Abstract
Background
Across sub-Saharan Africa, men's levels of HIV-testing remain inadequate relative to women’s. Men are less likely to access anti-retroviral therapy and experience higher levels of morbidity and mortality once initiated on treatment. More frequent HIV-testing by men at continued risk of HIV-infection is required to facilitate earlier diagnosis. This study explored the frequency of HIV-testing among a rural population of men and the factors associated with more frequent HIV-testing.
Methods
We conducted a secondary analysis of a population-based survey in three rural district in Zambia, from February-November, 2013. Households (N = 300) in randomly selected squares from 42 study sites, defined as a health facility and its catchment area, were invited to participate. Individuals in eligible households were invited to complete questionnaires regarding demographics and HIV-testing behaviours. Men were defined as multiple HIV-testers if they reported more than one lifetime test. Upon questionnaire completion, individuals were offered rapid home-based HIV-testing.
Results
Of the 2376 men, more than half (61 %) reported having ever-tested for HIV. The median number of lifetime tests was 2 (interquartile range = 1-3). Just over half (n = 834; 57 %) of ever-testers were defined as multiple-testers. Relative to never-testers, multiple-testers had higher levels of education and were more likely to report an occupation. Among the 719 men linked to a spouse, multiple-testing was higher among men whose spouse reported ever-testing (adjusted prevalence ratio = 3.02 95 % CI: 1.37-4.66). Multiple-testing was higher in study sites where anti-retroviral therapy was available at the health facility on the day of a health facility audit. Among ever-testers, education and occupation were positively associated with multiple-testing relative to reporting one lifetime HIV-test. Almost half (49 %) of ever-testers accepted the offer of home-based HIV-testing.
Discussion
Reported HIV-testing increased among this population of men since a 2011/12 survey. Yet, only 35 % of all men reported multiple lifetime HIV-tests. The factors associated with multiple HIV-testing were similar to factors associated with ever-testing for HIV. Men living with HIV were less likely to report multiple HIV-tests and employment and education were associated with multiple-testing. The offer of home-based HIV-testing increased the frequency of HIV-testing among men.
Conclusion
Although men's levels of ever-testing for HIV have increased, strategies need to increase the lifetime frequency of HIV-testing among men at continued risk of HIV-infection.
Background
Annual HIV-testing and counselling (HTC) in high prevalence settings is recommended for individuals at continued risk of HIV infection to support early detection of HIV-infection and initiation of anti-retroviral therapy (ART) [1]. Mathematical models suggest that the provision of “high-quality” HTC services to all individuals will increase the HIV-prevention impact of HTC service delivery [2]. In settings where annual HTC is recommended, including Zambia, men’s levels of ever HIV-testing remain lower than is needed to link men testing HIV-positive into care [3–5]. Encouraging men to increase their lifetime frequency of HIV-testing may prove challenging [6].
Studies exploring risk factors for HIV-testing in sub-Saharan Africa highlight that age [3, 7–10], employment [4, 11], education [8, 10, 12] and socio-economic position [4, 12], marital status [8, 10], having heard of ART [4], community-level employment and HIV-knowledge [13] are associated with men ever-testing. Whether these factors also encourage men to test more frequently deserves exploration, to determine whether the expansion of HTC services has increased the frequency of HTC among men at risk of HIV-infection. Such evidence would support the development of strategies to reach men in need of annual HIV-testing.
This study describes the frequency of HIV-testing among a rural population of Zambian men and explores the factors associated with frequent HIV-testing. We hypothesized that, relative to never-testers, the factors associated with multiple HIV-testing would be similar to ever-testing for HIV. Among men with a history of ever HIV-testing, we hypothesized that men reporting frequent HIV-testing would differ in socio-demographic characteristics from men reporting one lifetime HIV-test. We also explore whether an offer of home-based HIV-testing in a research setting increases the frequency of testing among men with a history of HIV-testing.
Methods
We analyzed data collected for a stepped-wedge cluster randomized trial (CRT): the Better Health Outcomes through Mentoring and Assessment (BHOMA) trial, which aims to strengthen the healthcare system [14]. Details of the intervention are published elsewhere [14, 15]. Briefly, BHOMA was implemented in 42 clusters, defined as a health facility and its catchment area, in three districts in Lusaka Province, Zambia. BHOMA aims to reduce age-adjusted all-cause and under-5 mortality, and is being evaluated through three rounds of household surveys [14]. Increasing HIV-testing is not a primary or secondary objective. However, health facilities were equipped with diagnostics and essential medicines [14], healthcare workers provided with protocols to guide adult visits alongside recruitment of community health workers to increase demand for health services [14]. The majority of BHOMA study sites were rural (n = 34, 81 %). Data for the present analysis were from the mid-line evaluation (February-November, 2013) after intervention implementation in all sites.
In each cluster, squares of 900 m2 were marked within a 3.8 km of the health facility [3, 14]. Computer-generated randomization was used to determine which squares would be visited and the order of visitation. All households in randomly selected squares where the survey was started were visited until 300 households were enumerated in each cluster.
Data collection
Data collection tools included: household enumeration, and household and individual questionnaires. Due to financial constraints, households were either invited to complete a partial (household enumeration and questionnaire only) or full survey (household members asked to complete an individual questionnaire and offered measurements including blood glucose and pressure, and HIV-testing). Systematic random sampling was used to select households for participation in the full survey, with every 2.5th household offered the full survey (n = 6788; 57 %). Personal digital assistants (PDAs) informed research assistants which survey to offer a household prior to visitation. Data to estimate BHOMA’s primary outcome were obtained from household enumeration Repeat visits were only conducted if entire households were absent. Questionnaires were adapted from Demographic and Health Surveys (DHS) and administered using PDAs. Household questionnaires included questions on asset ownership and housing material. Individuals aged 15–59 years were eligible for the individual questionnaire. After questionnaire completion, individuals were offered voluntary HIV-testing (Determine™ HIV-1/2).
Statistical analysis
We restricted analyses to men. Outcomes of interest included i) never-testing, ii) ever-testing (defined as testing and receiving the result of an HIV-test), and iii) multiple-testing (defined as reporting >1 lifetime HIV-test). Ever-testers reporting one lifetime HIV-test were defined as one-time testers. Men self-reporting that they were living with HIV were defined as multiple-testers if their first HIV-test was before the test in which they received their HIV-positive diagnosis.
We described never- and ever-testing among men with complete data on variables of interest: age, religion, marital status, occupation, education, head of household, history of TB-treatment, ever HIV-tested and household socioeconomic position (SEP). Among ever-testers, we described the proportion reporting one and multiple HIV-tests. We described acceptance of home-based HIV-testing.
We described the distribution of the outcomes by socio-demographic characteristics, temporary migrancy (defined as being absent ≥1 month in the 6 months preceding the survey), and a history of TB-treatment. During household enumeration, females were asked what the number of their spouse was as listed on the enumeration form. Using this number, females were linked to their spouse. For men linked to a spouse who completed a questionnaire, we described outcomes by whether the spouse was pregnant, reported having children or ever HIV-tested. At cluster-level, we described outcomes by ART availability at the local health facility, HIV-prevalence, whether or not ≥50 % of men reported employment and whether 25 % of men listed 3+ ways to prevent HIV-infection.
Data on whether unexpired ART was available at health facilities was obtained from a health facility audit (conducted in 2012) [16]. A household SEP indicator was developed using principal components analysis (PCA) [3]. PCA was conducted on households with no missing data, regardless of whether households completed the full or partial survey, whether an eligible man was present and without taking account of district or rural/urban residence. SEP scores were divided into quintiles.
We estimated minimally-adjusted associations between independent variables and outcomes controlling for age, urban residence and a fixed effect for the three districts. Factors significant at the p ≤ 0.1 level in minimally-adjusted models were included in multivariable models based on the framework in Fig. 1. Socio-demographic factors were not adjusted for the more proximal factors likely to mediate their effect. Associations with community-level characteristics were estimated without adjustment for individual-level variables. Spousal characteristics were explored among the sub-set of men linked to a spouse. Multivariable models included a continuous variable for cluster size.
Fig. 1.
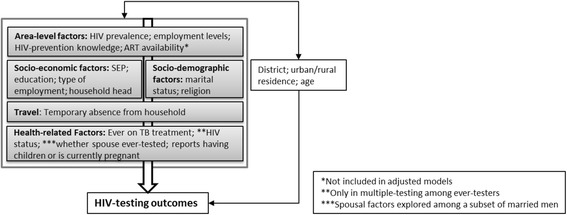
Framework illustrating the expected causal relationship between independent variables and HIV-testing
We fit random effects logistic regression models in Stata 13.0 to adjust for geographic clustering. We checked the reliability of model estimates by running the quadchk command. For age, education and SEP we conducted a test assuming linear trend if there appeared to be a linear association. Due to the high prevalence of the outcomes, we estimated associations with prevalence ratios (PRs) using marginal standardization to estimate PRs, and the delta method to estimate 95 % confidence intervals (95 % CI). We used the likelihood ratio test (LRT) to estimate p-values.
Missing data
Survey non-participation was high due to men being absent at the time of the household survey. We used Heckman-type selection models to investigate the null hypothesis that outcomes were “missing at random” conditional on covariates available for non-participants [17–19]. We identified three variables that we theorized would be associated with survey participation but not HIV-testing: time (morning, afternoon, evening), day (Monday-Thursday, Friday, Saturday-Sunday) and season (rainy, cool/dry, hot/dry) of the survey. These variables were included in a random effects model controlling for variables crudely associated with participation to investigate whether they were independently associated with participation (Appendix 1: Table 4) [18]. Characteristics of eligible participants were randomly distributed by time but not day of the survey (Appendix 3: Table 6). Time was entered in the model as a selection variable. Data available on non-participants and associated with HIV-testing in a 2011/12 survey [3] or theorized to be associated were included in the outcome model. We assessed evidence for the null hypothesis using rho and its p-value [18]. Estimates of association between independent variables and outcome were obtained by adjusting for variables as described in Fig. 1. Cluster-level variables were not adjusted for proximal factors. We investigated whether adjustment for variables included in the Heckman models affected estimates of association.
Table 4.
Characteristics of eligible men & Factors Associated with Participation (N = 5797)
| Characteristic | Detail | Distribution (n, col %) | Participants (n, row %) | Crude PRa (95 % CI) |
|---|---|---|---|---|
| All men | 5797 | - | 2463 (42.5) | - |
| Age category | 15-24 | 2303 (39.7) | 934 (40.6) | 1.0 |
| 25-60 | 3494 (60.3) | 1529 (43.8) | 1.08 (1.02-1.15) | |
| Head of household | No | 2590 (44.7) | 921 (35.6) | 1.0 |
| Yes | 3207 (55.3) | 1542 (48.1) | 1.34 (1.25-1.43) | |
| Present continuously previous 6mths | No | 223 (3.8) | 116 (52.0) | 1.0 |
| Yes | 5574 (96.2) | 2347 (42.1) | 0.80 (0.69-0.91) | |
| SEP | Lowest | 957 (16.5) | 463 (43.4) | 1.0 |
| Low | 1038 (17.9) | 471 (45.4) | 0.96 (0.87-1.06) | |
| Middle | 1206 (20.8) | 536 (44.4) | 0.93 (0.84-1.02) | |
| High | 1275 (22.0) | 517 (40.6) | 0.84 (0.76-0.93) | |
| Highest | 1321 (22.8) | 476 (36.0) | 0.74 (0.66-0.83) | |
| Household size | 1-5 | 2736 (47.2) | 1325 (48.4) | 1.0 |
| 6–10 | 2711 (46.8) | 1029 (38.0) | 0.78 (0.73-0.83) | |
| >10 | 350 (6.0) | 109 (31.1) | 0.64 (0.53-0.75) | |
| Urban cluster | No | 4751 (82.0) | 2042 (43.0) | 1.0 |
| Yes | 1046 (18.0) | 421 (40.3) | 0.94 (0.75-1.13) | |
| District | Kafue | 2106 (36.3) | 886 (42.1) | 1.0 |
| Chongwe | 2810 (48.5) | 1122 (39.9) | 0.95 (0.81-1.10) | |
| Luangwa | 881 (15.2) | 455 (51.7) | 1.23 (1.01-1.45) |
Table 6.
Distribution of characteristics of eligible men by selection variables (N = 5797)
| Characteristic | Detail | Monday-Thursday | Friday | Saturday-Sunday | p-valuea |
|---|---|---|---|---|---|
| Household Head | No | 1704 (65.8) | 401 (15.5) | 485 (18.7) | 0.83 |
| Yes | 2121 (66.1) | 530 (16.5) | 556 (17.3) | ||
| Present continuously in previous 6mth | No | 159 (71.3) | 37 (16.6) | 27 (12.1) | 0.08 |
| Yes | 3666 (65.8) | 894 (16.0) | 1014 (18.2) | ||
| SEP Group | Lowest | 603 (63.0) | 157 (16.4) | 197 (20.6) | <0.01 |
| Low | 595 (57.3) | 180 (17.3) | 263 (25.3) | ||
| Middle | 801 (66.4) | 186 (15.4) | 219 (18.2) | ||
| High | 877 (68.8) | 197 (15.5) | 201 (15.8) | ||
| Highest | 949 (71.8) | 211 (16.0) | 161 (12.2) | ||
| Household Size | 1-5 | 1817 (66.4) | 438 (16.0) | 481 (17.6) | 0.49 |
| 6–10 | 1758 (64.9) | 449 (16.6) | 504 (18.6) | ||
| >10 | 250 (71.4) | 44 (12.6) | 56 (16.0) | ||
| Urban cluster | No | 3104 (65.3) | 750 (15.8) | 897 (18.9) | 0.43 |
| Yes | 721 (68.9) | 181 (17.3) | 144 (13.8) | ||
| District | Kafue | 1507 (71.6) | 287 (13.6) | 312 (14.8) | 0.09 |
| Chongwe | 1792 (63.8) | 464 (16.5) | 554 (19.7) | ||
| Luangwa | 526 (59.7) | 180 (20.4) | 175 (19.9) | ||
| Characteristic | Detail | Morning | Early pm | Late pm | p-value |
| Household Head | No | 1261 (48.7) | 1183 (45.7) | 146 (5.6) | 0.28 |
| Yes | 1517 (47.3) | 1522 (47.5) | 168 (5.2) | ||
| Present continuously in previous 6mth | No | 112 (50.2) | 99 (44.4) | 12 (5.4) | 0.47 |
| Yes | 266 (47.8) | 2606 (46.8) | 302 (5.4) | ||
| SEP Group | Lowest | 457 (47.8) | 451 (47.1) | 49 (5.1) | 0.95 |
| Low | 475 (45.7) | 510 (49.1) | 53 (5.1) | ||
| Middle | 574 (47.6) | 565 (46.9) | 67 (5.6) | ||
| High | 617 (48.4) | 593 (46.5) | 65 (5.1) | ||
| Highest | 655 (50.0) | 586 (44.4) | 80 (6.1) | ||
| Household Size | 1-5 | 1288 (47.1) | 1307 (47.8) | 141 (5.2) | 0.31 |
| 6–10 | 1326 (48.9) | 1247 (46.0) | 138 (5.1) | ||
| >10 | 164 (46.9) | 151 (43.14) | 35 (10.0) | ||
| Urban cluster | No | 2279 (48.0) | 2194 (46.2) | 278 (5.9) | 0.55 |
| Yes | 499 (47.7) | 511 (48.9) | 36 (3.4) | ||
| District | Kafue | 993 (47.2) | 980 (46.5) | 133 (6.3) | 0.42 |
| Chongwe | 1352 (48.1) | 1318 (46.9) | 140 (5.0) | ||
| Luangwa | 433 (49.2) | 407 (46.2) | 41 (4.7) |
a p-value adjusted for clustering by study sites
Ethics statement
BHOMA was approved by the University of Zambia Bioethics Committee, the London School of Hygiene and Tropical Medicine Ethics Committee and the institutional review boards at the University of Alabama at Birmingham (Birmingham, AL, USA) and University of North Carolina (Chapel Hill, NC, USA) [14]. Individuals were informed of the study objectives and asked for written informed consent. Consent was obtained from a parent/guardian for individuals aged 15–17 years.
Results
Sample population
Of 5145 households invited to complete the full survey, 5144 consented. In these households, 6202 eligible men were enumerated of whom 29 did not have full data available and 376 were listed as absent in the month of or the month preceding the survey, leaving 5797 (93 %) men defined as eligible to participate. Among these men, 42 % (n = 2463) participated (Fig. 2). Participation ranged from 22–65 % (median: 42 %; inter-quartile range (IQR): 34–51 %) across study sites. Men of highest SEP were less likely to participate than men of lowest SEP (PR = 0.74, 95 % CI: 0.66-0.83; Appendix 1: Table 4). Men listed as a household head were more likely to participate (PR = 1.34 95 % CI: 1.25-1.43).
Fig. 2.
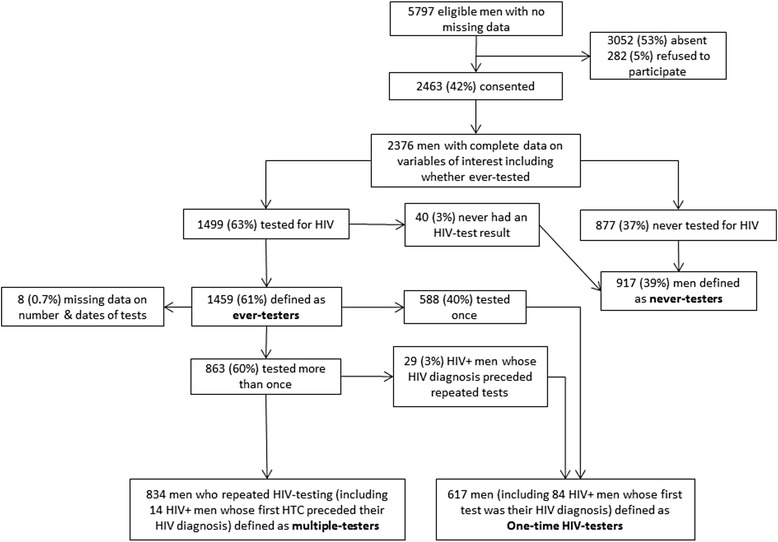
Flow diagram of study participation and frequency of HIV-testing
Frequency of HIV-testing
Among participating men, 37 % (n = 877/2376) reported never-testing for HIV, and 2 % (n = 40/2376) tested but never received the result of an HIV-test. Overall, 61 % (n = 1459) of men ever-tested. Ever-testing ranged from 44–87 % (median = 62 %; IQR = 56–67 %) across study sites. Among ever-testers, 7 % (n = 98) reported themselves HIV-positive. The number of lifetime HIV-tests ranged from 1–25 (median = 2; IQR = 1–3).
Just over half of ever-testers (57 %; n = 834/1459) were defined as multiple-testers (Fig. 2). Among ever-testers, levels of multiple-testing were 62 % in Kafue district and 55 % in Chongwe and Luangwa. There was evidence for correlation between multiple-testing among ever-testers and ever-testing in Chongwe district (Chongwe r = 0.54; p = 0.01; Kafue r = 0.05; p = 0.86; Luangwa r = 0.34; p = 0.46; Fig. 3). Multiple-testing ranged from 27– 83 % (median = 57 %; IQR: 48–68 %) across study sites and was clustered by study site (intra-cluster correlation coefficient (ICC) = 0.05 95 % CI: 0.03-0.11; p < 0.01). Just over half (57 %) the men living with HIV reported one-lifetime HIV-test. An estimated 14 % HIV-tested prior to the test in which they received an HIV-positive diagnosis.
Fig. 3.
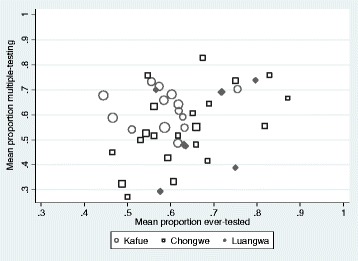
Scatter plot of correlation between multiple-testing among ever-testers and ever-testing at cluster-level
The median numbers of years between first and most recent HIV-test was 2 (IQR: 1–4). Half (n = 422; 51 %) of multiple-testers and 29 % (n = 176) of one-time testers reported their first HIV-test between 2009 and 2011. Sixty-percent (n = 504) of multiple-testers and 31 % (n = 191) of one-time testers tested within 12 months of the survey. Over half of one-time (n = 341; 55 %) and multiple-testers (n = 498; 60 %) reported their most recent HIV-test at the local health facility.
Factors associated with multiple HIV-testing
Relative to never-testers, multiple-testing was higher among men aged 30–39 relative to men 20–29 (65 % vs 53 %; adjPR = 1.25 95 % CI: 1.12-1.39; Table 1), men with complete secondary/higher education relative to men with no/primary education (65 % vs 43 %; adjPR = 1.59 95 % CI: 1.38-1.81) and among men reporting service/professional employment relative to men reporting no employment (70 % vs 33 %; adjPR = 1.29 95 % CI: 1.08-1.50; Table 1). Multiple-testing was higher among married/cohabiting men relative to single men (61 % vs 32 %; adjPR = 1.23 95 % CI: 1.03-1.43) and among Protestant men (52 %) relative to men of no religion (33 %; adjPR = 0.69 95 % CI: 0.47-0.90). There was weak evidence that men of middle SEP were more likely to report multiple-testing relative to men of lowest SEP (adjPR = 1.19 95 % CI: 1.02-1.37). Having a spouse who reported ever-testing was associated with multiple-testing (adjPR = 3.02 95 % CI: 1.37-4.66) with little evidence that having children was associated (p = 0.20). There was little evidence that multiple-testing differed by cluster-level employment or HIV-knowledge. Multiple-testing was higher in sites where ART was available on the day of the audit (52 % vs 43 %; adjPR = 1.29 95 % CI: 1.12-1.45).
Table 1.
Distribution of characteristics by never- and multiple-testers and factors associated with multiple HIV-testing relative to never-testers (N = 1751)
| Details | Multiple-testers & never-testers (n, col %) | Never-Testers (n, row %) | Multiple Testers (n, row %) | Minimally-adjusted PR (95 % CI)b | Adjusted PR (95 % CI)c | p-valued | |
|---|---|---|---|---|---|---|---|
| Age | 15-19 | 387 (22.1) | 328 (84.8) | 59 (15.2) | 0.28 (0.21-0.36) | 0.29 (0.22-0.37) | <0.01 |
| 20-29 | 553 (31.6) | 259 (46.8) | 294 (53.2) | 1.0 | 1.0 | ||
| 30-39 | 391 (22.3) | 137 (35.0) | 254 (65.0) | 1.23 (1.09-1.36) | 1.25 (1.12-1.39) | (<0.01) | |
| ≥40 | 420 (24.0) | 193 (46.0) | 227 (54.0) | 1.02 (0.89-1.14) | 1.04 (0.91-1.16) | ||
| Head of household | No | 680 (38.8) | 472 (69.4) | 208 (30.6) | 1.0 | 1.0 | 0.06 |
| Yes | 1071 (61.2) | 445 (41.5) | 626 (58.5) | 1.28 (1.09-1.46) | 1.15 (0.97-1.32) | ||
| Religion | Protestant | 684 (39.1) | 332 (48.5) | 352 (51.5) | 1.0 | 1.0 | 0.01 |
| Catholic | 435 (24.8) | 229 (52.6) | 206 (47.4) | 0.89 (0.78-1.00) | 0.91 (0.80-1.01) | ||
| SDA | 257 (14.7) | 122 (47.5) | 135 (52.5) | 1.01 (0.88-1.15) | 0.98 (0.85-1.11) | ||
| Other | 305 (17.4) | 187 (61.3) | 118 (38.7) | 0.78 (0.66-0.90) | 0.83 (0.71-0.96) | ||
| None | 70 (4.0) | 47 (67.1) | 23 (32.9) | 0.59 (0.38-0.79) | 0.69 (0.47-0.90) | ||
| Marital status | Single | 762 (43.5) | 522 (68.5) | 240 (31.5) | 1.0 | 1.0 | 0.02 |
| Married/cohabiting | 913 (52.1) | 361 (39.5) | 552 (60.5) | 1.33 (1.12-1.53) | 1.23 (1.03-1.43) | ||
| Ever married | 76 (4.3) | 34 (44.7) | 42 (55.3) | 1.26 (0.94-1.59) | 1.30 (0.99-1.61) | ||
| Education | No/Primary | 745 (42.5) | 426 (57.2) | 319 (42.8) | 1.0 | 1.0 | |
| Incomplete secondary | 649 (37.1) | 366 (56.4) | 283 (43.6) | 1.29 (1.13-1.44) | 1.26 (1.11-1.40) | <0.01 | |
| Secondary or higher | 357 (20.4) | 125 (35.0) | 232 (65.0) | 1.61 (1.41-1.81) | 1.59 (1.38-1.81) | (<0.01) | |
| Occupation | None | 802 (45.8) | 536 (66.8) | 266 (33.2) | 1.0 | 1.0 | <0.01 |
| Agriculture (others land) | 378 (21.6) | 182 (48.1) | 196 (51.9) | 1.17 (0.99-1.34) | 1.11 (0.94-1.27) | ||
| Agriculture (own land) | 357 (20.4) | 134 (37.5) | 223 (62.5) | 1.39 (1.19-1.60) | 1.30 (1.11-1.48) | ||
| Services/Professional | 214 (12.2) | 65 (30.4) | 149 (69.6) | 1.53 (1.30-1.77) | 1.29 (1.08-1.50) | ||
| SEP Group | Lowest | 343 (19.6) | 184 (53.6) | 159 (46.4) | 1.0 | 1.0 | 0.06 |
| Low | 331 (18.9) | 188 (56.8) | 143 (43.2) | 1.01 (0.83-1.18) | 0.99 (0.93-1.14) | ||
| Middle | 365 (20.8) | 179 (49.0) | 186 (51.0) | 1.24 (1.03-1.44) | 1.19 (1.02-1.37) | ||
| High | 361 (20.6) | 189 (52.4) | 172 (47.6) | 1.21 (1.01-1.42) | 1.09 (0.92-1.26) | ||
| Highest | 351 (20.0) | 177 (50.4) | 174 (49.6) | 1.32 (1.08-1.55) | 1.04 (0.84-1.25) | ||
| Present continuously previous 6mths | No | 87 (5.0) | 50 (57.5) | 37 (42.5) | 1.0 | - | - |
| Yes | 1664 (95.0) | 867 (52.1) | 797 (47.9) | 1.12 (0.85-1.40) | |||
| History of TB treatment | No | 1702 (97.2) | 899 (52.8) | 803 (47.2) | 1.0 | - | - |
| Yes | 49 (2.8) | 18 (36.7) | 31 (63.3) | 1.15 (0.88-1.43) | - | - | |
| Spousal Characteristics (N = 511) | |||||||
| Currently pregnant | No | 447 (87.6) | 179 (40.0) | 268 (60.0) | 1.0 | - | - |
| Yes | 63 (12.4) | 19 (30.2) | 44 (69.8) | 1.16 (0.95-1.38) | |||
| Has Children | No | 27 (5.3) | 14 (51.9) | 13 (48.2) | 1.0 | 1.0 | 0.20 |
| Yes | 483 (94.7) | 183 (37.9) | 300 (62.1) | 1.34 (0.76-1.91) | 1.26 (0.76-1.76) | ||
| Wife previously HIV-tested | No | 56 (11.0) | 46 (82.1) | 10 (17.9) | 1.0 | 1.0 | <0.01 |
| Yes | 454 (89.0 | 151 (33.3) | 303 (66.7) | 3.53 (1.50-5.57) | 3.02 (1.37-4.66) | ||
| Cluster-level Characteristics | |||||||
| >50 % of men employed | No | 684 (39.1) | 379 (55.4) | 305 (44.6) | 1.0 | - | - |
| Yes | 1067 (60.9) | 538 (50.4) | 529 (49.6) | 1.10 (0.91-1.29) | - | - | |
| >25 % mention 3+ ways to prevent HIV infection | No | 878 (50.1) | 435 (49.5) | 443 (50.5) | 1.0 | - | - |
| Yes | 873 (49.9) | 482 (55.2) | 391 (44.8) | 0.90 (0.76-1.04) | - | - | |
| HIV Prevalence | <10 % | 1381 (78.9) | 704 (51.0) | 677 (49.0) | 1.0 | 1.0 | 0.14 |
| 10 %+ | 370 (21.1) | 213 (57.6) | 157 (42.4) | 0.87 (0.70-1.03) | 0.87 (0.70-1.04) | ||
| ART Available at Health Facilitya | No | 858 (51.8) | 491 (57.2) | 367 (42.8) | 1.0 | 1.0 | <0.01 |
| Yes | 799 (48.2) | 381 (47.7) | 418 (52.3) | 1.30 (1.12-1.48) | 1.29 (1.12-1.45) | ||
a94 missing data N = 1657; bAdjusted for age, urban/rural residence and district; cAdjusted for variables higher in the conceptual framework (Fig. 1); dFor adjusted model and based on LRT, p-value in brackets is assuming linear trend
eever-married means either widowed, separated or divorced
Relative to one-time testers, multiple-testers were less likely to be aged 15–19 (adjPR compared to 20–29: 0.63 95 % CI: 0.49-0.77; Table 2). Among men working on their own land, 70 % reported multiple-testing relative to 48 % of men reporting no employment (adjPR = 1.45 95 % CI 1.27-1.63). There was little evidence of an association with marital status, a history of TB treatment or household SEP, with weak evidence that multiple-testing differed by being household head, pregnancy status of the spouse, or having children (Table 2). Men living with HIV were less likely to report multiple-tests prior to diagnosis (14 % vs 61 % among HIV-negative men; adjPR = 0.22; 95 % CI: 0.11-0.33). There was little evidence for an association with ART availability or cluster-level employment. Multiple-testing was lower in clusters with higher levels of HIV-prevention knowledge (53 % vs 62 %; adjPR =0.86 95 % CI: 0.74-0.98).
Table 2.
Distribution of characteristics by one- and multiple-testers and factors associated with multiple HIV-testing relative to one-time testers (N = 1451)
| Details | Men with a history of ever-testing | One-time Testers (n, row %) | Multiple Testers (n, row %) | Minimally-adjusted PR (95 % CI)b | Adjusted PR (95 % CI)c | p-valued | |
|---|---|---|---|---|---|---|---|
| Age | 15-19 | 159 (11.0) | 100 (62.9) | 59 (37.1) | 0.62 (0.48-0.76) | 0.63 (0.49-0.77) | <0.01 (0.03) |
| 20-29 | 489 (33.7) | 195 (39.9) | 294 (60.1) | 1.0 | 1.0 | ||
| 30-39 | 405 (27.9) | 151 (37.3) | 254 (62.7) | 1.06 (0.95-1.18) | 1.05 (0.94-1.16) | ||
| ≥40 | 398 (27.4) | 171 (43.0) | 227 (57.0) | 0.95 (0.84-1.06) | 0.95 (0.84-1.06) | ||
| Head of household | No | 424 (29.2) | 216 (50.9) | 208 (49.1) | 1.0 | 1.0 | 0.06 |
| Yes | 1027 (70.8) | 401 (39.0) | 626 (61.0) | 1.15 (1.00-1.30) | 1.13 (0.98-1.28) | ||
| Religion | Protestant | 575 (39.6) | 223 (38.8) | 352 (61.2) | 1.0 | 1.0 | 0.15 |
| Catholic | 386 (26.6) | 180 (46.6) | 206 (53.4) | 0.87 (0.77-0.98) | 0.89 (0.79-0.99) | ||
| SDA | 235 (16.2) | 100 (42.6) | 135 (57.4) | 0.90 (0.78-1.03) | 0.89 (0.77-1.01) | ||
| Other | 208 (14.3) | 90 (43.3) | 118 (56.7) | 0.92 (0.79-1.05) | 0.99 (0.86-1.11) | ||
| None | 47 (3.2) | 24 (51.1) | 23 (48.9) | 0.78 (0.54-1.02) | 0.86 (0.62-1.09) | ||
| Marital status | Single | 475 (32.7) | 235 (49.5) | 240 (50.5) | 1.0 | - | - |
| Married/cohabiting | 905 (62.4) | 353 (39.0) | 552 (61.0) | 1.15 (0.99-1.31) | |||
| Ever married | 71 (4.9) | 29 (40.8) | 42 (59.2) | 1.13 (0.87-1.40) | |||
| Education | No/Primary | 589 (40.6) | 270 (45.8) | 319 (54.2) | 1.0 | 1.0 | |
| Incomplete secondary | 515 (35.5) | 232 (45.0) | 283 (55.0) | 1.08 (0.96-1.21) | 1.11 (0.99-1.24) | <0.01 (<0.01) | |
| Secondary or higher | 347 (23.9) | 115 (33.1) | 232 (66.9) | 1.26 (1.11-1.41) | 1.29 (1.13-1.44) | ||
| Occupation | None | 555 (38.2) | 289 (52.1) | 266 (47.9) | 1.0 | 1.0 | <0.01 |
| Agriculture (others land) | 351 (24.2) | 155 (44.2) | 196 (55.8) | 1.11 (0.96-1.27) | 1.10 (0.95-1.26) | ||
| Agriculture (own land) | 320 (22.1) | 97 (30.3) | 223 (69.7) | 1.46 (1.28-1.65) | 1.45 (1.27-1.63) | ||
| Services/Professional | 225 (15.5) | 76 (33.8) | 149 (66.2) | 1.27 (1.09-1.46) | 1.19 (1.00-1.37) | ||
| SEP Group | Lowest | 263 (18.1) | 104 (39.5) | 159 (60.5) | 1.0 | - | - |
| Low | 264 (18.2) | 121 (45.8) | 143 (54.2) | 0.91 (0.77-1.04) | |||
| Middle | 336 (23.2) | 150 (44.6) | 186 (55.4) | 0.90 (0.77-1.04) | |||
| High | 311 (21.4) | 139 (44.7) | 172 (55.3) | 0.92 (0.78-1.06) | |||
| Highest | 277 (19.1) | 103 (37.2) | 174 (62.8) | 1.05 (0.88-1.21) | |||
| Present continuously previous 6mths | No | 60 (4.1) | 23 (38.3) | 37 (61.7) | 1.0 | - | - |
| Yes | 1391 (95.9) | 594 (42.7) | 797 (57.3) | 0.91 (0.72-1.09) | |||
| History of TB treatment | No | 1383 (95.3) | 580 (41.9) | 803 (58.1) | 1.0 | 1.0 | 0.62 |
| Yes | 68 (4.7) | 37 (54.4) | 31 (45.6) | 0.76 (0.55-0.97) | 1.06 (0.84-1.28) | ||
| HIV Status | Negative | 1297 (93.0) | 507 (39.1) | 790 (60.9) | 1.0 | 1.0 | <0.01 |
| Positive | 98 (7.0) | 84 (85.7) | 14 (14.3) | 0.20 (0.10-0.31) | 0.22 (0.11-0.33) | ||
| Spousal Characteristics (N = 517) | |||||||
| Currently pregnant | No | 443 (85.7) | 175 (39.5) | 268 (60.5) | 1.0 | -- | -- |
| Yes | 74 (14.3) | 30 (40.5) | 44 (59.5) | 0.97 (0.77-1.17) | |||
| Has Children | No | No | 20 (3.9) | 13 (65.0) | 1.0 | - | - |
| Yes | Yes | 497 (96.1) | 299 (60.2) | 0.93 (0.60-1.26) | |||
| Wife previously HIV-tested | No | 24 (4.6) | 14 (58.3) | 10 (41.7) | 1.0 | 1.0 | 0.32 |
| Yes | 493 (95.4) | 191 (38.7) | 303 (61.3) | 1.44 (0.75-2.12) | 1.19 (0.73-1.66) | ||
| Cluster-level Characteristics | |||||||
| >50 % of men employed | No | 578 (39.8) | 273 (47.2) | 305 (52.8) | 1.0 | 1.0 | 0.29 |
| Yes | 873 (60.2) | 344 (39.4) | 529 (60.6) | 1.15 (0.97-1.34) | 1.09 (0.92-1.25) | ||
| >25 % mention 3+ ways to prevent HIV infection | No | 711 (49.0) | 268 (37.7) | 443 (62.3) | 1.0 | 1.0 | 0.05 |
| Yes | 740 (51.0) | 349 (47.2) | 391 (52.8) | 0.84 (0.72-0.95) | 0.86 (0.74-0.98) | ||
| HIV Prevalence | <10 % | 1160 (79.9) | 483 (41.6) | 677 (58.4) | 1.0 | - | - |
| 10 %+ | 291 (20.1) | 134 (46.0) | 157 (54.0) | 0.96 (0.79-1.13) | |||
| ART Available at Health Facilitya | No | 676 (49.3) | 309 (45.7) | 367 (54.3) | 1.0 | - | - |
| Yes | 694 (50.7) | 276 (39.8) | 418 (60.2) | 1.09 (0.95-1.24) | |||
a81 missing data N = 1370; bAdjusted for age, urban/rural residence and district; cAdjusted for variables higher in the conceptual framework (Fig. 1); dFor adjusted model and based on LRT, p-value in brackets is assuming linear trend
eN=1395 as 56 men were missing data on self-reported HIV status
Acceptance of an offer of home-based HIV-testing
Almost half of never- and ever-testers accepted the offer of home-based HIV-testing (48 %; n = 449 & 49 %; n = 719, respectively). Acceptance among ever-testers was clustered by study site (median: 48.0 % IQR: 40.0-54.7 %; ICC = 0.06 95 % CI 0.03-0.11; p < 0.01). Acceptance was similar among multiple- (n = 422; 51 %) and one-time testers (n = 292; 47 %; adjPR = 1.05 95 % CI: 0.93-1.17; Table 3). Among men reporting themselves HIV-negative or who did not know their HIV-status, 3 % tested HIV-positive at this test.
Table 3.
Acceptance of an offer of home-based HIV-testing by socio-demographic characteristics and factors associated with acceptance among ever-testers (N = 1459)
| Details | Distribution (n, col %) | HBHTC (n, row %) | Minimally-Adjusted PR (95 % CI)d | Adjusted PR (95 % CI)e | p-valuef | |
|---|---|---|---|---|---|---|
| Ever tested | N = 1459 | 719 (49.0) | - | - | - | |
| Age | 15-19 | 159 (10.9) | 89 (56.0) | 0.98 (0.82-1.14) | 0.97 (0.81-1.14) | 0.01 (<0.01) |
| 20–29 | 492 (33.7) | 272 (55.6) | 1.0 | 1.0 | ||
| 30–39 | 408 (28.0) | 187 (46.2) | 0.83 (0.72-0.95) | 0.84 (0.73-0.95) | ||
| ≥40 | 400 (27.4) | 166 (41.7) | 0.75 (0.65-0.86) | 0.76 (0.65-0.87) | ||
| Household Head | No | 425 (29.1) | 239 (56.2) | 1.0 | 1.0 | 0.02 |
| Yes | 1034 (70.9) | 480 (46.4) | 0.88 (0.76-1.00) | 0.85 (0.74-0.97) | ||
| Education | None/primary | 591 (40.5) | 285 (48.4) | 1.0 | 1.0 | 0.32 |
| Some secondary | 517 (35.4) | 274 (53.2) | 1.05 (0.92-1.18) | 1.05 (0.92-1.19) | ||
| Complete secondary/higher | 351 (24.1) | 155 (44.7) | 0.89 (0.76-1.03) | 0.94 (0.79-1.10) | ||
| Occupation | None | 557 (38.2) | 298 (53.7) | 1.0 | - | - |
| Agriculture (others land) | 352 (24.1) | 170 (48.4) | 0.98 (0.84-1.12) | |||
| Agriculture (own land) | 322 (22.1) | 150 (46.9) | 0.95 (0.80-1.10) | |||
| Services/Professional | 228 (15.6) | 96 (42.7) | 0.85 (0.70-1.00) | |||
| Religion | Protestant | 577 (40.0) | 267 (46.4) | 1.0 | - | - |
| Catholic | 390 (26.7) | 204 (52.8) | 1.06 (0.92-1.20) | |||
| SDA | 235 (16.1) | 107 (45.5) | 0.96 (0.80-1.12) | |||
| Other | 210 (14.4) | 112 (53.8) | 1.08 (0.91-1.25) | |||
| None | 47 (3.2) | 24 (51.1) | 1.03 (0.71-1.34) | |||
| Marital status | Single | 477 (32.7) | 259 (54.5) | 1.0 | - | - |
| Married/cohabiting | 910 (62.4) | 419 (46.3) | 0.96 (0.81-1.10) | |||
| Ever married | 72 (4.9) | 36 (50.7) | 1.12 (0.85-1.40) | |||
| Household SEP Group | Lowest | 264 (18.1) | 143 (54.4) | 1.0 | 1.0 | 0.29 (0.13) |
| Low | 265 (18.2) | 137 (51.9) | 1.01 (0.84-1.18) | 1.00 (0.83-1.18) | ||
| Middle | 337 (23.1) | 172 (51.2) | 1.02 (0.85-1.18) | 1.01 (0.84-1.17) | ||
| High | 313 (21.5) | 150 (48.2) | 0.98 (0.81-1.15) | 0.97 (0.80-1.14) | ||
| Highest | 280 (19.2) | 112 (40.4) | 0.81 (0.64-0.99) | 0.82 (0.64-1.01) | ||
| Present continuously in previous 6mths | No | 60 (4.1) | 38 (63.3) | 1.0 | 1.0 | 0.04 |
| Yes | 1399 (95.9) | 676 (48.6) | 0.79 (0.62-0.96) | 0.79 (0.62-0.95) | ||
| History of TB treatment | No | 1391 (95.3) | 689 (49.8) | 1.0 | 1.0 | 0.71 |
| Yes | 68 (4.7) | 25 (36.8) | 0.80 (0.56-1.04) | 0.95 (0.68-1.21) | ||
| HIV statusa | Negative | 1305 (93.0) | 675 (51.7) | 1.0 | 1.0 | <0.01 |
| Positive | 98 (7.0) | 21 (21.4) | 0.47 (0.29-0.64) | 0.49 (0.30-0.68) | ||
| History of multiple HIV-testingb | No | 617 (42.5) | 292 (47.3) | 1.0 | 1.0 | 0.41 |
| Yes | 834 (57.5) | 422 (50.6) | 1.10 (0.98-1.22) | 1.05 (0.93-1.17) | ||
| Spouse Characteristics (N = 520) | ||||||
| Currently pregnant | No | 446 (85.8) | 231 (51.8) | 1.0 | 1.0 | 0.12 |
| Yes | 74 (14.2) | 30 (40.5) | 0.78 (0.56-1.00) | 0.82 (0.60-1.03) | ||
| Wife Reports having ≥1 Child | No | 21 (4.0) | 10 (47.6) | 1.0 | - | - |
| Yes | 499 (96.0) | 252 (50.4) | 1.10 (0.58-1.62) | |||
| Wife previously HIV-tested | No | 24 (4.6) | 17 (70.8) | 1.0 | 1.0 | 0.01 |
| Yes | 496 (95.4) | 245 (49.3) | 0.67 (0.50-0.84) | 0.66 (0.50-0.81) | ||
| Cluster-level Factors | ||||||
| >50 % of men employed | No | 581 (39.8) | 326 (56.4) | 1.0 | 1.0 | 0.30 |
| Yes | 878 (60.2) | 388 (44.4) | 0.88 (0.75-1.01) | 0.92 (0.78-1.06) | ||
| >25 % mention 3+ ways to prevent HIV infection | No | 715 (49.0) | 307 (43.2) | 1.0 | 1.0 | 0.13 |
| Yes | 744 (51.0) | 407 (55.0) | 1.16 (0.99-1.32) | 1.12 (0.95-1.30) | ||
| HIV Prevalence | <10 % | 1165 (79.8) | 576 (49.7) | 1.0 | - | - |
| >10 % | 294 (20.2) | 138 (47.4) | 0.98 (0.81-1.16) | |||
| ART Available at Health Facilityc | No | 680 (49.4) | 338 (50.0) | 1.0 | - | - |
| Yes | 697 (50.6) | 328 (47.3) | 0.97 (0.82-1.12) | |||
Number missing data: b8 men missing data on dates of first and last test N=1451 a 56 missing HIV status data; N = 1395; c82 missing data; dAdjusted for age, urban/rural residence and district; eAdjusted for variables higher in the conceptual framework (Fig. 1); fFor adjusted model & based on LRT, p-value in brackets is for assuming linear trend
Acceptance was lower among men aged ≥40 years relative to men aged 20–29 (42 % vs 56 %; adjPR = 0.76; 95 % CI: 0.65-0.87). There was little evidence that acceptance was associated with occupation, education, religion or marital status. Men present continuously in the 6 months preceding the survey were less likely to accept the offer relative to men with a period of being absent (adjPR = 0.79 95 % CI: 0.62-0.95) as were men whose spouse ever-tested (adjPR = 0.66 95 % CI: 0.50-0.81). Acceptance was lower among men listed as a household head (adjPR relative to men not a head = 0.85 95 % CI: 0.74-0.97) and among men of highest SEP (40 %) relative to men of lowest SEP (54 %; adjPR = 0.82 95 % CI: 0.64-1.01) with some evidence for a linear trend with SEP (p = 0.14). There was little evidence that cluster-level employment, HIV-prevalence or ART availability were associated with acceptance. There was weak evidence that acceptance was higher in clusters with higher HIV-prevention knowledge (55 % vs 43 %; adjPR = 1.12 95 % CI: 0.95-1.30).
Heckman-type selection modelling
Participation was somewhat higher among men visited on Saturday/Sunday (48 %) relative to Monday-Thursday (41 %) and among men visited in the afternoon (45 %) relative to the morning (41 %; adjPR = 1.08 95 % CI 1.01-1.14; (Appendix 2: Table 5)). There was little evidence for unobserved factors influencing survey participation and HIV-testing outcomes (ever-testing: rho = −0.12 95 % CI:-0.93 to 0.88; p = 0.88; multiple-testing: rho = 0.20 95 % CI:-0.87 to 0.94; p = 0.80) however, confidence intervals were very wide. Results were similar when day of the week was included in the selection models Estimates of association between independent variables and multiple-testing were similar when adjusting for variables included in the Heckman-type selection model.
Table 5.
Distribution of eligible men by selection variables and association between participation and selection variables (N = 5797)
| Selection Variables | Description | Distribution | Participants | Crude PR | Adjusted PRa | p-valueb |
|---|---|---|---|---|---|---|
| Time of Survey | Morning (630–1159) | 2778 (47.9) | 1140 (41.0) | 1.0 | 1.0 | 0.02 |
| Afternoon (12–1559) | 2705 (46.7) | 1204 (44.5) | 1.09 (1.02-1.16) | 1.08 (1.01-1.14) | ||
| Late pm (16–1830) | 314 (5.4) | 119 (37.9) | 0.92 (0.78-1.06) | 0.94 (0.80-1.07) | ||
| Day of Survey | Mon-Thurs | 3825 (66.0) | 1581 (41.3) | 1.0 | 1.0 | <0.01 |
| Friday | 931 (16.1) | 378 (40.6) | 0.97 (0.89-1.06) | 0.97 (0.88-1.05) | ||
| Sat-Sun | 1041 (18.0) | 504 (48.4) | 1.15 (1.06-1.24) | 1.14 (1.05-1.23) | ||
| Season of Survey | Rainy (Dec-Apr) | 1566 (27.0) | 694 (44.3) | 1.0 | 1.0 | 0.20 |
| Cool, dry (May-Aug) | 2646 (45.6) | 1123 (42.5) | 0.92 (0.78-1.05) | 0.93 (0.81-1.05) | ||
| Cool, hot (Sept-Nov) | 1586 (27.4) | 646 (40.7) | 0.89 (0.72-1.05) | 0.87 (0.73-1.01) |
aAdjusted for household head, whether present all months in previous 6, household SEP and size, urban/rural residence and district; bFor adjusted PR and based on LRT
Discussion
In this large, population-based survey of predominantly rural men, 61 % (95 % CI: 58-64 %) of men were defined as ever-testers. Over half the men with a history of HIV-testing reported more than one lifetime HIV-test. Factors associated with multiple-testing were similar to factors associated with ever-testing [3, 8, 10, 12]. The offer of home-based HIV-testing increased the lifetime frequency of HIV-testing among half of one-time and never-testers.
Limitations of this study are that, as data were cross-sectional, temporal relationships cannot be inferred. Data were self-reported and collected retrospectively. Men may have over-reported HIV-testing and there are likely to be errors in recalling dates of HIV-tests. As a secondary analysis of data collected for an unrelated CRT, the study had limited capacity to explore whether men were HIV-testing annually as data were collected on years since first and most recent test, and number of HIV-tests. Nonetheless, most multiple-testers first tested in 2009 or later, suggesting that recent expansions of HTC services, including PITC, have increased men’s frequency of HIV-testing. In the absence of data on sexual behaviours we had limited ability to explore whether multiple-testers were at increased risk of HIV-infection. However, we found that few men living with HIV reported HIV-testing prior to diagnosis. Although this measure is subject to limitations as multiple-testing was inferred from date of first HIV-test and of HIV-diagnosis, with almost 60 % of HIV-positive men reporting one lifetime test, findings suggest that a high proportion of men continue to be diagnosed on their first HIV-test. Further exploration of multiple-testing behaviours alongside data on sexual behaviours is needed. Some 60 % of married men were linked to their spouse; associations with spousal characteristics may be biased if characteristics of spouses linked differed from those not linked. Generalisability may be limited as the health system strengthening intervention, implemented in all sites at the time of data collection, may have contributed to increased frequency of HIV-testing.
Finally, outcomes were at risk of bias due to non-participation. Studies have shown that Heckman-type selection models can be used to correct HIV-prevalence estimates where refusal to HIV-test is high [18, 20]. We used Heckman-models as we theorised that non-participation, largely due to absence, may be related to HIV-testing behaviours. The models suggested that there was no evidence for unobserved factors associated with participation and HIV-testing outcomes. However, we had limited ability to model selection due to limited individual-level data on non-participants. The selection variables were weak predictors of participation and may not be valid exclusion restrictions [21], as survey timing within clusters was not randomly determined. Aspects of survey conduct may independently affect outcomes [18]; hence our estimates of correlation (rho) between outcome and participation had little precision.
Despite limitations, this study includes a large population of rural Zambian men whose multiple-testing behaviours have been understudied to date. The study provides important insights into the contribution of expanded HIV-testing service delivery to increasing men’s lifetime frequency of HIV-testing.
To our knowledge, there are relatively few population-based surveys exploring the factors associated with multiple-testing. In a 2007 population-based survey conducted in communities in Soweto, South Africa, 50 % of male ever-testers reported more than one lifetime test [4]. Multiple-testing was higher among individuals who had heard of ART [4]. In our study, multiple-testing was higher in clusters where ART was available at the health facility suggesting that expanded ART availability contributes not only to ever-testing [22], but to increased frequency of HIV-testing. In South and Central Province, Zambia (2010/11), 36 % of men ever-tested among whom 50 % reported >1 lifetime HIV-test [23]. In a 2012 nationally representative survey, 63 % of Kenyan males aged 15–64 years ever-tested with a median of 3 tests (IQR: 2–4) per person [10].
By the time of this study, HIV-testing services had been expanded across Zambia, couples HTC was recommended in ANC [24] and there was increased service promotion. Men whose spouse ever-tested were more likely to report multiple-tests. Similar to other settings, these findings suggest that HTC in ANC has provided men with access to HTC and may provide frequent access to HTC [12, 25]. Yet, few men attend ANC [25]. Considering the risk of HIV transmission among sero-discordant cohabiting/married couples, there remains a need to strengthen the delivery of HTC services to men through ANC [26–28].
Similar to a survey in South Africa, multiple-testers in this study were more likely to have complete secondary/higher education [4]. Employed men in this study were more likely to report multiple HIV-tests unlike in South Africa [4]. Formal employment may provide access to HTC services through the workplace thereby removing opportunity and financial costs of accessing facility-based HTC [29]. Alternatively, employed men may be encouraged by their employer or motivated by their role as providers to access health services [30]. Men of lower socioeconomic markers may face unique barriers to accessing HTC services that influence their frequency of HIV-testing. Lower health literacy likely contributes to lower levels of multiple-testing among men with less education. Other contributing factors, such as ability to access available services, stigma associated with HIV-testing within social networks or as experienced from healthcare workers, may also influence men’s frequency of HIV-testing [31]. Understanding why socioeconomic factors continue to influence men’s HIV-testing behaviors in the context of expanded service availability, the need for regular HIV-testing by socioeconomic factors and how to encourage men with lower levels of education or no formal employment to regularly test for HIV needs exploration.
Evidence suggests that men continue to access care at later stages of HIV-infection [32]. Regular-testing facilitates earlier diagnosis and opportunities to provide risk reduction counselling to HIV-negative individuals at higher risk of infection. In a facility-based cohort in South Africa, repeat-testers were less likely to be HIV-infected relative to first-time testers [33]. . In Uganda, South Africa and Zimbabwe, studies found that individuals at lower risk of HIV are more likely to ever- or repeat-test [4, 12, 34]. Conversely, in serological surveys in Tanzania, high-risk individuals were more likely to repeatedly accept VCT [35]. In this study, 40 % of ever-testers reported one-lifetime HIV-test and few men living with HIV tested before their diagnoses. With investment in delivering community-based HTC [36], there is a need to monitor whether those in greatest need of annual HIV-testing are accessing services and the effects of frequent HIV-testing on sexual behaviors [37]. Traditional “know your status” messaging may require reframing to emphasize the importance of annual HIV-testing if at ongoing risk of HIV-infection.
Home-based HIV-testing increased the lifetime frequency of HIV-testing among men in this study. As in other studies, there was little evidence that acceptance differed by markers of SEP [38, 39]. The relatively high refusal in our study relative to others [3, 40] likely reflects service delivery in the context of research, where the priority was data collection, rather than the acceptability of a home-based HTC programme [3]. In this study, multiple-testing was lower, but acceptance of home-based HIV-testing higher, in communities with higher HIV-prevention knowledge. These findings contribute to suggestions that poor accessibility influences men’s uptake of HTC services [38, 41]. Home-based HTC remains an important strategy to increase the frequency of HIV-testing among rural Zambian men with less access to services [3, 39]. However, with most men not home during household visits, a cost-effective strategy for offering regular home-based HIV-testing in rural settings requires exploration [40].
Conclusion
Effective strategies to reach men with HTC services are available [28], and levels of ever-testing increased among this population of men [3]. However, only 35 % of all men reported multiple HIV-tests and few men living with HIV reported HIV-testing before being diagnosed. More effective implementation and delivery of available HTC services is required to reach men in need of frequent HTC [42]. Novel alternatives to encourage never-testers to access existing HTC services should be explored [28]. These strategies could include self-testing and incentivised testing, shown to be acceptable and feasible among men [43–45]. Additional research to investigate models for delivery, yield of these strategies and whether they are effective at increasing HIV-testing among HIV-negative men at high risk of infection is required [46, 47].
Acknowledgements
BH was supported by an ESRC award ES/J500021/1
Abbreviations
- HIV
Human Immunodeficiency Virus
- HTC
HIV testing and counselling
- ART
Anti-retroviral therapy
- CRT
Cluster randomised trial
- BHOMA
Better Health Outcomes through Mentoring and Assessment
- PDAs
Personal digital assistant
- DHS
Demographic and Health Surveys
- SEP
Socioeconomic position
- TB
Tuberculosis
- PCA
Principal components analysis
- PR
Prevalence ratio
- adjPR
Adjusted prevalence ratio
- LRT
Likelihood ratio test
- ICC
Intra-cluster correlation
- IQR
Interquartile range
Appendix
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BH conceived this study, conducted the analyses, interpreted the data and drafted the first manuscript. JH conceived the study, advised on data analyses and made original text contributions. JL and HW participated in data interpretation and made original text contributions . JL also provided guidance on data analyses. AS, MT and WM participated in data acquisition and preparation for analyses. MvH participated in the Heckman analyses and the interpretation of these findings. JS and HA conceived and designed the CRT and made original text contributions All authors provided critical revisions to the manuscript and approved the final draft.
Authors’ information
Not applicable.
Availability of data and materials
Not applicable.
Contributor Information
B. Hensen, Phone: +44 207 299 4736, Email: Bernadette.hensen@lshtm.ac.uk
JJ Lewis, Email: james.lewis@lshtm.ac.uk.
A. Schaap, Email: ab@zambart.org.zm
M. Tembo, Email: Margaret@zambart.org.zm
M. Vera-Hernández, Email: m.vera@ucl.ac.uk
W. Mutale, Email: wmutale@yahoo.com
HA Weiss, Email: Helen.Weiss@lshtm.ac.uk.
J. Hargreaves, Email: James.hargreaves@lshtm.ac.uk
JSA Stringer, Email: jeffrey_stringer@med.unc.edu.
H. Ayles, Email: Helen@zambart.org.zm
References
- 1.Cremin I, Nyamukapa C, Sherr L, Hallett TB, Chawira G, Cauchemez S, et al. Patterns of self-reported behaviour change associated with receiving voluntary counselling and testing in a longitudinal study from Manicaland, Zimbabwe. AIDS & Behavior. 2010;14(3):708–15. doi: 10.1007/s10461-009-9592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallett TB, Dube S, Cremin I, Lopman B, Mahomva A, Ncube G, et al. The role of testing and counselling for HIV prevention and care in the era of scaling-up antiretroviral therapy. Epidemics. 2009;1(2):77–82. doi: 10.1016/j.epidem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Hensen B, Lewis JJ, Schaap A, Tembo M, Mutale W, Weiss HA, et al. Factors Associated with HIV-Testing and Acceptance of an Offer of Home-Based Testing by Men in Rural Zambia. AIDS Behav. 2015;19(3):492–504. doi: 10.1007/s10461-014-0866-0. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh KK, Madiba P, De Bruyn G, Lurie MN, Coates TJ, Gray GE. Who gets tested for HIV in a South African urban township? Implications for test and treat and gender-based prevention interventions. J Acquir Immune Defic Syndr. 2011;56(2):151–65. doi: 10.1097/QAI.0b013e318202c82c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): Rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment – a study protocol for a cluster randomised trial. Trials. 2014;15:57. doi: 10.1186/1745-6215-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawley C, Wringe A, Isingo R, Mtenga B, Clark B, Marston M, et al. Low Rates of Repeat HIV Testing Despite Increased Availability of Antiretroviral Therapy in Rural Tanzania: Findings from 2003–2010. PLoS One. 2013;8(4):e62212. doi: 10.1371/journal.pone.0062212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huchko MJ, Montandon M, Nguti R, Bukusi EA, Cohen CR. The Association of HIV Counseling and Testing with HIV Risk Behaviors in a Random Population-based Survey in Kisumu, Kenya. AIDS Behav. 2011;15:718–24. doi: 10.1007/s10461-009-9649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peltzer K, Matseke G, Mzolo T, Majaja M. Determinants of knowledge of HIV status in South Africa: results from a population-based HIV survey. BMC Public Health. 2009;9:174. doi: 10.1186/1471-2458-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranzer K, van Schaik N, Karmue U, Middelkoop K, Sebastian E, Lawn SD, et al. High prevalence of self-reported undiagnosed HIV despite high coverage of HIV testing: A cross-sectional population based sero-survey in South Africa. PLoS One. 2011;6(9):e25244. doi: 10.1371/journal.pone.0025244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng’ang’a A, Waruiru W, Ngare C, Ssempijja V, Gachuki T, Njoroge I, et al. The Status of HIV Testing and Counseling in Kenya: Results From a Nationally Representative Population-Based Survey. J Acquir Immune Defic Syndr. 2014;66(Suppl 1):S27–36. doi: 10.1097/QAI.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bwambale FM, Ssali SN, Byaruhanga S, Kalyango JN, Karamagi CAS. Voluntary HIV counselling and testing among men in rural western Uganda: implications for HIV prevention. BMC Public Health. 2008;8:263. doi: 10.1186/1471-2458-8-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage AJ, Ali D. Factors associated with self-reported HIV testing among men in Uganda. AIDS Care. 2005;17(2):153–65. doi: 10.1080/09540120512331325635. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson R, Elfstrom KM, Winter A. Community Influences on Married Men’s Uptake of HIV Testing in Eight African Countries. AIDS Behav. 2012;17(7):2352–66. doi: 10.1007/s10461-012-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stringer JSA, Chisembele-Taylor A, Chibwesha CJ, Chi HF, Ayles H, Manda H, et al. Protocol-driven primary care and community linkages to improve population health in rural Zambia: the Better Health Outcomes through Mentoring and Assessment (BHOMA) project. BMC Health Serv Res. 2013;13(Suppl 2):S7. doi: 10.1186/1472-6963-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutale W, Godfrey-Faussett P, Tembo Mwanamwenge M, Kasese N, Chintu N, Balabanova D, et al. Measuring Health System Strengthening: Application of the Balanced Scorecard Approach to Rank the Baseline Performance of Three Rural Districts in Zambia. PLoS One. 2013;8(3):e58650. doi: 10.1371/journal.pone.0058650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutale W, Stringer JS, Chintu N, Chilengi R, Tembo Mwanamwenge M, Kasese N, et al. Application of Balanced Scorecard in the Evaluation of a Complex Health System Intervention: 12 Months Post Intervention Findings from the BHOMA Intervention: A Cluster Randomised Trial in Zambia. PLoS One. 2014;9(4):e93977. doi: 10.1371/journal.pone.0093977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckman JJ. Sample selection bias as a specification error. Economtrica. 1979;47:153–61. doi: 10.2307/1912352. [DOI] [Google Scholar]
- 18.Barnighausen T, Bor J, Wandira-Kazibwe S, Canning D. Correcting HIV Prevalence Estimates for Survey Nonparticipation Using Heckman-type Selection Models. Epidemiology. 2011;22(1):27–35. doi: 10.1097/EDE.0b013e3181ffa201. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimons E, Malde B, Mesnard A, Vera-Hernandez M. Nutrition, information, and household behaviour: experimental evidence from Malawi. IFS Working Paper. 2014;14:2. [Google Scholar]
- 20.Hogan DR, Salomon JA, Canning D, Hammitt JK, Zaslavsky AM, Barnighausen T. National HIV prevalence estimates for sub-Saharan Africa: controlling selection bias with Heckman-type selection models. Sex Transm Infect. 2012;88:i17–23. doi: 10.1136/sextrans-2012-050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnighausen T, Bor J, Wandira-Kazibwe S, Canning D. Interviewer identity as exclusion restriction in epidemiology. Epidemiology. 2011;22(3):446. doi: 10.1097/EDE.0b013e3182117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warwick Z. The influence of antiretroviral therapy on the uptake of HIV testing in Tutume. Botswana Int J STD AIDS. 2006;17(7):479–81. doi: 10.1258/095646206777689189. [DOI] [PubMed] [Google Scholar]
- 23.Gari S, Malungo JRS, Martin-Hilber A, Musheke M, Schindler C, Merten S. HIV Testing and Tolerance to Gender Based Violence: A Cross-Sectional Study in Zambia. PLoS One. 2013;8(8):e71922. doi: 10.1371/journal.pone.0071922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Zambian Ministry of Health and National AIDS Council. National Protocol Guidelines. Integrated Prevention of Mother-to-Child Transmission of HIV/AIDS. Lusaka, Zambia, 2007.
- 25.Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20(6):700–9. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 26.Curran K, Baeten JM, Coates TJ, Kurth A, Mugo NR, Celum C. HIV-1 Prevention for HIV-1 Serodiscordant Couples. Curr HIV/AIDS Rep. 2012;9(2):160–70. doi: 10.1007/s11904-012-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 28.Hensen B, Taoka S, Lewis JJ, Weiss H, Hargreaves J. Systematic review of strategies to increase men's HIV-testing in sub-Saharan Africa. AIDS. 2014;28(14):2133–45. doi: 10.1097/QAD.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett EL, Dauya E, Matambo R, Cheung YB, Makamure B, Bassett MT, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Medicine / Public Library of Science. 2006;3(7):e238. doi: 10.1371/journal.pmed.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men's use of HIV services in Zimbabwe. Glob Health. 2011;7:13. doi: 10.1186/1744-8603-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aarnio P, Aarnio P, Olsson P, Chimbiri A, Kulmala T. Male involvement in antenatal HIV counseling and testing: exploring men’s perceptions in rural Malawi. AIDS Care. 2009;21(12):1537–46. doi: 10.1080/09540120902903719. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman S, Wu Y, Lahuerta M, Kulkarni SG, Nuwagaba-Biribonwoha H, El Sadr W, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS. 2014;28:2429–38. doi: 10.1097/QAD.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regan S, Losina E, Chetty S, Giddy J, Walensky RP, Ross D, et al. Factors Associated with Self-Reported Repeat HIV Testing after a Negative Result in Durban, South Africa. PLoS ONE. 2013;8(4):e62362. doi: 10.1371/journal.pone.0062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherr L, Lopman B, Kakowa M, Dube S, Chawira G, Nyamukapa C, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21(7):851–60. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]
- 35.Isingo R, Wringe A, Todd J, Urassa M, Mbata D, Maiseli G, et al. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Tropical Med Int Health. 2012;17(8):E15–25. doi: 10.1111/j.1365-3156.2011.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Service Delivery Approaches to HIV Testing and Counselling (HTC): a Strategic HTC Policy Framework. Geneva, 2012.
- 37.Fonner VA, Denison J, Kennedy CE, O’Reilly K, Sweat M. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;9:CD001224-CD. doi: 10.1002/14651858.CD001224.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51(2):185–93. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutale W, Michelo C, Jurgensen M, Fylkesnes K. Home-based voluntary HIV counselling and testing found highly acceptable and to reduce inequalities. BMC Public Health. 2010;10:347. doi: 10.1186/1471-2458-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabapathy K, van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of Home-Based Voluntary HIV Testing in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med. 2012;9:12. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obermeyer CM, Osborn M. The Utilization of Testing and Counseling for HIV: A Review of the Social and Behavioral Evidence. Am J Public Health. 2007;97(10):1762–74. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dovel K, Yeatman S, Watkins S, Poulin M. Men’s heightened risk of AIDS-related death: the legacy of gendered HIV testing and treatment strategies. AIDS. 2015;29(10):1123–5. doi: 10.1097/QAD.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nglazi MD, van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker L-G. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. JAIDS. 2012;59(3):e28–34. doi: 10.1097/QAI.0b013e31824445f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8:10. doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee R, Cui RR, Muessig KE, Thirumurthy H, Tucker JD. Incentivizing HIV/STI Testing: A Systematic Review of the Literature. AIDS Behav. 2014;18:905–12. doi: 10.1007/s10461-013-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson C, Baggaley R, Forsythe S, Van Rooyen H, Ford N, Napierala Mavedzenge S, et al. Realizing the Potential for HIV Self-Testing. AIDS Behav. 2014;18:S1. doi: 10.1007/s10461-014-0832-x. [DOI] [PubMed] [Google Scholar]
- 47.Kranzer K, Govindasamy D, van Schaik N, Thebus E, Davies N, Zimmermann MA, et al. Incentivized recruitment of a population sample to a mobile HIV testing service increases the yield of newly diagnosed cases, including those in need of antiretroviral therapy. HIV Medicine. 2012;13(2):132–7. doi: 10.1111/j.1468-1293.2011.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


