Abstract
We propose a new type of confocal microscope using Fresnel incoherent correlation holography (FINCH). Presented here is a confocal configuration of FINCH using a phase pinhole and point illumination that is able to suppress out-of-focus information from the recorded hologram and hence combine the super-resolution capabilities of FINCH with the sectioning capabilities of confocal microscopy.
Confocal imaging is commonly used for microscopy due to its ability to provide optical sectioning, improved contrast, and high-image resolution [1]. The concept of confocal microscopy was developed by Minsky in 1955 but found widespread use in biology only a few decades later. The reason for this delay is probably due to technological limitations at that time, as confocal imaging requires scanning over the entire imaged target [2,3]. Though confocal holographic systems that do not require scanning had been developed [4,5], they are unfortunately not suitable for fluorescence imaging [5], which is commonly practiced in microscopy for biological applications. In this Letter, we present, for the first time to our knowledge, a confocal configuration of Fresnel incoherent correlation holography (FINCH) [6]. FINCH is readily suitable for fluorescence microscopy and offers resolutions beyond the Rayleigh limit [7] but lacks the optical sectioning capabilities that are most important whenever thick objects are being imaged. These highly sought-after capabilities exist in the hereby proposed system and are achieved with the added cost of target scanning. These costs, however, can be mitigated to a large degree if a proper scanning methodology is used [1,8].
Before discussing the proposed confocal FINCH system, the working concept of FINCH is first briefly presented. A schematic configuration of a dual-lens FINCH system [9,10] is shown in Fig. 1(a). It is assumed that the object is spatially incoherent; thus light beams that are emitted or scattered from two different object points cannot interfere with each other, and the system is analyzed by considering a single point source object. In Fig. 1(a), a spherical light beam is emitted from the source point ao, located at the front focal plane of the objective lens Lo, and propagates into the FINCH system. An input polarizer P1 is set at a 45° angle to the active axis of a spatial light modulator (SLM), SLM1, which allows the formation of two in-parallel imaging systems in a common-path singlechannel configuration. The SLM modulates the phase of only the polarization components of the beam that are in parallel to its active axis. Polarization components of the input beam that are perpendicular to its active axis are not modulated; for them, the SLM is a transparent element. The system can thus be considered as two imaging systems, each acting with one of two orthogonal polarization components of light. In these systems, the input beam of light is collected by the objective lens Lo and then further concentrated by the lens Lc. In one of the two systems the SLM does not influence the beam, and an image is formed at the image point a2; in the other, a converging diffractive lens is displayed on SLM1 and the beam is concentrated into the image point a1.
Fig. 1.
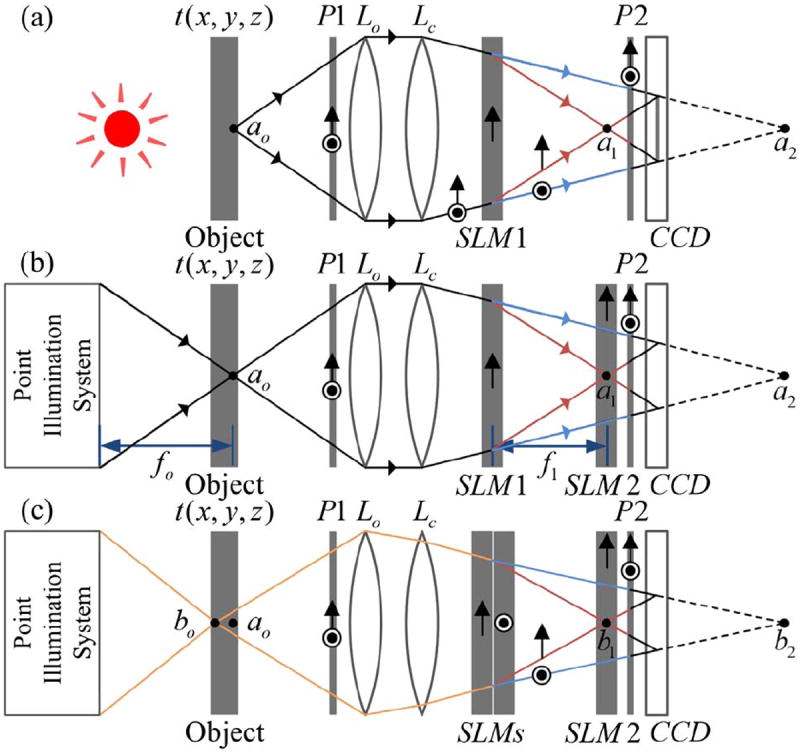
Schematics of FINCH recorders: (a) a dual-lens FINCH system; (b), (c) the proposed confocal FINCH systems. P1 and P2, polarizers; Lo, objective lens; Lc, converging lens; SLM1 and SLM2, spatial light modulators; CCD, charge-coupled device.
To record a hologram of the maximum achievable resolution [7,9], a charge-coupled device (CCD) is positioned between the two image points, a1 and a2, so that a perfect overlap is achieved between the beam diverging from the image point a1 and the beam converging toward the image point a2. Note that interference can occur between these two beams, since they originate from the same point source, ao, granted that the maximal optical path difference between the two is shorter than the coherence distance of the light source [9]. Further, note that the output polarizer P2 is essential and is used to project the polarization components of the two beams into a common orientation. Usually, P2 is also set at a 45° angle to the active axis of SLM1, but other angles can be used to control the relative intensity of the two beams [11]. The intensity of the two-beam interference pattern is recorded by the CCD, giving rise to a 0th order term and two other terms attributed to the holographic image of ao and its twin. A phase-shifting procedure [6,7,9-11], utilizing SLM1, requires at least three exposures and is performed so that only the holographic image term remains. The spatial incoherence of the object ensures that the final recorded FINCH hologram is a summation over the intensities of all point source interference patterns. The recorded object can then be reconstructed from the hologram through a digital Fresnel propagation to a specific reconstruction distance, zr [9]. An additional feature of FINCH is that out-of-focus points are also recorded in the hologram. This, on one hand, can be advantageous when refocusing to planes of different depths is required, but on the other hand, it can impose noise and artifacts over the observed in-focus image. Next, we present a FINCH-based method that can section any desired plane out of the three-dimensional (3D) object distribution.
In a confocal FINCH system [Fig. 1(b)], a second SLM, SLM2, is positioned in the x y plane in which the image point a1 is formed. A diffractive optical element, hereby referred to as a “phase pinhole,” is displayed on SLM2. This phase pinhole is considered as one of the main innovations of the presented work. The phase pinhole at the scanning point (m, n) is described by the following equation:
| (1) |
with , , and , and is composed of the actual pinhole part, exp(iφk), which is a circular area of radius r1 set to a uniform phase modulation, surrounded by a mask of an axicon, exp(iαr), where α is a parameter proportional to the axicon angle, denotes the circular aperture of SLM2, with a clear disk of radius R2, a and b are scanning intervals in the x and y axes, respectively, and is the starting point of the scanning. In the confocal FINCH system, for every scanning point (m, n), the uniform phase modulation at the pinhole region is set to three different φk values, usually 0°, 120°, and 240°, and SLM1 is no longer used for the phase-shifting procedure. Since the phase is changed only within the phase pinhole, any information carried by a wave that passes through SLM2 outside the phase pinhole and does not eventually interfere with the wave modulated by the phase pinhole is lost after the phase-shifting procedure. Overall, the proposed phase pinhole can be considered as a regular pinhole for the polarization components parallel to the active axis of SLM2 and as a clear aperture (of SLM2 dimensions) for the orthogonal polarization components. Accordingly, the proposed phase pinhole can perform properly even without displaying an axicon. Yet, by incorporating the axicon, light outside of the circular pinhole is actually deflected outside the sensor area. This, in turn, diminishes the amount of light that would otherwise reach the CCD and is only later removed digitally via phase shifting. Thus, the dynamic range of the CCD can be better exploited when the axicon is present. Eventually, we are left mostly with the information of the interference between light that passes through the phase pinhole of SLM2, with its orthogonal counterparts that are imaged at the point a2.
The above described phase pinhole can efficiently achieve optical sectioning, as will be demonstrated promptly. However, better results can be achieved by incorporating a point illumination system, forming a complete confocal FINCH system. In a confocal FINCH system [Fig. 1(b)], the object points outside the scanning spot are eliminated from the recorded hologram. First, due to the point illumination, any of the object points that are not lit (i.e., are not within the cone of light) will not be recorded. Second, all points that are focused onto the region of SLM2 but fall outside of the phase pinhole are also rejected from the hologram. Points that fall partly within the phase pinhole will be attenuated in the recorded hologram, as their recorded fringe patterns will have a very limited aperture, rejecting most of their intensity. Most importantly, points whose images are out of focus on SLM2 are either completely eliminated from the recorded hologram (if their light does not propagate through the phase pinhole) or their intensity is greatly diminished, first due to the out-of-focus illumination and then due to an additional rejection of most of the information, since only a small part of their cone of light can propagate through the phase pinhole. This mechanism allows optical sectioning, with a tradeoff; unlike a regular FINCH system [Fig. 1(a)], which records holograms that contain the complete 3D information of the wide-field illuminated scene, here only a single point in space is properly imaged in a single recorded hologram, and thus a scanning mechanism is required over the entire object.
Scanning of the entire object can be performed either mechanically, by translating the object in the x, y, and z axes, or electronically, without any mechanical intervention. In order to electronically control the position of the point illumination, one can introduce into the illumination system an additional SLM, for example, or other beam steerers (acousto-optical or electro-optical). As the object is scanned over the x y plane, the imaged point over SLM2 is formed at different positions, and so the phase pinhole mask is electronically centered to that point. For each scanning position (m, n), a single hologram is recorded (extracted from three exposures by a complete phase-shifting procedure). From each hologram a single point is reconstructed using the Fresnel diffraction integral formula for a single output point (a complete convolution is unnecessary), and all points from all of the recorded holograms can then be combined into a single image. Alternatively, as performed in the reported experiments, from each hologram a reconstruction of the entire input plane can be calculated; then, for each single pixel of the hologram, the maximum intensity value from all reconstructions is chosen, and a complete reconstruction of a specific x y plane is formed. In practice, just like in conventional confocal microscopy [1,8], many points can be imaged in parallel. This can be achieved, for example, by point illuminating multiple points on the same x y plane simultaneously, while a phase pinhole mask of multiple pinholes is displayed over SLM2. Of course, the distance between the illuminated points must be chosen so that proper optical sectioning can be achieved.
It is also possible to electronically scan at different depths (z positions) by controlling the focal length of the converging lens displayed on SLM1, but this will not allow a perfect overlap of the interfering beams on the CCD plane, and thus a resolution reduction is expected. A mechanical movement of the object at the z axis may then be preferred. This is a bearable cost, since this movement is only necessary once an x y plane scan is completed. Note that alternative FINCH configurations can be used to achieve perfect overlap at different z distances without a mechanical movement. This can be achieved by incorporating another SLM, placed with its active axis perpendicular to the active axis of SLM1 [see Fig. 1(c), in which two SLMs are stacked together and a perfect overlap can be achieved for any desired point source object]. This SLM can even replace the lens Lc if needed.
In Fig. 1(b), the point ao is located on the optical axis and on the back and front focal planes of the point illumination system and the objective lens, respectively. Consider an arbitrary point located on the optical axis at a distance zo from ao. According to McCutchen’s theorem, the complex amplitude at that point, (0, 0, zo), is given by , where the right-hand-side term is attributed to a onedimensional Fourier transform of the radial aperture distribution Hill, of the illumination system, fo is its focal distance, λ is the central wavelength, and ρ is the variable of Hill equal to r2 [12]. The point (0, 0, zo) is imaged into two image points, (0, 0, z1 = zo ma,1) and (0, 0, z2 = zo ma,2), with complex amplitudes of b1 hill (zo) and b2 hill (zo), respectively, where b1 and b2 are constants, ma, 1 and ma, 2 represent the axial magnifications of the corresponding imaging systems, and the planes of z1,2 = 0 are at the imaged points a1 and a2, respectively. The waves associated with these two points interfere on the CCD plane, forming a fringe pattern from which the point (0, 0, zo) can be reconstructed. The interference pattern can be described as
| (2) |
where is a quadratic phase function. The left-hand-side term in Eq. (2) is attributed to the wave associated with the image point (0, 0, z1 = zo ma,1), a distance l1 from the CCD, following its multiplication by the phase mask of the form of Eq. (1), which effectively multiplies the wave by a complex amplitude proportional to , with Hdet affiliated with the imaging system forming that point and f1 as its focal distance. Note that in the case of clear circular apertures, Hill and Hdet are Rect functions, so hill and hdet are Sinc functions. The right-hand-side term in Eq. (2) is attributed to the wave associated with the image point (0, 0, z2), a distance l2 from the CCD.
Following a phase-shifting procedure, only one of the cross terms in Eq. (2) is left:
| (3) |
where , zr = l1 l2/(l1 + l2) is the reconstruction distance and denotes the circular aperture of the hologram, with a clear disk of radius RH. This radius is determined by the overlap area of the two interfering waves. The recorded point can then be reconstructed from the hologram through a digital Fresnel propagation to the reconstruction distance zr [9]:
| (4) |
where *Q(1/zr) denotes a Fresnel propagation (here, * denotes a two-dimensional convolution) and J inc (r) ≜ J1 (r)/r is the Bessel function of the first kind and of order one. Comparing Eq. (4) above with Eq. (5) of Ref. [9], we conclude that the transverse resolution of the proposed system, attributed to the Jinc term, is similar to a regular FINCH system; however, the longitudinal separation is much better in the proposed system and is comparable to that of a conventional confocal microscope, due to the zo-dependent terms |hill (zo)|2 hdet (ma,1zo).
In order to demonstrate the optical sectioning capabilities of the proposed system, the experimental setup described in Fig. 2 was used. A beam splitter, BS1, was used as a beam combiner, with two resolutions charts (negative National Bureau of Standards 1963A), RC1 and RC2, positioned at a distance of 30 and 31 cm (for the x y plane scan of RC1) or 29 and 30 cm (for the scan of RC2) away from the objective lens, respectively. Together, the two charts can be considered as a 1 cm thick object. The resolution charts were back-illuminated using two LEDs (Thorlabs LED635L, 170 mW, λ = 632.8 nm, Δλ = 15 nm). Diffuser sheets were attached to the charts in order to imitate a scattering/illuminating object. The focal lengths of the objective lens Lo and the lens Lc were chosen as fobj = 30 cm and fc = 150 cm, respectively. For simplicity, we have replaced these two lenses with an equivalent lens with a focal length of fe = 25 cm, implying that d1 = 0 cm (Fig. 2). Other parameters in the system were: d2 = 10 cm, d3 = 66.3 cm, and zh = 90 cm (which is the distance from SLM1 to the CCD and is the parameter that determines the transverse magnification of FINCH as MT = zh/fobj). The two SLMs (Holoeye PLUTO, 1920 × 1080 pixels, 8 μm pixel pitch, phase-only modulation) were positioned at similar orientations, with their active axes in parallel, while the two polarizers, P1 and P2, were set at a 45° angle to these axes, and in parallel to each other. Since the two SLMs are reflective, two beam splitters, BS2 and BS3, were incorporated into the system so that the two SLMs were positioned orthogonally to the optical axis. This causes a loss of a large amount of light, which can be avoided by either by positioning the SLMs at a small angle, eliminating the beam splitters but requiring a careful compensation in the SLM displayed masks, or by using transmissive components (e.g., SLM1 can be replaced with a liquid crystal gradient index lens [13]).
Fig. 2.
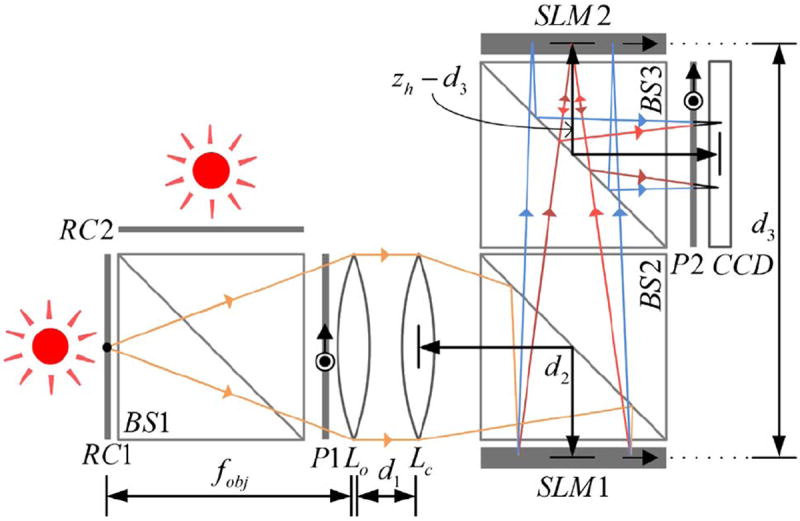
Experimental setup: RC1 and RC2, resolution charts; BS1, BS2, and BS3, beam splitters; P1 and P2, polarizers; Lo, objective lens; Lc, converging lens; SLM1 and SLM2, spatial light modulators; CCD, charge-coupled device.
In the experiments, we compared the results of a regular FINCH system (setting the phase mask of SLM2 to a constant zero modulation) and an optical sectioning FINCH system (with a phase pinhole displayed on SLM2). The phase pinhole radius was r1 = 44 μm, surrounded by an axicon of parameter α = 2π · 13 · 10−3/λ, and the scanning over a grid of size 81 × 61 points on each plane was performed with pinhole displacements in the x and y axes of 40 μm. The exposure time was set to 1.25 s, implying a scanning time of roughly 5 h per plane. Note that in the reported experiments we do not use the proposed point illumination, and the entire object is illuminated at all times. This is to emphasize the contribution of the phase pinhole, which can be considered the most genuine part and newest contribution of this work, whereas scanning illumination systems are widely used in confocal microscopy [8]. It should be noted that such systems, in which the sectioning is performed solely by the phase pinhole, can be useful for imaging situations in which the observed scene cannot be illuminated as required.
The experimental results are presented in Fig. 3. Reconstruction results from regular FINCH holograms are presented in Figs. 3(a) and 3(b), for the resolution targets closest to the objective (RC1) and farthest from it (RC2), respectively. Here, the out-of-focus targets greatly diminish the quality of the reconstruction. The equivalents of the reconstructions of Figs. 3(a) and 3(b), resulting from the phasepinhole-incorporated FINCH, are presented in Figs. 3(c) and 3(d), respectively. Unlike regular FINCH holography, the out-of-focus targets are highly attenuated, and so the in-focus targets appear with much detail, high contrast, and weak background artifacts. Hence, the optical sectioning capability of the proposed system is clearly demonstrated. It should be noted that once a single point illumination is incorporated into the system, the sectioning capabilities are expected to be further enhanced. Here, the out-of-focus target is illuminated with similar intensity to the in-focus target, unlike the case of point illumination, where object points will exhibit an illumination of less intensity that declines farther away from the location of the illuminated point.
Fig. 3.
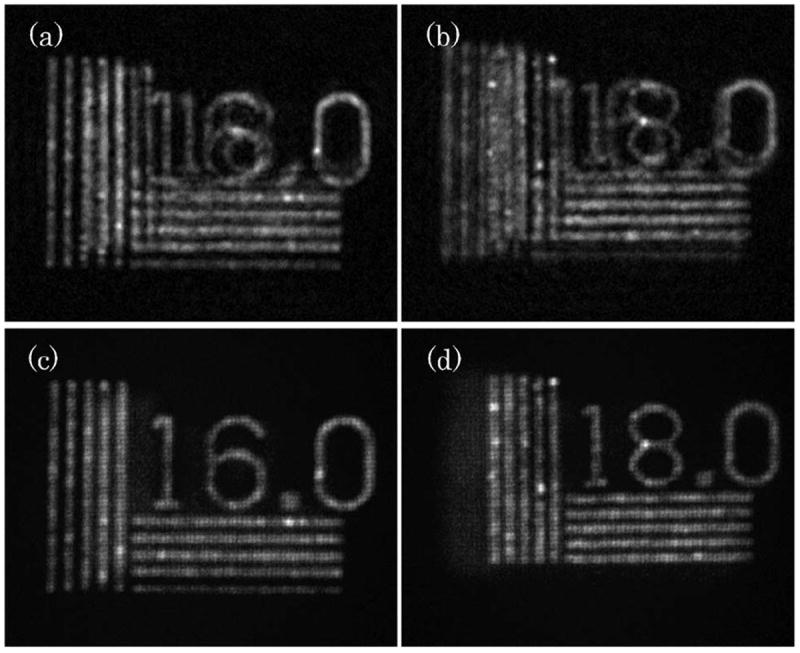
Experimental results: (a) FINCH reconstruction of a 16.0 cycles/mm resolution chart located 30 cm away from the objective lens, with the 18.0 cycles/mm resolution chart at 31 cm; (b) FINCH reconstruction of a 18.0 cycles/mm resolution chart located 30 cm away from the objective lens, with the 16.0 cycles/mm resolution chart at 29 cm; (c), (d) the optical sectioning FINCH equivalents of (a) and (b), respectively.
To conclude, a new confocal FINCH configuration capable of optical sectioning has been proposed in this Letter, and a simpler configuration of FINCH capable of sectioning has been demonstrated and compared with regular FINCH. The proposed system combines the super-resolution capabilities of FINCH with the sectioning capabilities of confocal microscopy. Together with the notable capabilities of FINCH for fluorescent microscopy, parallel illumination arrangements, and nonmechanical scanning schemes [8], we expect the proposed system to have an important role in microscopy in general and biological microscopy in particular. Other potential applications for the proposed system might include high lateral resolution tomography that is also capable of simultaneous imaging of passive reflecting surfaces and fluorescent objects.
Acknowledgments
FUNDING INFORMATION
National Institutes of Health (NIH) (U54GM105814); The Israel Ministry of Science and Technology (MOST); The Israel Science Foundation (ISF) (439/12).
References
- 1.Webb RH. Rep Prog Phys. 1996;59:427. [Google Scholar]
- 2.Minsky M. Scanning. 1988;10:128. [Google Scholar]
- 3.Amos WB, White JG. Biol Cell. 2003;95:335. doi: 10.1016/s0248-4900(03)00078-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun P-C, Leith EN. Appl Opt. 1994;33:597. doi: 10.1364/AO.33.000597. [DOI] [PubMed] [Google Scholar]
- 5.Chmelík R, Harna Z. Opt Eng. 1999;38:1635. [Google Scholar]
- 6.Rosen J, Brooker G. Opt Lett. 2007;32:912. doi: 10.1364/ol.32.000912. [DOI] [PubMed] [Google Scholar]
- 7.Rosen J, Siegel N, Brooker G. Opt Express. 2011;19:26249. doi: 10.1364/OE.19.026249. [DOI] [PubMed] [Google Scholar]
- 8.Verveer PJ, Hanley QS, Verbeek PW, Van Vliet LJ, Jovin TM. J Microsc. 1998;189:192. [Google Scholar]
- 9.Katz B, Rosen J, Kelner R, Brooker G. Opt Express. 2012;20:9109. doi: 10.1364/OE.20.009109. [DOI] [PubMed] [Google Scholar]
- 10.Kelner R, Rosen J, Brooker G. Opt Express. 2013;21:20131. doi: 10.1364/OE.21.020131. [DOI] [PubMed] [Google Scholar]
- 11.Brooker G, Siegel N, Wang V, Rosen J. Opt Express. 2011;19:5047. doi: 10.1364/OE.19.005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCutchen CW. J Opt Soc Am. 1964;54:240. [Google Scholar]
- 13.Brooker G, Siegel N, Rosen J, Hashimoto N, Kurihara M, Tanabe A. Opt Lett. 2013;38:5264. doi: 10.1364/OL.38.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]


