Dixon JB, Chapman L, O'Brien P. Marked improvement in asthma after Lap-Band surgery for morbid obesity. Obes Surg. 1999;9:385–389. doi: 10.1381/096089299765552981. [DOI] [PubMed] [Google Scholar]
- 29.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, et al. Weight loss and asthma control in severely obese asthmatic females. Respir Med. 2008;102:102–108. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Reddy RC, Baptist AP, Fan Z, Carlin AM, Birkmeyer NJ. The effects of bariatric surgery on asthma severity. Obes Surg. 2011;21:200–206. doi: 10.1007/s11695-010-0155-6. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi C, Gargioni S, Gardinazzi A, Canonica GW, Passalacqua G. Impact of bariatric surgery on pulmonary function and nitric oxide in asthmatic and non-asthmatic obese patients. J Asthma. 2011;48:553–557. doi: 10.3109/02770903.2011.587581. [DOI] [PubMed] [Google Scholar]
- 33.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106:651–660. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva PL, de Mello MT, Cheik NC, Sanches PL, Correia FA, et al. Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. Pediatr Pulmonol. 2012;47:8–17. doi: 10.1002/ppul.21502. [DOI] [PubMed] [Google Scholar]
- 37.Kouba J, Velsor-Friedrich B, Militello L, Harrison PR, Becklenberg A, et al. Efficacy of the I Can Control Asthma and Nutrition Now (ICAN) pilot program on health outcomes in high school students with asthma. The Journal of school nursing: the official publication of the National Association of School Nurses. 2013;29:235–247. doi: 10.1177/1059840512466110. [DOI] [PubMed] [Google Scholar]
- 38.Stream AR, Sutherland ER. Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012;12:76–81. doi: 10.1097/ACI.0b013e32834eca41. [DOI] [PubMed] [Google Scholar]
- 39.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol (1985) 2010;108:729–734. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Xiao L. Association of general and central obesity and atopic and nonatopic asthma in US adults. J Asthma. 2013;50:395–402. doi: 10.3109/02770903.2013.770014. [DOI] [PubMed] [Google Scholar]
- 41.Chinn S, Downs SH, Anto JM, Gerbase MW, Leynaert B, et al. Incidence of asthma and net change in symptoms in relation to changes in obesity. Eur Respir J. 2006;28:763–771. doi: 10.1183/09031936.06.00150505. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 43.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 44.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 Suppl. 2007;4:S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 45.Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–121. doi: 10.2147/JAA.S49960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Marcos, Castro-Rodriguez JA, Weinmayr G, Panagiotakos DB, Priftis KN, et al. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24:330–338. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 47.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, et al. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–543. doi: 10.3945/ajcn.111.032623. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Strub P, Lavori PW, Buist AS, Camargo CA, Jr, et al. DASH for asthma: a pilot study of the DASH diet in not-well-controlled adult asthma. Contemp Clin Trials. 2013;35:55–67. doi: 10.1016/j.cct.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernández Romero A, Matta Campos J, Mora Nieto A, del Rivero L, Andrés Dionicio AE, et al. [Clinical symptom relief in obese patients with persistent moderate asthma secondary to decreased obesity]. Rev Alerg Mex. 2008;55:103–111. [PubMed] [Google Scholar]